A Sustainable Approach to Dyed Cotton Fabric Stripping Using Ozone
Abstract
1. Introduction
2. Experimental
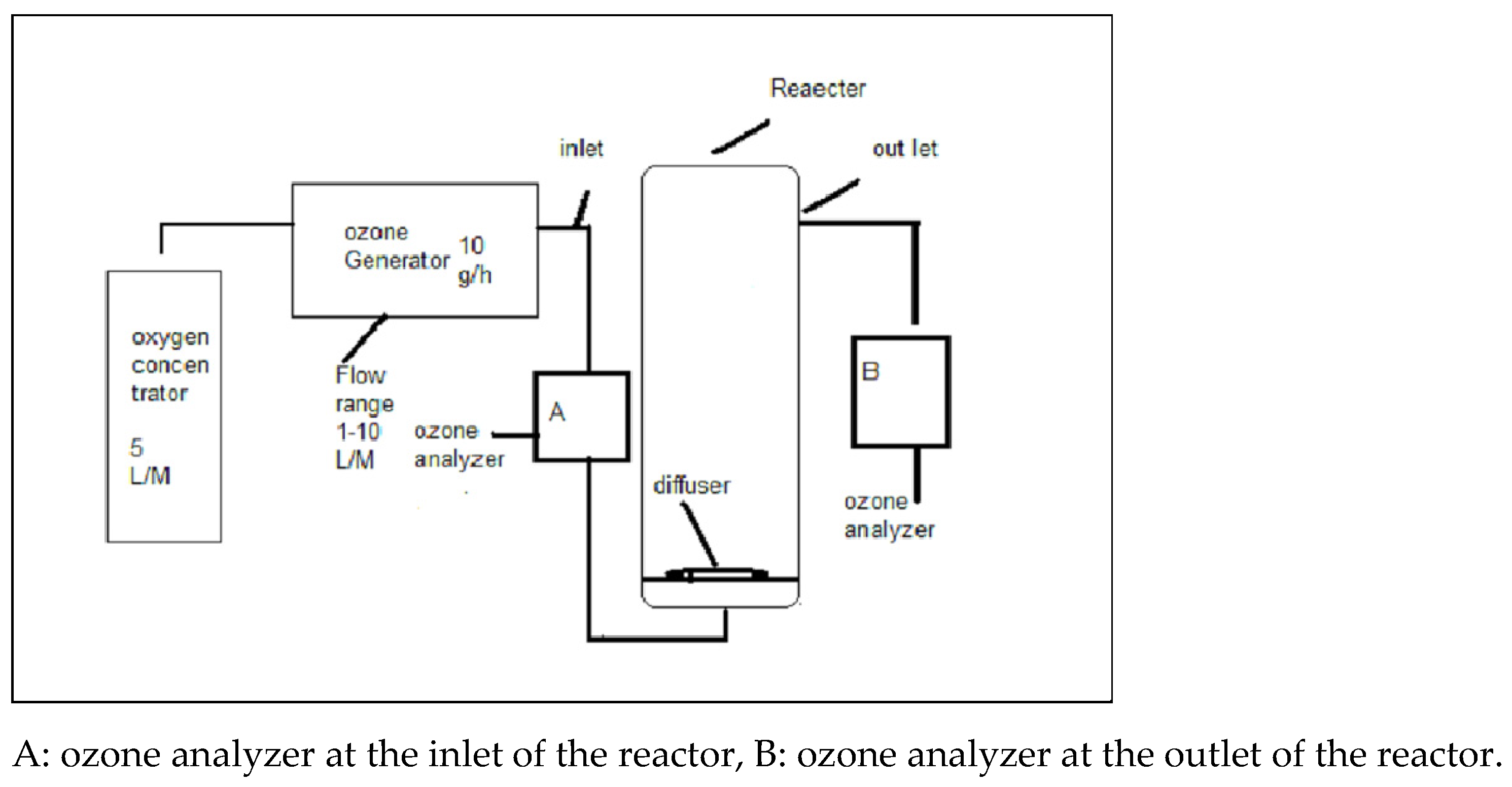
2.1. Apparatus Setup
2.2. Dyeing Procedure
2.3. Procedure for Ozone Treatment
2.4. Fabric Evaluation
2.5. Characteristics of Effluents
2.6. Statistical Analysis
3. Results and Discussion
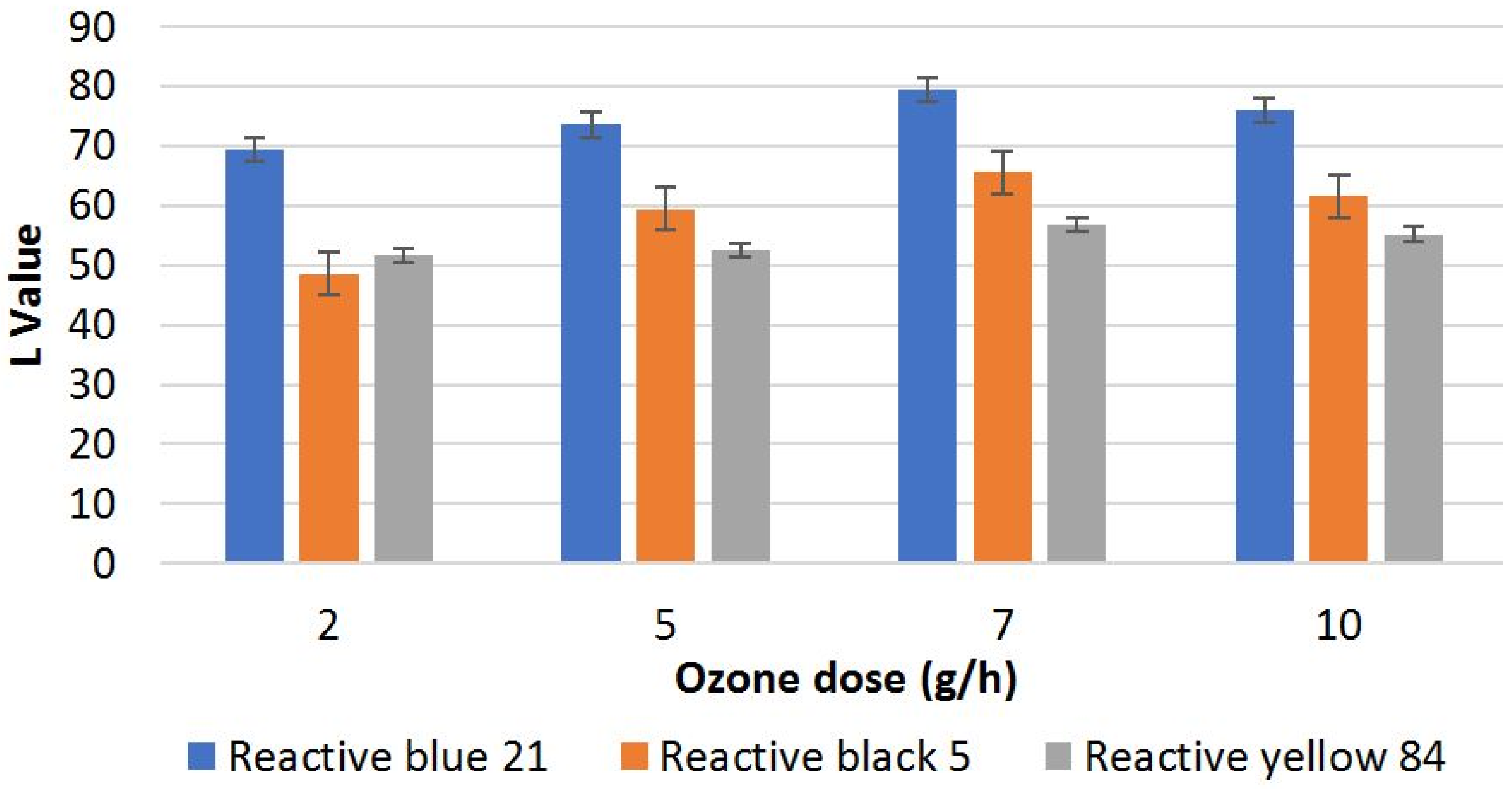
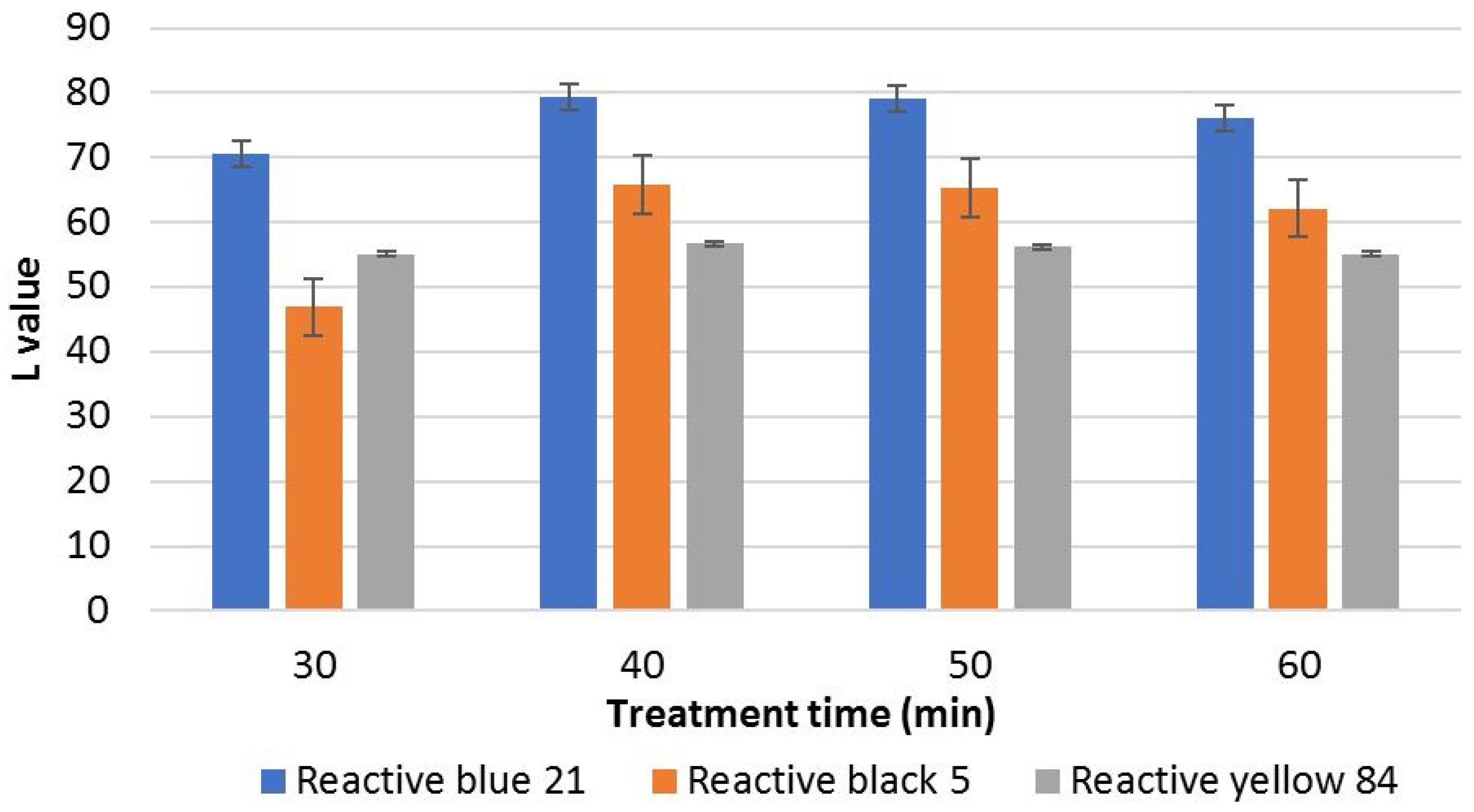
3.1. Effect of Ozonation Process on Lightness of Ozone-Stripped Fabric
3.2. Effect of Ozonation Process on Bursting Strength of Ozone-Stripped Fabric
3.3. Effect of Multiple Reuses of Ozone Stripping Bath on the Decolorization and Effluent Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tzanov, T.; Costa, S.; Guebitz, G.M.; Cavaco-Paulo, A. Dyeing in catalase-treated bleaching baths. Color. Technol. 2001, 117, 1–5. [Google Scholar] [CrossRef]
- Oğulata, R.T.; Balci, O. Investigation of the stripping process of the reactive dyes using organic sulphur reducing agents in alkali condition. Fibers Polym. 2007, 8, 25–36. [Google Scholar] [CrossRef]
- Wijannarong, S.; Aroonsrimorakot, S.; Thavipoke, P.; Kumsopa, C.; Sangjan, S. Removal of Reactive Dyes from Textile Dyeing Industrial Effluent by Ozonation Process. APCBEE Procedia 2013, 5, 279–282. [Google Scholar] [CrossRef]
- Eren, H.A.; Ozturk, D. The evaluation of ozonation as an environmentally friendly alternative for cotton preparation. Text. Res. J. 2010, 81, 512–519. [Google Scholar] [CrossRef]
- Islam, S.; Shaikh, I.A.; Firdous, N.; Ali, A.; Sadef, Y. A new approach for the removal of unfixed dyes from reactive dyed cotton by Fenton oxidation. J. Water Reuse Desalination 2019, 9, 133–141. [Google Scholar] [CrossRef]
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 608–826. [Google Scholar] [CrossRef] [PubMed]
