Abstract
The reduction and safe disposal of sewage sludge remains an urgent problem worldwide. In this work, biochar prepared from co−pyrolysis of sewage sludge and cotton stalk at different mix ratios and different pyrolysis temperatures was prepared using a novel microwave-assisted auger reactor. The obtained biochar samples were mixed with selected soil samples at different mix ratios for a short−term plant−growing test to examine their abilities as a soil conditioner on nitrogen fixation and retention. The addition of biochar could increase the total nitrogen in the soil to 0.3951% compared to 0.0403% in the untreated soil, while the concentration of available nitrogen could be increased to 114.45 mg·kg−1 compared to 47.95 mg·kg−1 in the untreated soil. Moreover, the introduction of biochar to the soil also contributed to the growth of corn seedlings, which grew at a rate of 3.41 cm·d−1 compared to 3.03 cm·d−1 in untreated soil. The results show that the addition of biochar can enrich total soil nitrogen before and after incubation and promote the growth of corn seedlings, providing a potential route for the safe disposal and resource recovery of sewage sludge.
1. Introduction
With the growth of population and social development, the annual production of sewage sludge (SS) is gradually rising [1]. In China alone, as an example, up to 5192 wastewater treatment facilities were built by the end of 2017, and at the end of 2020 it was estimated that 60 million tons of sewage sludge with a water content of about 80% will be produced annually [2]. Currently, the major disposal methods for sewage sludge include landfill [3], incineration, [4] and direct use for agriculture purpose [5]. However, sludge contains a large number of parasites, pathogenic bacteria, and viruses, and certain sludge contains a large number of heavy metals [6], which can cause serious secondary environmental pollution if disposed of improperly. Landfilling and agriculture usage can cause groundwater contamination, and some of the harmful substances contained in the sludge may poison the human body through the food chain [7], while the incineration method has superior ability in terms of safety but is very costly. Therefore, finding an economically feasible way to manage sewage sludge while meeting environmental needs is attracting increasing interest worldwide.
Pyrolysis technology attracts many researchers’ attention due to its potential ability of meeting both environmental and economic needs. The biochar generated from pyrolysis has a great potential for different applications [8,9]. Returning biochar to the soil can effectively achieve carbon fixation and help alleviate the environmental pressure caused by the emission of CO2, methane, NOx, and other gases [10]. However, biochar obtained from pyrolyzed sludge alone may have high heavy metal content, which poses potential ecological risk, limiting its application [11].
As a relatively new pyrolysis method, microwave pyrolysis technology has received much attention. Unlike conventional heating methods, microwave irradiation forms a heat transition from the core of the feedstock to the surface, which is different from the conductive/convective heating mode of passing energy from the surface to the core [12,13,14]. In addition, microwave pyrolysis has the advantages of relatively short heat treating time, lower energy consumption, efficient energy transfer, and selective heating ability [15,16,17]. This makes microwave pyrolysis potentially better for the treatment of sewage sludge to make high quality biochar [18]. Furthermore, co-pyrolysis of high-moisture sewage sludge with other dry agricultural and forestry waste can alleviate the difficulty in pretreating (e.g., drying) the sewage sludge [19]. The introduction of biomass can also lower the heavy metal content in the biochar and thus reduce the potential environmental hazard of heavy metal leaching after biochar application with increased binding of heavy metals to biochar [20]. Recent studies showed that soil application of different biochar obtained by microwave co-pyrolysis can improve soil acidity as well as alkalinity [21], improve the fertility of poor soils [22], and increase the fertility retention capacity and cation exchange properties of soils with high soil fertility [23,24,25]. It can also improve the physical structure of the soil to increase the water permeability and water retention capacity [26,27]. In addition, the application of biochar helps to absorb and fix pollutants such as heavy metal [28]. The pyrolytic conversion process eliminates the pathogenic microorganisms contained in the sewage sludge and results in a significant reduction in the amount of solid waste [29,30].
In this work, the sewage sludge, a waste contaminant in urgent need of safe disposal, and the cotton straw, a biomass widely available worldwide and in need of effective disposal, are co-pyrolyzed in a microwave-assisted reactor to prepare biochar. The aim of introducing cotton stalk is to improve the surface properties and the safety of the prepared biochar by enriching the surface functional groups and diluting the heavy metal within the biochar [31]. This ensures that the prepared biochar is safe for agricultural use as a potential soil conditioner. Microwave-assisted co-pyrolysis, as possible technology is explored for the safe disposal of municipal sludge and the effective resource utilization of sludge biochar at the same time.
2. Material and Methods
2.1. Material Preparation
Sewage sludge (SS) was obtained from a local wastewater treatment plant located in Beijing, which was received as a filter cake with approximately 80% water content. The filter cake was crushed into small chunks and then dried in an oven with the temperature set to 105 °C until constant weight. Dried sewage sludge was then finely crushed and sieved. Sludge particles from 2 mm to 0.105 mm were collected in bags for subsequent pyrolysis tests. Dried and crushed cotton stalk was purchased from a local company. The received cotton stalks were sieved after drying. Cotton stalk with a particle size between 1 mm and 0.105 mm were selected and used for the co-pyrolysis test. The mixture of sludge and cotton stalk was obtained by mechanically mixing the two previously prepared materials thoroughly. The mixing ratio of the two materials was 17:3 (sludge: stalk) and 7:3, marked as 85SS and 70SS, respectively.
The soil sample employed in this experiment was collected from the Changping campus of Beijing University of Chemical Technology (Beijing, China). The soil was a typical cinnamon soil with compact texture, easy agglomeration, and poor soil fertility. It is one of the major soil types in Beijing. The soil sample was flattened in an open area for air drying and dead branches and broken leaves were removed. After being sieved through a 4 mm screen to remove small stones, soil samples were transferred to an indoor storage unit with relatively constant temperature and humidity for subsequent experiments.
2.2. Experimental Apparatus and Procedure
In this work, a novel microwave-assisted auger moving bed reactor was employed, with the schematic diagram shown in Figure 1. The experimental device consisted of four units: feeding unit, microwave pyrolysis unit, biochar collection unit, and gas purification unit. The microwave reactor was equipped with a programmable logic controller (PLC) automatic temperature control system to control the reactor temperature.
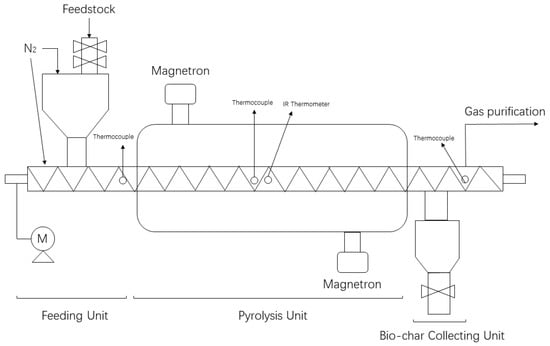
Figure 1.
Schematics diagram of microwave assisted auger moving bed.
