Environmental Impact of the Recycling of Ni-Co-Containing Saggars—A LCA Case Study in China
Abstract
1. Introduction
2. Methodology
2.1. LCA Goal and Scope Definition
2.2. Life-Cycle Inventory
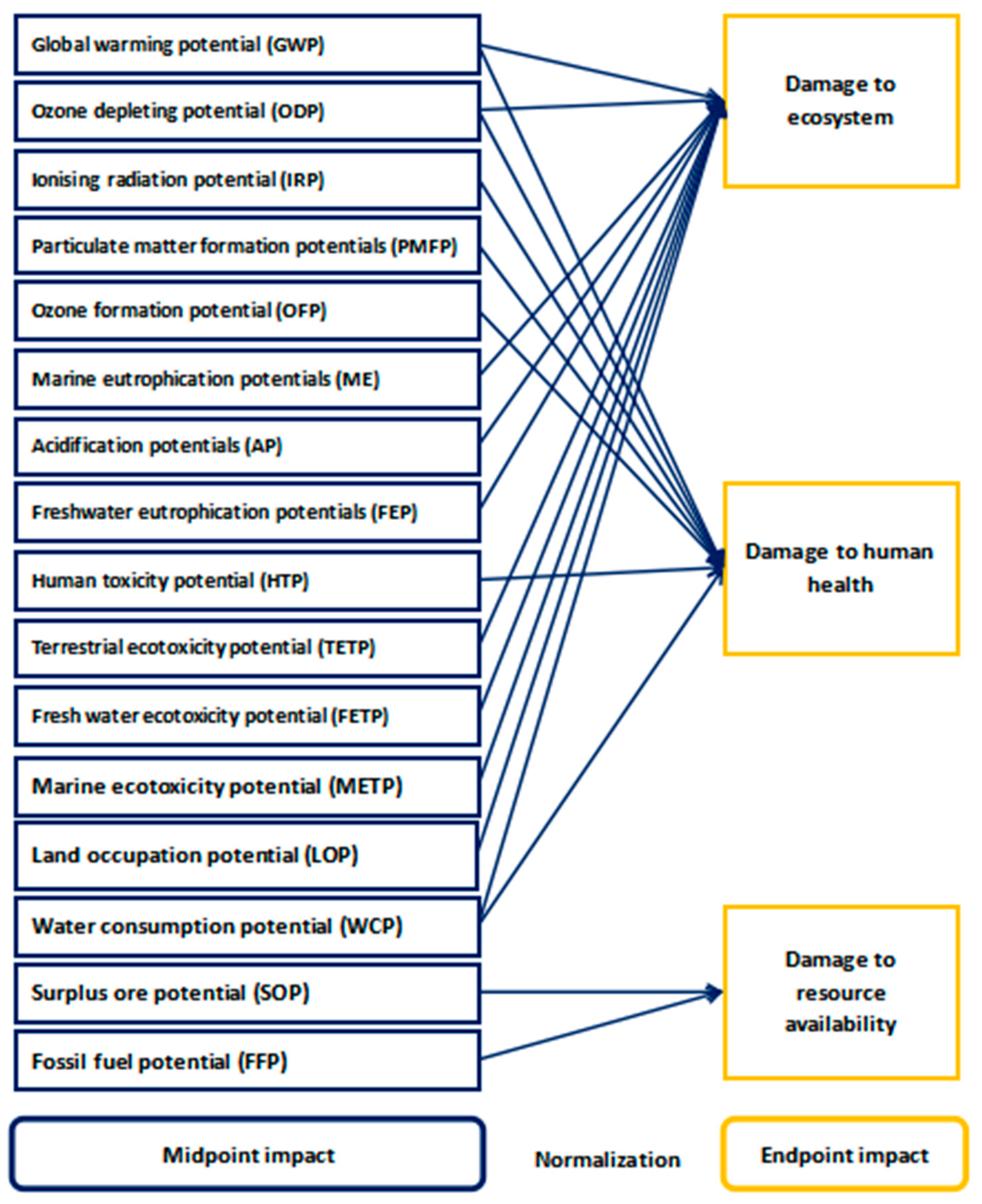
2.3. Life Cycle Impact Assessment Method
3. Results
3.1. Life Cycle Inventory Assessment Results
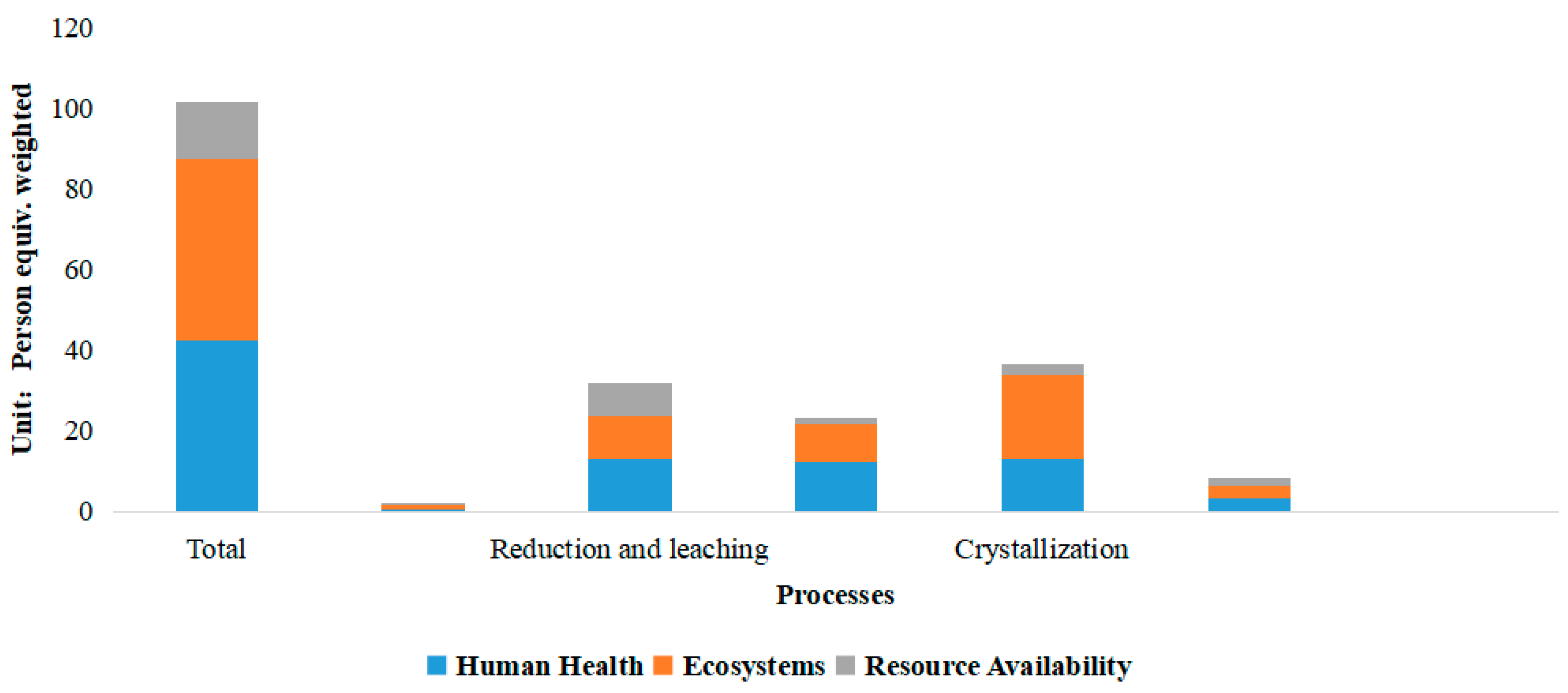
3.2. Life Cycle Impact Assessment Results
3.3. Uncertainty Analysis
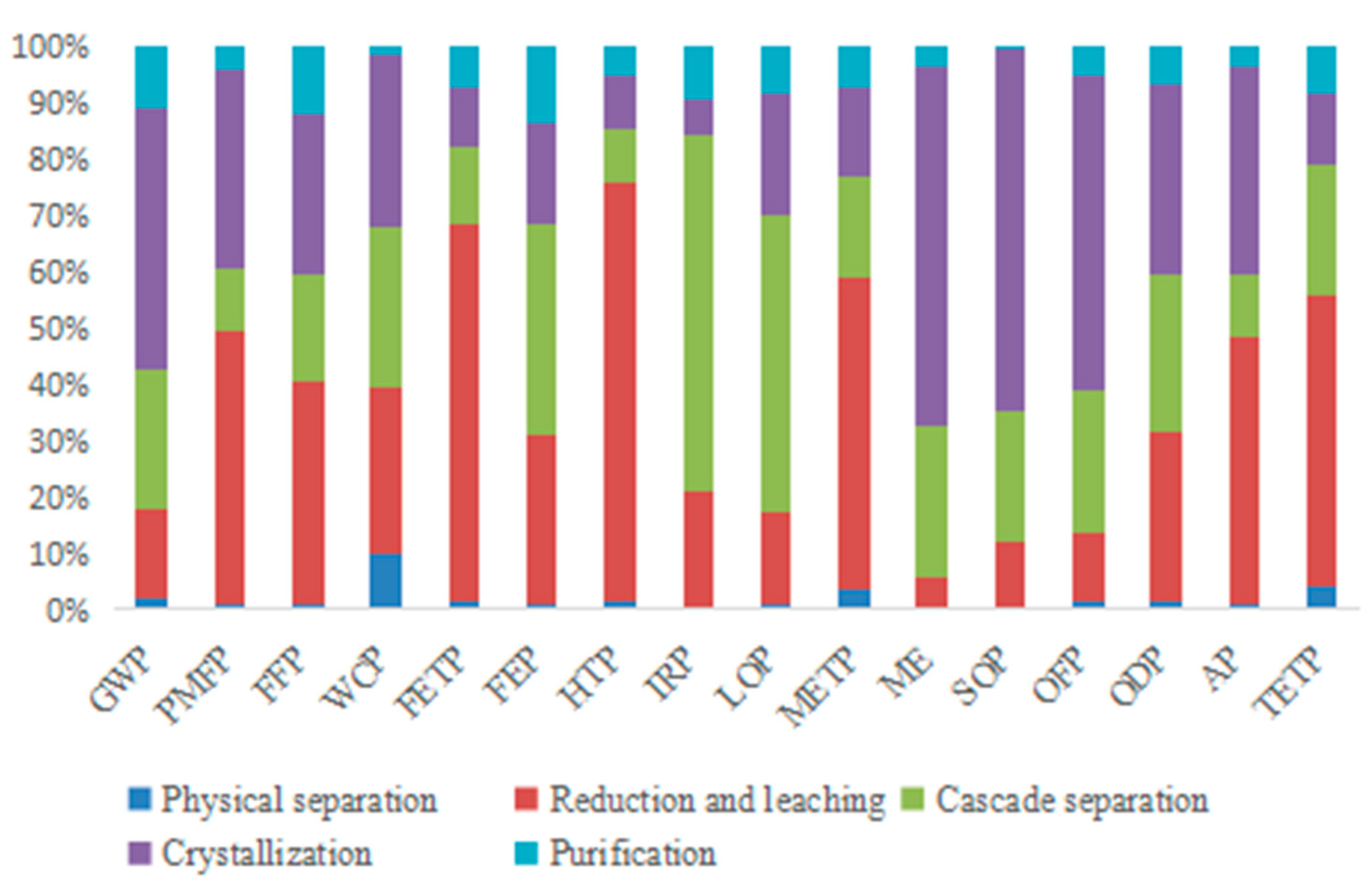
3.4. Sensitivity Analysis Results
4. Conclusions and Discussion
- Improve the energy utilization structure and reduce carbon emissions. Increase the use of clean energy and increase the efficiency of heat energy utilization in the crystallization process;
- Improve the accuracy of the use of key raw materials, including sulfuric acid, potassium carbonate, potassium hydroxide, and ammonia. In the reductive leaching process, sulfuric acid is used more accurately. In the crystallization and cascade separation process, the degree of supersaturation of potassium carbonate and potassium hydroxide input is strictly controlled. Control the input of ammonia in the purification process. In addition, new materials that are more environmentally friendly should be explored;
- Strengthen the air emission in the two processes of reduction and leaching and crystallization. Acid mist leakage in the reduction and leaching process should be reduced, the waste gas generated in the crystallization process should be treated, or a more environmentally friendly crystallization method should be used.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Government Website. Available online: http://www.gov.cn/guowuyuan/zfgzbg.html (accessed on 5 March 2021).
