Abstract
Climate change is causing a decline in the availability of crucial resources such as water and fertile soil, leading to a detrimental impact on crop yields. Basil (Ocimum basilicum L.), an annual aromatic plant used worldwide for culinary, cosmetic, and medicinal purposes, is especially at risk due to its high water demand and specific growing requirements typical of the Mediterranean climate. In Italy, basil is an essential part of any herb garden and is also commonly found in households as a potted plant. Nowadays, the conventional practice of growing basil in pots with peat as the primary medium is not environmentally sustainable, which underscores the need for alternative and sustainable cultivation techniques to ensure the continued growth of this majestic king of herbs. A greenhouse factorial experiment was conducted to study the impact of peat replacement and reduced water supply on the growth and biochemical traits of basil plants. The study included two cultivars (‘Genovese’ and ‘Valentino’), three substrate mixes with phytoremediated dredged sediment (TS) at varying volume percentages (0%, 12.5%, and 25%), and three levels of water irrigation (normal, reduced by 25%, and reduced by 37.8%). Increasing the TS percentage resulted in higher germination rates and greater biomass production in both cultivars compared with the control. ‘Valentino’ potted plants produced a higher yield than those of ‘Genovese’, while the latter had higher pigment contents due to its genetic characteristics. All combinations of substrates mixed with the highest water amounts of irrigation positively impacted seed germination and biomass-based outputs, while control peat with reduced water availability led to lower plant yields and germination capacity. Substrates with TS had suitable physicochemical characteristics for plant development. Our findings suggest that using peat–TS mixtures can produce quality results comparable to or even better than conventional soilless culture using only peat. Thus, replacing peat with moderate percentages of TS can be an effective and sustainable practice for recycling phytoremediated sediments.
1. Introduction
Currently, climate change is a major challenge affecting the global food supply, as extreme weather events might have significant negative impacts on major crop yields. Droughts and water scarcity are a global problem that is not limited to traditionally dry areas. Nearly 29% of European territory suffers from water scarcity and about 38% of the European population is affected by water stress. The situation is expected to worsen, and the problem is especially critical in southern Europe, where water is primarily used for food production [1]. Changes in temperature and rainfall patterns will impact the resources accessible in this area and will influence the growth and development of several food crops that are part of the traditional Mediterranean diet, such as basil (Ocimum basilicum L.).
Basil is one of the most important annual aromatic leaf species cultivated worldwide for culinary, medicinal, and cosmetic use [2,3]. In Italy, where it is considered the ‘king of the herbs’ due to its pleasant aroma and flavour, it is common practice to grow a basil plant in one’s home or garden, and even people without a garden hold a basil plant on their balcony or kitchen windowsill. Once the weather becomes warmer, it is typical to remove the growing tips or pluck fresh leaves of basil and sprinkle them on a variety of dishes.
Fresh basil leaves, dried basil, and basil paste are the most offered commercial forms of this aromatic herb in the market. Fresh basil leaves can be purchased either pre-packaged or loose. Fresh basil is also found at the supermarket in bunches or in small potted plants all year round. Each pot is seeded with dozens of plants that start their lives under very controlled greenhouse conditions, with factors such as air humidity, temperature, substrate moisture, and nutrient levels regularly monitored to ensure they meet the standards required by supermarkets [4,5].
To keep up with the growing consumer demand for fresh basil, many growers are turning to soilless greenhouse cultivation systems. Compared with conventional soil systems, these methods provide ideal conditions for maximising yield and quality. Additionally, they offer the advantage of early and uniform growth, as the plants are provided with an equal and well-adjusted supply of nutrient solution, while allowing a very high efficiency of water and fertiliser use. As a result, more and more growers are adopting these systems as a way to ensure a consistent supply of high-quality basil throughout the year [6].
In recent years, there has been growing interest in using alternative substrates for soilless basil cultivation. This is due to concerns about the sustainability of peat moss, which is the most used substrate for basil cultivation. Peat moss is a non-renewable resource harvested from natural peatlands, which are vital carbon sinks. Their destruction can release large amounts of greenhouse gases into the atmosphere. Thus, by reducing reliance on peat moss, growers can help to ensure the long-term sustainability of the basil industry while minimising their impact on the environment. In addition to using alternative substrates, irrigation regimes can also be adjusted for soilless basil cultivation. Different watering schedules can be used to optimise water and nutrient uptake, which can lead to higher yields and improved plant health [7,8].
Basil is a relatively low-maintenance species with a very short spring–summer cycle. It grows well in all types of soils, but does its best in well-drained, moist, nutrient rich soil with neutral pH; overly wet or dry soil can have a negative impact on basil growth. Basil is a warm-weather plant that flourishes in hot and dry conditions, but it also needs protection from strong winds and extreme temperatures. Basil is a high-water-demand species, but the water needs can vary significantly depending on the variety. Some varieties have been reported to be more tolerant and able to maintain physiological functions and yield production under water stress [9].
There are many different varieties of basil, each with its own unique characteristics and uses. When selecting a variety of basil to grow, it is important to consider the specific culinary or medicinal use of the herb, as well as the growing conditions required for each variety. ‘Valentino’ and ‘Genovese’ are two popular types of basil commonly used in Italy [10]. When it comes to flavour, ‘Valentino’ basil is known for having a slightly sweeter and milder taste without mintiness, while ‘Genovese’ basil has a stronger and more pungent flavour; its taste is pungent but leans toward a clove and mint back end. In terms of appearance, ‘Valentino’ basil has larger, lighter green curled drooping leaves that are more delicate in texture compared with ‘Genovese’ basil, which has smaller and darker green leaves. ‘Valentino’ basil is also known for being a bushier plant that produces a higher yield of leaves, while ‘Genovese’ basil has a taller, more upright growth habit with good branching. Both types of basil are commonly used in Italian cuisine, but they are often used in different dishes. ‘Genovese’ basil is the classic Italian basil that most people think about when talking about this aromatic herb. It is often used in salads, sandwiches, and as a garnish, while ‘Valentino’ basil is commonly used for sauces, particularly pesto.
The dredged sediment, which has a fine texture dominated by silt and clay, undergoes phytoremediation through a preliminary mixing with sandy soil to achieve a sandy loam composition. This is followed by the application of compost, allowing the selected fast-growing plant species (shrub and grass) with high salinity tolerance to establish and grow in the subsequent phytoremediation phase. This three-year process reduces electrical conductivity, both through salt accumulation in the plants and the dilution effect of rainwater. At the same time, the total microbial activity is increased, thus promoting the degradation of organic contaminants. Nevertheless, compared with soil, sediment is typically less homogeneous in its composition and particle size, has an alkaline pH, is lower in organic matter, and richer in heavy metals [11].
This study aimed to explore the partial substitution of peat moss with a phytoremediated sediment for the cultivation of basil in a soilless environment with varying irrigation levels. Previous studies have shown that using sediment-based growing media resulted in successful growth of pomegranate (Punica granatum L.), strawberry (Fragaria × ananassa Duch), and salad (Lactuca sativa L.), while also being safe for human consumption [12,13,14]. However, medicinal plants, such as Ocimum spp., have been frequently used as a biomarker to detect the presence of heavy metals, pesticides, and other toxic compounds in contaminated soils. The sensitivity of Ocimum spp. to soil pollution is attributed to their high bioaccumulation potential and their ability to produce various secondary metabolites, such as phenolic compounds, flavonoids, and alkaloids. These compounds are known to play a crucial role in the plant’s defence mechanisms against environmental stress [15,16,17]. In the next sections, we report physiological response and nutraceutical composition of ‘Genovese’ and ‘Valentino’ basil cultivars grown in a controlled greenhouse using treated sediment combined with peat moss. Additionally, we discuss the effect of the cultivar, substrate mixes, and water supply on basil performances.
2. Materials and Methods
2.1. Plant Material and Greenhouse Experimental Design
This study was conducted in a greenhouse at the Vivai Simoncini farm (Pescia, PT, Italy) in Spring 2021. The average minimum and maximum temperatures in the greenhouse were 20 ± 3 °C and 30 ± 3 °C day/night, respectively. The photoperiod consisted of 15 h of natural daylight and 9 h of darkness. The minimum relative humidity during the day was 60%, while the maximum relative humidity during the night was 90%.
Basil cultivars ‘Genovese’ and ‘Valentino’ (CV), both widely cultivated in the Italian peninsula, were used in this study.
Basil seeds were sown manually (23 per pot) in 1 L plastic pots on 12 May 2021, and the experiment was terminated on 21 June 2021 (after 40 days). In this experiment, a combination of peat moss and treated sediment from the port of Leghorn (Italy) was used as a growing medium for soilless cultivation. The sediment had been treated through phytoremediation for 3 years and further reclaimed through 3 months of landfarming [11]. The tested substrate mixes (SM) were prepared with three different ratios (v/v) of pure peat moss (Pe) and treated sediment (TS): (i) 100% Pe, considered as the control (TS0); (ii) 87.5% Pe and 12.5% TS (TS12.5); (iii) 75% Pe and 25% TS (TS25). Percentages of the TS were chosen based on results from a preliminary test. All plants received similar cultural practices except for irrigation, with all pots being initially hand irrigated with sprinkling water after sowing and subsequent irrigations carried out according to planned schemes. Water was applied via subirrigation below the soil surface to raise the water table into or near the plant root zone, with three raised benches receiving different amounts of water (IR): (i) normal (IR1 = 3.6 L day−1); (ii) low, reduced by 25% (IR2 = 2.7 L day−1); (iii) very low, reduced by 37.8% (IR3 = 2.25 L day−1). The pH of incoming water was adjusted to 6.0–6.3. The experiment was conducted in a complete randomised block design with three replicated blocks, each block consisting of 6 pots (each containing 23 seeds) per SM × IR × CV combination, resulting in a total of 162 pots per cultivar.
Before the cultivation, each pot was fertilised with 4 g of Osmocote Exact fertiliser Standard 3–4 M (16-9-12 + 2 MgO + TE by ICL, Milano, Italy). Weeds were controlled by hand during plant growth as required.
2.2. Plant Material and Greenhouse Experimental Design
Before sowing, the substrate mixes used in the study were analysed. Samples were collected from the three blocks to obtain approximately 500 g of material. The physico-biochemical parameters including humidity, volatile solid, porosity, bulk density, pH, electrical conductivity (EC), ammonia (N-NH4+), nitrates (N-NO3−), total organic carbon (TOC), total nitrogen content (TN), total phosphorus content (TP), enzymatic activities such as β-Glu, Acid-P, Buty-E, Aryl-S, and element concentration (Ca, Mg, Na, K, Fe, Mn, Cu, Zn, Ni, Cr, Pb, Cd) were determined as described in Table 1.

Table 1.
Physicochemical properties of the substrate mixes (TS0, TS12.5, and TS25) used for potted basil cultivation.
2.3. Germination
Number and percentage of germinated seeds and mean germination time were evaluated for each cultivar grown on the tested substrates with different water supplies. Germination was assessed every day and continued until there were no newly germinated seeds for three consecutive days. Rising of the cotyledons above the ground was the criterion for germination assessment. Cumulative seed germination percentage (Gmax), representing the total seeds germinated at the end of the trial and the mean germination time (MGT), was calculated for each SM × IR × CV combination. The MGT was computed using the formula cited by Ellis and Roberts [29]:
where n is the number of seeds newly germinated at time D; D is days from the beginning of the germination test; Σn is final germination.
MGT = Σ(n × D)/Σn
2.4. Biomass Production
After 40 days of culture, the aerial part of all plants was harvested for above-ground biomass production and other destructive plant analyses. Yield performance was assessed as the amount of basil produced by a single commercial unit of a basil pot that is typically sold in supermarkets. This measurement was based on the yield of fresh and dry biomass from a basil pot that was obtained by sowing 23 seeds per pot. Prior to separating the leaves from the stems of each pot unit, the weight of individual plants was also computed to assess the growth of ‘Genovese’ and ‘Valentino’ basil cultivars, independent of their germination capacity. The leaves harvested from every single pot were also used to determine leaf area using a leaf area meter (WinDIAS Image Analysis System, Delta-T Devices Ltd, Cambridge, UK) following the procedure of Massa et al. [30]. The aerial part of each block was weighed, a 5 g sample of fresh leaves was immediately freeze-dried, and the powder was stored at −20 °C until extraction and analysis of primary metabolites. Leaf discs were also taken for pigment analysis. The remaining portions of leaves and stems were dried in a forced-air oven at 75 °C until a constant weight was achieved to determine the dry matter percentage. The percentage of water in the tissue (water content, %) was determined following oven desiccation for 48 h. The percent of water in the tissue was calculated as 100 (FW − DW)/FW, where FW is the fresh weight of the aerial part and DW is the dry weight of the aerial part. Dry leaf samples were used for further analysis and evaluation of the plant’s chemical composition and characteristics.