- Adeel, S.; Salman, M.; Usama, M.; Rehman, F.-U.; Ahmad, T.; Amin, N. Sustainable Isolation and Application of Rose Petals Based Anthocyanin Natural Dye for Coloration of Bio-Mordanted Wool Fabric. J. Nat. Fibers 2021, 19, 6089–6103. [Google Scholar] [CrossRef]
- Agrawal, P.B.; Nierstrasz, V.A.; Klug-Santner, B.G.; Gübitz, G.M.; Lenting, H.B.M.; Warmoeskerken, M.M.C.G. Wax removal for accelerated cotton scouring with alkaline pectinase. Biotechnol. J. 2007, 2, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Arooj, F.; Ahmad, N.; Chaudhry, M.N. A Pilot-Scale Application of Ozone to Bleach Raw Cotton Fabric Using Various Additives. Ozone Sci. Eng. 2014, 37, 203–215. [Google Scholar] [CrossRef]
- Baig, U.; Khatri, A.; Ali, S.; Sanbhal, N.; Ishaque, F.; Junejo, N. Ultrasound-assisted dyeing of cotton fabric with natural dye extracted from Marigold flower. J. Text. Inst. 2020, 112, 801–808. [Google Scholar] [CrossRef]
- Hareem, T.; Arooj, F.; Kashif, S.U.R.; Farooq, Z. Economic Viability of Pilot-Scale Application of Ozone in Cotton Bleaching with Multiple Reuse of Water. J. Int. Ozone Assoc. 2018, 41, 197–203. [Google Scholar] [CrossRef]
- Chatha, S.; Mallhi, A.; Hussain, A.; Asgher, M.; Nigam, P. A Biological Approach for Color-Stripping of Cotton Fabric Dyed with C.I. Reactive Black 5 Using Fungal Enzymes from Solid State Fermentation. Curr. Biotechnol. 2014, 3, 166–173. [Google Scholar] [CrossRef]
- Iglesias, S.C. Degradation and Biodegradability Enhancement of Nitrobenzene and 2.4-dichlorophenol by Means of Advanced Oxidation Processes Based on Ozone. Ph.D. Thesis, Universidad de Barcelona, Barcelona, Spain, 2002; pp. 37–39. [Google Scholar]
- Bin, A.K.; Roustan, M. Basic Chemical Engineering Concepts for the Design of Ozone Gas-Liquid Reactors. In Proceedings of the 17th World Congress & Exhibition: Ozone and Related Oxidants, Innovative & Current Technologies, Strasbourg, France, 22–25 August 2005; pp. 99–131. [Google Scholar]
- Perincek, S.; Bahtiyari, M.; Körlü, A.; Duran, K. Ozone treatment of Angora rabbit fiber. J. Clean. Prod. 2008, 16, 1900–1906. [Google Scholar] [CrossRef]