- Yang, Z.; Lu, J.; Bian, D.; Zhang, W.; Yang, X.; Xia, J. Stepwise Co-Precipitation to Synthesize Li Ni1/3Co1/3Mn1/3O2 One-Dimensional Hierarchical Structure for Lithium-Ion Batteries. J. Power Sources 2014, 272, 144–151. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Q.; Li, L.; Feng, C. Synthesis and conditions of MnxNiyCozCO3, a precursor of ternary cathode material. Inorg. Chem. Ind. 2015, 47, 75–77. [Google Scholar]
- Ministry of Industry and Information Technology of the People’s Republic of China. Public Comment on the “New Energy Automobile Industry Development Plan 2021–2035” Draft for Comment. Available online: https://www.miit.gov.cn/gzcy/yjzj/art/2020/art_d4d031ed0f04438d9fc9e08ab13173c7.html (accessed on 3 December 2019).
- Xing, J.; Chen, Q.; Zhang, Y.; Long, T.; Zheng, G.; Wang, K. Related mineral demand forecast under the development of global nem energy automobile. China Min. Mag. 2019, 28, 66–71. [Google Scholar]
- USGS. Mineral Commodity Summaries 2021. Available online: https://pubs.er.usgs.gov/publication/mcs2021 (accessed on 1 February 2021).
- Zhai, P.; Chen, L.; Yin, Y.; Li, S.; Ding, D.; Ye, G. Interactions between mullite saggars refractories and Li-ion battery cathode materials during calcination. J. Eur. Ceram. Soc. 2018, 384, 2145–2151. [Google Scholar] [CrossRef]
- Li, J.; Chen, B.; Zhou, H. Exploration on efficient and comprehensive utilization of retired power battery. Battery China network: The first international summit on power battery application in 2016. J. Inorg. Mater. 2016, 31, 773–778. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Liu, L. Solving spent lithium-ion battery problems in China: Opportunities and challenges. Renew. Sustain. Energy Rev. 2015, 52, 1759–1767. [Google Scholar] [CrossRef]
- Shi, H. Recovery and Reuse of LiCo, Ni, MnO2 Cathode Material in Waste Lithium Ion Battery; Zhengzhou University: Zhengzhou, China, 2017; pp. 31–34. [Google Scholar]
- Wang, L. Research on the Recovery and Recycling of Nickel from Waste Nickel-Hydrogen Batteries; Kunming University of Science and Technology: Kunming, China, 2017; pp. 31–36. [Google Scholar]
- Yu, M. Study on Synthesizing Waste Nickel, Cobalt and Manganese Ternary Materials and Related Fine Chemicals by High Value of Cyclic Leaching Process; Beijing University of Chemical Technology: Beijing, China, 2018. [Google Scholar]
- Porvali, A.; Ojanen, R.; Wilson, B.; Serna-Guerrero, R.; Lundstrom, M. Nickel Metal Hydride Battery Waste: Mechano-hydrometallurgical Experimental Study on Recycling Aspects. J. Sustain. Metal. 2020, 16, 78–90. [Google Scholar] [CrossRef]
- Temporelli, A.; Carvalho, M.; Girardi, P. Life Cycle Assessment of Electric Vehicle Batteries: An Overview of Recent Literature. Energies 2020, 13, 2864. [Google Scholar] [CrossRef]
- Richa, K.; Babbitt, C.; Gaustad, G. Eco-Efficiency Analysis of a Lithium-Ion Battery Waste Hierarchy Inspired by Circular Economy. J. Ind. Ecol. 2017, 21, 715–730. [Google Scholar] [CrossRef]
