Modulation of Antioxidant Defense Mechanisms and Morpho-Physiological Attributes of Wheat through Exogenous Application of Silicon and Melatonin under Water Deficit Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Detail
2.2. Imposition of Water Holding Capacity
2.3. Photosynthetic Pigments and Relative Water Contents
2.4. Determination of Oxidative Stress Indicators
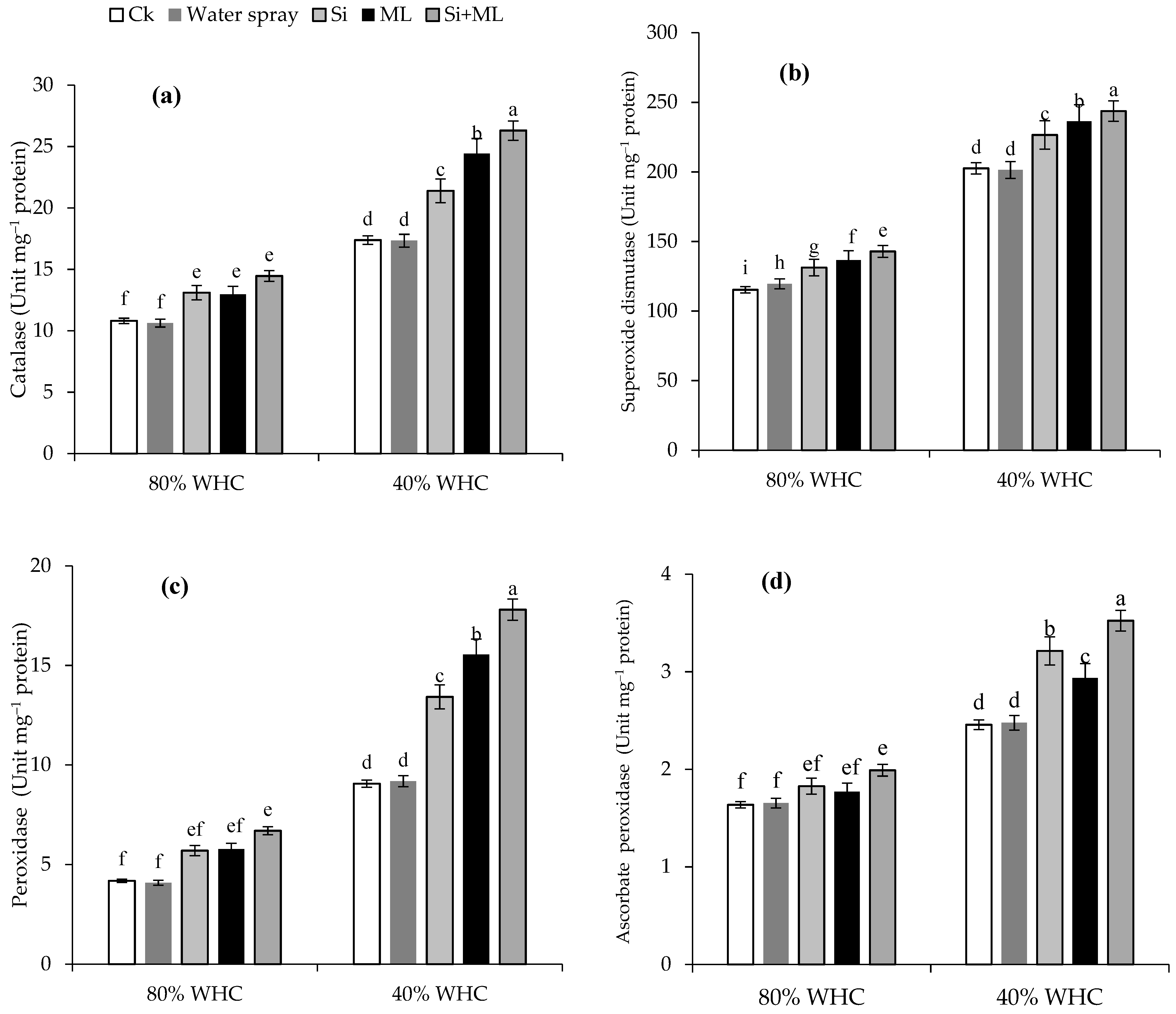
2.5. Enzymatic Antioxidants Activities
2.6. Osmolytes Determination
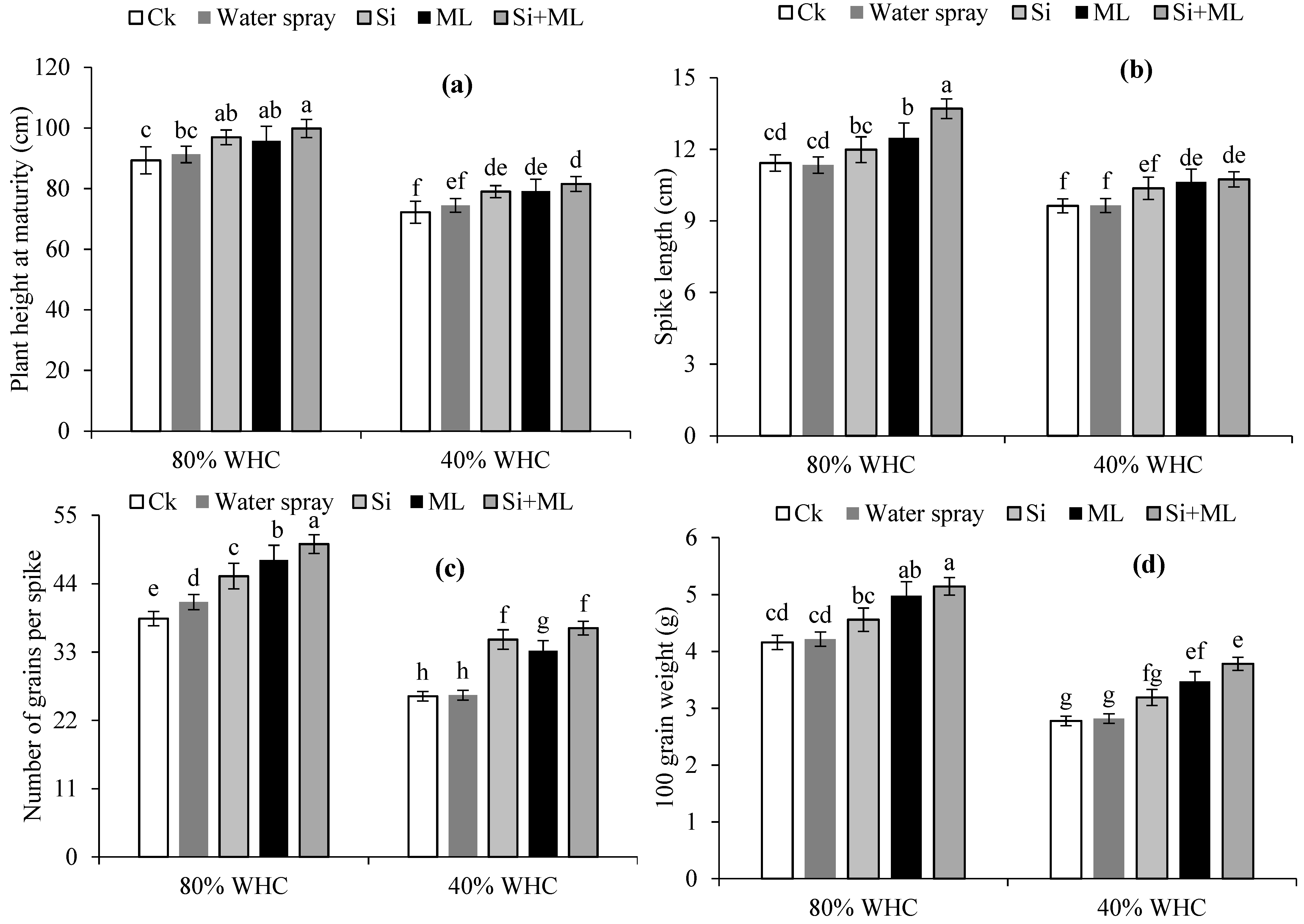
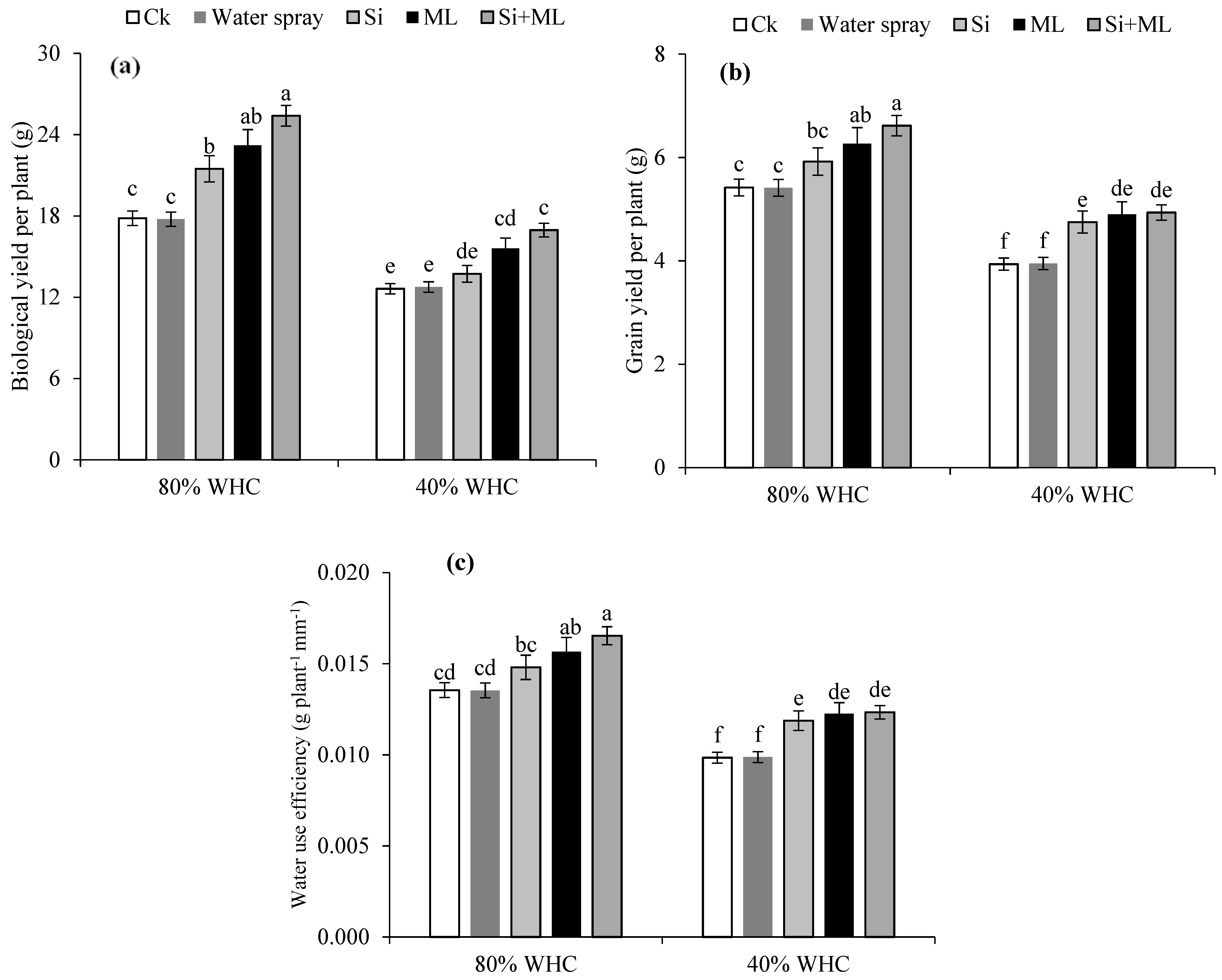
2.7. Yield and Yield-Related Traits
2.8. Water Use Efficiency
2.9. Statistical Analysis
3. Results
3.1. Photosynthetic Pigments and Relative Water Contents
3.2. Lipid Peroxidation Indicator