Throughout the pyrolysis experiment, nitrogen was constantly blown into the reactor to maintain an inert gas atmosphere. The feedstock enters the feed bin through the feed port and was conveyed into the reactor by the rotating auger. It was determined by tests that when the auger rotates at 1 rpm, the processing capacity of SS (pure sewage sludge), 85SS, or 70SS was about 2 kg per hour. The biochar was collected through the discharge bin and the pyrolysis gas was discharged through the exhaust port at the end of the purification system. In this work, the rotation speed of the auger was set to 1 rpm and the pyrolysis time of feedstock at such rotation speed was 30 min. The resulted biochar was marked as SC, 85SC, and 75SC for pyrolysis products of SS, 85SS, and 75SS, respectively.
2.3. Plant Cultivation Method
In this work, biochar prepared from three different sludge-stalk mixture ratios at 450, 550, 650, and 750 °C were selected for soil tests, while the application ratios were chosen to be 1%, 3%, and 5% by weight. Corn was chosen as the experimental plant for soil tests.
Planting experiments were carried out in a constant temperature incubator with a planting cycle of 13 days, including 5 days for seedling and 8 days for growing stage. Seeds were planted in seedling trays for germination, using both original soil and soil with different biochars added. After the seedling period, plants of about 2.5 cm in height were transplanted to the respective pots according to the soil used for further growing tests. During the whole planting process, the plants were watered with tap water at a temperature of 30 °C during the day and 25 °C at night at the seedling stage, and 25 °C during the day and 18 °C during the night at the growing stage [32]. A series of control groups were set up and watered with reverse osmosis water (RO water) and tap water throughout the experiment. Soil samples collected during this stage were named as SCS450-1 for soil with 1%wt SC450 biochar added, and 85SCS750-5 for soil with 5%wt 85SC biochar added. Other soil samples were named accordingly. Soils collected after the full plantation cycle are named as “after incubation”, while freshly prepared soils are named as “before incubation”.
2.4. Analytical Methods
The proximate analysis of sewage sludge and cotton stalk was carried out strictly in accordance with the standard analysis procedure for solid biomass fuel industry (GB/T 28731-2012) [33]. The ultimate analysis of soil sample, sewage sludge, corn stalk, and biochar was determined by a CHNS analyzer (VARIO EL cube, Frankfurt, Germany). The XRD (X-ray diffraction, Bruker D8-Advance with Cu K radiation (30 kV and 15 mA) and XRF (X-ray fluorescence, LAB CENTRE XRF-1800, SHIMADZU, Kyoto, Japan) analyses of biochar and SS were carried out to measure the crystal structure and the major metal and nonmetal contents of samples. FTIR (Fourier transform infrared spectroscopy, Nicolet 6700, Thermo fisher, Waltham, MA, USA) of biochar samples were carried out to detect surface condition and functional groups.
Soil total nitrogen measurement was carried out following the soil testing standard Section 24: determination of total nitrogen in soil by automatic nitrogen test method (NY/T 1121.24-2012) [34], and the determination of the alkali hydrolyzed nitrogen follows the part of the alkali hydrolysable nitrogen in the same standard.
Total nitrogen includes both organic and inorganic nitrogen present in the soil and is the sum of the two. The nitrogen that can be absorbed by plants includes inorganic nitrogen and organic nitrogen with simple structure, but the proportion of those types of nitrogen within total soil nitrogen is very small, and most of the nitrogen belongs to organic nitrogen which cannot be absorbed easily. However, this organic nitrogen can be converted into inorganic nitrogen after being processed by microorganisms such as nitrogen-fixing microorganisms [32,35]. The amount of soil total nitrogen content can represent the soil nitrogen storage and supply capacity, and therefore soil total nitrogen content becomes one of the important indicators in the investigation of soil fertility. In this work, total nitrogen of all samples was measured by an automatic Kjeldahl nitrogen detector (K9860, Hanon, China), with the specific procedure given in Appendix A.
Available nitrogen is the portion of total soil nitrogen that can be taken up by plants, for example, inorganic nitrogen and organic nitrogen with a simple structure that can be easily absorbed. Since it is susceptible to the influence of soil, water, and heat conditions, as well as biological activities, the content of alkaline nitrogen can only represent the amount of nitrogen that can be absorbed by plants over a short term, reflecting the short-term nitrogen supply capacity of the soil. The measurement method is given in Appendix A.
3. Results and Discussion
3.1. Impacts of Pyrolysis Temperature and Mix Ratio on Biochar Yield
The yields of biochar obtained by SS and cotton stalk at different mix ratios after pyrolysis are shown in Figure 2. Temperature appears to have a significant effect on the yield of biochar. With increasing temperature from 450 to 750 °C, the SC yield decreased from 56.39 wt% to 38.88 wt%; 85SC yield decreased from 49.62 wt% to 35.16 wt%; and 70SC yield decreased from 43.25 wt% to 31.41 wt%. Meanwhile, under the same pyrolysis temperature, the solids yield decreased with increasing amounts of added cotton stalk. As shown in Table 1, cotton stalk has lower ash content and higher volatile content than SS. As the amount of cotton stalk increases, the volatile content of the mixture increases, the overall content of ash decreases, and more gas vapor is produced during pyrolysis, leading to a decrease in the amount of biochar [32,35].
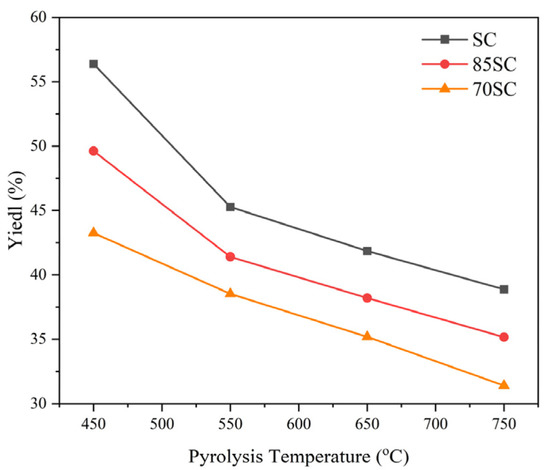
Figure 2.
Yield of biochar at different temperatures and mix ratios (0, 15%, and 30% cotton stalk).

Table 1.
Proximate and Ultimate analysis results of feedstock and biochar.