- Yang, J.; Gua, F.; Guo, J. Environmental feasibility of secondary use of electric vehicle lithium-ion batteries in communication base stations. Resour. Conserv. Recycl. 2020, 156, 104713. [Google Scholar] [CrossRef]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles-a Critical issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- Unterreiner, L.; Juelch, V.; Reith, S. Recycling of Battery Technologies—Ecological Impact Analysis Using Life Cycle Assessment. Energy Procedia 2016, 99, 229–234. [Google Scholar] [CrossRef]
- Raugei, W.; Winfield, P. Prospective LCA of the production and EoL recycling of a novel type of Li-ion battery for electric vehicles. J. Clean. Prod. 2019, 213, 926–932. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Y.; Yu, Y. Superior “green” electrode materials for secondary batteries: Through the footprint family indicators to analyze their environmental friendliness. Environ. Sci. Pollut. R 2019, 26, 36538–36557. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Syed, J.; Mehmood, C. Mass burden and estimated flux of heavy metals in Pakistan coast: Sedimentary pollution and eco-toxicological concerns. Environ. Sci. Pollut. Res. 2015, 22, 4316–4326. [Google Scholar] [CrossRef]
- El-Alfy, M.; El-Amier, A.; El-Eraky, T. Landuse/cover and eco-toxicity indices for identifying metal contaminationin sediments of drains, Manzala Lake, Egypt. Heliyon 2020, 6, e03177. [Google Scholar] [CrossRef]
- Liu, B. Lithium-Ion Battery Cathode Material Saggars Application Research; Qilu University of Technology: Jinan, China, 2015; pp. 23–25. [Google Scholar]
- Santoyo-Castelazo, E.; Azapagic, A. Sustainability assessment of energy systems: Integrating environmental, economic and social aspects. J. Clean. Prod. 2014, 80, 119–138. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management-Life Cycle Assessment-Principles and Framework. ISO: Geneva, Switzerland, 2006.
- Herrmann, I.; Moltesen, A. Does it matter which Life Cycle Assessment (LCA) tool you choose?—A comparative assessment of SimaPro and GaBi. J. Clean. Prod. 2015, 86, 163–169. [Google Scholar] [CrossRef]
- Saynajoki, A.; Heinonen, J.; Junnila, S.; Horvath, A. Can life-cycle assessment produce reliable policy guidelines in the building sector? Environ. Res. Lett. 2017, 12, 13001. [Google Scholar] [CrossRef]
- Emami, N.; Heinonen, J.; Marteinsson, B.; Saynajoki, A.; Junnonen, J.-M.; Laine, J.; Junnila, S. A life cycle assessment of two residential buildings using two different LCA database-software combinations: Recognizing uniformities and inconsistencies. Buildings 2019, 9, 20. [Google Scholar] [CrossRef]
- Huijbregts, M.; Steinmann, Z.; Elshout, P. ReCiPe2016: A harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Wang, F.; Deng, Y.; Yuan, C. Life cycle assessment of lithium oxygen battery for electric vehicles. J. Clean. Prod. 2020, 264, 121339. [Google Scholar] [CrossRef]