2.5. SPAD-Readings
Shortly before biomass analysis and pigment determination, SPAD measurements were taken using a hand-held dual-wavelength chlorophyll meter Minolta SPAD-502 (Minolta Camera Co., Ltd., Osaka, Japan). To ensure accurate readings, the selected leaves were first cleaned of any surface dust. For each block, SPAD readings were taken on each side of the midrib of 5 fully expanded leaves. Careful attention was paid to ensure that the SPAD meter sensor completely covered the leaf lamina and that there was no interference from the veins or midribs while recording the SPAD readings. The instrument stored and automatically averaged the readings to generate one reading per plot.
2.6. Determination of Photosynthetic Pigments and Malondialdehyde MDA
In order to determine the amount of photosynthetic pigment (chlorophylls a and b, carotenoids) and phenols, the method of Lichtenthaler and Buschmann [31] was used. Specifically, fresh frozen leaf discs were obtained from the middle portion of fully expanded leaves taken immediately following ‘Genovese’ and ‘Valentino’ basil harvest. A sample for each substrate × water regime replicate was considered. The absorbances of chlorophyll a and b were assessed using a spectrophotometer (Thermo Evolution 300 UV-Visible Spectrophotometer, Thermo Fisher Scientific, Waltham, MA, USA) at 665.2 nm and 652.4 nm, respectively, while carotenoids and phenols were read at wavelengths of 470 nm and 320 nm, respectively. The amounts of all pigments were expressed as mg g−1 of leaf FW.
Furthermore, basil leaf samples were analysed for malondialdehyde (MDA) content by adapting the procedure used by Heath and Packer [32]. Fresh leaf tissue weighing 500 mg was homogenised in 0.1% trichloroacetic acid (TCA), and the resulting homogenate was centrifuged at 11,320× g for 5 min. Then, 1 mL of the supernatant was mixed with 4 mL of 0.5% thiobarbituric acid in 20% TCA and heated at 95 °C for 30 min, followed by immediate cooling in an ice bath. After centrifugation at 5700× g for 10 min, the absorbance of the supernatant was measured at 532 nm. The MDA content was calculated using an extinction coefficient of 155 mM−1 cm−1 and expressed as nmol g-1 dry weight (DW).
2.7. Leaf Nutrients, Heavy Metal, and Organic Contaminant Contents
Foliar concentration of mineral elements and organic micropollutants were analysed in 500 mg oven-dried samples in compliance with official analytical protocol standards adopted at the international, European, and Italian levels. To determine the concentration of nutrients (Ca, K, Mg, Mn, Na, P) and heavy metals (Cd, Cr, Cu, Fe, Ni, Pb, Zn), pulverised dried leaves of each sample underwent mineralisation through microwave-assisted acid digestion (Ethos 1, Milestone s.r.l., Sorisole, Bergamo, Italy) using 25 mL of H2O2:HNO3 1:3 (v/v) and were subsequently analysed by ICP. To account for potential metal contamination of reagents and reactors, blanks were prepared for each analytical batch according to UNI EN 13804 [33], UNI EN 13805 [34], and UNI EN 17294-2 [35] standards. Total nitrogen levels were analysed according to CEN/TS 15407 [36], while hydrocarbons with carbon numbers ranging from 10 to 40 (C10–40) were observed using UNI EN 14039 [37]. Polycyclic aromatic hydrocarbons (PAHs), which are a group of organic pollutants that can be harmful to human health and the environment, were analysed using the PPA 338 rev 1 [38] standard, with the High-Performance Liquid Chromatography-Fluorescence Detector (HLPC-FLD) technique. The PAHs that were explored included anthracene, benzo(a) anthracene, benzo(b)fluoranthene, benzo(g,h,i)perylene, benzo(k)fluoranthene, chrysene, dibenzo(a,h)anthracene, phenanthrene, fluoranthene, and fluorene. These estimates provide valuable information about the chemical composition of the sample and can be used to assess the quality and safety of plant material.
2.8. Nutraceutical Analysis
Freeze-dried leaf samples from each block belonging to the same SM × IR × CV combination were used for detection of primary metabolites. All analyses were conducted in triplicate.
2.8.1. Quantification of Soluble Sugar
A high-performance anion exchange chromatography with pulsed amperometric detection, HPAEC-PAD, system was used to quantify sugars in the given sample. To prepare the sample, 1 g of leaves was homogenised in 25 mL of water at 30 °C and extracted using sonication for 20 min. The suspensions were then centrifuged, and the supernatant was filtered and injected for analysis. A Dionex system (Dionex Corp., Sunnyvale, CA, USA) equipped with a pulsed amperometric detector (model ED40) and an injection loop of 10 µL Dionex CarboPac PA100 analytical column (250 mm × 4 mm i.d.) was used for the carbohydrate analysis. Moreover, a guard CarboPac PA100 column (50 mm × 4 mm i.d.) was also used to optimise identification and quantification. The eluent used for the analysis was 40 mM NaOH + 1 mM Ba(CH3COO)2 at a flow rate of 0.5 mL min−1. The eluent was obtained using carbonate-free 50 wt% NaOH and degassed water continuously sparged with purified N2. The detection potential was set at the gold working electrode, EDET + 0.10 V vs. Ag|AgCl, in accordance with Cataldi et al. [39].
2.8.2. Quantification of Organic Acid
To prepare the basil extract for organic acid quantification, fresh homogenised samples were diluted 1:10 in acidic water containing 0.5% formic acid. The extract was purified using a solid phase extraction (SPE) C18 cartridge, and succinic acid (d4) was added as an internal standard (IS) to achieve a final concentration of 5 ng µL−1. The analysis was carried out using an HPLC system with a Column X Terra RP18 (particle size 5 µm, 4.6 × 150 mm at 18 °C). The mobile phases were acidic H2O (0.5% formic acid) (phase A) and acidic MeOH (0.5% formic acid + IPA 10%) (phase B) with varying flow rates. During organic acid quantification, the flow rate was kept constant at 530 µL min−1, while the percentage of mobile phases A and B were formulated at different time intervals, as follows: 98% of phase A and 2% of phase B from 0.0 to 1.5 min; 95% phase A and 5% phase B at 6.5 min; 85% phase A and 15% phase B 7.0 min; 50% of phase A and 50% of phase B between 8.5 and 9.0 min; return to the original conditions of 98% phase A and 2% phase B from 10.0 to 22.0 min. Detection was performed using a triple quadrupole SCIEX 4000 Qtrap with Turbo V ESI source and multiple reaction monitoring (MRM) in negative mode.
2.8.3. Determination of Total Phenolic Content
The Folin–Ciocalteu method, as described by Duarte-Almeida et al. [40], was employed to determine the total phenolic content (TPC) of basil extracts. In brief, 0.1–0.5 mL of extract was mixed with 0.1 mL of Folin–Ciocalteu and 6 mL of distilled water. The solution was then blended with 2 mL of 15% Na2CO3 solution and 10 mL of distilled water at room temperature. The mixture was kept in the dark at 37 °C for 2 h, followed by measurement of the absorbance at 750 nm. As standard, gallic acid (Sigma-Aldrich, St. Louis, MO, USA) was used and the total phenolic content was expressed as milligrams of gallic acid equivalents per 100 g of fresh basil leaves (mg GAE 100 g−1 FW).
2.8.4. Determination of Antioxidant Power
The method used by Chang et al. [41] was employed to evaluate the antioxidant activity of the samples based on their ability to donate hydrogen or scavenge radicals of 2,2-diphenyl-1-picrylhydrazyl (DPPH). To conduct the analysis, 5 g of each fresh basil sample was mixed with 1 mL of freshly prepared 1 mM DPPH ethanolic solution and allowed to stand for 30 min before spectrophotometric detection at 517 nm using a Shimadzu UV-1900 Spectrophotometer (Shimadzu, Kyoto, Japan). The DPPH scavenging activity was calculated using the following formula:
where A_blank is the absorbance of the blank (containing all reagents except the sample) and A_sample is the absorbance of the sample (DPPH solution plus extract).
DPPH scavenging activity (%) = [(A_blank − A_sample)/A_blank] × 100
2.9. Statistical Analysis
Data were processed with ANOVA analysis of variance using SPSS for Windows v25 (SPSS Inc., Chicago, IL, USA) to assess the significance of the main factors and of interactions. Significant differences were tested at p ≤ 0.01 using Duncan’s multiple range test. Before statistical analysis, percent values were subjected to arcsine transformation and the original values of all transformed data were presented. Furthermore, the entire dataset presented in Tables S1–S3 was subjected to a multivariate statistical analysis approach to simultaneously analyse the impacts associated with the incorporation of TS in the substrate (SM), the amount of water given during irrigation (IR), and the plant genotype (CV). For this purpose, a correlation analysis was carried out in accordance with Pearson correlation coefficient on all plant variables to select and remove the highly associated ones using the R-package corrplot v. 0.92 [42]. Subsequently, a Principal Component Analysis (PCA) was accomplished on the chosen traits with R-packages FactoMineR v. 2.7 [43] and factoextra v. 1.0.7 [44].
3. Results
3.1. Physicochemical Properties of the Substrate Mixes
The physicochemical properties of the substrate mixes are reported in Table 1. The pH and EC values of substrate mixes containing TS were higher than those of peat, rising linearly with increasing TS percentage in the SM (Table 1), in relation to the presence of alkaline elements (Ca, Mg, and Na) despite peat. These results are consistent with previous reports [12,45,46]. However, the pH values of the substrates were moderately acidic, ranging from 5.3 in TS0 to 5.8 in TS25.
The electrical conductivity (EC) increased with higher TS in the substrate mix. Higher values of EC were observed with the addition of TS probably due to the marine origin of the sediment, namely the influence of the conductive saltwater environment from which it is derived. Concerning the mineral N, both the N-NH4+ and N-NO3− concentrations were highest in pure peat (TS0) and decreased with increasing TS fraction. Similarly, the highest values of total organic carbon (TOC) and total nitrogen (TN) were found in the peat-based substrate as compared with sediment-based media, with values reduced by more than half in the TS25. Humidity, volatile solid, and porosity followed the same TS0 > TS12.5 > TS25 trend. Bulk density (BD) and phosphorus availability (TP) showed an opposite trend, with significantly higher values in the sediment-based substrates compared with those in the control.
In general, the availability of calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), and iron (Fe) was highest in TS25 and lowest in TS0. Regarding the availability of trace metals, zinc (Zn), manganese (Mn), and lead (Pb) were below the detection limit (0.01 mg kg−1) in peat substrate, while found in high concentrations in substrates containing 12.5% and 25% TS (102.6–137.7 mg kg−1; 1106.3–1538.0 mg kg−1; 25.6–35.8 mg kg−1, respectively). Chromium (Cr) was minimal in the peat, whereas the copper (Cu) and nickel (Ni) values were about one-sixth to -eighth lower than in TS25.
Concerning substrate enzyme assay, β-glucosidase (β-Glu) and butyrate esterase (Buty-E) activity behaved similarly and were not influenced by the substrate mix. Phosphatase activity (Acid-P) showed significantly lower values in TS25 compared with TS0 and TS12.5 (1320.9 vs. 1665.0 and 1684.4 µmol g−1 h−1). On the other hand, the enzyme activity related to S cycles (Aryl-S) content was minimum in the control (Pe) compared with sediment-based substrates (33.5 vs. 67.1 and 61.6 µmol g−1 h−1).
3.2. Plant Development and Growth
The significance of single and combined effects of the studied factors are given in Table 2. ANOVA analysis showed that most basil parameters were significantly affected (p ≤ 0.01) by the main effect of substrate mix (SM), irrigation volume (IR), and cultivar (CV), and by some of their two-way order interactions.

Table 2.
Effect of substrate mix (SM), applied irrigation volume (IR), and cultivar (CV) on germination and biomass parameters of potted basil.
3.2.1. Germination
According to Table 2, the germination rate was highest in the substrate containing 25% TS (53.0%) and when the highest water dose was applied (55.8%). Notably, no significant differences were found between cultivars. On the other hand, MGT showed an opposite trend, with higher values scored for control seedlings (17.0 d) and under reduced irrigation conditions (17.7 d).
A significant SM × IR interaction occurred for both Gmax and MGT. A detrimental impact on germinability was observed when seeds were sown in pure peat under reduced water input. Specifically, Gmax was minimum (22.5%) and MGT value was maximum (18.8 d) for seeds obtained in TS0 with water input IR3, but values did not differ significantly even for seeds grown in pure peat under IR2.
3.2.2. Biomass Production
ANOVA analysis showed that SM, IR, and CV factors exerted a significant effect on the studied growth parameter with only a few exceptions, being the substrate without effect on Plant FW while the cultivar did not affect Plant FW, SFW, and TDW (Table 2).