- Roncero, M.; Queral, M.; Colom, J.F.; Vidal, T. Why Acid pH Increases the Selectivity of the Ozone Bleaching Processes. Ozone Sci. Eng. 2003, 25, 523–534. [Google Scholar] [CrossRef]
- Bilińska, L.; Blus, K.; Gmurek, M.; Ledakowicz, S. Brine Recycling from Industrial Textile Wastewater Treated by Ozone. By-Products Accumulation. Part 1: Multi Recycling Loop. Water 2019, 11, 460. [Google Scholar] [CrossRef]
- Khan, H.; Ahmad, N.; Yasar, A.; Shahid, R. Advanced oxidative decolourization of. Red cl-5B: Effect of dye concentration, process optimization and reaction kinetics. Pol. J. Environ. Stud. 2010, 19, 83–92. [Google Scholar]
- Rizvi, H.; Ahmad, N.; Yasar, A.; Bokhari, K.; Khan, H. Disinfection of UASB treated municipal wastewater by H2O2, UV, ozone, PAA, H2O2/sunlight and.advanced oxidation processes: Regrowth potential of pathogens. Pol. J. Environ. Stud. 2013, 22, 1153–1161. [Google Scholar]
- Prabaharan, M.; Rao, J.V. Study on Ozone Bleaching of Cotton Fabric–Process Optimisation, Dyeing and Finishing Properties. Color. Technol. 2001, 117, 98–103. [Google Scholar] [CrossRef]
- Perincek, S.; Bahtiyari, M.; Korlu, E.; Duran, K. Ozone bleaching of jute fabrics. AATCC Rev. 2007, 7, 34–39. [Google Scholar]
- Shatalov, A.; Pereira, H. Polysaccharide degradation during ozone-based TCF bleaching of non-wood organosolv pulps. Carbohyd. Polym. 2007, 67, 275–281. [Google Scholar] [CrossRef]
- Cogo, E.; Albet, J.; Malmary, G.; Coste, C.; Molinier, J. Effect of reaction medium on ozone mass transfer and applications to pulp bleaching. Chem. Eng. J. 1999, 73, 23–28. [Google Scholar] [CrossRef]
- Shaikh, I.A.; Ahmad, N. A pilot scale study to remove hydrolyzed reactive dyes using direct injection of ozone in jet dyeing machine. AATCC Rev. 2013, 13, 41–46. [Google Scholar]
