The Effect of Sequential and Simultaneous Supplementation of Waste-Derived Volatile Fatty Acids and Methanol as Alternative Carbon Source Blend for Wastewater Denitrification
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbon Source Characteristics
2.2. Post-Denitrifying MBBR Operation
- Denitrification rate was calculated as follows:
- Denitrification capacity
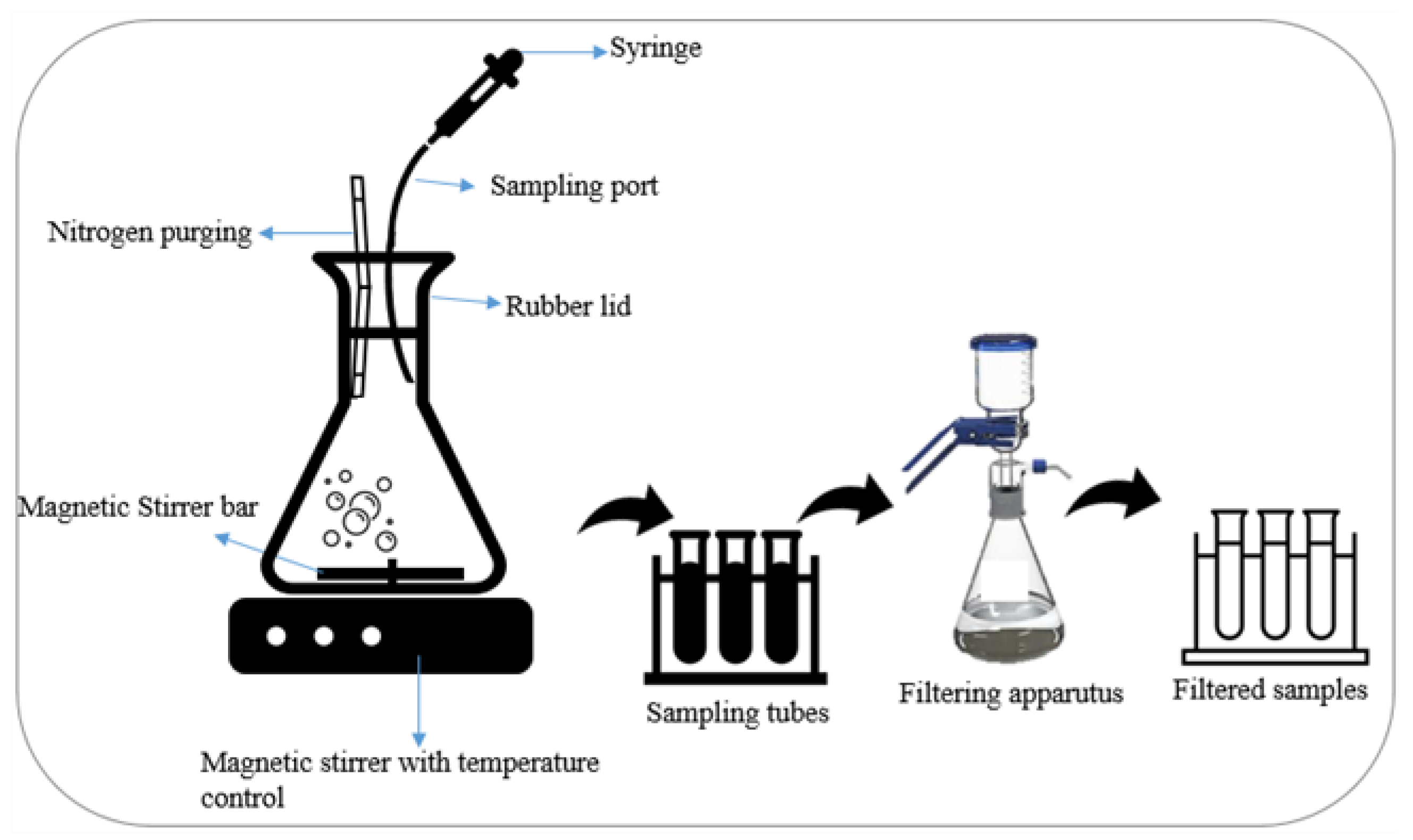
2.3. Sampling and Analytical Methods