Yield of potted basil plants, as expressed by both total fresh weight of basil biomass and leaf area, was lower for plants obtained in pure peat substrate and increased with increasing sediment fraction in the substrate. On the other hand, LFW, SFW, TFW, and LA progressively decreased with decreasing daily water supply. When evaluating each pot of basil as a separate unit, ‘Valentino’ produced a greater aboveground fresh biomass than ‘Genovese’, primarily associated with the significantly higher LFW component in the ‘Valentino’ pot compared with the ‘Genovese’ pot (31.3 g pot−1 versus 23.8 g pot−1). On the other hand, there was no significant difference in TDW between the two cultivars. The reason for this discrepancy in DW between ‘Genovese’ and ‘Valentino’ tissues can be explained by two factors: the higher water content (WC) in ‘Valentino’ tissues, as well as the greater contribution of leaves to total DW. On average, leaves counted for three and four times more than the stem in ‘Genovese’ and ‘Valentino’, respectively. Specifically, ‘Valentino’ had significantly higher LDW (2.7 g pot−1) compared with ‘Genovese’ (2.3 g pot−1), consistent with the differences observed in LFW values. In contrast, ‘Valentino’ had significantly lower SDW (0.6 g pot−1) than ‘Genovese’ (0.8 g pot−1), likely because its stem tissues were more tender and less leathery than those of ‘Genovese’.
Focusing now on the fresh weight per plant, there were no discernible differences between the substrates and cultivars; it is only the volume of water that played a critical role in determining the variances in plant growth and development of ‘Valentino’ and ‘Genovese’ basil (5.0 g plant−1, 3.9 plant−1, and 2.6 plant−1 for IR1, IR2, and IR3, respectively).
The interaction between SM and IR had a significant impact on the growth and biomass production of the basil plants, with the only exception being TDW. The basil plants that were grown with a higher water amount exhibited the highest TFW, which amounted to 55.6 g pot−1, 62.9 g pot−1, and 58.6 pot−1 in the TS0, TS12.5, and TS25 substrates, respectively. Water shortening was always detrimental for plant biomass production; however, the effect of water lessening was much more evident on plants grown on TS0 and TS12.5 compared with TS25. Indeed, TFW values consistently decreased by 69% and 74% in TS0 and by 60% and 68% in TS12.5 under IR2 and IR3, respectively, whereas under the same conditions in TS25 the TFW was reduced by only 28% and 32%. The same trend was observed for LA and the other growth parameters referred to the basil pot (LFW, SFW, LDW, TDW), although more pronounced in fresh weight than dry weight.
The situation changed when turning back to plant FW. In this scenario, the plants cultivated on TS0 and TS12.5 with IR1, and on TS25 with IR2, exhibited the highest values (5.7 g plant−1, 5.3 g plant−1, and 5.1 g plant−1, respectively). On the other hand, the most noticeable decrease was observed in TS12.5 when the irrigation volume was minimised (2.1 g plant−1).
3.3. SPAD Readings, Photosynthetic Pigments, and MDA Content
Table 3 shows the SPAD values, Chl a, Chl b, total Chls, carotenoid, and MDA contents in basil leaves, according to the tested substrates mixes, irrigation doses, and cultivars. Some clear trends were observed despite significant differences found for some parameters. Increasing the amount of water used for irrigation led to higher levels of Chls and phenols and lower levels of carotenoids. However, the substrate composition did not have a noticeable effect on plant physiology, while the choice of cultivar did, with lower values of SPAD, Chls, and carotenoids and higher levels of phenols found in the ‘Valentino’ cultivar. Additionally, reduced water conditions under IR3 resulted in a significant increase in lipid peroxidation, as measured by MDA activity, while no differences were observed among substrates and cultivars.

Table 3.
Effect of substrate mix (SM), applied irrigation volume (IR), and cultivar (CV) on leaf pigment and phenols content, SPAD readings, and oxidative stress (MDA) of potted basil.
3.4. Leaf Nutrients and Heavy Metal and Organic Contaminant Contents
Table 4 shows the effects of the substrate, irrigation volume, and cultivar on the elemental composition and high-molecular weight hydrocarbons (C10–40) of basil leaf tissue. Regarding heavy metals, Cd, Cr, Ni, Pb, Cu, and Zn were not detected in basil leaves, while Fe was found at very low concentrations. Finally, among the numerous organic pollutants searched for, only high-molecular weight hydrocarbons were detected. Plants grown on pure peat showed higher Mn and lower Na than those grown on sediment-based substrates. The impact of irrigation on the nutrient content of crops, specifically Ca, Mn, Mg, Na, and total N, was disordered and lacked a precise correlation with the amount of water supplied to the plant. ‘Valentino’ plants had a significantly higher uptake of Ca, Fe, Na, and C10–40 compared with ‘Genovese’ (13.5 vs. 10.2 g kg−1, 89.7 vs. 47.8 mg kg−1, 1019.5 vs. 693.8 mg kg−1, 1.9 vs. 1.1 g kg−1, respectively, for Ca, Fe, Na, and C10–40).

Table 4.
Effect of substrate mix (SM), applied irrigation volume (IR), and cultivar (CV) on leaf elemental composition and high-molecular weight hydrocarbons (C10–40) of potted basil.
3.5. Nutraceutical Analysis
The amounts of total soluble sugars, organic acids, total polyphenols, and the antiradical activity found in basil leaves, as affected by SM, IR, and CV, are presented in Table 5. All the nutraceutical parameters were influenced by the type of substrate used for basil cultivation, whereas the irrigation volume and cultivar only had a significant effect on certain qualitative attributes.

Table 5.
Effect of substrate mix (SM), applied irrigation volume (IR), and cultivar (CV) on leaf total soluble sugars, organic acids, total polyphenols, and DPPH scavenging activity of potted basil.
Significant variations in leaf sugar content were detected among the different substrates, irrigation volumes, and cultivars. Our study revealed that an increase in TS in the substrate mix, as well as a decrease in water supply, led to a rise in sugar content in leaves. Of the soluble carbohydrates, only glucose and fructose were predominant (up to 2.2 and 3.7 mg·g−1 FW, respectively), while sucrose was only a minor component (up to 0.2 mg g−1 FW). Compared with ‘Genovese’, a greater amount of total sugars was observed in the leaf tissue of ‘Valentino’. Furthermore, we observed that the presence of TS in the substrate resulted in a significant increase in sugar content under the most severe water shortening as compared with normal irrigation conditions (2.9–2.8 mg g−1 vs. 1.6–1.5 mg g−1 and 3.6–4.9 mg g−1 vs. 1.3–3.7 mg g−1, in leaves of ‘Genovese’ and ‘Valentino’ obtained on TS12.5 and TS25, respectively) (Table S2).
Regarding organic acids, malic acid was by far the most predominant organic acid found in mature leaves of basil plants, followed at a distance by citric and shikimic acids. Ascorbic acid was below the threshold limit of 1 mg g−1, whereas quinic acid was not detected. When grown on TS25 substrate, basil leaves exhibited a significant increase in malic acid content; such increase was more pronounced under reduced water availability, although the differences between water regimes were not statistically significant. There was a considerable cultivar-dependent variation in the malic acid content, with the lowest value recorded in ‘Genovese’ (48.79 vs. 60.73 mg 100 g−1) (Table 5). Increasing the TS percentage in the substrate mix led to a significant decrease in citric acid and shikimic acid levels, whereas a decrease in water availability resulted in a considerable increase in both acid concentrations. However, statistical significance was only observed in the case of citric acid. Shikimic acid was found to a greater extent in the ‘Genovese’ than in the ‘Valentino’ leaf tissue (4.50 vs. 4.14 mg 100 g−1).
On average, the total polyphenolic content ranged from 156 to 456 mg GAE 100 g−1 FW for samples of ‘Valentino’ TS12.5 × IR1 and ‘Genovese’ TS0 × IR3, respectively (Table S2). Specifically, ‘Valentino’ exhibited a significant decrease in polyphenol values compared with ‘Genovese’, with an average of 222.4 mg GAE 100 g−1 FW, almost half the value of that of ‘Genovese’ (417.56 mg GAE 100 g−1 FW). A decrease in water supply led to a build-up of polyphenolic compounds, while the effect of different levels of TS in the substrate was not clear, as values were in the order TS0 > TS25 > TS12.5.
Substrate composition significantly affected the antioxidant activity of the basil plants determined using the DPPH method. Lower DPPH values were found in TS12.5 (56.80%) and TS25 (59.79%) substrates, whereas control plants grown on TS0 showed the highest antioxidant activity (79.47%). Compared with ‘Valentino’, ‘Genovese’ showed a higher DPPH-scavenging activity (68.17% vs. 62.53%), exhibiting the maximum value in TS0 with IR3 (Table S2).
3.6. Principal Component Analysis (PCA)
PCA was carried out on all the studied plant variables (Tables S1–S3). Prior to this, a correlation matrix was achieved in order to detect and remove plant pairwise parameters that showed a high association (Figure S1). In more detail, four traits (LA, LDW, Chl a, and shikimic acid) were discarded for the multivariate analysis, revealing a Pearson correlation coefficient (r) greater than 0.95 with other variables: LA with TFW; LDW with TDW; Chl a with TChls; and shikimic acid with DPPH. Regarding PCA, the first three PCs described 33.48%, 24.90%, and 15,12% of the total variation, respectively, for a cumulative explained variation of 73.50%. All biomass parameters (i.e., TFW, Plant FW, SFW, SDW, TDW, and WC) were positively related to the PC1, as well as Gmax, calcium, magnesium, and sodium contents. On the other hand, leaf pigments (i.e., carotenoids), nutritional and nutraceutical variables (i.e., DPPH, total polyphenols, citric acid, and manganese), along with TMG, had a significant negative impact on PC1 (Table 6). Chlorophyll parameters (i.e., TChls and SPAD) and total polyphenols were significant and positively correlated on PC2, while leaf nutrients, such as iron and sodium, as well as MDA and C10–40, were negatively and highly associated with PC2.

Table 6.
PCA loadings of the first two PCs. The percentage of variation for each PC as well as the cumulative percentage of variation are accounted. * Variables with component loading used to interpret the PCs: threshold = 0.198.
Figure 1 provides a biplot of the PCA obtained using the first two PCs for the 26 considered variables. This biplot shows the correlation between SM × IR × CV variables measured on potted basil plants. A clear separation between the two cultivars was observed, with most ‘Genovese’ basils located in the middle-top of the plot, while all ‘Valentino’ samples were plotted in the middle-bottom. Being located in the top part of the plot, ‘Genovese’ resulted positively associated with TChls, TPC, and SPAD, while ‘Valentino’, being located in the bottom part of the figure, resulted greatly influenced by MDA, Fe, Na, and C10–40.
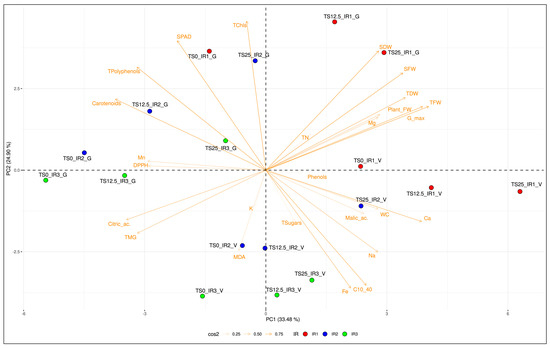
Figure 1.
PCA-biplot of the 26 selected variables of Ocimum basilicum in accordance with all the treatments analysed (SM × IR × CV). Samples are coloured based on the different amount of irrigation volume (IR) they received.
The two cultivars were mainly grouped based on the amount of irrigation volume they received (IR). When analysing each cultivar separately, all ‘Valentino’ IR1 treatments were clustered in the middle-right section of the plot. These treatments were positively affected by growth parameters such as Gmax, SFW, TDW, TFW, and plant FW on PC1. As for IR2 and IR3 treatments, they gradually moved further downwards and towards the core of the plot. IR3 was more influenced by the negative association of PC2, but the order of TS25, TS12.5, and TS0 always remained the same from right to left. As for ‘Genovese’, all IR1 treatments were clustered in the middle-top section of the biplot, while IR2 and IR3 treatments were gradually shifted towards the middle-left section. The TS order (i.e., TS25, TS12.5, and TS0) remained the same from right to left. Interestingly, in both cultivars, TS25 × IR2 was plotted near the basil plants that were cultivated with the normal irrigation volume required (IR1).
4. Discussion
4.1. Effect of Substrate Composition
The bulk density (BD) values of TS-based substrates exhibited consistent results, being either slightly higher (TS25) or lower (TS12.5) than the maximum threshold value of 0.4 g cm−3, which is considered ideal for a substrate. The BD value of a substrate is an essential parameter that influences the growth and development of plants, as it reflects the density of the substrate, which, in turn, determines the substrate’s porosity. The porosity of the substrate affects its capacity to hold and transport water and nutrients to the plant roots, and most importantly, it impacts the substrate’s ability to facilitate the movement of air through it. As air movement is crucial for healthy root growth, the substrate’s porosity can significantly influence the growth and quality of plants [47,48].
All macro- and micro-element concentrations were positively and significantly affected by the presence of TS in the media, suggesting that sediment is a good source of mineral nutrients for plants. Leaf nutrient analysis is important to understand the effects of treatments on the basil’s nutritional value. None of the macronutrients (Ca, Fe, K, and Mg) differed between substrates. Although greater amounts of P and K were available in the TS25 substrate and it is likely plants have taken them up, resulting in additional growth and yield, it did not affect the nutrient content in the leaves. The tested substrates differed only in their Mn and Na contents. Acidic substrates tend to have higher levels of Mn available for plant uptake, while Na uptake by plants is generally low in acidic media due to the limited availability of Na in the soil solution [49,50].