- Nabeela, F.; Shaikh, I.A.; Islam, S.; Arooj, F. Multiple Reuse of Electrocoagulation Treated Reactive Dyeing Wash-Off: Colorimetric Properties and Water Saving. J. Environ. Anal. Chem. 2021, 8, 1–6. [Google Scholar]
- Eren, H.A. Simultaneous after clearing and decolorization by ozonation after disperse dyeing of polyester. Color. Technol. 2007, 123, 224–229. [Google Scholar] [CrossRef]
- Shaikh, I.A.; Ahmed, F.; Sahito, A.R.; Pathan, A.A. In-situ Decolorization of Residual Dye Effluent in Textile Jet Dyeing Machine by Ozone. Pak. J. Anal. Environ. Chem. 2014, 15, 1–6. [Google Scholar]
- Senthilkumar, M.; Muthukumar, M. Studies on the Possibility of Recycling Reactive Dye Bath Effluent after Decoloration Using Ozone. Dye. Pigment. 2007, 72, 251–255. [Google Scholar] [CrossRef]
- Kıcık, H.; Eren, H.A. Application of ozone gas for the stripping of fabric ink-jet-printed with reactive dyes. Color. Technol. 2017, 133, 485–490. [Google Scholar] [CrossRef]
- Arooj, F.; Ahmed, N.; Shaikh, I.A. Application of Ozone in Stripping of Cotton Fabric Dyed with Reactive Dyes. Ozone Sci. Eng. 2019, 42, 319–330. [Google Scholar] [CrossRef]
- Atav, R.; Yüksel, M.F.; Özkaya, S.; Buğdaycı, B. The Use of Ozone Technology for Color Stripping and Obtaining Vintage Effect on Reactive Dyed Cotton Fabrics. J. Nat. Fibers 2021, 19, 6648–6658. [Google Scholar] [CrossRef]
- Yigit, I.; Eren, S.; Eren, H.A. Ozone utilization for discharge printing of reactive dyed cotton. Color. Technol. 2018, 134, 13–23. [Google Scholar] [CrossRef]
- Powar, A.S.; Perwuelz, A.; Behary, N.; Hoang, L.; Aussenac, T. Application of Ozone Treatment for the Decolorization of the Reactive-Dyed Fabrics in a Pilot-Scale Process—Optimization through Response Surface Methodology. Sustainability 2020, 12, 471. [Google Scholar] [CrossRef]
- Arooj, F. Application of Advanced Oxidation Process for Bleaching Textile Materials; College of Earth and Environmental Sciences, University of the Punjab: Lahore, Pakistan, 2014. [Google Scholar]
