Desalination of Saline Irrigation Water Using Hydrophobic, Metal–Polymer Hydrogels
Abstract
1. Introduction
1.1. Benefits of Chemical Desalination
- (i)
- The use of crown ether desalination (patent US2011/0147314A1 [26,27]. Crown ether technology is currently focused on the recovery of Li+ ions from water. The same technology can also be used to selectively recover Na+, K+, and Li+ ions from a water body [28,29,30]. It is being evaluated for use in the recovery of Cs+ and Mg2+ ions [31,32,33]. The ion recovery process from the absorbent is energy intensive (US2011/0147314A1). This technology tethers the Na+ ion to the absorbent site and forces the Cl− ion to become a spectator ion. Polymer desalination can reverse the adsorption site charges to tether the Cl− ions and maintain the Na+ ion as a spectator ion;
- (ii)
- Functionalization of the surface of a particle with negative charged or positive charged sites. Negatively charged sites attract Na+ ions and positively charged sites attract Cl− ions. The use of a charged particle in water remediation has been the focus of substantive patent activity (e.g., JP5405454B2; RU2463256C2; US9617175B2). The application of this adsorption technology to water desalination is addressed in patents US8636906B2 and FR2983191A1 [34];
- (iii)
- The use of hydrophilic polymers. These polymers actively adsorb water but not Na+ ions and Cl− ions. The desalination requires recovery and dehydration of the hydrated hydrophilic polymers to release desalinated water. This approach produces a waste brine and is not considered further;
- (iv)
- The use of hydrophobic polymers, which preferentially adsorb Na+ and Cl− ions from water was first outlined in patent US9617175B2. This approach was largely ignored in the academic literature until 2022 [18,34]. The discovery, in 2013 (GB2520775A), that these polymers abstract Na+ and Cl− ions from water and sequester them within dead-end pores (Equation (1)) has since been confirmed by patent US10919784B2 and academic publications [18,34]. These polymers combine chemical adsorption with chemical separation [18,34].
1.2. Ion Removal Selectivity
1.3. Entrained, Hydrophobic, Hydrogel, and Spherical Polymers
1.4. Purpose of This Study
1.5. Summary of the Study Results
2. Materials and Methods
2.1. Background Information, Data, and Statistical Methodology
2.1.1. Polymer Terminology
2.1.2. Primary Data Sets
2.1.3. Statistical Methodology Used
- PCC = 0.9 to 1.0 (R2 = 0.81 to 1.00): Interpretation = very strong correlation
- PCC = 0.7 to 0.89 (R2 = 0.49 to 0.79): Interpretation = strong correlation
- PCC = 0.4 to 0.69 (R2 = 0.16 to 0.47): Interpretation = moderate correlation
- PCC = 0.1 to 0.39 (R2 = 0.01 to 0.15): Interpretation = weak correlation
- PCC = 0.0 to 0.10 (R2 = 0.00 to 0.01): Interpretation = negligible correlation
2.2. Collection and Measurement of Data
2.2.1. Primary Water Composition Data Measurements
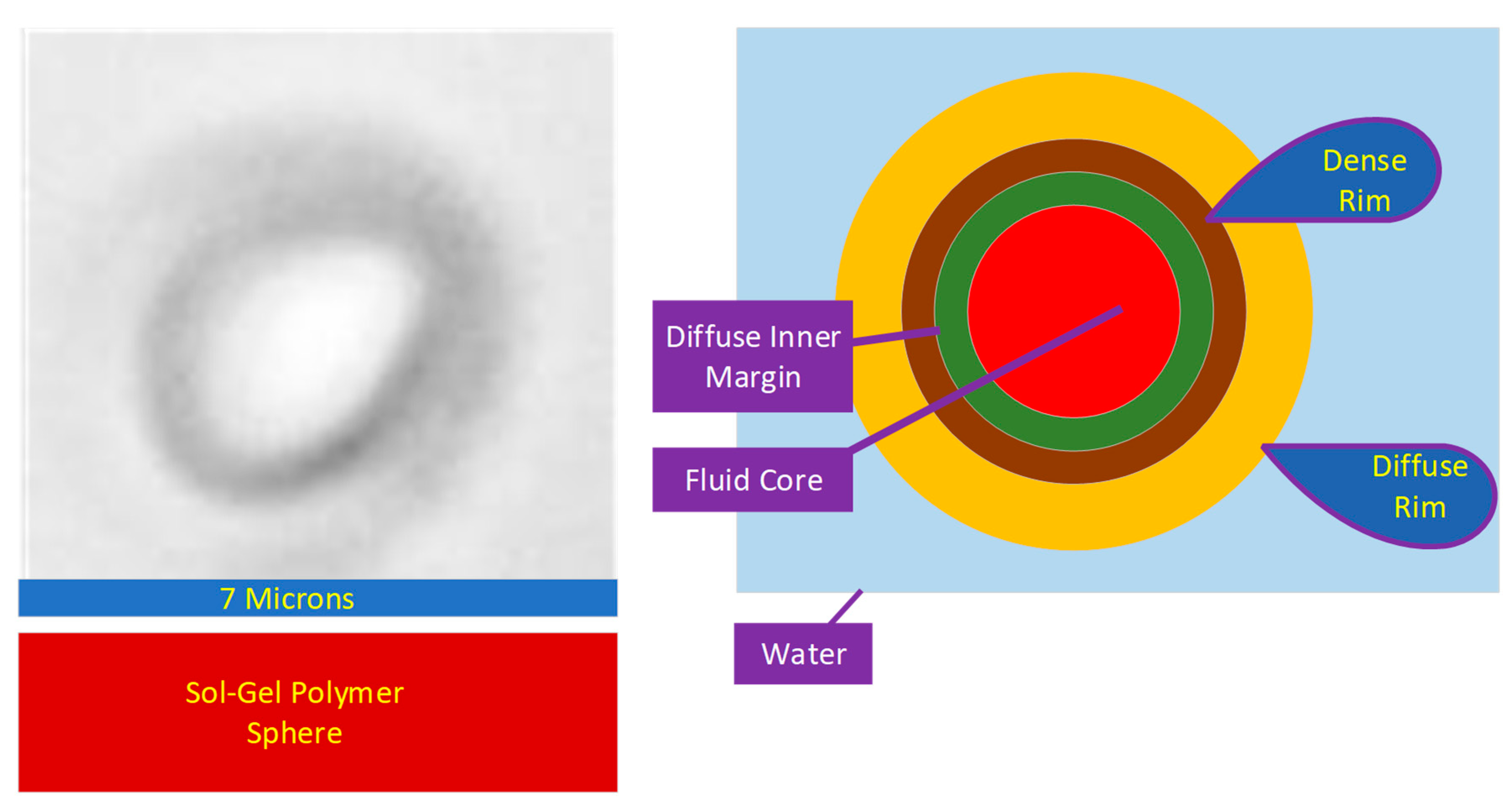
2.2.2. Photo Micrograph Data
2.2.3. Feed Water
2.2.4. Sol–Gel Polymer Formulations
2.3. Analysis of Polymer Data
2.3.1. Ostwald Ripening Model Analysis
2.3.2. Redox Model
2.3.3. Polymer Categories Evaluated
2.4. Crop Yield Parameters
3. Agricultural Crop Yields
3.1. Scenario 1
3.1.1. Measurement of Salinity
3.1.2. Scenario 1a
3.2. Scenario 2
3.3. Livestock
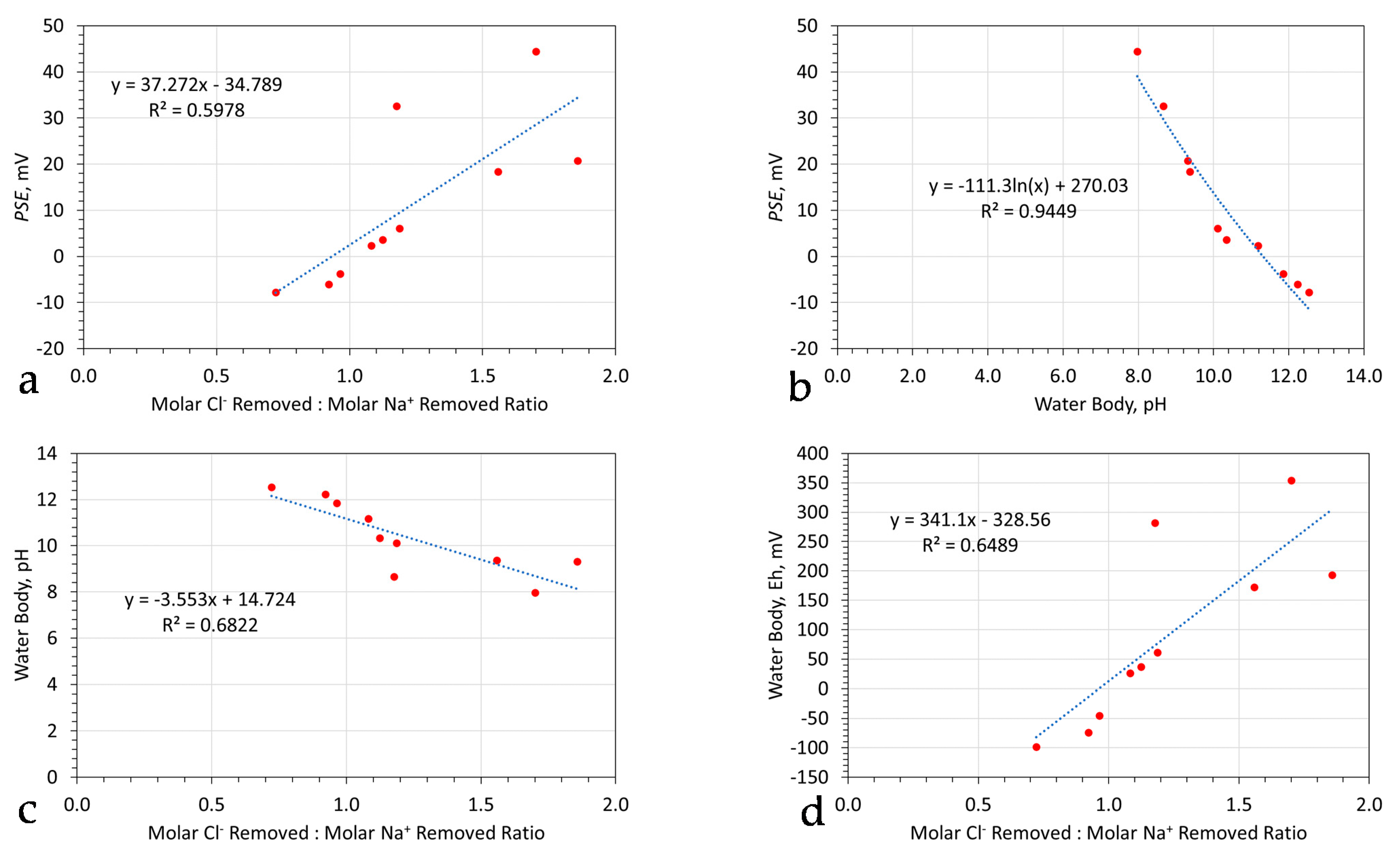
4. Changes in Redox Chemistry
- The median probabilities and mean values indicate that the amount of desalination is pH sensitive. An increase in pH results in a higher desalination than a decrease in pH;
- The median probabilities and mean values indicate that the proportion of Cl− ions removed will be higher than the proportion of Na+ ions removed.