3.2. Surface Properties of Biochar
To investigate the surface functional groups of biochar, FTIR spectra SC, 85SC, and 70SC were obtained. The FTIR spectra results are shown in Figure 3. The peak characteristics of the spectra from the same material at different pyrolysis temperatures were similar and the addition of cotton stalk to the sewage sludge has little impact on the types of functional groups. As shown in the three plots of Figure 3a–c, the strong and broad peak at 3424 cm−1 can be assigned to the stretching vibrations of N–H and O–H [36]. The weakening peak intensity near 3424 cm−1 was triggered by the enhanced decomposition, loss of hydrogen bonding, and instability of organic compounds with the increase in pyrolysis temperature. The weak peak at 2920 cm−1 can be assigned to the C-H stretching vibration, revealing the presence of alkanes in SC, 85SC, and 70SC. However, those peaks are not always distinct throughout the pyrolysis, and the addition of cotton stalk does not enhance its intensity. In addition, this peak intensity at 2920 cm−1 decreased for SC, 85SC, and 70SC biochar as the temperature increased from 450 °C to 750 °C, indicating that pyrolysis process at the higher temperature enabled more efficient break down of C–H bonds. The C=C stretching vibration and the N−H bending vibration around 1631 cm−1 showed the presence of olefins and amines [37]. The peaks of all biochar samples at 1631 cm−1 decrease in peak intensity as the pyrolysis temperature increases from 450 °C to 750 °C. Such a decrease may be caused by the loss of nitrogen as the pyrolysis temperature rises. The weak peak at 1432 cm−1 can be assigned to the O–H bending of carboxylic acids [10,38]. The broad absorption peak near 1043 cm−1 can be assigned to the stretching vibrations of C–F, C–O, C–N, S=O, and Si–O bonds, which indicates the presence of fluorine compounds, alkyl aryl ethers, amines, sulfoxides, and SiO2. The weak peak at 798 cm−1 corresponds to C–H and C=C bending vibrations of benzene and olefins, which may be formed from the decomposition of macromolecular organic compounds. The spectral peak at 606 cm−1 indicates the presence of C–Cl and C–Br stretching vibrations of inorganic and organic compounds, with the type and intensity of the absorption peaks diminishing with increasing temperature. The addition of cotton stalk also introduces more surface functional groups to biochar, which may help the biochar to immobilize heavy metals and improve its safe use as a soil conditioner.
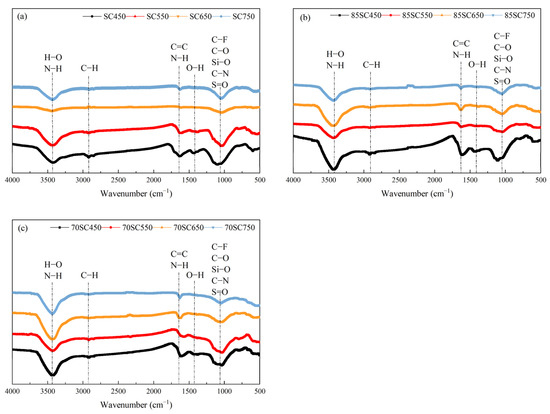
Figure 3.
FTIR spectra of biochar sample (a) SC, (b) 85SC, and (c) 70SC.
3.3. Crystal Structure of Biochar
XRD measurements were carried out on biochar samples to understand the crystalline morphology of metals and nonmetals in biochar. The XRD results in Figure 4 indicated that titanium suboxide (Ti4O7), silicon dioxide (SiO2), and magnetic pyrite (Pyrrhotite, Fe1−xS) may be present in SC, 85SC, and 70SC biochar. With more crystal structures in SC750, titanium sulfide (TiS) and gold mica (Phlogopite) can also be identified. In 85SC and 70SC, the diffraction peaks of magnetic pyrite increase in intensity as the pyrolysis temperature increases, unlike in SC where it is only present in SC750, probably due to the presence of cotton stalk. Ti4O7, peak around 26° to 27°, was resistant to temperatures below 600 °C, while the peak decreases when the temperature increases above 650 °C. The reaction of titanium with hydrogen sulfide at room temperature produces a protective layer on its surface that prevents further reaction of hydrogen sulfide with titanium [31,39] which starts to react with hydrogen sulfide at 600 °C to produce titanium sulfide, which explains why the peak of TiS is not prominent at 450 °C to 550 °C. SiO2, peak also around 26° to 27°, may increase in the quantity with increasing temperature due to the Si in ferrosilicon in SS that can be synthesized into SiO2 with O2 at higher temperatures. There may be SiO2 produced from other Si-containing rock-forming mineral elements during the pyrolysis process. Gold mica is present in the diffraction peak of SC450, but it has poor high-temperature resistance and the structure could be destroyed when the temperature increases above 550 °C, leading to the disappearance of this diffraction peak [40].
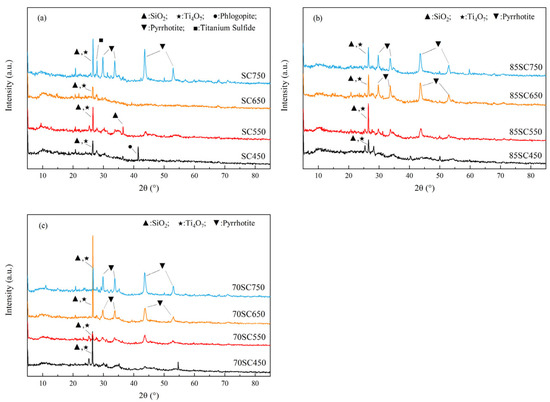
Figure 4.
XRD spectra for biochar sample (a) SC, (b) 85SC, and (c) 70SC.
3.4. Major Metal and Nonmetal Contents in Biochar
To better understand the composition of the major metallic and nonmetallic elements in the sludge and biochar, XRF was performed on the SS and biochar. Based on the XRF results, SS was then digested and the heavy metals in the SS were determined using ICP-MS to ensure they fall within the safe level. The XRF results of SS in Table 2 indicated that the SS sample contained mainly Fe and S. The other elements in abundance are P, Si, Al, Ti, Mg, Ca, and K. The SS also contained traces amounts of heavy metals such as Mn, Sr, Zn, and Cr, not abundant from the XRF analysis.

Table 2.
XRF result of sewage sludge and biochar samples.
The XRF characterization of SC is also given in Table 2, with the content of each element being given in the form of oxides. The major elements enriched in SC were essentially the same as those in SS. Those enriched elements came from solid contaminants in the wastewater effluent, from lime, organic polyelectrolytes and salts of trivalent iron, or aluminum that were added to the effluent during the treatment process to help the contaminants settle. Alkali and alkaline earth metals such as Na, K, Mg, and Ca were also found in SS and SC. Alkali metals can act as catalysts for pyrolysis reactions and have a considerable impact on product yields and composition. The content of S, P, Si, Ca, and K in SC increased as the pyrolysis temperature increased from 450 °C to 750 °C, indicating that most of these elements may be present in a stable inorganic form. The content of Fe in SC decreased as the pyrolysis temperature increased from 450 °C to 750 °C, suggesting that a small amount of Fe may be converted into volatile carbonyl iron. The irregular increase in the content of Al and Mg may be caused by the presence of a few elements in inorganic form that are difficult to decompose, or the impact brought by the increase or decrease in the content of other elements. The content of Ti shows an irregular decrease, probably because a small fraction of the element may exist in organic form and decompose during the pyrolysis process. However, it is noteworthy that it is difficult to detect the heavy metal elements in the SC biochar by XRF, which may be due to the immobilization of heavy metals by the biochar.