According to Nin et al.’s [46] findings, the presence of TS in the substrate had an impact on the Acid-P and Aryl-S enzymatic activities, which are linked to the P and S cycles. This is because microorganisms are known to optimise their metabolite production when faced with limited nutrient resources, as described by Fujita et al. [51]. Peat had the highest levels of acid phosphatase, indicating that P was the limiting nutrient under these conditions, as P availability is typically low in peatland [52]. Conversely, the trend for Aryl-S was the opposite, with higher values observed in TSs compared with peat. This may be due to microorganisms’ increased demand for S, which is likely a result of the high level of inorganic sulphur in the sediment [53]. Meanwhile, β-Glu and B-Est values in TSs did not differ significantly from those in peat, despite being lower in the former.
Some previous studies have explored how substrate composition affects the yield and content of active ingredients in greenhouse-cultivated basil. Mininni et al. [54] examined the impact of compost-enriched substrates on various productivity parameters and found that Posidonia-based compost could serve as a peat supplement, especially at a 30% rate. Ronga et al. [55] also discovered that adding spent coffee ground compost to the growing substrate led to increased plant growth, particularly at the foliar level. Burdina and Priss [56] observed that altering the proportions of peat and perlite used in substrate preparation significantly influenced basil’s productivity. According to Huang et al. [57], biochar and vermicompost had the potential to replace peat-based substrates for container-grown basil. Moreover, recent research by Kusnierek et al. [58] confirmed that the type and characteristics of growing media, specifically peat, coir, and wood fibre, have a notable impact on basil growth in hydroponic systems.
According to Kusnierek et al. [58], the pH level of the substrate may be a crucial factor affecting the development of basil plants in an ebb-and-flow system, as indicated by data from the chemical analysis of the substrate’s leachate. The authors suggested that the reduced growth observed in some of the wood fibre substrates may be due to excessively acidic conditions. Previous studies, such as Gillespie et al. [59], have also shown that nutrient uptake in basil leaf tissue decreases under acidic growth conditions. The relative nutrient availability to the plant root is well-known to vary at a given pH value. For instance, substrates with neutral to alkaline pH levels are known to limit the availability of micronutrients such as Zn, Mn, Cu, Bo, and Fe (except for Mo) and may result in deficiencies, as noted by Koc and Karayigit [60]. There are several studies available in the literature that support this observation, showing that plants grown on peat typically have a higher content of elements than those grown on compost-based substrates with higher pH levels [45,54]. Additionally, prior research has indicated that elevated levels of pH and EC in compost are the primary factors responsible for suppressing basil seed emergence [61,62].
In this context, by its nature, as observed in previous papers [45,46], marine sediment has an alkaline pH and could affect the nutrients’ uptake by plants; however, sediment-based mixtures composed of TS ≤ 25% in combination with peat had pH values somewhat higher than peat alone (5.3) but basically acidic, thus favouring the bioavailability of the metals in the soil [46,63]. Positively, in the current investigation, the pH and EC values observed in substrate mixes containing TS were deemed suitable for the growth of basil [64,65] and significantly lower than those reported by Ronga et al. [55]. Indeed, the composition of the substrate mix did not affect the rate of basil seedling emergence. Thus, TS seemed to be a valid alternative for agricultural media composition, even if its dilution with peat is more advisable due to its high pH and bulk density, as reported by other authors [66]. Hence, we prepared substrates’ mixtures by displacing commercial peat from 12.5 to 25% vol. with aliquots of TS for basil production. This aliquot allowed EC values to be close to the optimal value of 0.5 dS m−1 indicated by Abad et al. [67] for growing media.
Our data definitively demonstrate that SM containing TS led to greater basil biomass production compared with control plants. These results align with previous research from authors such as Bustamante et al. [68], Castillo et al. [69], Moore [70], and Ribeiro et al. [71], who observed that replacing a portion of peat with compost led to a clear increase in plant biomass. While compost may contain growth-promoting substances, the nutrient availability in both the TS and the compost may be responsible for the enhanced plant development, as noted by Bernal-Vicente et al. [72].
The presence of contaminants in sediments, particularly heavy metals, is an important aspect to consider since they are typically found in higher concentrations in dredged material compared with peat [11]. In our study, TS12.5 and TS25 had a considerably higher heavy metal content than peat, but it is worth noting that the overall heavy metal content in the sediment-based substrate dwindled by 36.4% in TS12.5 and 39.2% in TS25 by the end of the experiment. The Zn contents, initially particularly high and close to the threshold limit imposed by the Italian regulations (L.D. 75/2010) [73], were reduced at the end of June by approximately 74% on average. The decrease in Zn concentration in the TS-based substrate at the end of the experiment might be due to the transfer, as well as the accumulation of soluble and bioavailable Zn in plants, as reported by [17,74]. Moreover, Stancheva et al. [75] observed that Cd, Pb, and Zn concentrations reduced in media after O. basilicum harvest. From the same perspective, a considerable relationship between the metals’ content and their uptake by vegetation was found in a forsaken mining area in Romania, which was characterised by metal levels above intervention limits [76]. In our study, however, element analyses of basil leaves at the end of cultivation showed the absence of Cu, Zn, Ni, Cr, and Pb in the vegetal tissues. As a matter of fact, no major side effects attributable to the high concentration of Cr, Ni, Pb, and Zn, such as reduced biomass production, leaf spots, foliar chlorosis, delayed stem growth, etc., were detected on growth or photosynthetic activity. Rather, water more than substrate composition appeared to exert the greatest influence on basil growth and, to some extent, on the content of chlorophyll, carotenoids, phenols, and MDA. In this context, the chlorophyll values, which were between 0.60 and 0.63 µg mg−1 FW, were slightly higher than those reported by Abbasifar et al. [77], but consistent with the findings of earlier studies such as that by El-Kereti et al. [78]. It is advisable that the substrate mixes employed were adequate for the development of a healthy chlorophyll content relative to the plant’s growth, as no notable distinction was detected in the levels of chlorophylls and carotenoids among the various SMs tested. Furthermore, the presence of organic carbon and organic matter in the soil plays a crucial role in determining the bioavailability of metals, as they can limit the mobility of metals in the substrate by complexing them [54,79]. Although the total organic carbon (TOC) in TS12.5 and TS25 was significantly lower compared with that of peat substrate, it was still abundantly above the legal limits set by Italian Legislative Decrees (L.D.) 75/2010 [73]. Therefore, it is reasonable to assume that the TOC levels were sufficient to hinder the bioavailability of metals. This process might have also facilitated the growth and development of plants by enabling them to absorb trace amounts of metals as essential microelements. The tolerance mechanisms for metals in plants were indicated by the greater development of basil plants, in terms of both seed germination and plant growth, in sediment-based media. It has been reported that the addition of minute quantities of Cd, Pb, and Ni to the soil resulted in an increase in both plant yield and essential oil content of Ocimum basilicum when compared with the control samples [80]. Mentha pulegium L. grown on contaminated soil with Cu and Zn also showed similar results [81].
Additionally, the sediment-based substrates (TS12.5 and TS25) that were tested contained varying amounts of metals that might have exerted an antagonistic and competing effect on the uptake of certain metals, thus affecting their toxicity levels [82]. Earlier studies on soils contaminated by industry [75] and mining [17] showed that Cd, Co, Cr, and Pb primarily accumulate in the root, whereas Cu, Ni, and Zn can be found in either the root or the flower of basil. According to Prasad et al. [80], the reason for the higher concentration of Cr, Cd, Pb, and Ni in sweet basil roots compared with shoot tissues may be attributed to a regulatory mechanism that avoids the translocation of these metals from root to shoot. Zayed et al. [83] demonstrated that the translocation of Cr from roots to shoots is limited, while Angelova et al. [84] evidenced a reduced movement of Pb towards the aboveground parts of the plant due to a barrier effect of actively growing roots. Since sweet basil does not absorb large quantities of contaminants from soil and sequester them in the aboveground biomass, it is unlikely to be classified as a hyperaccumulator plant for such heavy metals.
Finally, the incorporation of TS into the substrate mixtures not only affected the biomass parameters, but also had an impact on some biochemical traits of basil plants. Substrate physico-biochemical properties are known to strongly affect the quality and yield of crops, as reported by Nissim-Levi et al. [85]. The impact of substrate composition, including the quantity and quality of certain elements, on molecules essential for human health, such as phenolic acids, has been examined. In the case of basil, Nurzyńska-Wierdak et al. [86] have shown that alterations in potassium levels can have a notable effect on the phenolic profile and antioxidant potential of leaves. In our study, the amount of total phenolic compounds of basil leaves was higher than the values reported by Kwee and Niemeyer [87] and Barickman et al. [88], but comparable to those from Stagnari et al. [89] and Ghasemzadeh et al. [90]. The SM treatments induced differences in both TPC values and radical scavenging activity. The presence of the TS always reduced TPC and DPPH concentrations in basil leaves, however, via significantly raising the values of the total soluble sugars and malic acid.
4.2. Effect of Water Amount Given with Irrigation
The scarcity of water is a critical issue for various crop species, and is particularly significant in Mediterranean regions where limited water resources act as a major constraint. Plant growth and yield are adversely affected by water stress in these regions, and the extent of reduction is determined by its severity [91] and the plant genotype [92]. Abiotic stress as a result of increased fluctuations in temperature and water stress affects the productivity of many important crops, including basil [93,94]. In particular, extreme water stress during seedling growth might reduce the yield of basil. In our study, the greater observed biomass production can be related to the higher percentage of germination obtained under the same operating conditions, as indicated by the high correlation found between growth parameters and germination in the biplot (Figure 1). It is well-known that water is an essential factor for proper seed imbibition and germination. The end of this phase is marked by an asymptotic approach to a final hydration level, which depends on soil–water potential, soil conductivity to water, seed–soil contact, and seed composition [95]. A more detailed analysis of the impact on the growth of individual plants, as shown by the plant FW, eliminated any disparities between substrates and cultivars. The only factor that accounted for differences among the various SM × IR treatments was water. This finding is in line with the fact that basil is a crop that thrives in warm weather and requires substantial amounts of water. As a result, it is typically cultivated at high temperatures and with frequent watering.
Chlorophyll concentration is one of the key factors in determining the intensity of photosynthesis and dry matter production. Chlorophyll content determined with a chlorophyll meter, e.g., SPAD, has been proposed as a good indicator of green colour and the stay green characteristic in several plant species, and also for stress tolerance index [96,97]. In our study, the SPAD-assessed chlorophyll content of basil was in agreement with earlier studies on sweet basil, as reported by Saha et al. [4] and Kalamartzis et al. [9]. Our findings showed slightly higher chlorophyll content than Abbasifar et al. [77] and slightly lower SPAD values than that proposed by Kashani et al. [98] and Ontiveros-Capurata et al. [99]. In addition, we found that there were discrepancies in leaf chlorophyll content across various volumes of water used to irrigate the basil crop, although the mean leaf chlorophyll, as measured by SPAD, did not exhibit a significant difference. According to Azia and Stewart [100], the relationship between SPAD readings and extractable chlorophyll was stronger when expressed on a leaf area basis rather than a fresh weight basis. This may be due to the uneven distribution of chlorophyll within the leaf, indicating that the SPAD meter could be more accurate in predicting chlorophyll content in basil leaves if extractable chlorophyll is expressed on a leaf area basis [101].
The effects of stress factors on the content and composition of pigments in plants have been extensively documented in the global literature [102,103,104]. Water stress affects enzyme activity, metabolic processes, mineral composition, and the uptake of essential mineral elements such as nitrogen, magnesium, zinc, and iron, which are vital constituents of chlorophyll molecules [105,106]. Moreover, water stress can induce oxidative stress, indirectly altering photosynthetic metabolism [107]. Previous studies have reported a reduction in chlorophyll content in plants under water stress, coupled with an increase in carotenoid concentration [108,109,110,111,112]. Similarly, our study revealed that basil plants exposed to water stress conditions exhibited a significant reduction in the concentrations of total Chls, Chl a, and Chl b, accompanied by an increase in carotenoid levels. Specifically, the Chl a and Chl b concentrations declined by 16% and 18%, respectively, from IR1 to IR3, which is not to be considered a substantial drop, despite the observed statistically significant differences in chlorophyll content. This finding aligns with prior research on other thermophilic plants [113]. Moreover, Kalamartzis et al. [9] reported that the cultivar, rather than irrigation, influenced basil, indicating that chlorophyll content might not be an ideal criterion for selecting cultivars that can tolerate water stress.