5. Polymer Sphere Growth
- (i)
- (ii)
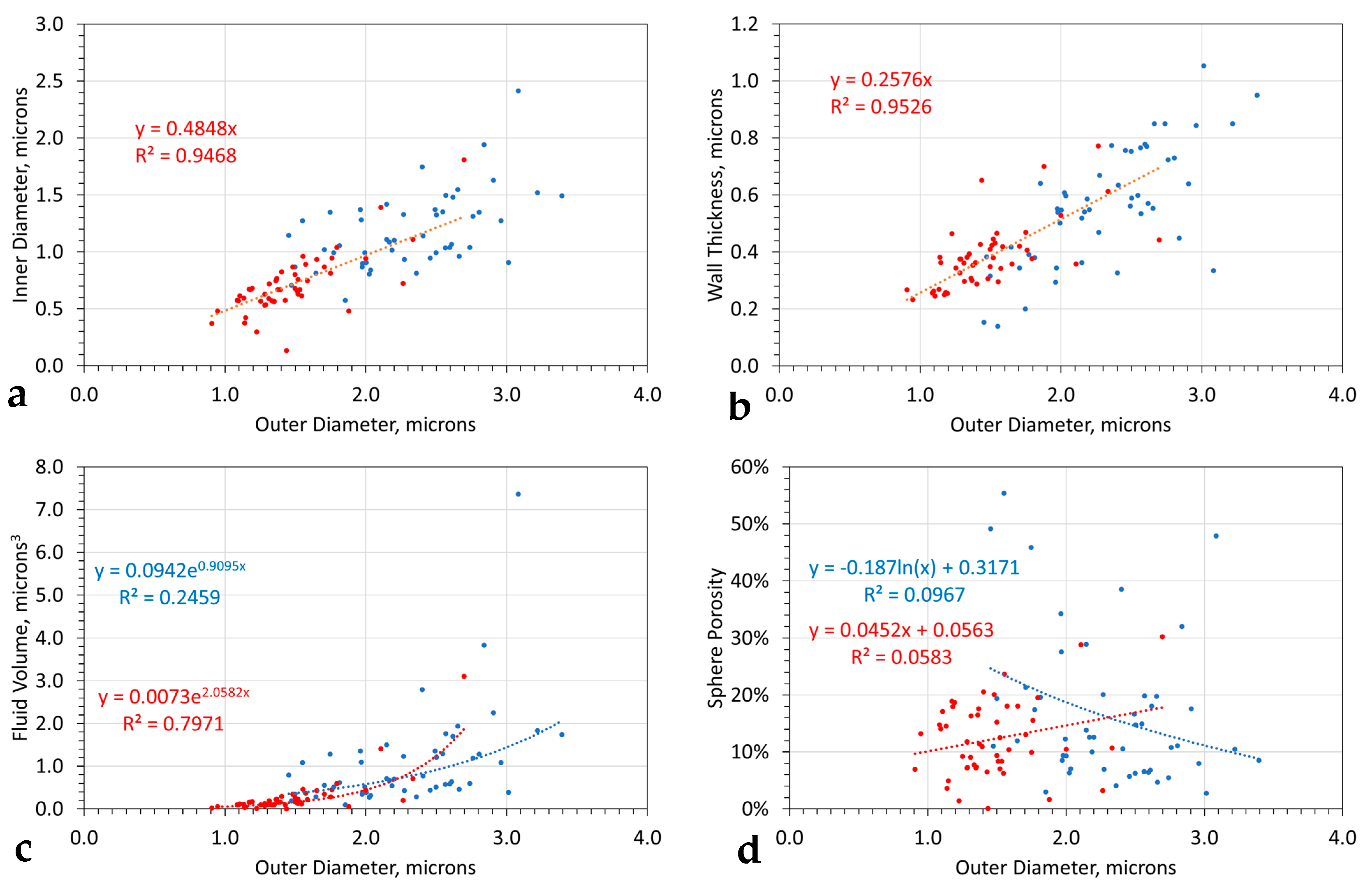
- The aggregated spheres are larger than the individual entrained spheres (Figure 4);
- (iii)
- Both groups of spheres adhere to the same statistical relationship between (i) the outer sphere diameter and the inner sphere diameter (Figure 4a), (ii) the outer sphere diameter and sphere wall thickness (Figure 4b), and (iii) the outer sphere diameter and fluid volume (Figure 4c). The aggregated spheres have a larger porosity variance for specific size than the entrained spheres (Figure 4d).
6. Discussion
6.1. Hydrogel Characteristic
- Networks of polymers forming colloidal gels, where water is the dispersion medium;
- A water swollen cross-linked polymeric network produced by one or more monomers;
- Insoluble polymeric material that is able to swell and retain a significant fraction of water within its structure.
6.1.1. Hydrophobic Hydrogels
- They can contain up to 99.6% water;
- Their structure consists of hydrophobic skin and water-trapped micropores;
- They can exhibit selective water absorption from concentrated saline solutions;
- They can exhibit rapid water release in response to small pressure changes (chemical, osmotic, or physical);
- The gels expand with time.
6.1.2. Significance of Hydrogen
- A gas (hydrogen) sphere, surrounded by or partially surrounded by hydrophilic FeOOH (Figure 1). Hydrophobicity is created by the surface tensions associated with hydrogen gas;
- A fluid (hydrogen + water) sphere surrounded by hydrophilic FeOOH. The fluid core of the sphere contains a gas–water contact. Hydrophobicity may be partial and is created by the surface tensions associated with hydrogen gas.
6.2. Significance of MnO2 in Fe(a,b,c)@MnO2 Polymers
- The Fe(a,b,c) form as nano-micron-sized hollow spheres;
- The spheres then aggregate around one or more MnO2 particles to form hydrated colloids containing one or more MnO2 particles surrounded by Fe(a,b,c) hollow spheres containing water, hydrogen, Na+, and Cl− ions.