Similar to SC, 85SC and 70SC in Table 2 are rich in Fe and S, followed by P, Si, Al, Ti, Mg, Ca, and K, with more Cl elements and increased proportions of Si, Mg, Ca, and K than SC. It is possible that the addition of cotton stalk enriched Si, Mg, Ca, and K during pyrolysis, while some Cl was retained during low temperature pyrolysis and converted to gaseous form at higher temperatures. Alkali and alkaline earth metals such as Na, K, Mg, and Ca are also found in 85SC and 70SC. The Si and Al contents in 85SC and 70SC increase and then decrease with increasing pyrolysis temperature from 450 °C to 750 °C, indicating that most of those elements may be present in stable inorganic form. Similar to SC, no heavy metal elements contained in SS are detected in the XRF results of 85SC and 70SC. This may be caused by a combination of the dilution of heavy metal concentrations by the addition of cotton stalks and the immobilization of heavy metals by biochar.
To assess the safe soil application of biochar, ICP-MS of SS was performed on the heavy metal elements presented in the SS XRF result. The test results showed that the SS contained 43.87 mg·kg−1 Cr, 100.92 mg·kg−1 Zn, 192.16 mg·kg−1 Mn, and 115.16 mg·kg−1 Sr. Also, dried corn seedlings grown in soil blended with 5% biochar were ablated and ICP-MS tests were carried out. The results showed that the plants grown in the biochar-applied soil were almost free of those heavy metals contained in the biochar samples, suggesting the biochar samples are environmentally safe.
3.5. Co-Pyrolysis Biochar as a Soil Conditioner
3.5.1. Ultimate Analysis of Biochar
Ultimate analyses were carried out on SS and biochar samples. The results of soil, sludge, SC, 85SC, and 70SC are shown in Table 1. The soil is seen to contain little nitrogen, while SS contains much more nitrogen than soil, laying the foundation for biochar as a possible soil conditioner. As the N-containing compounds in the feedstock can be converted to NH3, HCN [21,35,41], and other nitrogen-containing small molecules such as pyridine and get removed with the pyrolysis gas, the percentage of elemental N in SC, 85SC, and 70SC gradually decreased with the increase of pyrolysis temperature. Moreover, with the addition of cotton stalk with lower N content, the N content of 85SC and 70SC decreased compared to SC. The C content increased with increasing the cotton stalk fraction, and C content in SC, 85SC, and 70SC samples changed little with the increase of pyrolysis temperature from 450 °C to 750 °C. H element may be extracted into small molecules such as H2, CH4, and other light hydrocarbons during the pyrolysis process, leading to the lower H content in 85SC and 70SC than in SC. The S content in SC, 85SC, and 70SC increased gradually with increasing pyrolysis temperature, which may be triggered by the formation of highly stable organic or inorganic sulfur compound during pyrolysis and the loss of other elements in SC, 85SC, and 70SC. The addition of cotton stalk, which was almost free of S element, decreases the content of S elements in 85SC and 70SC. In general, although the addition of cotton stalk reduces the N concentration in 85SC and 70SC, the samples SC, 85SC, and 70SC still contain much more nitrogen than the soil, which makes them great candidates as soil conditioners.
3.5.2. Impacts of Biochar on Corn Growing
The application of biochar was also found to have a beneficial effect on the growth of corn seedlings during the incubation process. In the planting experiment of blank soil sample, reverse osmosis purified water (RO water) and tap water were used to water the crop, and the RO water and tap water groups were labeled as PWS (pure water sets) and TWS (tap water sets), respectively. The growth rates of plants cultured in soil with different biochar additions are shown in Figure 5. As shown in Figure 5a, the growth rate of TWS was 3.03 cm·d−1 during the culture growth period, while the plants cultured with tap water and pure water did not differ much in growth rate, and the growth rate of PWS was 0.125 cm·d−1 lower than that of TWS.
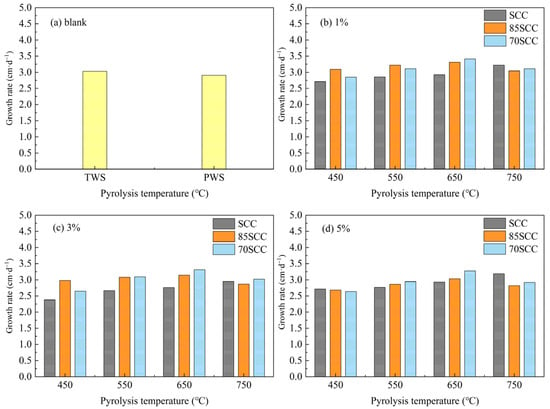
Figure 5.
(a–d) Growth rates of plants in soil with different biochar application ratios.
As shown in Figure 5b–d, the growth rate of corn seedlings in soils with different SC application ratios all increased gradually with the increase in biochar preparation temperature. In soils with different 85SC and 70SC application ratios, the growth rate of corn seedlings increased and then decreased with the increase of 85SC and 70SC preparation temperatures. In comparison with TWS result shown in Figure 5a, the growth rate of SCC was generally lower than that of TWS, and only SCC750-1 and SCC750-5 showed slightly higher growth rates. 85SCC had a better growth rate than TWS prepared at a pyrolysis temperature of 650 °C for all application ratios, and 85SCC650-1 had the highest growth rate of 3.313 cm·d−1. 70SCC prepared at a pyrolysis temperature of 650 °C had the highest growth rate. The growth rate of 70SCC prepared at a pyrolysis temperature of 650 °C also had a better growth rate and had the highest growth rate at 1% application ratio, reaching 3.413 cm·d−1. Overall, the biochar with cotton stalk addition and 1% application ratio showed a better growth rate than TWS. However, the impact of biochar application on corn growth in this work was limited to the three-leafed seedling stage due to the experimental conditions. Further research on the effects on corn plant growth and the effect on corn yield is needed on the future [42].
3.5.3. Impacts of Biochar on Soil Total Nitrogen
Total nitrogen includes both organic and inorganic nitrogen present in the soil and is the sum of both. The concentration of total nitrogen is an indication of the soil’s capacity to store and supply nitrogen and is therefore an important indicator of soil fertility [41,43,44]. The total nitrogen contents of the soil to which different percentages of biochar were applied are shown in Figure 6. It can be seen in Figure 6a that the total nitrogen content of both TWS and PWS increased a little after experiment, probably because the nitrogen-fixing microorganisms present in the soil immobilized more atmospheric nitrogen than what plants take up for growth. The reduction of ammonia to nitrogen increased the total nitrogen concentration. The increase in total soil nitrogen in the PWS sample was only slightly less than that in TWS.
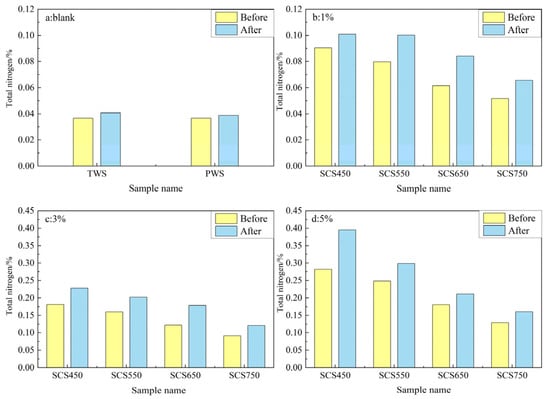
Figure 6.