3.3. Enzymatic Antioxidants Activities
3.4. Accumulation of Osmolytes
3.5. Plant Height, Yield and Yield Attributes
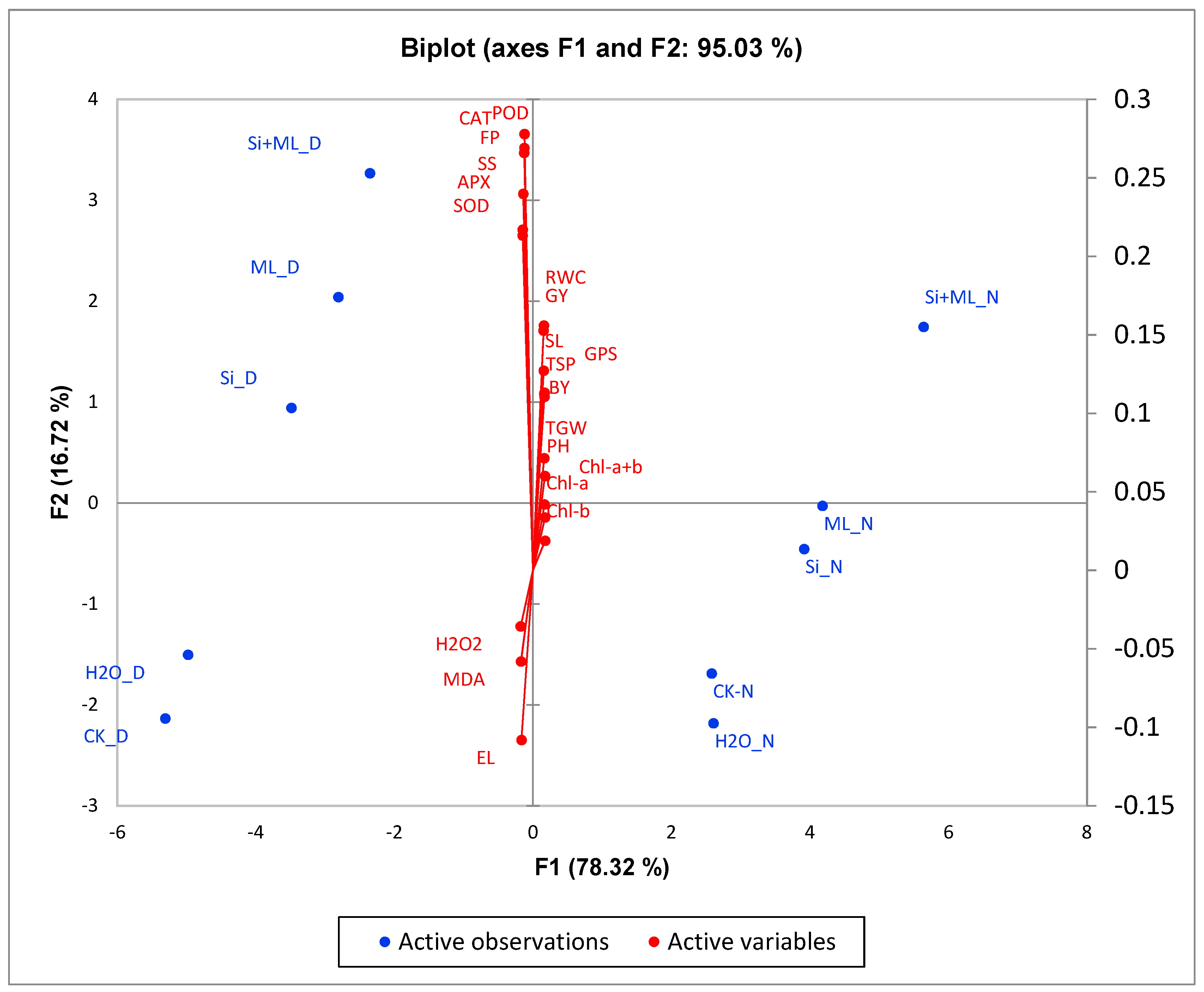
3.6. Principal Components Analyse Biplot
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Chowdhury, M.K.; Hasan, M.; Bahadur, M.; Islam, M.R.; Hakim, M.A.; Iqbal, M.A.; Javed, T.; Raza, A.; Shabbir, R.; Sorour, S. Evaluation of drought tolerance of some wheat (Triticum aestivum L.) genotypes through phenology, growth, and physiological indices. Agronomy 2021, 11, 1792. [Google Scholar] [CrossRef]
- World Health Organization (WHO). A Report about Drought Stress 2022. Available online: https://www.who.int/health-topics/drought#tab=tab_1 (accessed on 28 March 2023).