Carotenoids play a crucial role as accessory pigments for photosynthesis and also have a potential role in tissue protection by serving as biological antioxidants. They can quench free radicals and chlorophyll triplets, which could otherwise generate oxygen singlets that react with lipids, proteins, and other macromolecules, causing irreparable damage [109,114,115]. Our findings support the observation that the gradual increase in water stress levels leads to a protective mechanism against the stress, resulting in an increase in carotenoid concentration. Our data suggest that the decrease in chlorophyll during low-water conditions may be due to reduced metabolic functions, specifically in plant development and the production of metabolites aimed at protecting the plants, as evidenced by the increase in carotenoids. This is consistent with previous studies that have reported a greater increase in carotenoid concentration under water stress, such as those by Mibei et al. [116], Ahmadi et al. [117], and Mahdavikia et al. [111]. However, our results contradict the prior research conducted by Al-Huqail et al. [118], which found that basil experiences a decline in carotenoid content under any stress condition. Likewise, Barickman et al. [88] documented that both cold and heat stress negatively impaired the xanthophyll cycle pigments in basil plants.
Different physiological responses such as lipid peroxidation of membranes and the biosynthetic changes of secondary plant metabolites can be used to determine water stress in plants [119]. Water stress frequently induces harm to cell membranes. MDA is a crucial indicator of membrane system injuries and cellular metabolism deterioration [88]. Accordingly, our study revealed that when water intake was reduced to a maximum, there was an increase in MDA content.
Finally, water is an important factor that regulates several compounds in basil that have health benefits to humans, including sugars, organic acids, polyphenolics, and DPPH. The range of total soluble carbohydrates was between 0.8 and 4.9 mg g−1, with glucose and fructose being the predominant components. Sucrose, on the other hand, was only a minor constituent, consistent with the findings of Büchi et al. [120]. According to these authors, sweet basil leaves primarily utilise starch, with glucose and fructose to a lesser extent, as their primary non-structural storage carbohydrate. Soluble sugar content contributes to osmotic adjustment during stress and protects the structure of macromolecules and membranes during extreme dehydration [121]. In particular, proline, sucrose, and other organic sugars in quinoa contributed to osmotic adjustment during stress and protected the structure of macromolecules and membranes during extreme dehydration [122]. In this context, Mahdavikia et al. [111] reported an increase in soluble sugars under water deficit stress, which is consistent with our findings. Despite reducing the amount of water during irrigation, the total content of polyphenols at harvest remained unchanged, with no significant differences observed among the treatments. In fact, phenolics are typically associated with thermophilic plant defence mechanisms against heat and stress [123,124].
4.3. Effect of Plant Genotype (‘Genovese’ vs. ‘Valentino’)
Several papers have reported that water stress decreases plant growth and yield to varying degrees depending on the plant genotype and water deficit level [92,102,125,126,127]. In our study, we observed that there was a reduction in the growth characteristics of fresh basil due to reduced water availability, with significant differences in both cultivars. Cultivars did not differ in terms of germination rate, whereas the differences between cultivar growth, as demonstrated by higher TFW and LA values, were probably related to a greater leaf area expansion of ‘Valentino’ compact habitus compared with ‘Genovese’, as an intrinsic characteristic of the genotype behavioural pattern.
In general, ‘Genovese’ basil showed higher chlorophyll and carotenoid contents as well as SPAD values, whereas an opposite trend was shown for phenols, which were found in greater amounts in ‘Valentino’ basil. These results were confirmed in the PCA biplot, where ‘Genovese’ samples were clustered in the upper quadrants, while ‘Valentino’ treatments were in the bottom part of Figure 1. According to Ontiveros-Capurata et al. [99], the concentration of chlorophyll in basil leaves can be estimated with sufficient accuracy from SPAD readings and is also highly correlated to nitrogen concentration and fresh matter. Our data showed a good relationship between cultivars’ chlorophyll and SPAD values in accordance with Ontiveros-Capurata et al. [99], but these values were not related to fresh biomass production, which was found to be higher in ‘Valentino’ compared with ‘Genovese’.
During periods of underwater stress, plants may experience a decrease in chlorophyll levels and changes in leaf coloration, both of which are important visual quality indicators [128]. Scientific evidence indicates that basil undergoes physiological and biochemical changes in response to salt and water stress, which vary in magnitude depending on the genotype [86,129,130,131,132,133,134]. Among the cultivars studied, Bekhradi et al. [131] found that the ‘Genovese’ cultivar exhibited more pronounced changes than the Green Iranian cultivar, possibly due to a decrease in chlorophyll content and an increase in lipid peroxidation. Conversely, Mancarella et al. [132] found that ‘Genovese’ basil showed better water balance and photosynthesis preservation than ‘Napoletano’ basil, suggesting that ‘Genovese’ plants behave as early stage Na+ accumulators and have a better functioning ROS scavenging mechanism and photodamage protection. In our study, we observed a greater reduction in chlorophyll content and DPPH scavenging activity in ‘Valentino’ basil leaves compared with ‘Genovese’ basil leaves, but no differences in lipid peroxidation between the two cultivars. Based on these observations, it is reasonable to speculate that the dissimilarities observed in chlorophyll contents between the two Italian cultivars tested in this study are mainly attributable to their genotypic inherent coloration characteristics (Figure 2) rather than physiological stress effects.

Figure 2.
‘Valentino’ (left) and ‘Genovese’ (right) basil grown on TS12.5 under IR2.
The total polyphenols varied between the basil genotypes whether the plants were grown with or without TS and despite water availability. Specifically, ‘Genovese’ always had the maximum polyphenol content (417 vs. 222 mg 100 g−1) and displayed higher scavenging activity (68 vs. 62%) and shikimic acid content (4.5 vs. 4.1 mg 100 g−1). On the other hand, total soluble sugars and malic acid content in leaf tissue of ‘Genovese’ basil was reduced compared with ‘Valentino’ (2.1 vs. 2.5 mg g−1 and 48 vs. 60 mg 100 g−1, respectively). Similarly, Nurzyńska-Wierdak et al. [86] found that cultivar play an important role in the phenolic composition and antioxidant properties of basil. The study compared different cultivars and found that ‘Genovese’ had significantly higher total phenolic concentrations and antioxidant capacity than Sweet Thai and Dark Opal basil. Moreover, in basil leaves, malic acid is one of the primary organic acids that contribute to the overall taste and aroma of the herb, and its presence may fluctuate depending on the growing conditions and cultivar. According to Formisano et al. [133], ‘Genovese’ basil contains a higher concentration of malic acid compared with cultivars ‘Aroma 2’ and ‘Eleonora’.
4.4. Irrigation Set Points Based on Substrate Composition
The optimal amount of water required for crop growth and productivity is a crucial factor to consider. Given existing environmental threats such as water scarcity, it is important to determine the degree to which the amount of irrigation water can be reduced without affecting agronomic variables of commercial importance. The irrigation set points are influenced by the type of substrate used for plant cultivation, since the composition of the substrate affects its water-holding capacity, drainage, and aeration, which in turn affects the plant’s irrigation needs. As per research studies conducted by Caliskan et al. [135] and Doty [136], both the growth and morphology of basil were found to be significantly affected by the substrate type and volume of irrigation applied, as an adaptive response to the modified soil environment.
Regarding our research findings, it was noted that high IR1 mainly stimulated germination rate, biomass production, and leaf development area. Both water shortening treatments reduced plant growth (as recorded by the lower TFW and LA values of pot units), independently from the substrate type, as shown by each SM × IR2 × CV and SM × IR3 × CV combination, which were progressively shifted leftwards along PC1 when compared with the respective SM × IR1 × CV interaction (Figure 1). The reason for reduced growth in plants may be attributed to the limited water supply, which triggers several physiological changes in the plant. It is notorious that water stress lowers nutrient uptake and reduces photosynthesis, inducing a decrease in the production of energy and carbohydrates, which can negatively impact plant growth and ultimately lead to reduced yields and economic losses [137,138]. It has to be highlighted that in this work lower biomass productions were given by TS0 and TS12.5 under reduced IR2 and IR3 water availability and not by the most water stressed plant grown in TS25. The biplot effectively confirmed these results, showcasing how for each cultivar, both the TS0 × IR1 and TS12.5 × IR1 treatments were located to the right of their respective TS0s and TS12.5s samples that received lower irrigation volumes. Additionally, it is noticeable that both TS25s × IR2 were found near TS0s × IR1 treatments.
Kusnierek et al. [58] observed that basil plants experienced temporary water stress during the initial production phase when root development was underway, particularly in wood fibre substrates with inadequate capillary suction that contributed to delayed growth. In our operational conditions, basil roots in the peat-based substrate were likely adversely affected due to rehydration challenges following a period of water shortage. Similarly, in a prior study on Prunus laurocerasus it was proposed that the favourable leaf photosynthetic rate observed in plants grown in sediment-based media with limited water supply could be associated with enhanced stomatal conductance, possibly attributable to increased soil water retention capacity [46]. Mahdavikia et al. [111] noted that drought stress in basil cultivation can have adverse effects, but these can be mitigated by using biofertilisers and organic fertilisers that enhance water and mineral uptake. In our case, the higher levels of TS are believed to reduce the damage caused by increased water deficit due to their higher water retention capacity and balanced absorption of small amounts of essential microelements, such as metals.
For plant vegetative growth, nitrogen is a key element [139], and it is the most important nutrient for crop yield [140]. Bryson et al. [141] published recommended sweet basil tissue nutrient concentrations and, although these came from established plants grown outdoors in soil, these recommendations are still valuable for interpreting the results of our study with the two basil cultivars under greenhouse conditions. Sweet basil tissue N concentrations were within the recommended concentration range of 4% to 6% for all samples, except for TS12.5 × IR2, TS25 × IR2, and TS25 × IR3, which were slightly under the optimum range. Previous studies [142,143,144] have demonstrated a correlation between relative chlorophyll concentration measured by the SPAD meter and N concentration. In our study, despite observing a decrease in the SPAD index for the ‘Valentino’ cultivar compared with the ‘Genovese’ cultivar, the substrate mixes remained unaffected, further supporting that tissue N levels were within sufficient ranges. Our tissue samples had K concentrations of 4.5%, which is above the upper range of 2.0% for tissue K, regardless of substrate composition and water supply. Similarly, Walters and Currey [145] reported K concentrations ranging from 4.2 to 7.6%, postulating that this was likely due to an increase in K concentration in the nutrient solution from the addition of potassium carbonate to increase pH, which contributed to the luxury consumption of K. Although the Mg ranged from 2.5 to 3%, which is above the recommended range of 0.6% to 1.0%, there was no apparent connection with the starting substrate and applied water regime. On the other hand, Ca concentrations declined with increasing water deficit, but nearly all Ca concentrations across cultivars and substrates were within the recommended ranges.
The impact of the antagonistic relationship between cations, such as potassium (K) and sodium (Na), on the availability of Ca and Mg is also widely acknowledged in the literature [82,146]. In our study, water scarcity significantly increased Na levels in basil regardless of the substrate used. Previous research by Hniliekova et al. [147] has shown that high accumulation of sodium in plant leaf tissue can cause elevated malondialdehyde (MDA) levels, as confirmed by our findings. However, our study observed an interesting response in MDA levels. Specifically, basil grown under low IR3 conditions showed a significant increase in MDA content, while substrate type had no effect. This outcome suggests that the combination of low-water stress and peat substrate is more detrimental than the interaction of water stress and elevated TS occurrence.
Regarding the nutraceutical characteristics of the basil product, the general trend observed in the results was an increase in beneficial compounds such as carotenoids, polyphenols, citric acid, and sugars, along with a rise in DPPH percentage as water content decreased. More specifically, under conditions of reduced water availability, polyphenols increased in all SMs, citric acid in TS0s and TS12.5s, shikimic acid and DPPH in TS0s and TS25s, and basil leaves showed a significant increase in malic acid and sugars when grown on TS25 substrates (Table S2). Given the higher yields observed in substrates containing TS, it is plausible to suggest that incorporating TS up to 25% v/v into the substrate could result in good basil yields, even with a nearly 40% reduction in water intake, while maintaining high quality standards. This speculation is indirectly supported by a previous report from Bekhradi et al. [131], which stated that basil as a fresh herb can be cultivated with less water without compromising its quality characteristics.
5. Conclusions
In the present study, we tested whether the physiological and biochemical effects induced by the incorporation of treated sediments in the substrate mix allowed us to prime basil plants for enhanced tolerance to water stress of mild intensity, a condition that recurrently occurs during the growing season in the Mediterranean area. To our knowledge, this is the first research that investigated the suitability of TS as a substrate component in replacing peat aliquots to produce potted basil plants.