(a–d) Total nitrogen content of soil samples without (blank) and with (1%, 3%, and 5%) SC biochar addition.
As shown in Figure 6b–d, in soils with different biochar application ratios, the total nitrogen concentration is lower in soils applied with biochar produced at higher pyrolysis temperature, both before and after planting experiments, because of lower N content in biochar produced at higher temperature. Compared to the blank tests, the total nitrogen content in biochar-containing soils is higher in all cases, which can be attributed to the migration of nitrogen from the biochar to the soil and the promoted nitrogen-fixing microbial activity by the biochar [41,42]. When more nitrogen is adsorbed or retained during the incubation process, a greater increase in the total soil nitrogen concentration results after incubation. This result of increased total nitrogen concentration of soil after biochar application is consistent with the findings of other researchers [43,45,46]. Among all experiments, the maximum total nitrogen content of SCS-1, SCS-3, and SCS-5 soil after incubation increased by 1.60, 4.87, and 9.18 times, respectively, more than blank soil (PWS), and the total nitrogen concentration in the soil reached 0.101%, 0.2278%, and 0.3951%, respectively. It was also found that the total nitrogen content in biochar-added soil showed a gradual increase with the increase of applied biochar ratio.
The total nitrogen contents in soils mixed with 85SC and 70SC biochar are shown in Figure 7 and Figure 8. From Figure 7b–d and Figure 8b–d, it can be seen that the experimental data for the 85SC and 70SC blended soils follow a similar trend to the SC biochar blended soil in Figure 5, where the total nitrogen content increased with increasing biochar blending ratio. The maximum total N content of 85SC and 70SC soils after incubation increased 7.24 and 5.47 times more than the blank PWS soil, and the total N concentration in the soil reached 0.3198% and 0.2511%, respectively. The maximum increases in total nitrogen content for soils with 85SC and 70SC biochar at different ratios are generally smaller than those for soils with SC biochar, as expected, due to the lower N content of biochar derived from the sewage sludge and cotton stalk mixture. It is worth noting that the incubation of corn seedlings in this experiment was terminated at the three-leaf stage due to planting site limitations. At this early stage of incubation, almost all the nutrients consumed by corn seedling growth came from the seeds themselves and the consumption of soil nutrients by plant growth is negligible [32].
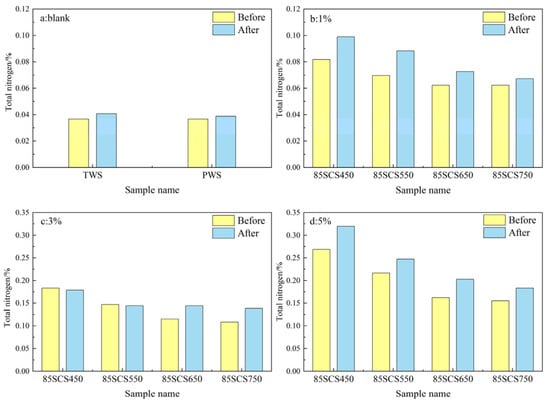
Figure 7.
(a–d) Total nitrogen content of soil samples without (blank) and with (1%, 3%, and 5%) 85SC biochar addition.
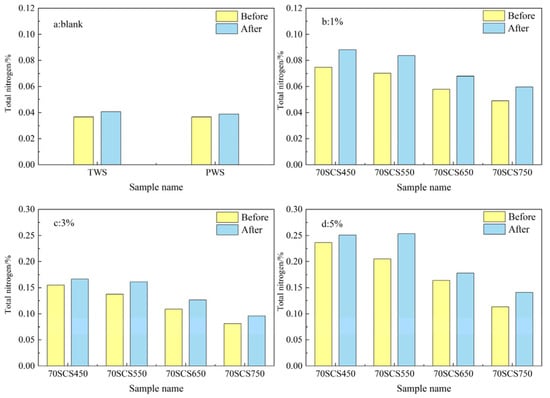
Figure 8.
(a–d) Total nitrogen content of soil samples without (blank) and with (1%, 3%, and 5%) 70SC biochar addition.
Overall, regardless of the biochar application ratio and the cotton stalk to sewage sludge mix ratio, the prepared biochar had a good performance in enriching soil total nitrogen, demonstrating its potential to be used for soil amendment.
3.5.4. Impacts of Biochar on Soil Available Nitrogen
Available nitrogen is the portion of total nitrogen that can be taken up by plants, usually in the inorganic and organic forms with a simple molecular structure. Meanwhile, due to its sensitivity to soil hydrothermal conditions and biological activity, the available nitrogen content can only reflect the recent nitrogen supply capacity of the soil [35,44]. The available nitrogen content of soils blended with different fractions of biochar derived from sewage sludge is shown in Figure 9. As shown in Figure 9a, the available nitrogen content of tested soil without biochar addition (both TWS and PWS) increased after incubation. With the addition of SC biochar, the available nitrogen both before and after incubation increased in all tested biochar ratios, with higher available nitrogen content for biochars prepared at lower pyrolysis temperatures. After incubation, the available nitrogen content of soil samples containing biochar decreased in most cases, probably because of (1) weakened nitrification in soil due to soil not being turned over for a long time, (2) reduced nitrate by denitrifying bacteria to nitrogen (N2) or nitrous oxide (N2O) gases, and (3) nitrogen uptake by plants. Similar results have been found by other researchers [42]. Although soil sample SCS450 showed the greatest reduction in available nitrogen content after incubation, the available nitrogen content was still higher than that of the other treated soils.
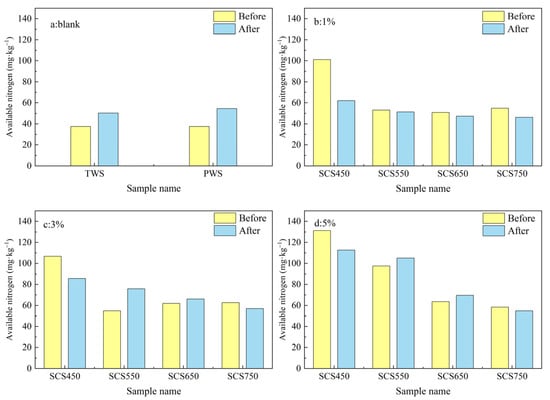
Figure 9.
(a–d) Available nitrogen content of soil samples blended with different fractions of biochar SC (0, 1%, 3%, and 5%wt).
Comparing soil samples with different application ratios of biochar prepared at the same pyrolysis temperature, it can be seen that the soil available nitrogen content shows a gradual increase with the increase of biochar application ratio before and after incubation, which is likely related to the change in soil porosity and oxygen content [27,47] with application ratio. The greatest increase in available nitrogen before and after incubation was observed for SCS450-5, which was 131.2 mg·kg−1 (3.50 times of PWS) before incubation and 112.7 mg·kg−1 (2.06 times of PWS) after incubation.