6.3. Selectivity Associated with Fe(a,b,c)@MnO2 Polymers
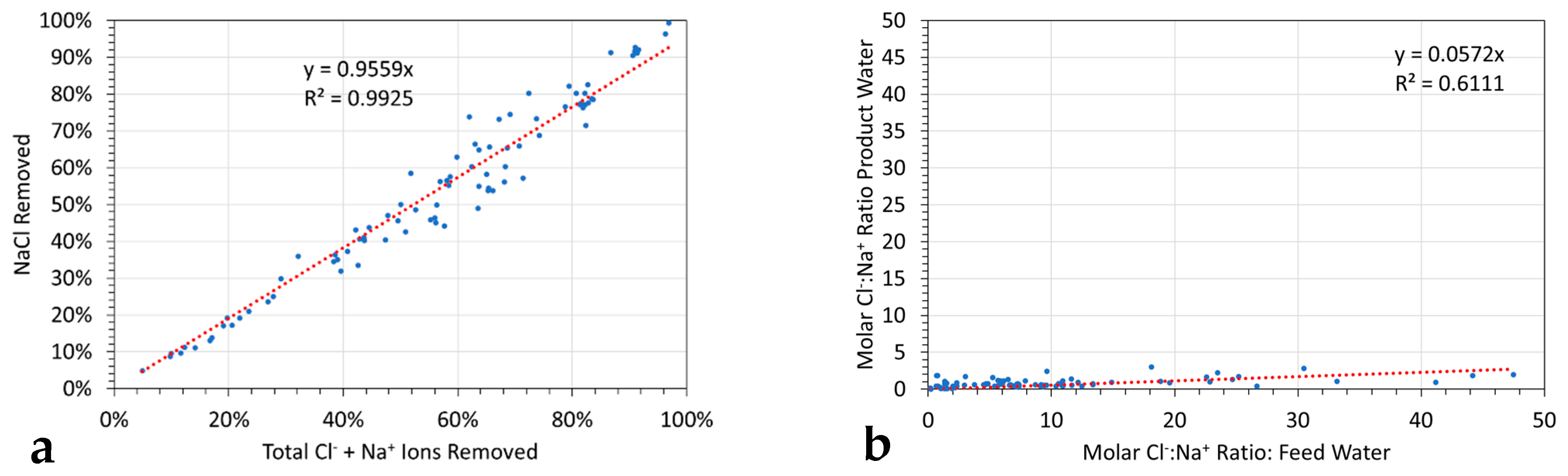
6.3.1. Redox Controls on Molar Removal Selectivity of Cl− ions and Na+ Ions
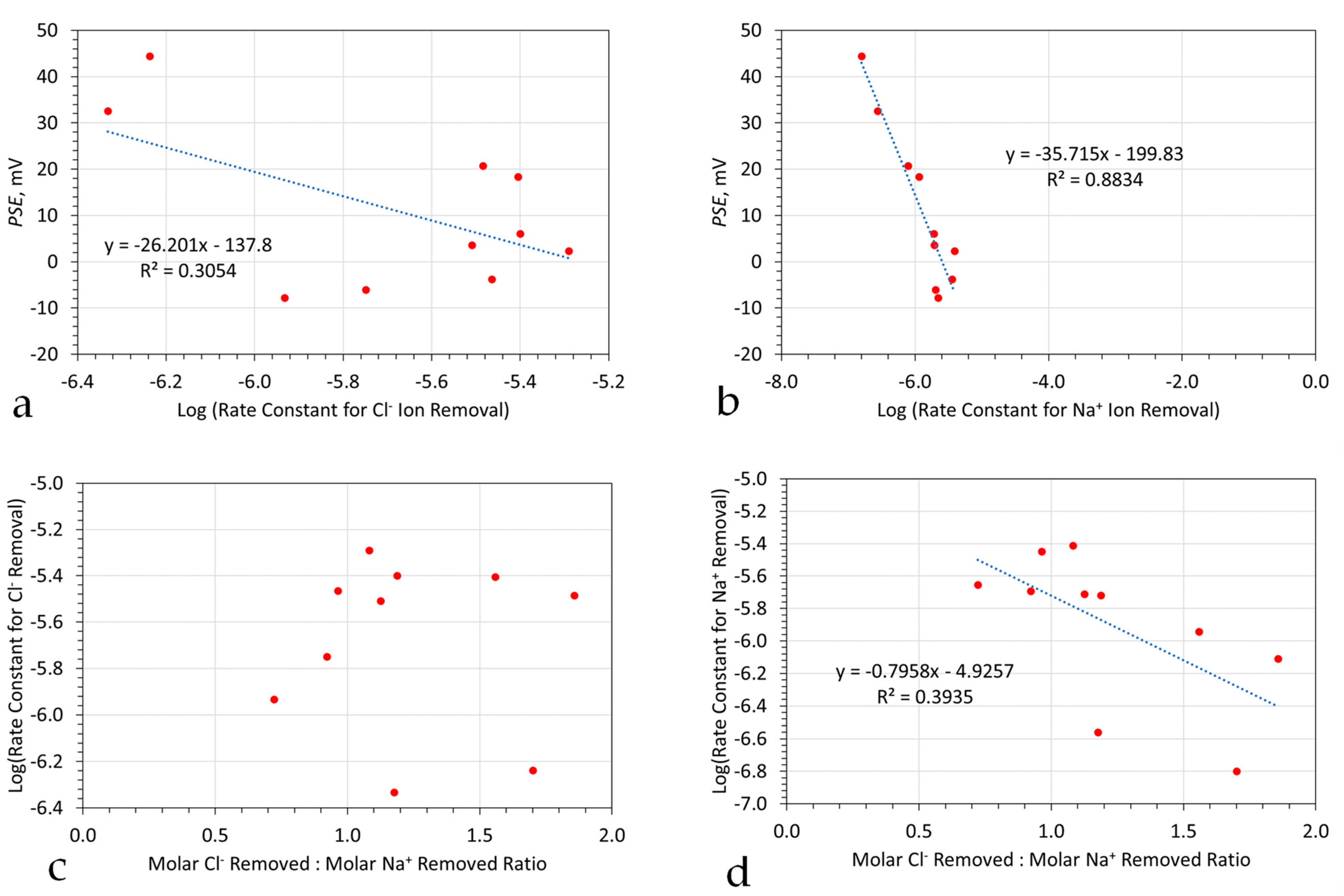
6.3.2. Redox Controls on the Ion Removal Rate Constant
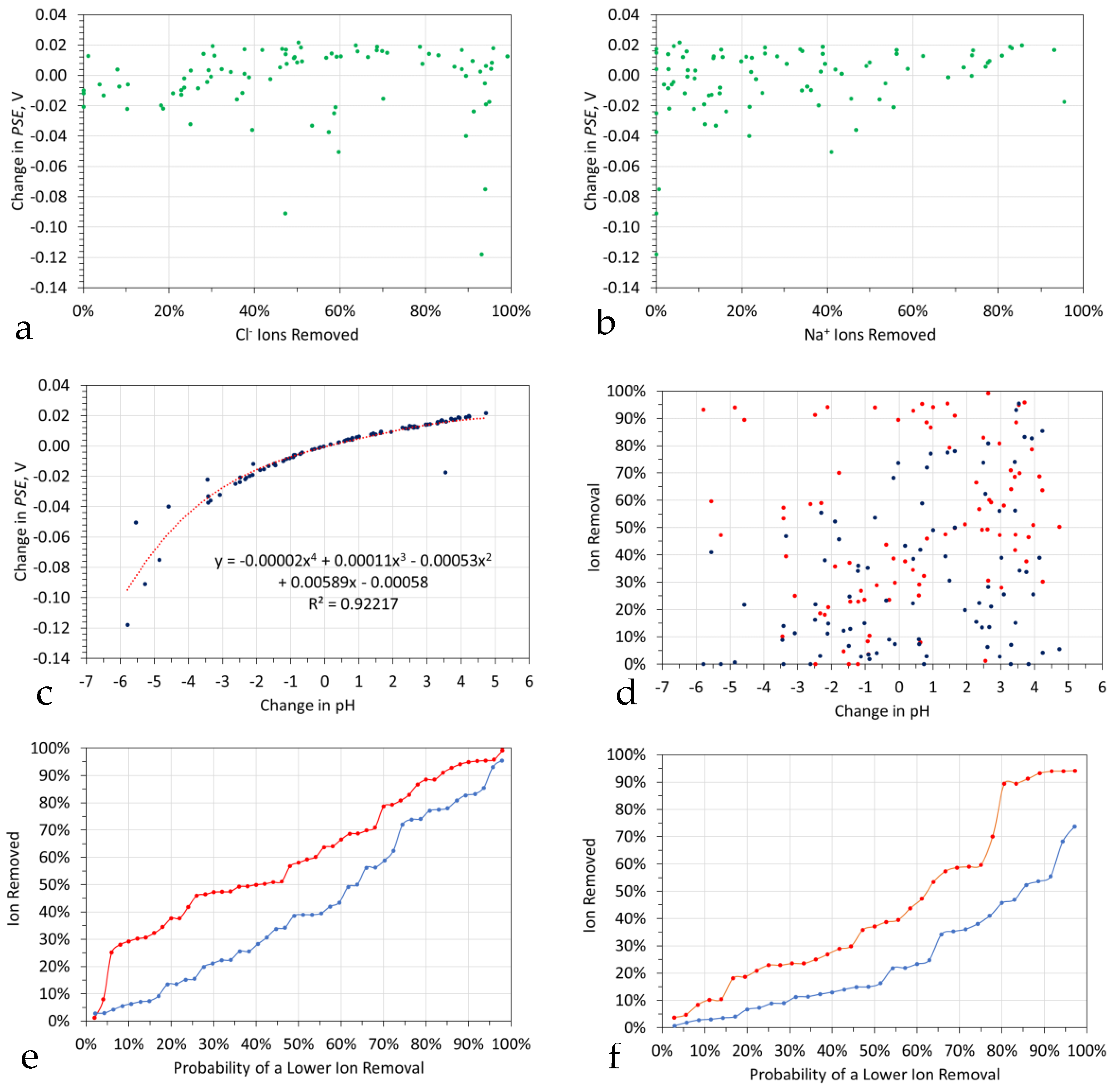
- A moderate, negative statistical regression relationship (R2 = 0.3) between the rate constant for Cl− ion removal and PSE (Figure 7a). The low R2 value results from the inclusion of two outliers. The R2 indicates a 55% dependency between the rate constant for Cl− ion removal and PSE;
- A strong negative statistical relationship between the rate constant for Na+ ion removal and PSE (Figure 7b). The R2 indicates a 94% dependency between the rate constant for Na+ ion removal and PSE;
- No significant statistical correlation between the rate constant for Cl− ion removal and the ratio of molar Na+ ion removed, is shown in Figure 7c.