Overall, the two sediment-based substrates exhibited suitable physicochemical properties and high levels of macro- and micro-nutrients, indicating their potential as a partial peat substitute in potting cultivation, particularly at a 25% rate. Additionally, the two compost-based substrates showed no issues with metal accumulation in basil cultivation. Compared with control plants, the use of sediment-based substrates containing SM positively correlated with seed germination and biomass-based outputs. Furthermore, basil plants grown on TS25 under reduced watering exhibited superior productive parameters than those grown on peat alone. Overall, control peat with reduced water availability was correlated with lower plant yields and germination capacity. These results indicate that plants grown on peat–TS mixtures exhibited quality indices that were comparable to or even better than those observed in conventional soilless culture using only peat. Therefore, replacing peat aliquots with TS in moderate percentages could be an excellent sustainable practice for recycling phytoremediated sediments. We are aware that excessive application of TS can lead to increased soil alkalinity and reduced crop yields; however, we suggest that adding an appropriate amount of TS in the substrate can provide plants with essential nutrients more effectively. Additionally, TS may improve hydraulic conductivity and substrate moisture, which can enhance plant growth and increase crop yields under water-limited environments. In conclusion, these findings encourage the use of phytoremediated sediments as a viable substitute for peat in potting cultivation, addressing the challenges associated with sediment accumulation near coastal areas and their disposal in landfills.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15097314/s1, Table S1: Effect of SM × CV × IR interaction on germination and biomass parameters of potted basil; Table S2: Effect of SM × CV × IR interaction on leaf photosynthetic pigments, SPAD-readings, MDA content, chemical constituents, and DPPH scavenging activity of potted basil; Table S3: Effect of SM × CV × IR interaction on leaf elemental composition and high-molecular weight hydrocarbons (C10–40) of potted basil; Figure S1: Correlation plot according to Pearson correlation coefficient (r) on the 32 basil plant variables.
Author Contributions
Conceptualisation, S.N.; methodology, S.N. and M.A.; software, S.N., L.B. and D.B.; validation, S.N., L.B. and D.B.; formal analysis, S.N., L.B., D.M. and D.B.; investigation, S.N., M.A., D.M. and D.B.; resources, S.N.; data curation S.N., L.B., M.A., D.M. and D.B.; writing—original draft preparation, S.N., L.B. and D.B.; writing—review and editing, S.N., L.B., M.A., D.M. and D.B.; funding acquisition, S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the European Life Project SUBSED “Sustainable substrates for agriculture from dredged remediated marine sediments: from ports to pots” (LIFE 17 ENV/IT/000347).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Environment Agency European Environment Agency. 2021. Available online: https://www.eea.europa.eu/highlights/water-stress-is-a-major/ (accessed on 15 March 2023).
- Bączek, K.; Kosakowska, O.; Gniewosz, M.; Gientka, I.; Węglarz, Z. Sweet Basil (Ocimum basilicum L.) Productivity and Raw Material Quality from Organic Cultivation. Agronomy 2019, 9, 279. [Google Scholar] [CrossRef]
- Jakovljević, D.; Stanković, M.; Warchoł, M.; Skrzypek, E. Basil (Ocimum L.) Cell and Organ Culture for the Secondary Metabolites Production: A Review. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 149, 61–79. [Google Scholar] [CrossRef]
- Saha, S.; Monroe, A.; Day, M.R. Growth, Yield, Plant Quality and Nutrition of Basil (Ocimum basilicum L.) under Soilless Agricultural Systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Tebow, J. Evaluating Silicon Foliar Sprays as a Strategy to Improve Postproduction Performance of Potted Basil (Ocimum basilicum L.). Bachelor’s Thesis, Bachelor of Science in Agricultural, Food and Life Sciences, AR, USA, May 2021. Available online: https://scholarworks.uark.edu/hortuht/9/ (accessed on 15 March 2023).
- Khater, E.-S.; Bahnasawy, A.; Abass, W.; Morsy, O.; El-Ghobashy, H.; Shaban, Y.; Egela, M. Production of Basil (Ocimum basilicum L.) under Different Soilless Cultures. Sci. Rep. 2021, 11, 12754. [Google Scholar] [CrossRef] [PubMed]
- Radácsi, P.; Inotai, K.; Sárosi, S.; Czövek, P.; Bernáth, J.; Németh, É. Effect of Water Supply on the Physiological Characteristic and Production of Basil (Ocimum basilicum L.). Eur. J. Hortic. Sci. 2010, 75, 193. [Google Scholar]
- dos Santos, J.F.; Coelho, M.A.; Cruz, J.L.; Soares, T.M.; Cruz, A.M.L. Growth, Water Consumption and Basil Production in the Hydroponic System under Salinity. Rev. Ceres 2019, 66, 45–53. [Google Scholar] [CrossRef]
- Kalamartzis, I.; Menexes, G.; Georgiou, P.; Dordas, C. Effect of Water Stress on the Physiological Characteristics of Five Basil (Ocimum Basilicum L.) Cultivars. Agronomy 2020, 10, 1029. [Google Scholar] [CrossRef]
- Sanbugaro, A. L’utilizzazione Alimentare del Basilico. Bachelor’s Thesis, Università degli Studi di Padova, Padova, Italy, 2008. [Google Scholar]
- Macci, C.; Peruzzi, E.; Doni, S.; Vannucchi, F.; Masciandaro, G. Landfarming as a Sustainable Management Strategy for Fresh and Phytoremediated Sediment. Environ. Sci. Pollut. Res. 2021, 28, 39692–39707. [Google Scholar] [CrossRef]
- Tozzi, F.; Pecchioli, S.; Renella, G.; Melgarejo, P.; Legua, P.; Macci, C.; Doni, S.; Masciandaro, G.; Giordani, E.; Lenzi, A. Remediated Marine Sediment as Growing Medium for Lettuce Production: Assessment of Agronomic Performance and Food Safety in a Pilot Experiment. J. Sci. Food Agric. 2019, 99, 5624–5630. [Google Scholar] [CrossRef]
- Tozzi, F.; Renella, G.; Macci, C.; Masciandaro, G.; Gonnelli, C.; Colzi, I.; Giagnoni, L.; Pecchioli, S.; Nin, S.; Giordani, E. Agronomic Performance and Food Safety of Strawberry Cultivated on a Remediated Sediment. Sci. Total Environ. 2021, 796, 148803. [Google Scholar] [CrossRef]
- Martínez-Nicolás, J.J.; Legua, P.; Hernández, F.; Martínez-Font, R.; Giordani, E.; Melgarejo, P. Effect of Phytoremediated Port Sediment as an Agricultural Medium for Pomegranate Cultivation: Mobility of Contaminants in the Plant. Sustainability 2021, 13, 9661. [Google Scholar] [CrossRef]
- Chand, S.; Singh, S.; Singh, V.K.; Patra, D.D. Utilization of Heavy Metal-Rich Tannery Sludge for Sweet Basil (Ocimum basilicum L.) Cultivation. Environ. Sci. Pollut. Res. 2015, 22, 7470–7475. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Romanowska-Duda, Z.; Lisowska, K.; Wolf, W.M. Heavy Metal Uptake by Herbs. V. Metal Accumulation and Physiological Effects Induced by Thiuram in Ocimum basilicum L. Water Air Soil Pollut. 2017, 228, 334. [Google Scholar] [CrossRef] [PubMed]
- Dinu, C.; Vasile, G.-G.; Buleandra, M.; Popa, D.E.; Gheorghe, S.; Ungureanu, E.-M. Translocation and Accumulation of Heavy Metals in Ocimum basilicum L. Plants Grown in a Mining-Contaminated Soil. J. Soils Sediments 2020, 20, 2141–2154. [Google Scholar] [CrossRef]
- UNI EN 13037 Soil Improvers and Growing Media—Determination of PH 2012. Available online: https://store.uni.com/en/uni-en-13037-2012 (accessed on 27 December 2022).
- UNI EN 13038 Soil Improvers and Growing Media—Determination of Electrical Conductivity 2012. Available online: https://store.uni.com/en/uni-en-13038-2012 (accessed on 27 December 2022).
- ISO 11464 Soil Quality—Pretreatment of Samples for Physico-Chemical Analysis 2006. Available online: https://www.iso.org/standard/37718.html (accessed on 22 March 2023).
- EPA Method 1684 Total, Fixed, and Volatile Solids in Water, Solids, and Biosolids 2001. Available online: https://settek.com/epa-method-1684/ (accessed on 22 March 2023).
- UNI EN 13041 Soil Improvers and Growing Media—Determination of Physical Properties—Dry Bulk Density, Air Volume, Water Volume, Shrinkage Value and Total Pore Space 2012. Available online: https://store.uni.com/en/uni-en-13041-2012-106028.html (accessed on 27 December 2022).
- ISO 13878 Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (“elemental Analysis”) 1998. Available online: https://www.iso.org/standard/23117.html (accessed on 22 March 2023).
- ISO 10694 Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis) 1995. Available online: https://www.iso.org/standard/18782.html (accessed on 22 March 2023).
- EPA Method 3050B Acid Digestion of Sediments, Sludges, and Soils 1996. Available online: https://settek.com/epa-method-3050b/ (accessed on 22 March 2023).
- EPA Method 6010C Inductively Coupled Plasma-Atomic Emission Spectrometry 2000. Available online: https://settek.com/epa-method-6010c/ (accessed on 22 March 2023).
- Marx, M.-C.; Wood, M.; Jarvis, S.C. A Microplate Fluorimetric Assay for the Study of Enzyme Diversity in Soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Vepsäläinen, M.; Kukkonen, S.; Vestberg, M.; Sirviö, H.; Niemi, R.M. Application of Soil Enzyme Activity Test Kit in a Field Experiment. Soil Biol. Biochem. 2001, 33, 1665–1672. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved Equations for the Prediction of Seed Longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Massa, D.; Malorgio, F.; Lazzereschi, S.; Carmassi, G.; Prisa, D.; Burchi, G. Evaluation of Two Green Composts for Peat Substitution in Geranium (Pelargonium zonale L.) Cultivation: Effect on Plant Growth, Quality, Nutrition, and Photosynthesis. Sci. Hortic. 2018, 228, 213–221. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4-3. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- UNI EN 13804 Foodstuffs-Determination of Elements and Their Chemical Species-General Considerations and Specific Requirements 2013. Available online: https://store.uni.com/en/uni-en-13804-2013 (accessed on 15 March 2023).
- UNI EN 13805 Foodstuffs-Determination of Trace Elements-Pressure Digestion 2014. Available online: https://store.uni.com/en/uni-en-13805-2014 (accessed on 15 March 2023).
- UNI EN ISO 17294-2 Water Quality-Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)-Part 2: Determination of Selected Elements Including Uranium Isotopes 2016. Available online: https://store.uni.com/en/en-iso-17294-2-2016 (accessed on 15 March 2023).
- UNI CEN/TS 15407 Solid Recovered Fuels-Method for the Determination of Carbon (C), Hydrogen (H) and Nitrogen (N) Content 2006. Available online: https://store.uni.com/cen-ts-15407-2006 (accessed on 15 March 2023).
- UNI EN 14039 Waste Characterization-Determination of Hydrocarbon Content in the Range C10 to C40 by Gas Chromatography 2005. Available online: https://store.uni.com/uni-en-14039-2005 (accessed on 15 March 2023).
- PPA 338 Rev 1. 2021. Available online: https://urly.it/3rh23 (accessed on 15 March 2023).