- Elkelish, A.; El-Mogy, M.M.; Niedbała, G.; Piekutowska, M.; Atia, M.A.; Hamada, M.M.; Shahin, M.; Mukherjee, S.; El-Yazied, A.A.; Shebl, M. Roles of Exogenous α-Lipoic Acid and Cysteine in Mitigation of Drought Stress and Restoration of Grain Quality in Wheat. Plants 2021, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hu, Y.; Wang, C.; Liu, W.; Ma, G.; Han, Q.; Ma, D. Effects of high temperature and drought stress on the expression of gene encoding enzymes and the activity of key enzymes involved in starch biosynthesis in wheat grains. Front. Plant Sci. 2019, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Kovalchuk, N.; Langridge, P.; Tricker, P.J.; Lopato, S.; Borisjuk, N. The impact of drought on wheat leaf cuticle properties. BMC Plant Biol. 2017, 17, 85. [Google Scholar] [CrossRef]
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Collin, B.; Doelsch, E.; Keller, C.; Cazevieille, P.; Tella, M.; Chaurand, P.; Panfili, F.; Hazemann, J.-L.; Meunier, J.-D. Evidence of sulfur-bound reduced copper in bamboo exposed to high silicon and copper concentrations. Environ. Pollut. 2014, 187, 22–30. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 2017, 110, 70–81. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Ashfaque, F.; Chhillar, H.; Irfan, M.; Khan, N.A. The intricacy of silicon, plant growth regulators and other signaling molecules for abiotic stress tolerance: An entrancing crosstalk between stress alleviators. Plant Physiol. Biochem. 2021, 162, 36–47. [Google Scholar] [CrossRef]
- Prychid, C.J.; Rudall, P.J.; Gregory, M. Systematics and biology of silica bodies in monocotyledons. Bot. Rev. 2003, 69, 377–440. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Lee, I.-J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef]
- Li, Z.-C.; Song, Z.-L.; Yang, X.-M.; Song, A.-L.; Yu, C.-X.; Wang, T.; Xia, S.; Liang, Y.-C. Impacts of silicon on biogeochemical cycles of carbon and nutrients in croplands. J. Integr. Agric. 2018, 17, 2182–2195. [Google Scholar] [CrossRef]
- Yan, G.-C.; Nikolic, M.; Ye, M.-J.; Xiao, Z.-X.; Liang, Y.-C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Butt, M.; Irfan, M.; Rizwan, M.S.; Ali, H.; Cheema, M.A. Interactive effect of biochar and silicon on improving morpho-physiological and biochemical attributes of maize by reducing drought hazards. J. Soil Sci. Plant Nutr. 2020, 20, 1819–1826. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Yue, Z.; Wang, J.; Jin, L.; Xu, Z.; Jin, N.; Zhang, B.; Lyu, J.; Yu, J. Application of Exogenous Silicon for Alleviating Photosynthetic Inhibition in Tomato Seedlings under Low− Calcium Stress. Int. J. Mol. Sci. 2022, 23, 13526. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Wasaya, A.; Hassan, J.; Yasir, T.A.; Ateeq, M.; Raza, M.A. Foliar Application of Silicon Improved Physiological Indicators, Yield Attributes, and Yield of Pearl Millet (Pennisetum glaucum L.) Under Terminal Drought Stress. J. Soil Sci. Plant Nutr. 2022, 22, 4458–4472. [Google Scholar] [CrossRef]
- Gokulraj, N.; Ravichandran, V.; Boominathan, P.; Soundararajan, R. Influence of Silicon on Physiology and Yield of Rice under Drought Stress. Madras Agric. J. 2018, 105, 524. [Google Scholar]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, L.; Higgs, D. Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J. Plant Nutr. 2006, 29, 1469–1480. [Google Scholar] [CrossRef]
- Crusciol, C.A.; Pulz, A.L.; Lemos, L.B.; Soratto, R.P.; Lima, G.P. Effects of silicon and drought stress on tuber yield and leaf biochemical characteristics in potato. Crop Sci. 2009, 49, 949–954. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Ahmad, Z.; Ashraf, M.Y.; Afzal, M.; Nawaz, F.; Nafees, M.; Jatoi, W.N.; Malghani, N.A.; Shah, A.N.; Manan, A. Silicon mitigates drought stress in wheat (Triticum aestivum L.) through improving photosynthetic pigments, biochemical and yield characters. Silicon 2021, 13, 4757–4772. [Google Scholar] [CrossRef]