6.3.3. Significance of pH and Eh Change
6.3.4. Relationship between Ion Removal and pH Change
6.3.5. Relationship between Ion Removal and Eh Change
6.3.6. Statistical Model
7. Implications
7.1. Agriculture
7.2. General Water Remediation
7.3. Catalysis
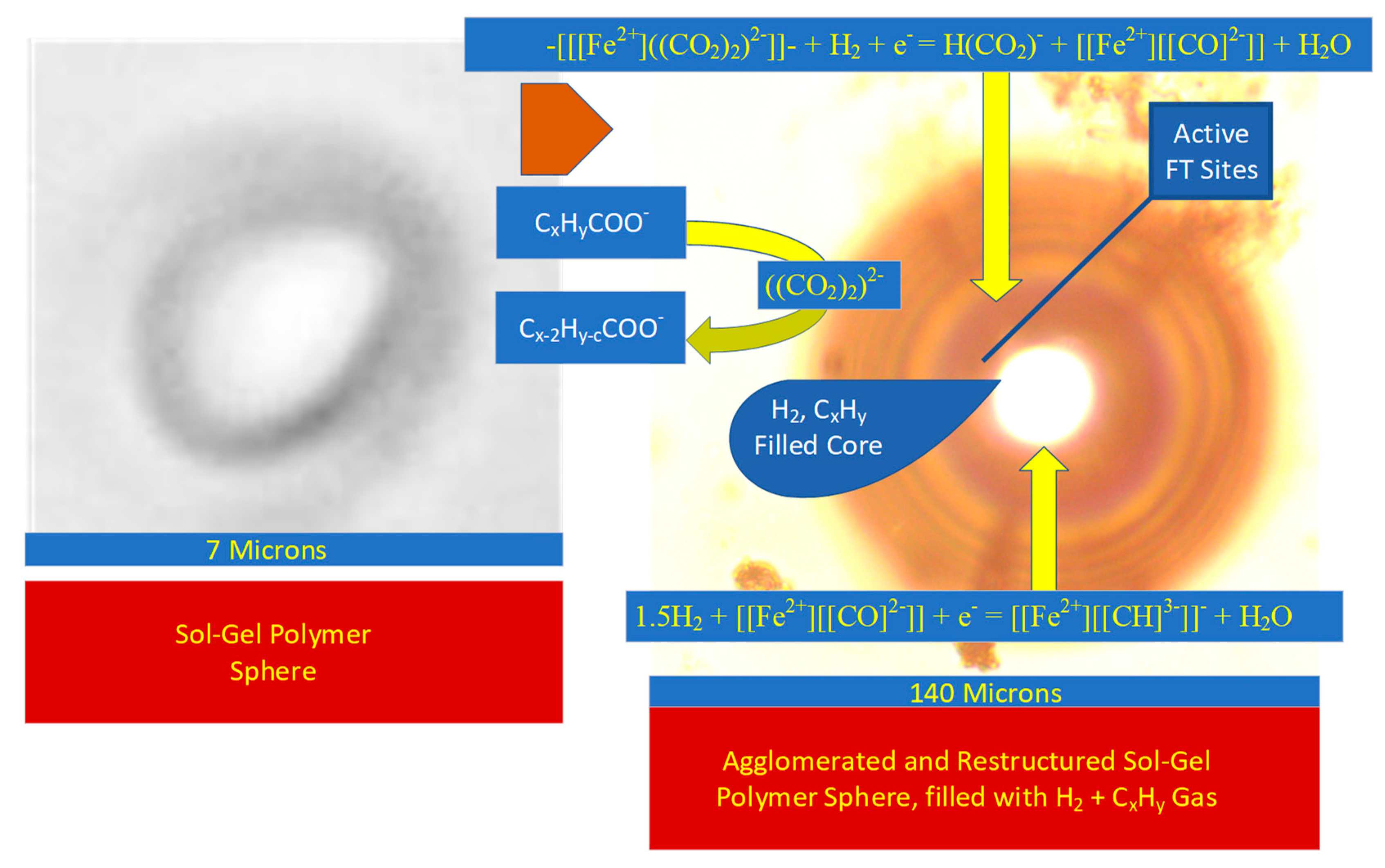
- The polymer spheres (Figure 9) agglomerate to form larger fluid-filled spheres;
- The outer –[Fe(a,b,c)]- polymer crystallites interact with R-COO− to form –[[[Fe2+]((CO2)2)2−]]- polymers (Figure 9);
- Over time, the Ostwald ripening effectively transports the –[[[Fe2+]((CO2)2)2−]]- polymers to the fluid–rim boundary (Figure 9); during this transport, the polymers are first hydrogenated to form –[[[Fe2+][[CO]2−]]- polymers. They are then further hydrogenated to form –[[[Fe2+][[CH]3−]]- polymers;
8. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebbers, R.; Adamchuk, V.I. Precision agriculture and food security. Science 2010, 327, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Shafi, U.; Mumtaz, R.; García-Nieto, J.; Hassan, S.A.; Zaidi, S.A.R.; Iqbal, N. Precision Agriculture Techniques and Practices: From Considerations to Applications. Sensors 2019, 19, 3796. [Google Scholar] [CrossRef] [PubMed]
- Pathak, H.S.; Brown, P.; Best, T. A systematic literature review of the factors affecting the precision agriculture adoption process. Precis. Agric. 2019, 20, 1292–1316. [Google Scholar] [CrossRef]
- Monteiro, A.; Santos, S.; Gonçalves, P. Precision Agriculture for Crop and Livestock Farming—Brief Review. Animals 2021, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, I.; Phadikar, S.; Majumder, K. State-of-the-art technologies in precision agriculture: A systematic review. J. Sci. Food Agric. 2019, 99, 4878–4888. [Google Scholar] [CrossRef]
- Rajak, R.K.; Pawar, A.; Pendke, M.; Shinde, P.; Rathod, S.; Devare, A. Crop recommendation system to maximize crop yield using machine learning technique. Int. Res. J. Eng. Technol. 2017, 4, 950–953. [Google Scholar]
- Singh, A.K. Precision Agriculture in India–Opportunities and Challenges. Indian J. Fertil. 2022, 18, 308–331. [Google Scholar]
- Perakis, K.; Lampathaki, F.; Nikas, K.; Georgiou, Y.; Marko, O.; Maselyne, J. CYBELE–Fostering precision agriculture & livestock farming through secure access to large-scale HPC enabled virtual industrial experimentation environments fostering scalable big data analytics. Comput. Netw. 2020, 168, 107035. [Google Scholar]
- García, R.; Aguilar, J.; Toro, M.; Pérez, N.; Pinto, A.; Rodríguez, P. Autonomic computing in a beef-production process for Precision Livestock Farming. J. Ind. Inf. Integr. 2023, 31, 100425. [Google Scholar] [CrossRef]
- Mishra, S.; Sharma, S.K. Advanced contribution of IoT in agricultural production for the development of smart livestock environments. Internet Things 2023, 22, 100724. [Google Scholar] [CrossRef]
- Shin, J.; Mahmud, M.; Rehman, T.U.; Ravichandran, P.; Heung, B.; Chang, Y.K. Trends and Prospect of Machine Vision Technology for Stresses and Diseases Detection in Precision Agriculture. AgriEngineering 2023, 5, 20–39. [Google Scholar] [CrossRef]
- Gokool, S.; Mahomed, M.; Kunz, R.; Clulow, A.; Sibanda, M.; Naiken, V.; Chetty, K.; Mabhaudhi, T. Crop Monitoring in Smallholder Farms Using Unmanned Aerial Vehicles to Facilitate Precision Agriculture Practices: A Scoping Review and Bibliometric Analysis. Sustainability 2023, 15, 3557. [Google Scholar] [CrossRef]
- Tamirat, T.W.; Pedersen, S.M.; Ørum, J.E.; Holm, S.H. Multi-stakeholder perspectives on field crop robots: Lessons from four case areas in Europe. Smart Agric. Technol. 2023, 4, 100143. [Google Scholar] [CrossRef]
- Tzanidakis, C.; Tzamaloukas, O.; Simitzis, P.; Panagakis, P. Precision Livestock Farming Applications (PLF) for Grazing Animals. Agriculture 2023, 13, 288. [Google Scholar] [CrossRef]
- Geipel, J.; Bakken, A.K.; Jørgensen, M.; Korsaeth, A. Forage yield and quality estimation by means of UAV and hyperspectral imaging. Precis. Agric. 2021, 22, 1437–1463. [Google Scholar] [CrossRef]
- Klokov, A.V.; Loktionov, E.Y.; Loktionov, Y.V.; Panchenko, V.A.; Sharaborova, E.S. A Mini-Review of Current Activities and Future Trends in Agrivoltaics. Energies 2023, 16, 3009. [Google Scholar] [CrossRef]
- Ali, A.; Hussain, T.; Tantashutikun, N.; Hussain, N.; Cocetta, G. Application of Smart Techniques, Internet of Things and Data Mining for Resource Use Efficient and Sustainable Crop Production. Agriculture 2023, 13, 397. [Google Scholar] [CrossRef]
- Antia, D.D.J. Desalination of Irrigation Water Using Metal Polymers. Water 2022, 14, 3224. [Google Scholar] [CrossRef]
- Awaad, H.A.; Mansour, E.; Akrami, M.; Fath, H.E.S.; Javadi, A.A.; Negm, A. Availability and Feasibility of Water Desalination as a Non-Conventional Resource for Agricultural Irrigation in the MENA Region: A Review. Sustainability 2020, 12, 7592. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Yip, N.Y.; Gilron, J.; Elimelech, M. Seawater desalination for agriculture by integrated forward and reverse osmosis: Improved product water quality for potentially less energy. J. Membr. Sci. 2012, 415–416, 1–8. [Google Scholar] [CrossRef]
- Bunani, S.; Yörükoğlu, E.; Yüksel, Ü.; Kabay, N.; Yüksel, M.; Sert, G. Application of reverse osmosis for reuse of secondary treated urban wastewater in agricultural irrigation. Desalination 2015, 364, 68–74. [Google Scholar] [CrossRef]
- Suwaileh, W.; Johnson, D.; Hilal, N. Membrane desalination and water re-use for agriculture: State of the art and future outlook. Desalination 2020, 491, 114559. [Google Scholar] [CrossRef]
- Tomaszewska, B.; Akkurt, G.G.; Kaczmarczyk, M.; Bujakowski, W.; Keles, N.; Jarma, Y.A.; Baba, A.; Bryjak, M.; Kabay, N. Utilization of renewable energy sources in desalination of geothermal water for agriculture. Desalination 2021, 513, 115151. [Google Scholar] [CrossRef]
- Colmenar-Santos, A.; Palomo-Torrejón, E.; Mur-Pérez, F.; Rosales-Asensio, E. Thermal desalination potential with parabolic trough collectors and geothermal energy in the Spanish southeast. Appl. Energy 2020, 262, 114433. [Google Scholar] [CrossRef]
- Caldera, U.; Breyer, C. Global potential for renewable energy powered desalination in the irrigation sector. In Energy Storage for Multigeneration; Academic Press: Cambridge, MA, USA, 2023; pp. 53–92. [Google Scholar]