- Cataldi, T.R.I.; Campa, C.; Casella, I.G.; Bufo, S.A. Determination of Maltitol, Isomaltitol, and Lactitol by High-PH Anion-Exchange Chromatography with Pulsed Amperometric Detection. J. Agric. Food Chem. 1999, 47, 157–163. [Google Scholar] [CrossRef]
- Maurício Duarte-Almeida, J.; Novoa, A.V.; Linares, A.F.; Lajolo, F.M.; Inés Genovese, M. Antioxidant Activity of Phenolics Compounds from Sugar Cane (Saccharum officinarum L.) Juice. Plant Foods Hum. Nutr. 2006, 61, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Lin, H.-Y.; Chang, C.-Y.; Liu, Y.-C. Comparisons on the Antioxidant Properties of Fresh, Freeze-Dried and Hot-Air-Dried Tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.R. Package “Corrplot”: (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 22 May 2022).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package ‘Factoextra’. Extr. Vis. Results Multivar. Data Anal. 2017, 76, 1–74. [Google Scholar]
- Tozzi, F.; Antonetti, M.; Prisa, D.; Burchi, G.; Turchi, A.; Macci, C.; Peruzzi, E.; Nin, S. Developing Patterns in Prunus laurocerasus Grown on Sediment Enriched Substrates. J. Soils Sediments 2022, 22, 2117–2127. [Google Scholar] [CrossRef]
- Nin, S.; Bonetti, D.; Antonetti, M.; Peruzzi, E.; Manzi, D.; Macci, C. Sediment-Based Growing Media Provides a Window Opportunity for Environmentally Friendly Production of Ornamental Shrubs. Agronomy 2022, 13, 92. [Google Scholar] [CrossRef]
- Bitterlich, M.; Franken, P.; Graefe, J. Arbuscular Mycorrhiza Improves Substrate Hydraulic Conductivity in the Plant Available Moisture Range under Root Growth Exclusion. Front. Plant Sci. 2018, 9, 301. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Akinci, S.; Bueyuekkeskin, T.; Eroğlu, A.; Erdoğan, B.E. The Effect of Humic Acid on Nutrient Composition in Broad Bean (Vicia faba L.) Roots. Not. Sci. Biol. 2009, 1, 81–87. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Miyabara, Y.; Kunito, T. Microbial Biomass and Ecoenzymatic Stoichiometries Vary in Response to Nutrient Availability in an Arable Soil. Eur. J. Soil Biol. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Giannini, V.; Peruzzi, E.; Masciandaro, G.; Doni, S.; Macci, C.; Bonari, E.; Silvestri, N. Comparison among Different Rewetting Strategies of Degraded Agricultural Peaty Soils: Short-Term Effects on Chemical Properties and Ecoenzymatic Activities. Agronomy 2020, 10, 1084. [Google Scholar] [CrossRef]
- Turner, B.L.; Condron, L.M.; France, C.A.M.; Lehmann, J.; Solomon, D.; Peltzer, D.A.; Richardson, S.J. Sulfur Dynamics during Long-Term Ecosystem Development. Biogeochemistry 2016, 128, 281–305. [Google Scholar] [CrossRef]
- Mininni, C.; Grassi, F.; Traversa, A.; Cocozza, C.; Parente, A.; Miano, T.; Santamaria, P. Posidonia oceanica (L.) Based Compost as Substrate for Potted Basil Production. J. Sci. Food Agric. 2015, 95, 2041–2046. [Google Scholar] [CrossRef]
- Ronga, D.; Pane, C.; Zaccardelli, M.; Pecchioni, N. Use of Spent Coffee Ground Compost in Peat-Based Growing Media for the Production of Basil and Tomato Potting Plants. Commun. Soil Sci. Plant Anal. 2016, 47, 356–368. [Google Scholar] [CrossRef]
- Burdina, I.; Priss, O. Effect of the Substrate Composition on Yield and Quality of Basil (Ocimum basilicum L.). J. Hortic. Res. 2016, 24, 109–118. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M.; Yu, P.; Zhou, C.; Liu, X. Biochar and Vermicompost Amendments Affect Substrate Properties and Plant Growth of Basil and Tomato. Agronomy 2020, 10, 224. [Google Scholar] [CrossRef]
- Woznicki, T.; Kusnierek, K.; Thomsen, M.; Sø nsteby, A. Basil (Ocimum basilicum L.) Growth Response to Wood Fiber Based Growing Media in Ebb-and-Flow System as a Function of the Electrical Conductivity of Nutrient Solution and Pot Size. In Proceedings of the II International Symposium on Growing Media, Soilless Cultivation, and Compost Utilization in Horticulture 1317, Ghent, Belgium, 22–27 August 2021; pp. 133–140. [Google Scholar]
- Gillespie, D.P.; Kubota, C.; Miller, S.A. Effects of Low PH of Hydroponic Nutrient Solution on Plant Growth, Nutrient Uptake, and Root Rot Disease Incidence of Basil (Ocimum basilicum L.). HortScience 2020, 55, 1251–1258. [Google Scholar] [CrossRef]
- Koç, E.; Karayiğit, B. Assessment of Biofortification Approaches Used to Improve Micronutrient-Dense Plants That Are a Sustainable Solution to Combat Hidden Hunger. J. Soil Sci. Plant Nutr. 2022, 22, 475–500. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Nabipour, M.; Azizi, M.; Gheisary, H.; Jalali, M.; Amini, Z. Effect of Kinds of Salt and Its Different Levels on Seed Germination and Growth of Basil Plant. World Appl. Sci. J. 2011, 15, 1039–1045. [Google Scholar]
- da Silva, T.I.; Nóbrega, J.S.; Figueiredo, F.R.A.; de Sousa, L.V.; da Silva Ribeiro, J.E.; Bruno, R.d.L.A.; Dias, T.J.; de Albuquerque, M.B. Ocimum basilicum L. Seeds Quality as Submitted to Saline Stress and Salicylic Acid. J. Agric. Sci. 2018, 10, 159–166. [Google Scholar] [CrossRef]
- Vasile, G.G.; Popa, D.E.; Buleandră, M.; David, I.G. An Experimental Design for the Optimization of the Extraction Methods of Metallic Mobile Fractions from Environmental Solid Samples. Environ. Monit. Assess. 2018, 190, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bugbee, G.J. Growth of Rhododendron, Rudbeckia and Thujia and the Leaching of Nitrates as Affected by the PH of Potting Media Amended with Biosolids Compost. Compost. Sci. Util. 1996, 4, 53–59. [Google Scholar] [CrossRef]
- Noguera, P.; Abad, M.; Puchades, R.; Maquieira, A.; Noguera, V. Influence of Particle Size on Physical and Chemical Properties of Coconut Coir Dust as Container Medium. Commun. Soil Sci. Plant Anal. 2003, 34, 593–605. [Google Scholar] [CrossRef]
- Tozzi, F.; Del Bubba, M.; Petrucci, W.A.; Pecchioli, S.; Macci, C.; García, F.H.; Nicolás, J.J.M.; Giordani, E. Use of a Remediated Dredged Marine Sediment as a Substrate for Food Crop Cultivation: Sediment Characterization and Assessment of Fruit Safety and Quality Using Strawberry (Fragaria x ananassa Duch.) as Model Species of Contamination Transfer. Chemosphere 2020, 238, 124651. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Burés, S. National Inventory of Organic Wastes for Use as Growing Media for Ornamental Potted Plant Production: Case Study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Paredes, C.; Moral, R.; Agulló, E.; Pérez-Murcia, M.D.; Abad, M. Composts from Distillery Wastes as Peat Substitutes for Transplant Production. Resour. Conserv. Recycl. 2008, 52, 792–799. [Google Scholar] [CrossRef]
- Castillo, J.E.; Herrera, F.; López-Bellido, R.J.; López-Bellido, F.J.; López-Bellido, L.; Fernández, E.J. Municipal Solid Waste (MSW) Compost as a Tomato Transplant Medium. Compost. Sci. Util. 2004, 12, 86–92. [Google Scholar] [CrossRef]
- Moore, K.K. Uses of Compost in Potting Mixes. HortTechnology 2005, 15, 58–60. [Google Scholar] [CrossRef]
- Ribeiro, H.M.; Romero, A.M.; Pereira, H.; Borges, P.; Cabral, F.; Vasconcelos, E. Evaluation of a Compost Obtained from Forestry Wastes and Solid Phase of Pig Slurry as a Substrate for Seedlings Production. Bioresour. Technol. 2007, 98, 3294–3297. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Vicente, A.; Ros, M.; Tittarelli, F.; Intrigliolo, F.; Pascual, J.A. Citrus Compost and Its Water Extract for Cultivation of Melon Plants in Greenhouse Nurseries. Evaluation of Nutriactive and Biocontrol Effects. Bioresour. Technol. 2008, 99, 8722–8728. [Google Scholar] [CrossRef] [PubMed]
- Government of Italy. Legislative Decree n. 75 of 29 April 2010: Reorganization and Review of the Fertilizer Regulations (Riordino e Revisione Della Disciplina in Materia Di Fertilizzanti). Gazzetta Ufficiale Della Repubblica Italiana. Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:decreto.legislativo:2010;75 (accessed on 15 March 2023).
- Shen, Z.J.; Xu, D.C.; Chen, Y.S.; Zhang, Z. Heavy Metals Translocation and Accumulation from the Rhizosphere Soils to the Edible Parts of the Medicinal Plant Fengdan (Paeonia ostii) Grown on a Metal Mining Area, China. Ecotoxicol. Environ. Saf. 2017, 143, 19–27. [Google Scholar] [CrossRef]
- Stancheva, I.; Geneva, M.; Markovska, Y.; Tzvetkova, N.; Mitova, I.; Todorova, M.; Petrovl, P. A Comparative Study on Plant Morphology, Gas Exchange Parameters, and Antioxidant Response of Ocimum basilicum L. And Origanum vulgare L. Grown on Industrially Polluted Soil. Turk. J. Biol. 2014, 38, 89–102. [Google Scholar] [CrossRef]
- Dinu, C.; Mihaela Ungureanu, E.; Geanina Vasile, G.; Kim, L.; Ionescu, I.; Ene, C.; Simion, M. Soil and vegetation pollution from an abandoned mining area situated in Hunedoara County, Romania. Rev. Chim. 2018, 69, 14–20. [Google Scholar] [CrossRef]
- Abbasifar, A.; Shahrabadi, F.; ValizadehKaji, B. Effects of Green Synthesized Zinc and Copper Nano-Fertilizers on the Morphological and Biochemical Attributes of Basil Plant. J. Plant Nutr. 2020, 43, 1104–1118. [Google Scholar] [CrossRef]
- El-Kereti, M.; El-feky, S.; Khater, M.; Osman, Y.; El-sherbini, E. ZnO Nanofertilizer and He Ne Laser Irradiation for Promoting Growth and Yield of Sweet Basil Plant. Recent. Pat. Food Nutr. Agric. 2014, 5, 169–181. [Google Scholar] [CrossRef]
- Prokop’ev, I.A.; Filippova, G.V.; Shein, A.A.; Gabyshev, D. V Impact of Urban Anthropogenic Pollution on Seed Production, Morphological and Biochemical Characteristics of Chamomile, Matricaria chamomila L. Russ. J. Ecol. 2014, 45, 18–23. [Google Scholar] [CrossRef]
- Prasad, A.; Kumar, S.; Khaliq, A.; Pandey, A. Heavy Metals and Arbuscular Mycorrhizal (AM) Fungi Can Alter the Yield and Chemical Composition of Volatile Oil of Sweet Basil (Ocimum basilicum L.). Biol. Fertil. Soils 2011, 47, 853–861. [Google Scholar] [CrossRef]
- Lajayer, H.A.; Savaghebi, G.; Hadian, J.; Hatami, M.; Pezhmanmehr, M. Comparison of Copper and Zinc Effects on Growth, Micro- and Macronutrients Status and Essential Oil Constituents in Pennyroyal (Mentha pulegium L.). Braz. J. Bot. 2017, 40, 379–388. [Google Scholar] [CrossRef]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Mitrović, M.; Pavlović, P. Ecological Potential of Plants for Phytoremediation and Ecorestoration of Fly Ash Deposits and Mine Wastes. Front. Environ. Sci. 2018, 6, 124. [Google Scholar] [CrossRef]
- Zayed, A.M.; Terry, N. Chromium in the Environment: Factors Affecting Biological Remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, R.; Todorov, G.; Ivanov, K. Heavy Metal Uptake by Rape. Commun. Soil Sci. Plant Anal. 2008, 39, 344–357. [Google Scholar] [CrossRef]
- Ada, N.-L.; Farkash, L.; Hamburger, D.; Ovadia, R.; Forrer, I.; Kagan, S.; Michal, O.-S. Light-Scattering Shade Net Increases Branching and Flowering in Ornamental Pot Plants. J. Hortic. Sci. Biotechnol. 2008, 83, 9–14. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Rożek, E.; Dzida, K.; Borowski, B. Growth Response to Nitrogen and Potassium Fertilization of Common Basil (Ocimum basilicum L.) Plants. Acta Sci. Pol. Hortorum Cultus 2012, 11, 275–288. [Google Scholar]
- Kwee, E.M.; Niemeyer, E.D. Variations in Phenolic Composition and Antioxidant Properties among 15 Basil (Ocimum basilicum L.) Cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Barickman, T.C.; Olorunwa, O.J.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Yield, Physiological Performance, and Phytochemistry of Basil (Ocimum basilicum L.) under Temperature Stress and Elevated CO2 Concentrations. Plants 2021, 10, 1072. [Google Scholar] [CrossRef]
- Stagnari, F.; Di Mattia, C.; Galieni, A.; Santarelli, V.; D’Egidio, S.; Pagnani, G.; Pisante, M. Light Quantity and Quality Supplies Sharply Affect Growth, Morphological, Physiological and Quality Traits of Basil. Ind. Crops Prod. 2018, 122, 277–289. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.Z.E.; Rahmat, A. Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation. Molecules 2016, 21, 1203. [Google Scholar] [CrossRef]
- Munns, R. Comparative Physiology of Salt and Water Stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Bie, Z.; Ito, T.; Shinohara, Y. Effects of Sodium Sulfate and Sodium Bicarbonate on the Growth, Gas Exchange and Mineral Composition of Lettuce. Sci. Hortic. 2004, 99, 215–224. [Google Scholar] [CrossRef]