The available nitrogen content of soils blended with biochar 85SC is presented in Figure 10. A similar trend is seen for the effect of pyrolysis temperature, as for biochar SC. For biochar prepared at low temperatures (450 and 550 °C), the available nitrogen content increased after incubation. However, for biochar prepared at high temperatures (750 °C), the available nitrogen decreased after incubation, similar to biochar SC. This suggests that biochar 85SC has a higher nitrogen fixation capability than biochar SC, which is likely related to the addition of cotton stalk in the feedstock for making biochar 85SC. Soil 85SCS450 showed the largest increase in available nitrogen content before and after incubation, probably because biochar 85SC contains more NH4+, which as a substrate promotes digestion by nitrifying bacteria [44]. Comparing against Figure 10a, it can be found that the available nitrogen content of soils containing 85SC biochar is higher than that of the blank test before incubation.
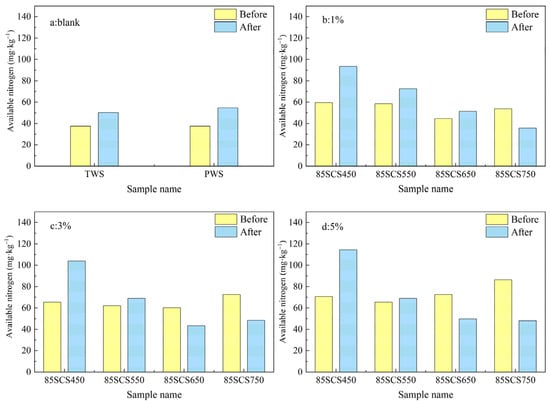
Figure 10.
(a–d) Available nitrogen content of soil samples blended with different fractions of biochar 85SC (0, 1%, 3%, and 5%wt).
The available nitrogen content of soils blended with biochar 70SC are shown in Figure 11. It is observed from Figure 11b–d that, unlike biochar SC and 85SC, the addition of biochar 70SC into the soil reduces the available soil nitrogen content in many cases compared to the blank soil (TWS), regardless of the mix ratios of 70SC and the pyrolysis temperatures. This may be caused by the addition of a large amount of low-N-containing cotton stalk in the pyrolysis process, which leads to the low nitrogen content in the biochar. It is also observed that the available soil nitrogen increased after incubation for all soil samples blended with different fractions of nitrogen. Figure 9, Figure 10 and Figure 11 together confirm that the nitrogen fixation process is promoted by biochar prepared from sewage sludge mixed with cotton stalk, although the nitrogen content in the biochar is reduced with the addition of cotton stalk. As noted earlier, the growth of corn seedlings hardly consumed nutrients from the soil, the change in soil available nitrogen concentration after incubation was thus mostly caused by the added biochar. The effect of biochar addition on soil available nitrogen concentration could be obtained by subtracting the TWS available nitrogen concentration from the available nitrogen concentration in soil after incubation. SCS450-5, 85SCS450-5 and 75SCS450-5 elevated the soil available nitrogen by 58.1 mg·kg−1, 59.85 mg·kg−1 and 11.9 mg·kg−1, respectively, after incubation. The specific reason on either the suppression of nitrogen fixation by sewage sludge biochar (SC) or the promotion of nitrogen fixation by biochar from sewage sludge and cotton stalk mixture should be explored in the future study.
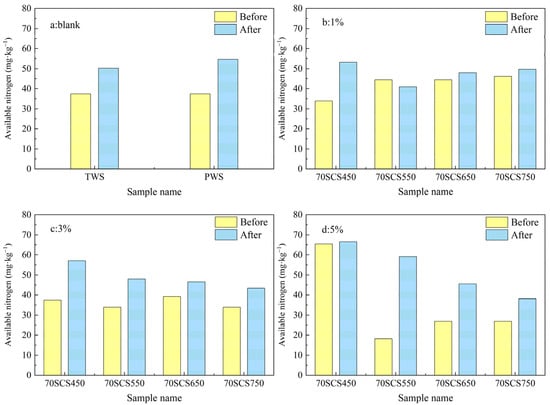
Figure 11.
(a–d) Available nitrogen content of soil samples blended with different fractions of biochar 70SC (0, 1%, 3%, and 5%wt).
Overall, biochar SC and 85SC have better ability to improve the soil available nitrogen. However, biochar 70SC will improve nitrification or nitrogen fixation, making the available soil nitrogen higher after incubation than before incubation.
4. Conclusions
In this work, a series of biochar samples were prepared in a novel microwave-assisted auger reactor by co-pyrolysis of sewage sludge and cotton stalk. Biochar application as a potential soil conditioner was examined systematically based on measured metal contents and nitrogen contents before and after incubation. The total nitrogen content of the soil with different biochar addition was found to be greater than that of the blank soil sample before and after incubation, and the total nitrogen content increased with increasing the application ratio. Nitrogen content in prepared biochar, and consequently biochar-containing soil, decreased with increasing the pyrolysis temperature. The nitrogen content in the biochar and biochar-containing soil also decreased as the cotton stalk content in the feedstock increased because of low nitrogen content in cotton stalk. The increase in total nitrogen content in the soils with biochar application may be caused by the release of nitrogen from the biochar and the promotion of nitrogen-fixing microbial activity by the biochar.
The content of soil available nitrogen with biochar applied was affected by multiple factors such as soil hydrothermal conditions, biological activity, and seed differences. It is observed that the addition of sewage sludge biochar increased the available nitrogen content of soil before incubation, but the available nitrogen became lower after incubation. In contrast, biochar made from mixture of sewage sludge and cotton stalk decreased the available nitrogen content in the soil before incubation in several tested samples, but the available nitrogen content increased after incubation. This suggests that mixing cotton stalk in sewage sludge pyrolysis improves the biochar performance in enhancing soil nitrification and/or nitrogen fixation, making biochar a potential soil conditioner.
Author Contributions
Conceptualization, J.Q.; formal analysis, H.Y.; investigation, J.Q., D.W. and Z.D.; methodology, D.W. and Z.D.; supervision, J.D. and X.B.; writing—original draft, J.Q.; writing—review & editing, J.D. and X.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from Beijing Advanced Innovation Center for Soft Matter Science and Engineering, and the Ministry of Science and Technology of the People’s Republic of China (Grant number: 2017YFE0124800).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A
Total nitrogen: Weigh 1 g of the sample (accurate to 0.001 g) into the bottom of the dry digestion tube, then add 2 g of mixed accelerator and mix well. Then add a small amount of RO water to moisten the sample, then add 5 mL of sulfuric acid and shake gently. The sample was transferred to the digestion device for digestion according to the program setting. After cooling, the digestion tube was placed on the automatic nitrogen determination instrument for distillation and titration. The total nitrogen content was calculated as mass fraction (%).