- AATCC (American Association of Textile Chemists and colorists). Technical Manual of Standard Test Methods for Textiles; AATCC: Durham, NC, USA, 2007; Volume 82. [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA American Water Works Association and Water Pollution Control Federation: Washington, DC, USA, 1998. [Google Scholar]
- Li, J.; Zou, J.; Zhang, S.; Cai, H.; Huang, Y.; Lin, J.; Li, Q.; Yuan, B.; Ma, J. Sodium tetraborate simultaneously enhances the degradation of acetaminophen and reduces the formation potential of chlorinated by-products with heat-activated peroxymonosulfate oxidation. Water Res. 2022, 224, 119095. [Google Scholar] [CrossRef] [PubMed]
- Konsowa, A. Decolorization of wastewater containing direct dye by ozonation in a batch bubble column reactor. Desalination 2003, 158, 233–240. [Google Scholar] [CrossRef]
- Cai, H.; Zou, J.; Lin, J.; Li, J.; Huang, Y.; Zhang, S.; Yuan, B.; Ma, J. Sodium hydroxide-enhanced acetaminophen elimination in heat/peroxymonosulfate system: Production of singlet oxygen and hydroxyl radical. Chem. Eng. J. 2021, 429, 132438. [Google Scholar] [CrossRef]
- Eren, S.; Gumus, B.; Eren, H.A. Colour Stripping of Reactive Dyed Cotton by Ozone Treatment. Color. Technol. 2016, 132, 466–471. [Google Scholar] [CrossRef]

| Sr. No | Dye Name | Chemical Structure | Chromophore Group | Anchor Group |
|---|---|---|---|---|
| 1 | CI Reactive blue 21 | 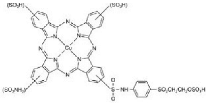 | Phthalocyanine | Vinylsulphone |
| 2 | CI Reactive black 5 | 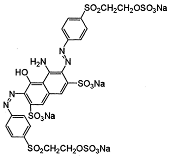 | Diazo | Vinylsulphone |
| 3 | CI Reactive yellow 84 | 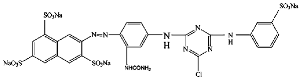 | Monoazo | Monochlorotriazine |
| Parameter | Mean Square | F-Value | p Value |
|---|---|---|---|
| Model | 109.043 | 22.052 | <0.001 |
| pH | 140.963 | 28.507 | <0.001 |
| Ozone dose | 95.580 | 19.329 | <0.001 |
| Treatment time | 78.820 | 15.940 | <0.001 |
| Dye type | 365.383 | 73.892 | 0.003 |