- Kiwan, S.; Alali, A.; Al-Safadi, M. A novel water freezing desalination plant integrated into a combined gas power cycle plant. Energy 2023, 263, 125983. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Ruan, Y.; Zhao, N.; Liu, W.; Zhuang, W.; Lu, X. Molecular insights into multilayer 18-crown-6-like graphene nanopores for K+/Na+ separation: A molecular dynamics study. Carbon 2019, 144, 32–42. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Huang, M.; Jiang, H.; Toghan, A. Crown ether interlayer-modulated polyamide membrane with nanoscale structures for efficient desalination. Nano Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Coterillo, R.; Gallart, L.E.; Fernandez-Escalante, E.; Junquera, J.; Garcia-Fernandez, P.; Ortiz, I.; Ibanez, R.; San-Roman, M.F. Selective extraction of lithium from seawater desalination concentrates: Study of thermodynamic and equilibrium properties using Density Functional Theory (DFT). Desalination 2022, 532, 115704. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, Q.; Zou, W.; Fang, J.; Yang, Z.; Xu, T. Dibenzo-15-crown-5-based Tröger’s Base membrane for 6Li+/7Li+ separation. Sep. Purif. Technol. 2023, 309, 122990. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; Ma, Z.; Qin, Y.; Lu, X.; Jin, W.; Zhu, Y. Assisting role of water molecules in ionic recognition by 18-crown-6 ether in aqueous solutions. J. Mol. Liq. 2023, 371, 121127. [Google Scholar] [CrossRef]
- Chaudhury, S.; Bhattacharyya, A.; Goswami, A. Electrodriven ion transport through crown ether–nafion composite membrane: Enhanced selectivity of Cs+ over Na+ by ion gating at the surface. Ind. Eng. Chem. Res. 2014, 53, 8804–8809. [Google Scholar] [CrossRef]
- Zahedi, M.M.; Ghasemi, S.M. Separation study of Mg+2 from seawater and RO brine through a facilitated bulk liquid membrane transport using 18-Crown-6. J. Water Reuse Desalination 2017, 7, 468–475. [Google Scholar] [CrossRef]
- Antia, D.D.J. Purification of Saline Water Using Desalination Pellets. Water 2022, 14, 2639. [Google Scholar] [CrossRef]
- Abuhabib, A.A.; Ghasemi, M.; Mohammad, A.W.; Rahman, R.A.; El-Shafie, A.H. Desalination of brackish water using nanofiltration: Performance comparison of different membranes. Arab. J. Sci. Eng. 2013, 38, 2929–2939. [Google Scholar] [CrossRef]
- Wang, B.; Wu, H.; Yu, L.; Xu, R.; Lim, T.T.; Lou, X.W. Template-free formation of uniform urchin-like α-FeOOH hollow spheres with superior capability for water treatment. Adv. Mater. 2012, 24, 1111–1116. [Google Scholar] [CrossRef]
- Cao, S.W.; Zhu, Y.J. Iron oxide hollow spheres: Microwave–hydrothermal ionic liquid preparation, formation mechanism, crystal phase and morphology control and properties. Acta Mater. 2009, 57, 2154–2165. [Google Scholar] [CrossRef]
- Liang, H.; Chen, W.; Wang, R.; Qi, Z.; Mi, J.; Wang, Z. X-shaped hollow α-FeOOH penetration twins and their conversion to α-Fe2O3 nanocrystals bound by high-index facets with enhanced photocatalytic activity. Chem. Eng. J. 2015, 274, 224–230. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, C.; Liu, Z.; Li, D. Exploration of amorphous hollow FeOOH@ C nanosphere on energy storage for sodium ion batteries. Int. J. Hydrog. Energy 2021, 46, 26457–26465. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic surfaces: Insights from theory and experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Z. Biomimetic Superhydrophobic Surfaces with Transition Metals and Their Oxides: A Review. J. Bionic Eng. 2017, 14, 401–439. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Missimer, T.M.; Maliva, R.G. Environmental issues in seawater reverse osmosis desalination: Intakes and outfalls. Desalination 2018, 434, 198–215. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Yang, D.R.; Hong, S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE International: Houston, TX, USA, 1974; p. 644. [Google Scholar]
- Refait, P.; Génin, J.M. The oxidation of ferrous hydroxide in chloride-containing aqueous media and Pourbaix diagrams of green rust one. Corros. Sci. 1993, 34, 797–819. [Google Scholar] [CrossRef]
- Refait, P.H.; Abdelmoula, M.; Génin, J.M. Mechanisms of formation and structure of green rust one in aqueous corrosion of iron in the presence of chloride ions. Corros. Sci. 1998, 40, 1547–1560. [Google Scholar] [CrossRef]
- Sagoe-Crentsil, K.K.; Glasser, F.P. “Green rust”, iron solubility and the role of chloride in the corrosion of steel at high pH. Cem. Concr. Res. 1993, 23, 785–791. [Google Scholar] [CrossRef]
- Génin, J.M.; Refait, P.; Simon, L.; Drissi, S.H. Preparation and Eh--pH diagrams of Fe (II)--Fe (III) green rust compounds; hyperfine interaction characteristics and stoichiometry of hydroxy-chloride,-sulphate and-carbonate. Hyperfine Interact. 1998, 111, 313–318. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Gao, Z.; Navrotsky, A.; Suib, S.L. Synthesis and anion exchange of tunnel structure akaganeite. Chem. Mater. 2001, 13, 4595–4602. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Refait, P. On the formation of β-FeOOH (akaganéite) in chloride-containing environments. Corros. Sci. 2007, 49, 844–857. [Google Scholar] [CrossRef]
- Post, J.E.; Buchwald, V.F. Crystal structure refinement of akaganeite. Am. Mineral. 1991, 76, 272–277. [Google Scholar]
- Scheck, J.; Lemke, T.; Gebauer, D. The Role of Chloride Ions during the Formation of Akaganéite Revisited. Minerals 2015, 5, 778–787. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Li, S.; Bundschuh, J. A review of the distribution, sources, genesis, and environmental concerns of salinity in groundwater. Environ. Sci. Pollut. Res. 2020, 27, 41157–41174. [Google Scholar] [CrossRef]
- Marandi, A.; Shand, P. Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Mondal, N.C.; Singh, V.P.; Singh, V.S.; Saxena, V.K. Determining the interaction between groundwater and saline water through groundwater major ions chemistry. J. Hydrol. 2010, 388, 100–111. [Google Scholar] [CrossRef]
- Ebbing, D.D.; Gammon, S.D. General Chemistry; Houghton Mifflin Company: New York, NY, USA, 2005; ISBN 0-618-399410. [Google Scholar]
- Heder, M. From NASA to EU: The evolution of the TRL scale in Public Sector Innovation. Innov. J. Public Sect. Innov. J. 2017, 22, 3. [Google Scholar]
- European Space Agency. Technology Readiness Levels Handbook for Space Applications; TRL Handbook Issue 1 Revision 6- September 2008 TEC-SHS/5551/MG/ap; European Space Agency: Paris, France, 2008. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Irrigation and Drainage Paper No. 29, Rev. 1, Reprinted 1989, 1994; Food and Agriculture Organization of the United Nations: Rome, Italy, 1994. [Google Scholar]
- Masters, D.G.; Benes, S.E.; Norman, H.C. Biosaline agriculture for forage and livestock production. Agric. Ecosyst. Environ. 2007, 119, 234–248. [Google Scholar] [CrossRef]
- Glauert, S. Livestock and Water Salinity; Farm Note: 249; Government of Western Australia, Department of Agriculture and Food: Perth, Australia, 2007. Available online: https://futurebeef.com.au/wp-content/uploads/2011/09/Livestock_and_water_salinity_DAFWA.pdf (accessed on 12 April 2023).