- Garcia-Sanchez, M.; Guereca, L. Environmental and social life cycle assessment of urban water systems: The case of Mexico City. Sci. Total Environ. 2019, 693, 133464. [Google Scholar] [CrossRef] [PubMed]


| Unit: kg | Total | Physical Separation | Reduction and Leaching | Cascade Separation | Crystallization | Purification |
|---|---|---|---|---|---|---|
| Total Flows | 281,487.69 | 4914.43 | 51,754.89 | 161,663.66 | 31,028.75 | 21,125.98 |
| Energy resources | 83.97 | 1.18 | 29.66 | 16.79 | 27.36 | 8.98 |
| Nonrenewable energy resources | 83.97 | 1.18 | 29.66 | 16.79 | 27.36 | 8.98 |
| Renewable energy resources | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Material resources | 141,317.35 | 2722.75 | 21,949.37 | 81,877.59 | 11,193.84 | 11,573.78 |
| Nonrenewable resources | 467.89 | 3.06 | 38.94 | 171.92 | 223.00 | 30.97 |
| Renewable resources | 141,849.23 | 2719.69 | 18,910.35 | 81,705.63 | 11,970.80 | 11,542.77 |
| Emissions | 141,860.19 | 2653.38 | 21,775.85 | 81,769.27 | 11,118.47 | 11,543.21 |
| Deposited goods | 336.30 | 2.92 | 88.02 | 109.67 | 120.81 | 14.88 |
| Emissions to air | 1569.12 | 48.14 | 263.72 | 674.41 | 434.28 | 148.56 |
| Emissions to freshwater | 131,349.77 | 2598.35 | 21,318.15 | 71,668.99 | 11,454.20 | 11,310.09 |
| Emissions to seawater | 605.00 | 3.97 | 105.97 | 316.20 | 109.18 | 69.68 |
| Emissions to agricultural soil | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Emissions to industrial soil | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Factor | Unit | Ni-Co-Containing Saggar Recovery | Alternative Production | Yang (2020) [16] LIB Secondary Use | Temporelli (2020) [14] NMC Production | Wang (2020) [30] NMC Production |
|---|---|---|---|---|---|---|
| GWP | kg CO2 eq. | 1.58 × 102 | 9.80 × 102 | 5.00 × 102 | 2.00 × 105 | 1.50 × 105 |
| PMFP | kg PM2.5 eq. | 3.19 × 10−1 | 1.27 × 100 | |||
| FFP | MJ eq. | 3.13 × 103 | 1.59 × 104 | 2.10 × 102 | 1.89 × 104 | |
| WCP | m3 | 9.89 × 10−1 | 6.67 × 100 | |||
| FETP | kg 1,4-DB eq. | 2.43 × 10−2 | 3.54 × 10−1 | 2.10 × 104 | ||
| FEP | kg P eq. | 2.67 × 10−4 | 1.94 × 10−3 | 1.10 × 100 | 4.00 × 102 | 3.60 × 102 |
| HTP | kg 1,4-DB eq. | 6.70 × 102 | 7.48 × 103 | 6.00 × 105 | ||
| IRP | kBq Co60 eq. to air | 1.50 × 101 | 1.73 × 102 | |||
| LOP | Annual crop eq.·y | 2.25 × 100 | 4.53 × 101 | |||
| METP | kg 1,4-DB eq. | 2.14 × 102 | 5.49 × 103 | 1.80 × 104 | ||
| ME | kg N eq. | 8.52 × 10−3 | 2.76 × 10−2 | 1.80 × 102 | ||
| SOP | kg Cu eq. | 1.78 × 100 | 1.40 × 102 | 2.70 × 102 | 7.50 × 102 | |
| OFP | kg NOx eq. | 7.69 × 10−1 | 8.99 × 100 | |||
| ODP | kg CFC-11 eq. | 5.73 × 10−5 | 3.71 × 10−4 | 1.00 × 100 | 1.50 × 10−2 | |
| AP | kg SO2 eq. | 1.10 × 100 | 4.22 × 100 | 2.00 × 103 | 3.00 × 103 | |
| TETP | kg 1,4-DB eq. | 3.49 × 101 | 1.11 × 103 |
| Unit | Mean | SD | CV (%) | |
|---|---|---|---|---|