| R2 | 0.991 | ||
| Adj. R2 | 0.946 |
| Parameters | Types of Reactive Dyes | |||||
|---|---|---|---|---|---|---|
| CI Reactive Blue 21 | CI Reactive Black 5 | CI Reactive Yellow 84 | ||||
| pH | L | ∆L | L | ∆L | L | ∆L |
| 3 | 79.61 | 31.24 | 65.59 | 51.67 | 60.81 | 11.49 |
| 5 | 73.81 | 25.44 | 59.53 | 45.61 | 57.1 | 7.78 |
| 7 | 63.05 | 14.68 | 54.58 | 40.66 | 54.56 | 5.24 |
| 9 | 62.07 | 13.7 | 54.02 | 40.1 | 51.8 | 2.48 |
| Ozone Dose (g/h) | ||||||
| 2 | 69.4 | 21.03 | 48.62 | 34.7 | 51.74 | 2.42 |
| 5 | 73.58 | 25.21 | 59.45 | 45.53 | 52.48 | 3.16 |
| 7 | 79.43 | 31.06 | 65.54 | 51.62 | 56.7 | 7.38 |
| 10 | 75.95 | 27.58 | 61.52 | 47.6 | 55.2 | 5.88 |
| Treatment Time (min) | ||||||
| 30 | 70.61 | 22.24 | 46.89 | 32.97 | 55.08 | 5.76 |
| 40 | 79.43 | 31.06 | 65.76 | 51.84 | 56.7 | 7.38 |
| 50 | 79.05 | 30.68 | 65.34 | 51.42 | 56.24 | 6.92 |
| 60 | 76.07 | 27.7 | 62.12 | 48.2 | 55.1 | 5.78 |
| Parameters | Bursting Strength (kPa) | ||
|---|---|---|---|
| CI Reactive Blue 21 | CI Reactive Black 5 | CI Reactive Yellow 84 | |
| pH | |||
| 3 | 862 ± 0.08 | 903 ± 0.21 | 825 ± 0.15 |
| 5 | 847 ± 0.38 | 890 ± 0.23 | 872 ± 0.34 |
| 7 | 825 ± 0.15 | 872 ± 0.34 | 790 ± 0.28 |
| 9 | 807 ± 0.21 | 903 ± 0.21 | 864 ± 0.26 |
| Ozone dose (g/h) | |||
| 2 | 882 ± 0.11 | 902 ± 0.21 | 885 ± 0.32 |
| 5 | 876 ± 0.08 | 898 ± 0.13 | 863 ± 0.25 |
| 7 | 862 ± 0.14 | 895 ± 0.26 | 825 ± 0.09 |
| 10 | 848 ± 0.23 | 876 ± 0.18 | 797 ± 0.18 |
| Treatment time (min) | |||
| 30 | 892 ± 0.08 | 905 ± 0.09 | 875 ± 0.26 |
| 40 | 862 ± 0.14 | 895 ± 0.13 | 825 ± 0.16 |
| 50 | 805 ± 0.15 | 861 ± 0.31 | 805 ± 0.39 |
| 60 | 800 ± 0.21 | 830 ± 0.05 | 792 ± 0.28 |
| Parameter | Mean Square | F-Value | p Value |
|---|---|---|---|
| Model | 1229.074 | 7.351 | <0.001 |
| pH | 120.134 | 0.719 | 0.576 |
| Ozone dose | 446.376 | 2.670 | 0.141 |
| Treatment time | 2648.171 | 15.839 | 0.003 |
| Dye type | 1185.679 | 7.092 | 0.026 |