- New South Wales Government, Department of Primary Industries (NSW DPI). Water Requirements for Sheep and Cattle; Prime Facts 326; NSW DPI: Sydney, Australia, 2014. Available online: https://www.dpi.nsw.gov.au/__da (accessed on 12 April 2023).
- Bottero, J.Y.; Manceau, A.; Villieras, F.; Tchoubar, D. Structure and mechanisms of formation of iron oxide hydroxide (chloride) polymers. Langmuir 1994, 10, 316–319. [Google Scholar] [CrossRef]
- Spiro, T.G.; Allerton, S.E.; Renner, J.; Terzis, A.; Bils, R.; Saltman, P. The Hydrolytic Polymerization of Iron(III). J. Am. Chem. Soc. 1966, 88, 2721–2726. [Google Scholar] [CrossRef]
- Dong, H.; Gao, B.; Yue, Q.; Sun, S.; Wang, Y.; Li, Q. Floc properties and membrane fouling of different monomer and polymer Fe coagulants in coagulation–ultrafiltration process: The role of Fe (III) species. Chem. Eng. J. 2014, 258, 442–449. [Google Scholar] [CrossRef]
- British Standards Institute. Quality management systems, BSI Handbook 25. In Statistical Interpretation of Data; British Standards Institute: London, UK, 1985; p. 318. ISBN 0580150712/9780580150715. [Google Scholar]
- Siegel, S. Nonparametric statistics. Am. Stat. 1957, 11, 13–19. [Google Scholar]
- Journel, A.G. Nonparametric estimation of spatial distributions. J. Int. Assoc. Math. Geol. 1983, 15, 445–468. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Interpretation of the Correlation Coefficient: A Basic Review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, T.H.; Ergün, T. The instability of the Pearson correlation coefficient in the presence of coincidental outliers. Financ. Res. Lett. 2015, 13, 243–257. [Google Scholar] [CrossRef]
- Antia, D.D.J. Partial Desalination of Saline Groundwater, including Flowback Water, to Produce Irrigation Water. Hydrology 2022, 9, 219. [Google Scholar] [CrossRef]
- Jain, S.C.; Hughes, A.E. Ostwald ripening in ionic crystals. J. Phys. Colloq. 1976, 37, C7–C463. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, D.; Li, D.; Jia, H.; Qin, W. Control of Ostwald ripening. Sci. China Mater. 2023, 66, 1249–1255. [Google Scholar] [CrossRef]
- Zhang, Y.; Bijeljic, B.; Gao, Y.; Goodarzi, S.; Foroughi, S.; Blunt, M.J. Pore-Scale Observations of Hydrogen Trapping and Migration in Porous Rock: Demonstrating the Effect of Ostwald Ripening. Geophys. Res. Lett. 2023, 50, e2022GL102383. [Google Scholar] [CrossRef]
- Antia, D.D.J. Hydrodynamic Decontamination of Groundwater and Soils Using ZVI. Water 2023, 15, 540. [Google Scholar] [CrossRef]
- Wu, B.; Tian, F.; Zhang, M.; Piao, S.; Zeng, H.; Zhu, W.; Liu, J.; Elnashar, A.; Lu, Y. Quantifying global agricultural water appropriation with data derived from earth observations. J. Clean. Prod. 2022, 358, 131891. [Google Scholar] [CrossRef]
- Negacz, K.; Malek, Ž.; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid. Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Rosa, L. Adapting agriculture to climate change via sustainable irrigation: Biophysical potentials and feedbacks. Environ. Res. Lett. 2022, 17, 063008. [Google Scholar] [CrossRef]
- Pison, G. World population: 8 billion today, how many tomorrow? Popul. Soc. 2022, 604, 1–4. [Google Scholar]
- Liu, S.; Wang, Y.; Zhang, G.J.; Wang, B.; Yu, L. Contrasting influences of biogeophysical and biogeochemical impacts of historical land use on global economic inequality. Nat. Commun. 2022, 13, 2479. [Google Scholar] [CrossRef]
- Wang, X. Managing Land Carrying Capacity: Key to Achieving Sustainable Production Systems for Food Security. Land 2022, 11, 484. [Google Scholar] [CrossRef]
- Zhao, H.; Di, L.; Sun, Z. WaterSmart-GIS: A Web Application of a Data Assimilation Model to Support Irrigation Research and Decision Making. ISPRS Int. J. Geo-Inf. 2022, 11, 271. [Google Scholar] [CrossRef]
- Misstear, B.; Banks, D.; Clark, L. Water Wells and Boreholes; John Wiley and Sons: Hoboken, NJ, USA, 2007; 498p. [Google Scholar]
- Lide, D. CRC Handbook of Chemistry and Physics, 2008–2009, 89th ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Rebello, L.R.B.; Siepman, T.; Drexler, S. Correlations between TDS and electrical conductivity for high-salinity formation brines characteristic of South Atlantic pre-salt basins. Water SA 2020, 46, 602–609. [Google Scholar]
- Guisbiers, G. Schottky Defects in Nanoparticles. J. Phys. Chem. C 2011, 115, 2616–2621. [Google Scholar] [CrossRef]
- Schilling, W. Properties of Frenkel defects. J. Nucl. Mater. 1994, 216, 45–48. [Google Scholar] [CrossRef]
- Song, X.; An, J.; He, C.; Zhou, J.; Xu, Y.; Ji, H.; Yang, L.; Yin, J.; Zhao, W.; Zhao, C. A bioinspired strategy towards super-adsorbent hydrogel spheres via self-sacrificing micro-reactors for robust wastewater remediation. J. Mater. Chem. A 2019, 7, 21386–21403. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, J.; Wang, Z.; Guo, H.L.; Hong, W.; Wang, X. Dynamic swelling performance of hydrophobic hydrogels. Chin. Chem. Lett. 2022, 33, 2178–2182. [Google Scholar] [CrossRef]
- Guo, H.; Nakajima, T.; Hourdet, D.; Marcellan, A.; Creton, C.; Hong, W.; Kurokawa, T.; Gong, J.P. Hydrophobic Hydrogels with Fruit-Like Structure and Functions. Adv. Mater. 2019, 31, 1900702. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Kawamura, S.; Hagayama, M. Acceleration of the oxidation of Fe2+ ions by Fe (III)-oxyhydroxides. Corros. Sci. 1980, 20, 963–971. [Google Scholar] [CrossRef]
- Kanie, K.; Muramatsu, A.; Suzuki, S.; Waseda, Y. Influence of sulfate ions on conversion of Fe (OH)3 gel to β-FeOOH and α-Fe2O3. Mater. Trans. 2004, 45, 968–971. [Google Scholar] [CrossRef]
- Antia, D.D.J. Conversion of Waste Synthesis Gas to Desalination Catalyst at Ambient Temperatures. Waste, 2023; in press. [Google Scholar]
- Antia, D.D.J. Remediation of Saline Wastewater Producing a Fuel Gas Containing Alkanes and Hydrogen Using Zero Valent Iron (Fe0). Water 2022, 14, 1926. [Google Scholar] [CrossRef]
- El-taweel, R.M.; Mohamed, N.; Alrefaey, K.A.; Husien, S.; Abdel-Aziz, A.B.; Salim, A.I.; Mostafa, N.G.; Said, L.A.; Fahim, I.S.; Radwan, A.G. A review of coagulation explaining its definition, mechanism, coagulant types, and optimization models; RSM, and ANN. Curr. Res. Green Sustain. Chem. 2023, 6, 100358. [Google Scholar] [CrossRef]
- Joshi, S.; Kathuria, H.; Verma, S.; Valiyaveettil, S. Functional catechol–metal polymers via interfacial polymerization for applications in water purification. ACS Appl. Mater. Interfaces 2020, 12, 19044–19053. [Google Scholar] [CrossRef]
- Khoe, G.H.; Robins, R.G. Polymerization reactions in hydrolyzed iron (III) solutions. J. Colloid Interface Sci. 1989, 133, 244–252. [Google Scholar] [CrossRef]
- Rose, J.; Manceau, A.; Masion, A.; Bottero, J.Y. Structure and mechanisms of formation of FeOOH (NO3) oligomers in the early stages of hydrolysis. Langmuir 1997, 13, 3240–3246. [Google Scholar] [CrossRef]
- Zhu, M.; Frandsen, C.; Wallace, A.F.; Legg, B.; Khalid, S.; Zhang, H.; Mørup, S.; Banfield, J.F.; Waychunas, G.A. Precipitation pathways for ferrihydrite formation in acidic solutions. Geochim. Cosmochim. Acta 2016, 172, 247–264. [Google Scholar] [CrossRef]