- Flores, R.A.; Arruda, E.M.; Souza Junior, J.P.d.; de Mello Prado, R.; Santos, A.C.A.d.; Aragão, A.S.; Pedreira, N.G.; da Costa, C.F. Nutrition and production of Helianthus annuus in a function of application of leaf silicon. J. Plant Nutr. 2019, 42, 137–144. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef]
- Debnath, B.; Li, M.; Liu, S.; Pan, T.; Ma, C.; Qiu, D. Melatonin-mediate acid rain stress tolerance mechanism through alteration of transcriptional factors and secondary metabolites gene expression in tomato. Ecotoxicol. Environ. Saf. 2020, 200, 110720. [Google Scholar] [CrossRef]
- Chen, L.; Lu, B.; Liu, L.; Duan, W.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Li, C. Melatonin promotes seed germination under salt stress by regulating ABA and GA3 in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2021, 162, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Boyko, E.; Golovatskaya, I.; Bender, O.; Plyusnin, I. Effect of short-term treatment of roots with melatonin on photosynthesis of cucumber leaves. Russ. J. Plant Physiol. 2020, 67, 351–359. [Google Scholar] [CrossRef]
- Kolář, J.; Johnson, C.H.; Macháčková, I. Exogenously applied melatonin (N-acetyl-5-methoxytryptamine) affects flowering of the short-day plant Chenopodium rubrum. Physiol. Plant. 2003, 118, 605–612. [Google Scholar] [CrossRef]
- Ahmad, S.; Su, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Javed, T.; Han, Q. Foliar application of melatonin delay leaf senescence in maize by improving the antioxidant defense system and enhancing photosynthetic capacity under semi-arid regions. Protoplasma 2020, 257, 1079–1092. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef]
- Qi, Z.-Y.; Wang, K.-X.; Yan, M.-Y.; Kanwar, M.K.; Li, D.-Y.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Zhou, J. Melatonin alleviates high temperature-induced pollen abortion in Solanum lycopersicum. Molecules 2018, 23, 386. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2020, 168, 256–277. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Khan, M.N.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mater. 2020, 398, 122882. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Ding, F. Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants. Antioxidants 2020, 9, 218. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef]
- Yao, J.W.; Ma, Z.; Ma, Y.Q.; Zhu, Y.; Lei, M.Q.; Hao, C.Y.; Chen, L.Y.; Xu, Z.Q.; Huang, X. Role of melatonin in UV-B signaling pathway and UV-B stress resistance in Arabidopsis thaliana. Plant Cell Environ. 2021, 44, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shi, Z.; Zhang, X.; Zheng, S.; Wang, J.; Mo, J. Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Hu, Z.; Fan, J.; Xie, Y.; Amombo, E.; Liu, A.; Gitau, M.M.; Khaldun, A.; Chen, L.; Fu, J. Comparative photosynthetic and metabolic analyses reveal mechanism of improved cold stress tolerance in bermudagrass by exogenous melatonin. Plant Physiol. Biochem. 2016, 100, 94–104. [Google Scholar] [CrossRef]
- Hu, W.; Cao, Y.; Loka, D.A.; Harris-Shultz, K.R.; Reiter, R.J.; Ali, S.; Liu, Y.; Zhou, Z. Exogenous melatonin improves cotton (Gossypium hirsutum L.) pollen fertility under drought by regulating carbohydrate metabolism in male tissues. Plant Physiol. Biochem. 2020, 151, 579–588. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Imran, M.; Shazad, R.; Bilal, S.; Imran, Q.M.; Khan, M.; Kang, S.-M.; Khan, A.L.; Yun, B.-W.; Lee, I.-J. Exogenous melatonin mediates the regulation of endogenous nitric oxide in Glycine max L. to reduce effects of drought stress. Environ. Exp. Bot. 2021, 188, 104511. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Hall, D.O.; Coombs, J. Techniques in Bioproductivity and Photosynthesis; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Rao, K.M.; Sresty, T. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Agarie, S.; Hanaoka, N.; Kubota, F.; Agata, W.; Kaufman, P.B. Measurement of cell membrane stability evaluated by electrolyte leakage as a drought and heat tolerance test in rice (Oryza sativa L.). J. Fac. Agric. 