Available nitrogen: Weigh 2 g of sample (accurate to 0.001 g) and 0.2 g of finely ground FeSO4-7H2O, spread them evenly in the outer chamber of the diffusion dish, and gently rotate the dish horizontally to make the sample spread evenly. Add 2 mL of 2% boric acid-indicator solution to the inner chamber of the diffusion dish, then spread the gum solution evenly on the edge of the outer chamber of the diffusion dish, cover the glass piece, and rotate it to ensure no air bubbles at the contact point. Move the glass sheet slowly to expose the outer chamber at the notch. Add 0.1 mL of Ag2SO4 saturated solution and 10 mL of 1.8 mol·L−1 NaOH solution from the small notch to the outer chamber with a pipette and cover tightly immediately. Carefully, rotate the diffusion dish horizontally to mix the solution with the soil, tie it with a rubber band, and put it in a 40 °C oven for 24 h. Then, titrate with 0.01 mol·L−1 H2SO4 standard solution, and titrate the end point when the solution turns light red. The available nitrogen of the sample was calculated using the following equation.
where V is the volume of H2SO4 standard solution used for titration of the sample (mL); V0 is the volume of H2SO4 standard solution used for titration reagent blank test (mL); c is the concentration of H2SO4 standard solution (0.01 mol·L−1); m is the mass of the sample (g).
References
- Racek, J.; Sevcik, J.; Chorazy, T.; Kucerik, J.; Hlavinek, P. Biochar—Recovery Material from Pyrolysis of Sewage Sludge: A Review. Waste Biomass Valorization 2020, 11, 3677–3709. [Google Scholar] [CrossRef]
- Bora, A.P.; Gupta, D.P.; Durbha, K.S. Sewage Sludge to Bio-Fuel: A Review on the Sustainable Approach of Transforming Sewage Waste to Alternative Fuel. Fuel 2020, 259, 116262. [Google Scholar] [CrossRef]
- Jin, J.; Wang, M.; Cao, Y.; Wu, S.; Liang, P.; Li, Y.; Zhang, J.; Zhang, J.; Wong, M.H.; Shan, S.; et al. Cumulative Effects of Bamboo Sawdust Addition on Pyrolysis of Sewage Sludge: Biochar Properties and Environmental Risk from Metals. Bioresour. Technol. 2017, 228, 218–226. [Google Scholar] [CrossRef]
- Hwang, I.H.; Ouchi, Y.; Matsuto, T. Characteristics of Leachate from Pyrolysis Residue of Sewage Sludge. Chemosphere 2007, 68, 1913–1919. [Google Scholar] [CrossRef]
- Dhote, L.; Kumar, S.; Singh, L.; Kumar, R. A Systematic Review on Options for Sustainable Treatment and Resource Recovery of Distillery Sludge. Chemosphere 2021, 263, 128225. [Google Scholar] [CrossRef] [PubMed]
- Fytili, D.; Zabaniotou, A. Utilization of Sewage Sludge in EU Application of Old and New Methods-A Review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Effect of Different Sewage Sludge Applications on Growth and Yield of Vigna radiata L. Field Crop: Metal Uptake by Plant. Ecol. Eng. 2010, 36, 969–972. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Li, L.Y. Biofuel Production by Co-Pyrolysis of Sewage Sludge and Other Materials: A Review. Environ. Chem. Lett. 2023, 21, 153–182. [Google Scholar] [CrossRef]
- Hu, M.; Hu, H.; Ye, Z.; Tan, S.; Yin, K.; Chen, Z.; Guo, D.; Rong, H.; Wang, J.; Pan, Z.; et al. A Review on Turning Sewage Sludge to Value-Added Energy and Materials via Thermochemical Conversion towards Carbon Neutrality. J. Clean. Prod. 2022, 379, 134657. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, S.; Xiao, B.; Yu, D.; Jia, X. Mechanism of Wet Sewage Sludge Pyrolysis in a Tubular Furnace. Int. J. Hydrogen Energy 2011, 36, 355–363. [Google Scholar] [CrossRef]
- Tomasi Morgano, M.; Leibold, H.; Richter, F.; Stapf, D.; Seifert, H. Screw Pyrolysis Technology for Sewage Sludge Treatment. Waste Manag. 2018, 73, 487–495. [Google Scholar] [CrossRef]
- Mketo, N.; Nomngongo, P.N.; Ngila, J.C. Rapid Total Sulphur Reduction in Coal Samples Using Various Dilute Alkaline Leaching Reagents under Microwave Heating: Preventing Sulphur Emissions during Coal Processing. Environ. Sci. Pollut. Res. 2017, 24, 19852–19858. [Google Scholar] [CrossRef]
- Salomatov, V.; Karelin, V.; Salomatov, V. Mathematical Models of Microwave Heating of a Coal Mass with Release of Absorbed Energy by the Heat Radiation Law. J. Eng. Thermophys. 2016, 25, 485–494. [Google Scholar] [CrossRef]
- Meda, V.; Orsat, V.; Raghavan, V. Microwave Heating and the Dielectric Properties of Foods. In The Microwave Processing of Foods, 2nd ed.; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Mushtaq, F.; Mat, R.; Ani, F.N. A Review on Microwave Assisted Pyrolysis of Coal and Biomass for Fuel Production. Renew. Sustain. Energy Rev. 2014, 39, 555–574. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T. A Review on the Microwave-Assisted Pyrolysis Technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330. [Google Scholar] [CrossRef]
- Kostas, E.T.; Beneroso, D.; Robinson, J.P. The Application of Microwave Heating in Bioenergy: A Review on the Microwave Pre-Treatment and Upgrading Technologies for Biomass. Renew. Sustain. Energy Rev. 2017, 77, 12–27. [Google Scholar] [CrossRef]
- Ren, X.; Shanb Ghazani, M.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and Opportunities in Microwave-Assisted Catalytic Pyrolysis of Biomass: A Review. Appl. Energy 2022, 315, 118970. [Google Scholar] [CrossRef]
- Siddiqui, M.I.; Rameez, H.; Farooqi, I.H.; Basheer, F. Recent Advancement in Commercial and Other Sustainable Techniques for Energy and Material Recovery from Sewage Sludge. Water 2023, 15, 948. [Google Scholar] [CrossRef]
- Huang, H.J.; Yang, T.; Lai, F.Y.; Wu, G.Q. Co-Pyrolysis of Sewage Sludge and Sawdust/Rice Straw for the Production of Biochar. J. Anal. Appl. Pyrolysis 2017, 125, 61–68. [Google Scholar] [CrossRef]