- Li, S.; Kang, Y. Polymerization mechanism of polyferric aluminum phosphatic sulfate (PFAPS) and its flocculation effect on simulated dye wastewater. Korean J. Chem. Eng. 2022, 39, 1831–1838. [Google Scholar] [CrossRef]
- Wang, C.; Sheng, Y.; Zhao, X.; Pan, Y.; Wang, Z. Synthesis of hydrophobic CaCO3 nanoparticles. Mater. Lett. 2006, 60, 854–857. [Google Scholar] [CrossRef]
- Wang, C.; Piao, C.; Zhai, X.; Hickman, F.N.; Li, J. Synthesis and character of super-hydrophobic CaCO3 powder in situ. Powder Technol. 2010, 200, 84–86. [Google Scholar] [CrossRef]
- Liu, L.; Yang, L.Q.; Liang, H.W.; Cong, H.P.; Jiang, J.; Yu, S.H. Bio-inspired fabrication of hierarchical FeOOH nanostructure array films at the air–water interface, their hydrophobicity and application for water treatment. ACS Nano 2013, 7, 1368–1378. [Google Scholar] [CrossRef]
- Suzaimi, N.D.; Goh, P.S.; Wong, K.C.; Malek, N.A.N.N.; Ismail, A.F.; Lim, J.W. Tailoring the substrate of thin film reverse osmosis membrane through a novel β-FeOOH nanorods templating strategy: An insight into the effects on interfacial polymerization of polyamide. J. Membr. Sci. 2022, 657, 120706. [Google Scholar] [CrossRef]
- Sipos, P.; Berkesi, O.; Tombácz, E.; Pierre, T.G.S.; Webb, J. Formation of spherical iron (III) oxyhydroxide nanoparticles sterically stabilized by chitosan in aqueous solutions. J. Inorg. Biochem. 2003, 95, 55–63. [Google Scholar] [CrossRef]
- Fan, H.; Song, B.; Yang, Z.; Li, Q. Fast inducing synthesis of spherical superparamagnetic β-FeOOH nanoparticles without aggregation. Chem. Lett. 2004, 33, 576–577. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Liu, C.; Zhang, M.; Ma, H.; Li, J. Fabrication of superhydrophobic spherical-like α-FeOOH films on the wood surface by a hydrothermal method. Colloids Surf. A Physicochem. Eng. Asp. 2012, 403, 29–34. [Google Scholar] [CrossRef]
- Krehula, S.; Musić, S. Influence of aging in an alkaline medium on the microstructural properties of α-FeOOH. J. Cryst. Growth 2008, 310, 513–520. [Google Scholar] [CrossRef]
- Wu, H.; Xia, G.; Yu, X. Recent Progress on Nanostructured Iron-based Anodes Beyond Metal-Organic Frameworks for Sodium-Ion Batteries. EnergyChem 2022, 5, 100095. [Google Scholar] [CrossRef]
- Kang, S.; Wang, G.; Fang, M.; Wang, H.; Wang, X.; Cai, W. Water bath synthesis and enhanced photocatalytic performances of urchin-like micro/nanostructured α-FeOOH. J. Mater. Res. 2015, 30, 1629–1638. [Google Scholar] [CrossRef]
- Lan, J.S.; Liu, L.; Zeng, R.F.; Qin, Y.H.; Liu, Y.; Jiang, X.Y.; Aihemaiti, A.; Ding, Y.; Zhang, T.; Ho, R.J. Rational modulation of coumarin–hemicyanine platform based on OH substitution for higher selective detection of hypochlorite. Chem. Commun. 2020, 56, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Mousset, E.; Oturan, N.; Oturan, M.A. An unprecedented route of OH radical reactivity evidenced by an electrocatalytical process: Ipso-substitution with perhalogenocarbon compounds. Appl. Catal. B Environ. 2018, 226, 135–146. [Google Scholar] [CrossRef]
- He, T.; Deng, L.; Lai, B.; Xu, S.; Wang, L.; Zhang, Y.; Zheng, D.; Hu, C. The performance of Fe2+/ClO− system in advanced removal of fulvic acid under mild conditions. J. Environ. Chem. Eng. 2022, 10, 107515. [Google Scholar] [CrossRef]
- Miyata, S. The syntheses of hydrotalcite-like compounds and their structures and physico-chemical properties—I: The systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Feng, X.; Long, R.; Wang, L.; Liu, C.; Bai, Z.; Liu, X. A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Sep. Purif. Technol. 2022, 284, 120099. [Google Scholar] [CrossRef]
- Sajid, M.; Jillani, S.M.S.; Baig, N.; Alhooshani, K. Layered double hydroxide-modified membranes for water treatment: Recent advances and prospects. Chemosphere 2022, 287, 132140. [Google Scholar] [CrossRef]
- Yu, L.; Xiao, J.; Huang, C.; Zhou, J.; Qiu, M.; Yu, Y.; Ren, Z.; Chu, C.W.; Yu, J.C. High-performance seawater oxidation by a homogeneous multimetallic layered double hydroxide electrocatalyst. Proc. Natl. Acad. Sci. USA 2022, 119, e2202382119. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Li, Z.; Zhu, Y.; Yu, H. Oil removal from oily water by a low-cost and durable flexible membrane made of layered double hydroxide nanosheet on cellulose support. J. Clean. Prod. 2018, 180, 307–315. [Google Scholar] [CrossRef]
- Lu, P.; Liang, S.; Qiu, L.; Gao, Y.; Wang, Q. Thin film nanocomposite forward osmosis membranes based on layered double hydroxide nanoparticles blended substrates. J. Membr. Sci. 2016, 504, 196–205. [Google Scholar] [CrossRef]
- Yang, J.; Wen, G.; Gou, X.; Song, H.; Guo, Z. A study on the manufacture of Kevlar membrane modified by inorganic nanoparticles with universal applicability in separating diffident types of emulsions. J. Membr. Sci. 2018, 563, 326–335. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, S.; Sun, Y.; Fang, X.; Wu, L. Fabrication, properties and applications of Janus particles. Chem. Soc. Rev. 2012, 41, 4356–4378. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, C.; Liu, J.; Qu, X.; Li, J.; Yang, Z. Large scale synthesis of Janus submicrometer sized colloids by seeded emulsion polymerization. Macromolecules 2010, 43, 5114–5120. [Google Scholar] [CrossRef]
- Antia, D.D.J. Catalytic Partial Desalination of Saline Water. Water 2022, 14, 2893. [Google Scholar] [CrossRef]
- Li, D.; Kang, Y.; Li, J.; Wang, X. Transformation and coagulation behaviors of iron (III) species of solid polymeric ferric sulfate with high basicity. Korean J. Chem. Eng. 2019, 36, 1499–1508. [Google Scholar] [CrossRef]
- Fronczyk, J.; Pawluk, K.; Michniak, M. Application of permeable reactive barriers near roads for chloride ions removal. Ann. Wars. Univ. Life Sci. SGGW Land Reclaim. 2010, 42, 249–259. [Google Scholar] [CrossRef]
- Fronczyk, J.; Pawluk, K.; Garbulewski, K. Multilayer PRBs—Effective technology for protection of the groundwater environment in traffic infrastructures. Chem. Eng. Trans. 2012, 28, 67–72. [Google Scholar]
- Hwang, Y.; Kim, D.; Shin, H.-S. Inhibition of nitrate reduction by NaCl adsorption on a nano-zero valent iron surface during concentrate treatment for water reuse. Environ. Technol. 2015, 36, 1178–1187. [Google Scholar] [CrossRef]
- Franco Gonzalez, F. Zero-valence iron nanoparticles applied in the desalination of sea water. In Proceedings of the 3rd International Congress on Water, Waste and Energy Management, Rome, Italy, 18–20 July 2016; p. 180. [Google Scholar]
- Bai, H.; Liu, Z.; Sun, D.D. Highly water soluble and recovered dextran coated Fe3O4 magnetic nanoparticles for brackish water desalination. Sep. Purif. Technol. 2011, 81, 392–399. [Google Scholar] [CrossRef]
- Dong, L.; Li, M.; Zhang, S.; Si, X.; Bai, Y.; Zhang, C. NH2-Fe3O4-regulated graphene oxide membranes with well-defined laminar nanochannels for desalination of dye solutions. Desalination 2020, 476, 114227. [Google Scholar] [CrossRef]