| R2 | 0.973 | ||
| Adj. R2 | 0.840 |
| No. | No. of Reuses | Fabric Lightness | Effluent Characteristics | |||
|---|---|---|---|---|---|---|
| L Value | pH | EC (µS/cm) | TDS (mg/L) | COD (mg/L) | ||
| Conventional stripping method | 11.2 | 5890 | 3980 | 2847 | ||
| 1 | 1st | 79.05 | 3.02 | 570 | 439 | 156 |
| 2 | 2nd | 79.61 | 3.19 | 620 | 487 | 340 |
| 3 | 3rd | 78.97 | 3.58 | 780 | 543 | 569 |
| 4 | 4th | 79.02 | 3.76 | 800 | 623 | 743 |
| 5 | 5th | 77.01 | 3.98 | 907 | 789 | 866 |
| 6 | 6th | 72.31 | 4.12 | 990 | 802 | 945 |
| 7 | 7th | 69.81 | 4.78 | 1360 | 856 | 1657 |
| Parameters | EC | TDS | pH | COD | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| EC | 1 | - | 0.902 | 0.006 | 0.987 | 0.000 | 0.933 | 0.000 |
| TDS | 0.902 | 0.006 | 1 | - | 0.944 | 0.001 | 0.907 | 0.005 |
| pH | 0.987 | 0.000 | 0.944 | 0.001 | 1 | - | 0.992 | 0.000 |
| COD | 0.993 | 0.000 | 0.907 | 0.005 | 0.992 | 0.000 | 1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulfiqar, A.; Arooj, F.; Aftab, M.; Rashid, M.; Luqman, M.; Kashif, S.u.R.; Naseer, R. A Sustainable Approach to Dyed Cotton Fabric Stripping Using Ozone. Sustainability 2023, 15, 7467. https://doi.org/10.3390/su15097467
Zulfiqar A, Arooj F, Aftab M, Rashid M, Luqman M, Kashif SuR, Naseer R. A Sustainable Approach to Dyed Cotton Fabric Stripping Using Ozone. Sustainability. 2023; 15(9):7467. https://doi.org/10.3390/su15097467
Chicago/Turabian StyleZulfiqar, Amna, Fariha Arooj, Mahwish Aftab, Muhammad Rashid, Muhammad Luqman, Saif ur Rehman Kashif, and Rahat Naseer. 2023. "A Sustainable Approach to Dyed Cotton Fabric Stripping Using Ozone" Sustainability 15, no. 9: 7467. https://doi.org/10.3390/su15097467
APA StyleZulfiqar, A., Arooj, F., Aftab, M., Rashid, M., Luqman, M., Kashif, S. u. R., & Naseer, R. (2023). A Sustainable Approach to Dyed Cotton Fabric Stripping Using Ozone. Sustainability, 15(9), 7467. https://doi.org/10.3390/su15097467