1995, 40, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Change, B.; Maehly, A. Assay of catalases and peroxidase. Methods Enzym. 1955, 2, 764–775. [Google Scholar]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Simaei, M.; Khavarinejad, R.; Saadatmand, S.; Bernard, F.; Fahimi, H. Interactive effects of salicylic acid and nitric oxide on soybean plants under NaCl salinity. Russ. J. Plant Physiol. 2011, 58, 783–790. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Tanner, C.B.; Bennett, J.M. Water-Use Efficiency in Crop Production. Am. Inst. Biol. Sci. 1984, 34, 36–40. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Dickey DA Principles and Procedures of Statistics: A Biometrical Approach; McGraw Hill: New York, NY, USA, 1997. [Google Scholar]
- Hoober, J.K. The Role of Chlorophyll b in Photosynthesis: Hypothesis. In Photosynthesis; Apple Academic Press: Palm Bay, FL, USA, 2016; pp. 104–118. [Google Scholar]
- Xu, H.; Vavilin, D.; Vermaas, W. Chlorophyll b can serve as the major pigment in functional photosystem II complexes of cyanobacteria. Proc. Natl. Acad. Sci. USA 2001, 98, 14168–14173. [Google Scholar] [CrossRef]
- Chakhchar, A.; Lamaoui, M.; Aissam, S.; Ferradous, A.; Wahbi, S.; El Mousadik, A.; Ibnsouda-Koraichi, S.; Filali-Maltouf, A.; El Modafar, C. Using chlorophyll fluorescence, photosynthetic enzymes and pigment composition to discriminate drought-tolerant ecotypes of Argania spinosa. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2018, 152, 356–367. [Google Scholar] [CrossRef]
- Manzoor, H.; Anjam, M.S.; Saeed, F.; Rasul, S.; Yousaf, S.; Kirn, A.; Qureshi, M.K.; Zafar, Z.U.; Ashraf, M.; Athar, H.-U.-R. Photosynthetic Efficiency and Antioxidant Defense Potential are Key Players in Inducing Drought Tolerance in Transgenic Tobacco Plants Over-Expressing AVP1. J. Plant Growth Regul. 2022, 41, 2653–2668. [Google Scholar] [CrossRef]
- Berry, J. 3.10 solar induced chlorophyll fluorescence: Origins, relation to photosynthesis and retrieval. Compr. Remote Sens. 2018, 3, 143–162. [Google Scholar]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Charagh, S.; Albaqami, M.; Sofi, P.A.; Zargar, S.M. Multidimensional role of silicon to activate resilient plant growth and to mitigate abiotic stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Consistent alleviation of abiotic stress with silicon addition: A meta-analysis. Funct. Ecol. 2016, 30, 1340–1357. [Google Scholar] [CrossRef]
- Abdel-Latif, A.; El-Demerdash, F. The ameliorative effects of silicon on salt-stressed sorghum seedlings and its influence on the activities of sucrose synthase and PEP carboxylase. J. Plant Physiol. Pathol. 2017, 5, 2–8. [Google Scholar] [CrossRef]
- dos Santos, M.S.; Sanglard, L.M.P.V.; Martins, S.C.V.; Barbosa, M.L.; de Melo, D.C.; Gonzaga, W.F.; DaMatta, F.M. Silicon alleviates the impairments of iron toxicity on the rice photosynthetic performance via alterations in leaf diffusive conductance with minimal impacts on carbon metabolism. Plant Physiol. Biochem. 2019, 143, 275–285. [Google Scholar] [CrossRef]
- Schaefer, M.; Hardeland, R. The melatonin metabolite N1-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J. Pineal Res. 2009, 46, 49–52. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Pandey, A.; Hamurcu, M.; Avsaroglu, Z.Z.; Ozbek, M.; Omay, A.H.; Elbasan, F.; Omay, M.R.; Gokmen, F.; Topal, A.; et al. Variability in Physiological Traits Reveals Boron Toxicity Tolerance in Aegilops Species. Front. Plant Sci. 2021, 12, 736614. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Ahuja, S.; Rai, G.K.; Kumar, S.; Mishra, D.; Kumar, S.N.; Rai, A.; Singh, B.; Chinnusamy, V.; Praveen, S. Silicon triggers the signalling molecules and stress-associated genes for alleviating the adverse effect of terminal heat stress in wheat with improved grain quality. Acta Physiol. Plant. 2022, 44, 30. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Hamurcu, M.; Khan, M.K.; Pandey, A.; Ozdemir, C.; Avsaroglu, Z.Z.; Elbasan, F.; Omay, A.H.; Gezgin, S. Nitric oxide regulates watermelon (Citrullus lanatus) responses to drought stress. Biotecholohy 2020, 10, 494. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Mushtaq, A.; Khan, Z.; Khan, S.; Rizwan, S.; Jabeen, U.; Bashir, F.; Ismail, T.; Anjum, S.; Masood, A. Effect of silicon on antioxidant enzymes of wheat (Triticum aestivum L.) grown under salt stress. Silicon 2020, 12, 2783–2788. [Google Scholar] [CrossRef]