- Liu, Q.; Bei, Y.; Zhou, F. Removal of lead (II) from aqueous solution with amino-functionalized nanoscale zero-valent iron. Open Chem. 2009, 7, 79–82. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Zhi, D.; Guo, B.; Zhou, Y.; Nie, J.; Huang, A.; Yang, Y.; Huang, H.; Luo, L. Applications of nanoscale zero-valent iron and its composites to the removal of antibiotics: A review. J. Mater. Sci. 2019, 54, 12171–12188. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Ma, J. Novel synthesis of carbon spheres supported nanoscale zero-valent iron for removal of metronidazole. Appl. Surf. Sci. 2016, 390, 50–59. [Google Scholar] [CrossRef]
- Jiang, Z.; Lv, L.; Zhang, W.; Du, Q.; Pan, B.; Yang, L.; Zhang, Q. Nitrate reduction using nanosized zero-valent iron supported by polystyrene resins: Role of surface functional groups. Water Res. 2011, 45, 2191–2198. [Google Scholar] [CrossRef]
- Ng, W.M.; Lim, J.K. Complex interplay between colloidal stability, transport, chemical reactivity and magnetic separability of polyelectrolyte-functionalized nanoscale zero-valent iron particles (nZVI) toward their environmental engineering application. Colloid Interface Sci. Commun. 2022, 46, 100582. [Google Scholar] [CrossRef]
- Wang, C.F.; Li, Y.Y.; Li, A.H.; Yang, N.; Wang, X.W.; Li, Y.M.; Zhang, Y. Degradation of COD in antibiotic wastewater by a combination process of electrochemistry, hydroxyl-functionalized ball-milled zero-valent iron/Fe3O4 and Oxone. Environ. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Ahmad, S.; Liu, X.; Tang, J.; Zhang, S. Biochar-supported nanosized zero-valent iron (nZVI/BC) composites for removal of nitro and chlorinated contaminants. Chem. Eng. J. 2022, 431, 133187. [Google Scholar] [CrossRef]
- Berry, C.C.; Curtis, A.S. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef]
- Huang, S.H.; Chen, D.H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Joneidi-Yekta, H.; Mohseni, M. Adsorption of anionic and cationic dyes from aqueous solution using gelatin-based magnetic nanocomposite beads comprising carboxylic acid functionalized carbon nanotube. Chem. Eng. J. 2017, 308, 1133–1144. [Google Scholar] [CrossRef]
- Ge, F.; Ye, H.; Li, M.M.; Zhao, B.X. Efficient removal of cationic dyes from aqueous solution by polymer-modified magnetic nanoparticles. Chem. Eng. J. 2012, 198, 11–17. [Google Scholar] [CrossRef]
- Peerless, J.S.; Gulyuk, A.V.; Milliken, N.J.; Kim, G.D.; Reid, E.; Lee, J.W.; Kim, D.; Hendren, Z.; Choi, Y.C.; Yingling, Y.G. Role of Nanoscale Morphology on the Efficiency of Solvent-Based Desalination Method. ACS EST Water 2023, 3, 400–409. [Google Scholar] [CrossRef]
- Barbosa, G.D.; Dach, E.; Liu, X.; Yip, N.Y.; Turner, C.H. Computational and experimental study of different brines in temperature swing solvent extraction desalination with amine solvents. Desalination 2022, 537, 115863. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, X.; Gong, X.; Ding, M.; Yu, J.; Zhang, S.; Ding, B. Environmentally friendly polyamide nanofiber membranes with interconnective amphiphobic channels for seawater desalination. ACS Appl. Mater. Interfaces 2022, 14, 35287–35296. [Google Scholar] [CrossRef]
- Foo, Z.H.; Stetson, C.; Dach, E.; Deshmukh, A.; Lee, H.; Menon, A.K.; Prasher, R.; Yip, N.Y.; Lienhard, J.H.; Wilson, A.D. Solvent-driven aqueous separations for hypersaline brine concentration and resource recovery. Trends Chem. 2022, 4, 1078–1093. [Google Scholar] [CrossRef]
- Asenath-Smith, E.; Estroff, L.A. Role of akaganeite (β-FeOOH) in the growth of hematite (α-Fe2O3) in an inorganic silica hydrogel. Cryst. Growth Des. 2015, 15, 3388–3398. [Google Scholar] [CrossRef]
- Žic, M.; Ristić, M.; Musić, S. Effect of phosphate on the morphology and size of α-Fe2O3 particles crystallized from dense β-FeOOH suspensions. J. Alloys Compd. 2008, 466, 498–506. [Google Scholar] [CrossRef]
- Domingo, C.; Rodríguez-Clemente, R.; Blesa, M. Morphological properties of α-FeOOH, γ-FeOOH and Fe3O4 obtained by oxidation of aqueous Fe (II) solutions. J. Colloid Interface Sci. 1994, 165, 244–252. [Google Scholar] [CrossRef]
- Constantinou, D.; Samanides, C.G.; Koutsokeras, L.; Constantinides, G.; Vyrides, I. Hydrogen generation by soluble CO2 reaction with zero-valent iron or scrap iron and the role of weak acids for controlling FeCO3 formation. Sustain. Energy Technol. Assess. 2023, 56, 103061. [Google Scholar] [CrossRef]
- Balić-Žunić, T.; Birkedal, R.; Katerinopoulou, A.; Comodi, P. Dehydration of blödite, Na2Mg (SO4)2 (H2O)4, and leonite, K2Mg(SO4)2 (H2O)4. Eur. J. Mineral. 2016, 28, 33–42. [Google Scholar] [CrossRef]
- Stoilova, D.; Wildner, M. Blödite-type compounds Na2Me (SO4)2· 4H2O (Me= Mg, Co, Ni, Zn): Crystal structures and hydrogen bonding systems. J. Mol. Struct. 2004, 706, 57–63. [Google Scholar] [CrossRef]
- Keester, K.L.; Eysel, W. New compounds MNa6 (SO4)4 with vanthoffite structure. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1977, 33, 306–307. [Google Scholar] [CrossRef]
- Souamti, A.; Kahlaoui, M.; Fezai, R.; Lozano-Gorrín, A.D.; Chehimi, D.B.H. Synthesis, characterization and electrical properties of Na6M (SO4)4 (M = Co, Ni, Cu) vanthoffite materials. Mater. Sci. Eng. B 2019, 244, 56–64. [Google Scholar] [CrossRef]


| X | SD | 0% | 25% | 50% | 75% | 100% | |
|---|---|---|---|---|---|---|---|
| Entrained Spheres | |||||||
| Outer Diameter | 1.472 | 0.352 | 0.907 | 1.260 | 1.397 | 1.568 | 2.697 |
| Inner Diameter | 0.706 | 0.266 | 0.134 | 0.569 | 0.667 | 0.809 | 1.811 |
| Fluid Volume | 0.277 | 0.468 | 0.001 | 0.097 | 0.155 | 0.277 | 3.108 |
| Porosity | 12.28% | 6.59% | 0.08% | 7.30% | 11.21% | 16.97% | 30.27% |
| Agglomerated Spheres | |||||||
| Outer Diameter | 2.310 | 0.476 | 1.453 | 1.975 | 2.317 | 2.617 | 3.393 |
| Inner Diameter | 1.179 | 0.333 | 0.573 | 0.950 | 1.095 | 1.349 | 2.413 |
| Fluid Volume | 1.081 | 1.158 | 0.099 | 0.449 | 0.688 | 1.286 | 7.360 |
| Porosity | 16.44% | 12.77% | 2.73% | 7.28% | 12.13% | 19.76% | 55.37% |
| Trial | Reaction Time, Minutes | Feed Water, Cl− g/L | Feed Water, Na+ g/L | Cl− Removal | Na+ Removal | Product Water, Salinity g/L | Desalination | Acid |
|---|---|---|---|---|---|---|---|---|
| F5 | 6 | 22.11 | 17.21 | 93.2% | 0.0% | 2.44 | 93.8% | Tartaric |
| F6 | 6 | 22.11 | 17.21 | 91.3% | 16.4% | 3.12 | 92.1% | Tartaric |
| F8 | 6 | 22.11 | 17.21 | 47.2% | 0.0% | 18.91 | 51.9% | Malic |
| F9 | 6 | 22.11 | 17.21 | 70.1% | 45.7% | 10.71 | 72.8% | Malic |
| F11 | 6 | 22.11 | 17.21 | 94.0% | 0.7% | 2.15 | 94.5% | Citric |
| F12 | 6 | 22.11 | 17.21 | 94.2% | 11.2% | 2.08 | 94.7% | Citric |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antia, D.D.J. Desalination of Saline Irrigation Water Using Hydrophobic, Metal–Polymer Hydrogels. Sustainability 2023, 15, 7063. https://doi.org/10.3390/su15097063
Antia DDJ. Desalination of Saline Irrigation Water Using Hydrophobic, Metal–Polymer Hydrogels. Sustainability. 2023; 15(9):7063. https://doi.org/10.3390/su15097063
Chicago/Turabian StyleAntia, David D. J. 2023. "Desalination of Saline Irrigation Water Using Hydrophobic, Metal–Polymer Hydrogels" Sustainability 15, no. 9: 7063. https://doi.org/10.3390/su15097063
APA StyleAntia, D. D. J. (2023). Desalination of Saline Irrigation Water Using Hydrophobic, Metal–Polymer Hydrogels. Sustainability, 15(9), 7063. https://doi.org/10.3390/su15097063