| Mass-Input | kg | 2.99 × 105 | 1.76 × 10−6 | 5.89 × 10−10 |
| Mass-Output | kg | 3.00 × 105 | 1.56 × 10−6 | 5.20 × 10−10 |
| Midpoint-GWP | kg CO2 eq. | 3.33 × 102 | 1.03 × 10−6 | 3.09 × 10−7 |
| Midpoint-PMFP | kg PM2.5 eq. | 6.74 × 10−1 | 1.82 × 10−6 | 2.70 × 10−4 |
| Midpoint-FFP | MJ eq. | 1.55 × 102 | 1.84 × 10−6 | 1.19 × 10−6 |
| Midpoint-WCP | m3 | 2.94 × 102 | 0.00 × 100 | 0.00 × 100 |
| Midpoint-FETP | kg 1,4 DB eq. | 5.13 × 10−2 | 1.77 × 10−6 | 3.45 × 10−3 |
| Midpoint-FEP | kg P eq. | 5.63 × 10−4 | 2.52 × 10−6 | 4.48 × 10−1 |
| Midpoint-HTP | kg 1,4-DB eq. | 5.82 × 100 | 4.60 × 10−7 | 7.90 × 10−6 |
| Midpoint-IRP | kBq Co60 eq. to air | 3.17 × 101 | 1.58 × 10−6 | 4.98 × 10−6 |
| Midpoint-LOP | Annual crop eq.·y | 4.75 × 100 | 5.64 × 10−7 | 1.19 × 10−5 |
| Midpoint-METP | kg 1,4-DB eq. | 4.51 × 102 | 0.00 × 100 | 0.00 × 100 |
| Midpoint-ME | kg N eq. | 1.80 × 10−2 | 1.35 × 10−6 | 7.50 × 10−3 |
| Midpoint-SOP | kg Cu eq. | 3.77 × 100 | 2.49 × 10−6 | 6.60 × 10−5 |
| Midpoint-OFP | kg NOx eq. | 8.17 × 10−1 | 1.64 × 10−6 | 2.01 × 10−4 |
| Midpoint-ODP | kg CFC-11 eq. | 1.21 × 10−4 | 2.49 × 10−6 | 2.06 × 100 |
| Midpoint-AP | kg SO2 eq. | 2.33 × 100 | 1.54 × 10−6 | 6.61 × 10−5 |
| Midpoint-TETP | kg 1,4-DB eq. | 7.37 × 101 | 7.75 × 10−7 | 1.05 × 10−6 |
| Endpoint | Weighted person equivalents | 4.50 × 102 | 2.16 × 10−6 | 4.80 × 10−7 |
| No. | Process | Material Flow | Environmental Impact (%) |
|---|---|---|---|
| 1 | Reduction and leaching | Sulfuric acid | 3.04 |
| 2 | Crystallization | Potassium carbonate | 2.03 |
| 3 | Crystallization | Steam | 1.22 |
| 4 | Cascade separation | Potassium hydroxide | 1.19 |
| 5 | Cascade separation | Potassium carbonate | 0.62 |
| 6 | Purification | Ammonia water | 0.52 |
| 7 | Crystallization | Electricity | 0.34 |
| 8 | Purification | Carbon dioxide | 0.21 |
| 9 | Physical separation | Electricity | 0.17 |
| 10 | Purification | Sulfuric acid | 0.14 |
| 11 | Purification | Sodium hydroxide | 0.13 |
| 12 | Reduction and leaching | Electricity | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Sun, Z.; Zhang, W.; Li, S. Environmental Impact of the Recycling of Ni-Co-Containing Saggars—A LCA Case Study in China. Sustainability 2023, 15, 7442. https://doi.org/10.3390/su15097442
Li Z, Sun Z, Zhang W, Li S. Environmental Impact of the Recycling of Ni-Co-Containing Saggars—A LCA Case Study in China. Sustainability. 2023; 15(9):7442. https://doi.org/10.3390/su15097442
Chicago/Turabian StyleLi, Zehong, Zhenhua Sun, Wenbiao Zhang, and Shaopeng Li. 2023. "Environmental Impact of the Recycling of Ni-Co-Containing Saggars—A LCA Case Study in China" Sustainability 15, no. 9: 7442. https://doi.org/10.3390/su15097442
APA StyleLi, Z., Sun, Z., Zhang, W., & Li, S. (2023). Environmental Impact of the Recycling of Ni-Co-Containing Saggars—A LCA Case Study in China. Sustainability, 15(9), 7442. https://doi.org/10.3390/su15097442