- Zafar, S.; Hasnain, Z.; Anwar, S.; Perveen, S.; Iqbal, N.; Noman, A.; Ali, M. Influence of melatonin on antioxidant defense system and yield of wheat (Triticum aestivum L.) genotypes under saline condition. Pak. J. Bot. 2019, 51, 1987–1994. [Google Scholar] [CrossRef]
- Li, D.; Batchelor, W.D.; Zhang, D.; Miao, H.; Li, H.; Song, S.; Li, R. Analysis of melatonin regulation of germination and antioxidant metabolism in different wheat cultivars under polyethylene glycol stress. PLoS ONE 2020, 15, e0237536. [Google Scholar] [CrossRef]
- Ghouri, F.; Ali, Z.; Naeem, M.; Ul-Allah, S.; Babar, M.; Baloch, F.S.; Chattah, W.S.; Shahid, M.Q. Effects of silicon and selenium in alleviation of drought stress in rice. Silicon 2022, 14, 5453–5461. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Zhang, J.; Hemat, M.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Osmolyte accumulation plays important roles in the drought priming induced tolerance to post-anthesis drought stress in winter wheat (Triticum aestivum L.). Environ. Exp. Bot. 2019, 166, 103804. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Khayatnezhad, M.; Gholamin, R. The effect of drought stress on the superoxide dismutase and chlorophyll content in durum wheat genotypes. Adv. Life Sci. 2021, 8, 119–123. [Google Scholar]
- Botyanszka, L.; Zivcak, M.; Chovancek, E.; Sytar, O.; Barek, V.; Hauptvogel, P.; Halabuk, A.; Brestic, M. Chlorophyll fluorescence kinetics may be useful to identify early drought and irrigation effects on photosynthetic apparatus in field-grown wheat. Agronomy 2020, 10, 1275. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Han, Z.; Feng, H.; Wang, Y.; Kang, J.; Han, X.; Wang, L.; Wang, C.; Li, H.; et al. Analysis of Physiological Indicators Associated with Drought Tolerance in Wheat under Drought and Re-Watering Conditions. Antioxidants 2022, 11, 2266. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Hussain, S.; Rasheed, U.; Hussain, J.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Mahmoud, S.F.; et al. Modulation of Antioxidant Defense Mechanisms and Morpho-Physiological Attributes of Wheat through Exogenous Application of Silicon and Melatonin under Water Deficit Conditions. Sustainability 2023, 15, 7426. https://doi.org/10.3390/su15097426
Sattar A, Sher A, Ijaz M, Ul-Allah S, Hussain S, Rasheed U, Hussain J, Al-Qahtani SM, Al-Harbi NA, Mahmoud SF, et al. Modulation of Antioxidant Defense Mechanisms and Morpho-Physiological Attributes of Wheat through Exogenous Application of Silicon and Melatonin under Water Deficit Conditions. Sustainability. 2023; 15(9):7426. https://doi.org/10.3390/su15097426
Chicago/Turabian StyleSattar, Abdul, Ahmad Sher, Muhammad Ijaz, Sami Ul-Allah, Sajjad Hussain, Umair Rasheed, Jamshad Hussain, Salem Mesfir Al-Qahtani, Nadi Awad Al-Harbi, Samy F. Mahmoud, and et al. 2023. "Modulation of Antioxidant Defense Mechanisms and Morpho-Physiological Attributes of Wheat through Exogenous Application of Silicon and Melatonin under Water Deficit Conditions" Sustainability 15, no. 9: 7426. https://doi.org/10.3390/su15097426
APA StyleSattar, A., Sher, A., Ijaz, M., Ul-Allah, S., Hussain, S., Rasheed, U., Hussain, J., Al-Qahtani, S. M., Al-Harbi, N. A., Mahmoud, S. F., & Ibrahim, M. F. M. (2023). Modulation of Antioxidant Defense Mechanisms and Morpho-Physiological Attributes of Wheat through Exogenous Application of Silicon and Melatonin under Water Deficit Conditions. Sustainability, 15(9), 7426. https://doi.org/10.3390/su15097426