- Kalisz, A.; Jezdinský, A.; Pokluda, R.; Sękara, A.; Grabowska, A.; Gil, J. Impacts of Chilling on Photosynthesis and Chlorophyll Pigment Content in Juvenile Basil Cultivars. Hortic. Environ. Biotechnol. 2016, 57, 330–339. [Google Scholar] [CrossRef]
- Walters, K.J.; Currey, C.J. Growth and Development of Basil Species in Response to Temperature. HortScience 2019, 54, 1915–1920. [Google Scholar] [CrossRef]
- Hadas, A. Germination and Seedling Establishment. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 130–137. [Google Scholar] [CrossRef]
- Fotovat, R.; Valizadeh, M.; Toorchi, M. Association between Water-Use Efficiency Components and Total Chlorophyll Content (SPAD) in Wheat (Triticum aestivum L.) under Well-Watered and Drought Stress Conditions. J. Food Agric. Environ. 2007, 5, 225. [Google Scholar]
- Dordas, C.A.; Papathanasiou, F.; Lithourgidis, A.; Petrevska, J.K.; Papadopoulos, I.; Pankou, C.; Gekas, F.; Ninou, E.; Mylonas, I.; Sistanis, I. Evaluation of Physiological Characteristics as Selection Criteria for Drought Tolerance in Maize Inbred Lines and Their Hybrids. Maydica 2018, 63, 14. [Google Scholar]
- Kashani, A.; Pirdashti, H.; Kashani, K. Effects of Four Year Sewage Sludge Application on Some Morphological Traits and Chlorophyll Content in Basil (Ocimum basilicum L.). J. Agric. Technol. 2013, 9, 451–460. [Google Scholar]
- Ontiveros-Capurata, R.E.; Juárez-López, P.; Mendoza-Tafolla, R.O.; Alia-Tejacal, I.; Villegas-Torres, O.G.; Guillén-Sánchez, D.; Cartmill, A.D. Relationship between Chlorophyll and Nitrogen Concentration, and Fresh Matter Production in Basil ‘Nufar’ (Ocimum basilicum) with Three Handheld Chlorophyll Meter Readings: SPAD, AtLEAF and MC-100. Rev. Chapingo Ser. Hortic. 2022, 28, 189–202. [Google Scholar] [CrossRef]
- Azia, F.; Stewart, K.A. Relationships between Extractable Chlorophyll and SPAD Values in Muskmelon Leaves. J. Plant Nutr. 2001, 24, 961–966. [Google Scholar] [CrossRef]
- Ruiz-Espinoza, F.H.; Murillo-Amador, B.; Garcia-Hernandez, J.L.; Fenech-Larios, L.; Rueda-Puente, E.O.; Troyo-Dieguez, E.; Kaya, C.; Beltran-Morales, A. Field Evaluation of the Relationship between Chlorophyll Content in Basil Leaves and a Portable Chlorophyll Meter (SPAD-502) Readings. J. Plant Nutr. 2010, 33, 423–438. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of Salinity Stress on Carotenoids, Anthocyanins, and Color of Diverse Tomato Genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic Response of Plants under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Esfandiari, E.; Shakiba, M.R.; Mahboob, S.A.; Alyari, H.; Shahabivand, S. The Effect of Water Stress on the Antioxidant Content, Protective Enzyme Activities, Proline Content and Lipid Peroxidation in Wheat Seedling. Pak. J. Biol. Sci. 2008, 11, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Attia, H.; Ouhibi, C.; Ellili, A.; Msilini, N.; Bouzaïen, G.; Karray, N.; Lachaâl, M. Analysis of Salinity Effects on Basil Leaf Surface Area, Photosynthetic Activity, and Growth. Acta Physiol. Plant 2011, 33, 823–833. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms Underlying Plant Resilience to Water Deficits: Prospects for Water-Saving Agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- Khaleghi, E.; Arzani, K.; Moallemi, N.; Barzegar, M. Evaluation of Chlorophyll Content and Chlorophyll Fluorescence Parameters and Relationships between Chlorophyll a, b and Chlorophyll Content Index under Water Stress in Olea europaea cv. Dezful. Int. J. Agric. Biosyst. Eng. 2012, 6, 636–639. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Seyyedi, S.M.; Ebrahimian, E.; Moghaddam, S.S.; Damalas, C.A. Exogenous Application of Gamma-Aminobutyric Acid (GABA) Alleviates the Effect of Water Deficit Stress in Black Cumin (Nigella sativa L.). Ind. Crops Prod. 2018, 112, 741–748. [Google Scholar] [CrossRef]
- Mahdavikia, H.; Rezaei-Chiyaneh, E.; Rahimi, A.; Mohammadkhani, N. Effects of Fertilizer Treatments on Antioxidant Activities and Physiological Traits of Basil (Ocimum basilicum L.) under Water Limitation Conditions. J. Med. Plants By-Prod. 2019, 8, 143–151. [Google Scholar]
- Yusuf, N.; Hamed, N.F.I. Effects of Water Deficit on the Growth and Chlorophyll Content of Capsicum frutescens. J. Sustain. Sci. Manag. 2021, 16, 148–158. [Google Scholar] [CrossRef]
- Olszewska, M.; Kobylinski, A.; Kurzeja, M. Effects of Different Proportions of Medicago Media Pers. in Mixtures with Dactylis glomerata L. on the Yield of Aboveground Biomass, Protein Yield and Relative Chlorophyll Content in Orchard Grass Leaves. Acta Sci. Polonorum Agric. 2016, 15, 15–24. [Google Scholar]
- Di Mascio, P.; Murphy, M.E.; Sies, H. Antioxidant Defense Systems: The Role of Carotenoids, Tocopherols, and Thiols. Am. J. Clin. Nutr. 1991, 53, 194S–200S. [Google Scholar] [CrossRef]
- Snodderly, D.M. Evidence for Protection against Age-Related Macular Degeneration by Carotenoids and Antioxidant Vitamins. Am. J. Clin. Nutr. 1995, 62, 1448S–1461S. [Google Scholar] [CrossRef] [PubMed]
- Mibei, E.K.; Ambuko, J.; Giovannoni, J.J.; Onyango, A.N.; Owino, W.O. Carotenoid Profiling of the Leaves of Selected African Eggplant Accessions Subjected to Drought Stress. Food Sci. Nutr. 2017, 5, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, J.; Pour-Aboughadareh, A.; Ourang, S.F.; Mehrabi, A.A.; Siddique, K.H.M. Wild Relatives of Wheat: Aegilops–Triticum Accessions Disclose Differential Antioxidative and Physiological Responses to Water Stress. Acta Physiol. Plant 2018, 40, 90. [Google Scholar] [CrossRef]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of Climate Temperature and Water Stress on Plant Growth and Accumulation of Antioxidant Compounds in Sweet Basil (Ocimum basilicum L.) Leafy Vegetable. Scientifica 2020, 2020, 3808909. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.; Ashraf, M.A.; Parveen, S.; Iqbal, M.; Hussain, I. Effect of Salt Stress on Different Growth and Biochemical Attributes in Two Canola (Brassica napus L.) Cultivars. Commun. Soil Sci. Plant Anal. 2014, 45, 669–679. [Google Scholar] [CrossRef]
- Büchi, R.; Bachmann, M.; Keller, F. Carbohydrate Metabolism in Source Leaves of Sweet Basil (Ocimum basilicum L.), a Starch-Storing and Stachyose-Translocating Labiate. J. Plant Physiol. 1998, 153, 308–315. [Google Scholar] [CrossRef]
- Farhoudi, R. Effect of Salt Stress on Physiological and Morphological Parameters of Rapeseed Cultivars. Adv. Environ. Biol. 2011, 5, 2501–2509. [Google Scholar]
- Prado, F.; Boero, C.; Gallardo, M.; Gonzalez, J. Effect of NaCl On Germination, Growth And Soluble Sugar Content In Chenopodium quinoa Wild Seeds. Bot. Bull. Acad. Sin. 2000, 41, 27–34. [Google Scholar]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants: An Overview. Plant Metab. Regul. Environ. Stress 2018, 153–167. [Google Scholar] [CrossRef]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of Phenols and Polyphenols in Plant Defense Response to Biotic and Abiotic Stresses. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 419–441. [Google Scholar]
- Ahanger, M.A.; Morad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant Growth under Drought Stress: Significance of Mineral Nutrients. Water Stress Crop Plants A Sustain. Approach 2016, 2, 649–668. [Google Scholar]
- Sun, Y.; Wang, C.; Chen, H.Y.H.; Ruan, H. Response of Plants to Water Stress: A Meta-Analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A Review on Drought Stress in Plants: Implications, Mitigation and the Role of Plant Growth Promoting Rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Trivellini, A.; Gordillo, B.; Rodriguez-Pulido, F.J.; Borghesi, E.; Ferrante, A.; Vernieri, P.; Quijada-Morin, N.; Gonzalez-Miret, M.L.; Heredia, F.J. Effect of Salt Stress in the Regulation of Anthocyanins and Color of Hibiscus Flowers by Digital Image Analysis. J. Agric. Food Chem. 2014, 62, 6966–6974. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Kwee, E.M.; Niemeyer, E.D. Potassium Rate Alters the Antioxidant Capacity and Phenolic Concentration of Basil (Ocimum basilicum L.) Leaves. Food Chem. 2010, 123, 1235–1241. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing Quality of Fresh Vegetables through Salinity Eustress and Biofortification Applications Facilitated by Soilless Cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Bekhradi, F.; Delshad, M.; Marín, A.; Luna, M.C.; Garrido, Y.; Kashi, A.; Babalar, M.; Gil, M.I. Effects of Salt Stress on Physiological and Postharvest Quality Characteristics of Different Iranian Genotypes of Basil. Hortic. Environ. Biotechnol. 2015, 56, 777–785. [Google Scholar] [CrossRef]
- Mancarella, S.; Orsini, F.; Van Oosten, M.J.; Sanoubar, R.; Stanghellini, C.; Kondo, S.; Gianquinto, G.; Maggio, A. Leaf Sodium Accumulation Facilitates Salt Stress Adaptation and Preserves Photosystem Functionality in Salt Stressed Ocimum basilicum. Environ. Exp. Bot. 2016, 130, 162–173. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; Kyriacou, M.C.; Rouphael, Y. Successive Harvests Modulate the Productive and Physiological Behavior of Three Genovese Pesto Basil Cultivars. Agronomy 2021, 11, 560. [Google Scholar] [CrossRef]
- Teliban, G.-C.; Burducea, M.; Mihalache, G.; Zheljazkov, V.D.; Dincheva, I.; Badjakov, I.; Popa, L.-D.; Bodale, I.; Vlăduț, N.-V.; Cojocaru, A. Morphological, Physiological and Quality Performances of Basil Cultivars under Different Fertilization Types. Agronomy 2022, 12, 3219. [Google Scholar] [CrossRef]
- Caliskan, O.; Kurt, D.; Temizel, K.E.; Odabas, M.S. Effect of Salt Stress and Irrigation Water on Growth and Development of Sweet Basil (Ocimum basilicum L.). Open Agric. 2017, 2, 589–594. [Google Scholar] [CrossRef]
- Doty, S.W.E. Hydroponic and Soilless Culture Systems and Transplant Practices Influence Production of Basil (Omicum basilicum L.). Master’s Thesis, Science in Horticulture, AR, USA, May 2020. Available online: https://www.proquest.com/openview/873c25e8b81539b43025246e38fc9627/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 15 March 2023).
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought Stress in Sunflower: Physiological Effects and Its Management through Breeding and Agronomic Alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C. Interactive Effects of Drought and Heat Stresses on Morpho-Physiological Attributes, Yield, Nutrient Uptake and Oxidative Status in Maize Hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef] [PubMed]
- Evert, R.F.; Eichhorn, S.E. Raven Biology of Plants, 8th ed.; W.H. Freeman Press: New York, NY, USA, 2013; p. 864. [Google Scholar] [CrossRef]
- Acharya, T.P.; Reiter, M.S.; Welbaum, G.; Arancibia, R.A. Nitrogen Uptake and Use Efficiency in Sweet Basil Production under Low Tunnels. HortScience 2020, 55, 429–435. [Google Scholar] [CrossRef]
- Bryson, G.M.; Mills, H.A.; Sasseville, D.N.; Jones, J.B.; Barker, A.V. Plant Analysis Handbook III: A Guide to Sampling, Preparation, Analysis, Interpretation and Use of Results of Agronomic and Horticultural Crop Plant Tissue; Micro-Macro Publishing, Inc.: Athens, GA, USA, 2014; p. 571. [Google Scholar]
- Bullock, D.G.; Anderson, D.S. Evaluation of the Minolta SPAD-502 Chlorophyll Meter for Nitrogen Management in Corn. J. Plant Nutr. 1998, 21, 741–755. [Google Scholar] [CrossRef]
- Choi, S.-T.; Park, D.-S.; Kang, S.-M.; Park, S.-J. Use of a Chlorophyll Meter to Diagnose Nitrogen Status of ‘Fuyu’Persimmon Leaves. HortScience 2011, 46, 821–824. [Google Scholar] [CrossRef]
- Gianquinto, G.; Sambo, P.; Bona, S. The Use of SPAD-502 Chlorophyll Meter for Dynamically Optimizing the Nitrogen Supply in Potato Crop: A Methodological Approach. In Proceedings of the IX International Symposium on Timing of Field Production in Vegetable Crops 607, Piracicaba, Brazil, 20–24 May 2001; pp. 197–204. [Google Scholar]
- Walters, K.J.; Currey, C.J. Effects of Nutrient Solution Concentration and Daily Light Integral on Growth and Nutrient Concentration of Several Basil Species in Hydroponic Production. HortScience 2018, 53, 1319–1325. [Google Scholar] [CrossRef]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and Antagonistic Interactions between Potassium and Magnesium in Higher Plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).