- Khan, S.; Chao, C.; Waqas, M.; Arp, H.P.H.; Zhu, Y.G. Sewage Sludge Biochar Influence upon Rice (Oryza sativa L) Yield, Metal Bioaccumulation and Greenhouse Gas Emissions from Acidic Paddy Soil. Environ. Sci. Technol. 2013, 47, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar Reduces the Bioavailability and Phytotoxicity of Heavy Metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Rehman, R.A.; Rizwan, M.; Qayyum, M.F.; Ali, S.; Zia-ur-Rehman, M.; Zafar-ul-Hye, M.; Hafeez, F.; Iqbal, M.F. Efficiency of Various Sewage Sludges and Their Biochars in Improving Selected Soil Properties and Growth of Wheat (Triticum aestivum). J. Environ. Manag. 2018, 223, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Strezov, V.; Yin Chan, K.; Nelson, P.F. Agronomic Properties of Wastewater Sludge Biochar and Bioavailability of Metals in Production of Cherry Tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Hoang, S.A.; Bolan, N.; Madhubashini, A.M.P.; Vithanage, M.; Perera, V.; Wijesekara, H.; Wang, H.; Srivastava, P.; Kirkham, M.B.; Mickan, B.S.; et al. Treatment Processes to Eliminate Potential Environmental Hazards and Restore Agronomic Value of Sewage Sludge: A Review. Environ. Pollut. 2022, 293, 118564. [Google Scholar] [CrossRef]
- Jien, S.H.; Wang, C.S. Effects of Biochar on Soil Properties and Erosion Potential in a Highly Weathered Soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef]
- Ibrahim, A.; Usman, A.R.A.; Al-Wabel, M.I.; Nadeem, M.; Ok, Y.S.; Al-Omran, A. Effects of Conocarpus Biochar on Hydraulic Properties of Calcareous Sandy Soil: Influence of Particle Size and Application Depth. Arch. Agron. Soil Sci. 2017, 63, 185–197. [Google Scholar] [CrossRef]
- Ibrahim, M.; Li, G.; Khan, S.; Chi, Q.; Xu, Y. Biochars Mitigate Greenhouse Gas Emissions and Bioaccumulation of Potentially Toxic Elements and Arsenic Speciation in Phaseolus vulgaris L. Environ. Sci. Pollut. Res. 2017, 24, 19524–19534. [Google Scholar] [CrossRef]
- Wang, X.; Deng, S.; Tan, H.; Adeosun, A.; Vujanović, M.; Yang, F.; Duić, N. Synergetic Effect of Sewage Sludge and Biomass Co-Pyrolysis: A Combined Study in Thermogravimetric Analyzer and a Fixed Bed Reactor. Energy Convers. Manag. 2016, 118, 399–405. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Zhu, G.; Yang, L.; Zhu, Y. The Effect of High Temperature on Syngas Production by Immediate Pyrolysis of Wet Sewage Sludge with Sawdust. J. Therm. Anal. Calorim. 2018, 132, 1783–1794. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Ruan, R.; Bilal, M.; Khan, N.A.; Awasthi, M.K.; Amer, M.A.; Leng, L.; Hamouda, M.A.; Li, J. Co-Pyrolysis of Sewage Sludge and Biomass for Stabilizing Heavy Metals and Reducing Biochar Toxicity: A Review. Environ. Chem. Lett. 2022, 21, 1231–1250. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; Mackowiak, C.L.; Comerford, N.B.; Moline, E.F.d.V.; Shirley, J.P.; Guimaraes, D.V. Pyrolysis Methods Impact Biosolids-Derived Biochar Composition, Maize Growth and Nutrition. Soil Tillage Res. 2017, 165, 59–65. [Google Scholar] [CrossRef]
- GB/T 28731-2012; Proximate Analysis of Solid Biofuels. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. Standardization Administration of China: Beijing, China, 2012.
- Wang, L.S.; Wang, R.J.; Lu, C.P.; Wang, J.; Huang, W. Rapid determination of moisture content in compound fertilizer usingvisible and near infrared spectroscopy combined with chemometrics. Infrared Phys. Technol. 2019, 102, 103045. [Google Scholar] [CrossRef]
- de la Rosa, J.M.; Rosado, M.; Paneque, M.; Miller, A.Z.; Knicker, H. Effects of Aging under Field Conditions on Biochar Structure and Composition: Implications for Biochar Stability in Soils. Sci. Total Environ. 2018, 613–614, 969–976. [Google Scholar] [CrossRef]
- Georgakopoulos, A.; Iordanidis, A.; Kapina, V. Study of Low Rank Greek Coals Using FTIR Spectroscopy. Energy Sources 2003, 25, 995–1005. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, C.; Siddiqui, A.R.; Mao, X.; Kang, Q.; Fu, J.; Deng, Z.; Song, Y.; Jiang, Z.; Zhang, T.; et al. Pyrolysis of Textile Dyeing Sludge in Fluidized Bed and Microwave-Assisted Auger Reactor: Comparison and Characterization of Pyrolysis Products. J. Hazard. Mater. 2018, 359, 454–464. [Google Scholar] [CrossRef]
- Fonts, I.; Azuara, M.; Gea, G.; Murillo, M.B. Study of the Pyrolysis Liquids Obtained from Different Sewage Sludge. J. Anal. Appl. Pyrolysis 2009, 85, 184–191. [Google Scholar] [CrossRef]
- Kim, Y.; Parker, W. A Technical and Economic Evaluation of the Pyrolysis of Sewage Sludge for the Production of Bio-Oil. Bioresour. Technol. 2008, 99, 1409–1416. [Google Scholar] [CrossRef]
- Yao, J.; Dong, Z.; Li, H.; Wang, X.; Ji, M.; Zhang, S. Influence of Sewage Sludge Addition on Huainan Coal Ash Fusion Characteristics. Appl. Mech. Mater. 2013, 295–298, 1720–1724. [Google Scholar]
- Brassard, P.; Godbout, S.; Raghavan, V. Soil Biochar Amendment as a Climate Change Mitigation Tool: Key Parameters and Mechanisms Involved. J. Environ. Manag. 2016, 181, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, Y.; Pan, G.; Hussain, Q.; Li, L.; Zheng, J.; Zhang, X. Effect of Biochar Amendment on Maize Yield and Greenhouse Gas Emissions from a Soil Organic Carbon Poor Calcareous Loamy Soil from Central China Plain. Plant Soil 2012, 351, 263–275. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of Biochar Amendment on Yield and Methane and Nitrous Oxide Emissions from a Rice Paddy from Tai Lake Plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s Effect on Crop Productivity and the Dependence on Experimental Conditions—A Meta-Analysis of Literature Data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Q.; Liu, G.; Cowie, A.L.; Bei, Q.; Liu, B.; Wang, X.; Ma, J.; Zhu, J.; Xie, Z. Effects of Different Biochars on Pinus Elliottii Growth, N Use Efficiency, Soil N2O and CH4 Emissions and C Storage in a Subtropical Area of China. Pedosphere 2017, 27, 248–261. [Google Scholar] [CrossRef]
- Yue, Y.; Cui, L.; Lin, Q.; Li, G.; Zhao, X. Efficiency of Sewage Sludge Biochar in Improving Urban Soil Properties and Promoting Grass Growth. Chemosphere 2017, 173, 551–556. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Song, L.; Song, X.; Hänninen, H.; Wu, J. Biochar Enhances Nut Quality of Torreya Grandis and Soil Fertility under Simulated Nitrogen Deposition. For. Ecol. Manag. 2017, 391, 321–329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).