3. Results and Discussion
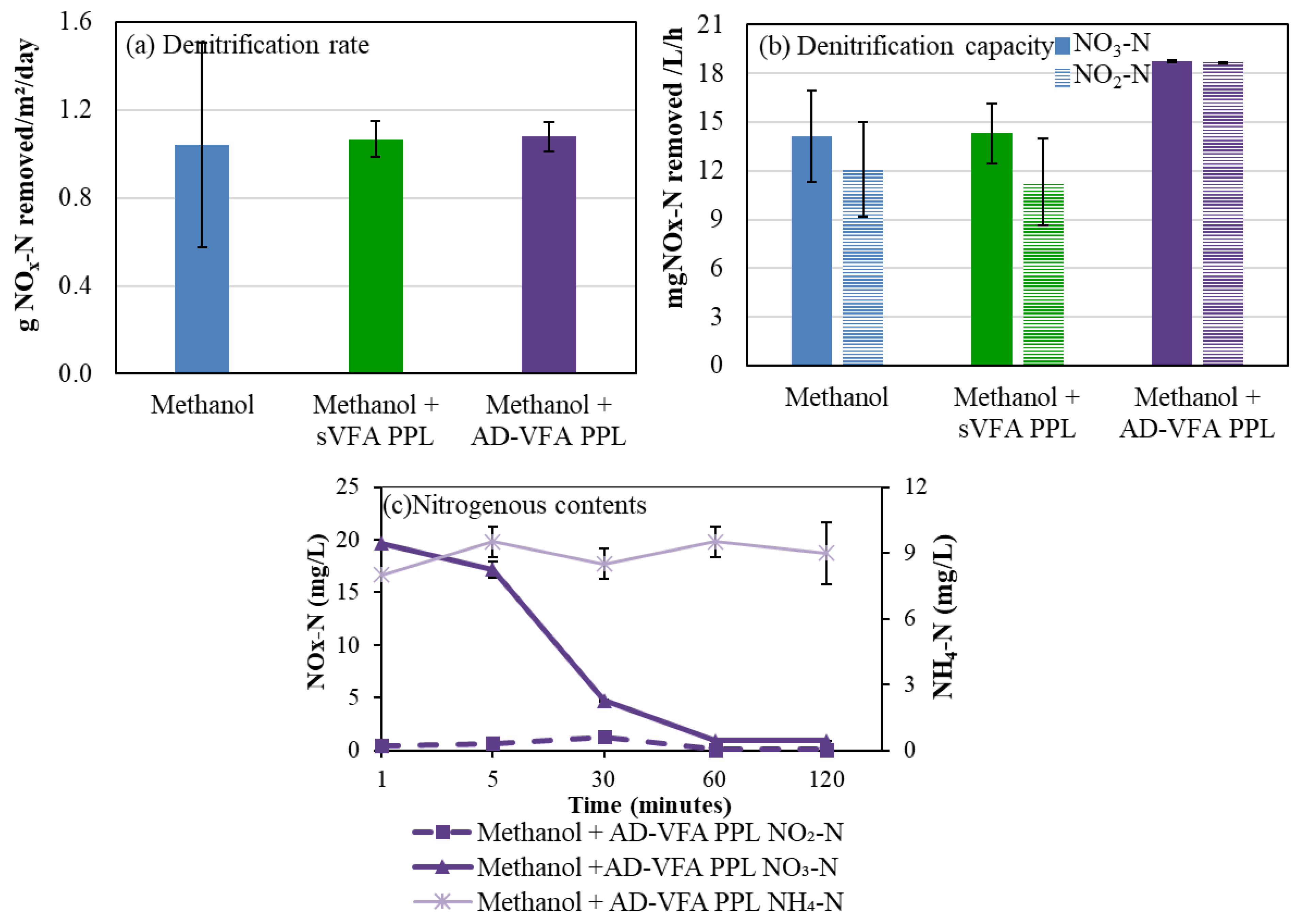
3.1. Source of VFA Effluents as the Sole Carbon Source on Denitrification
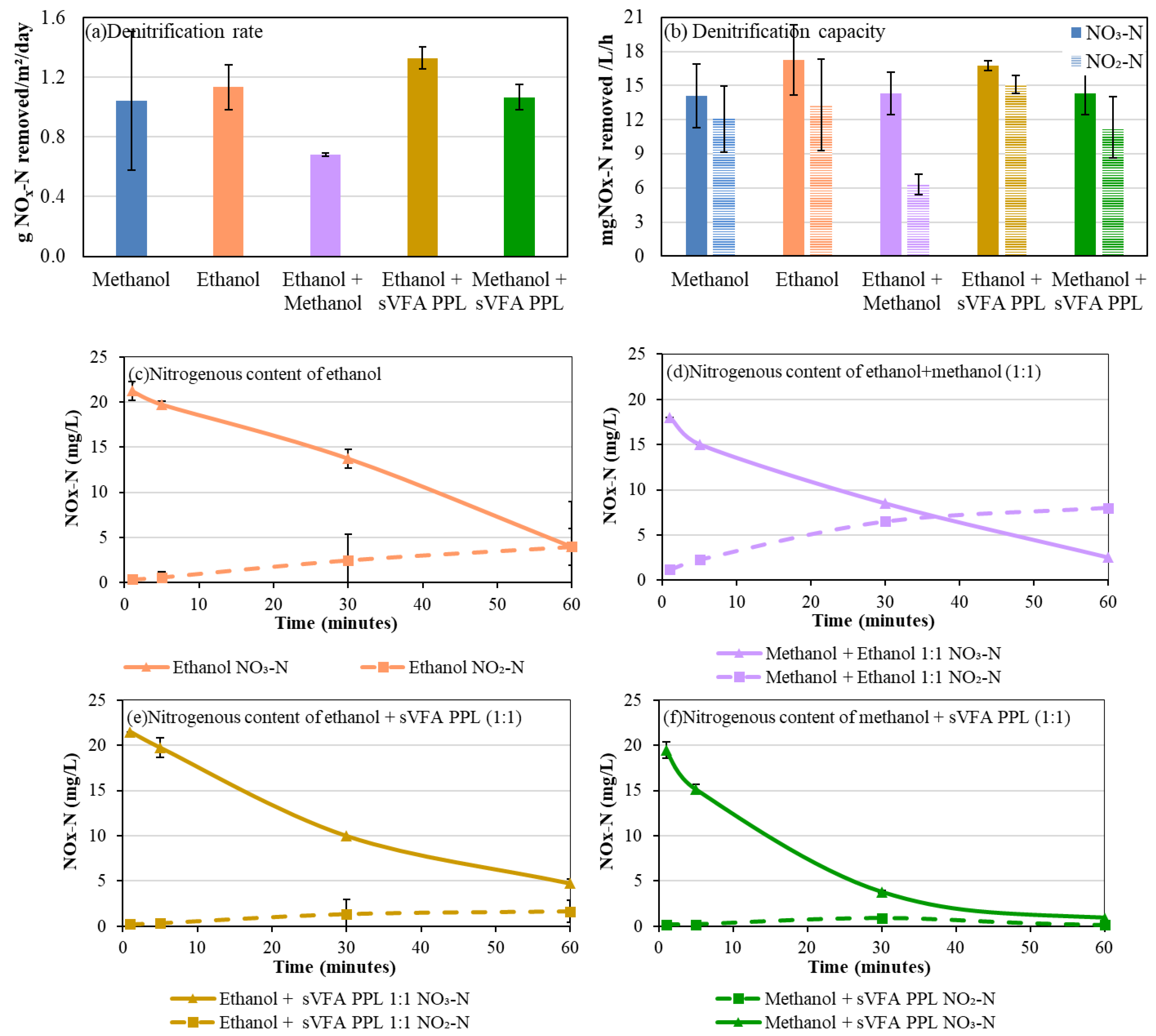
3.2. Effect of Co-Fed Carbon Sources on Denitrification
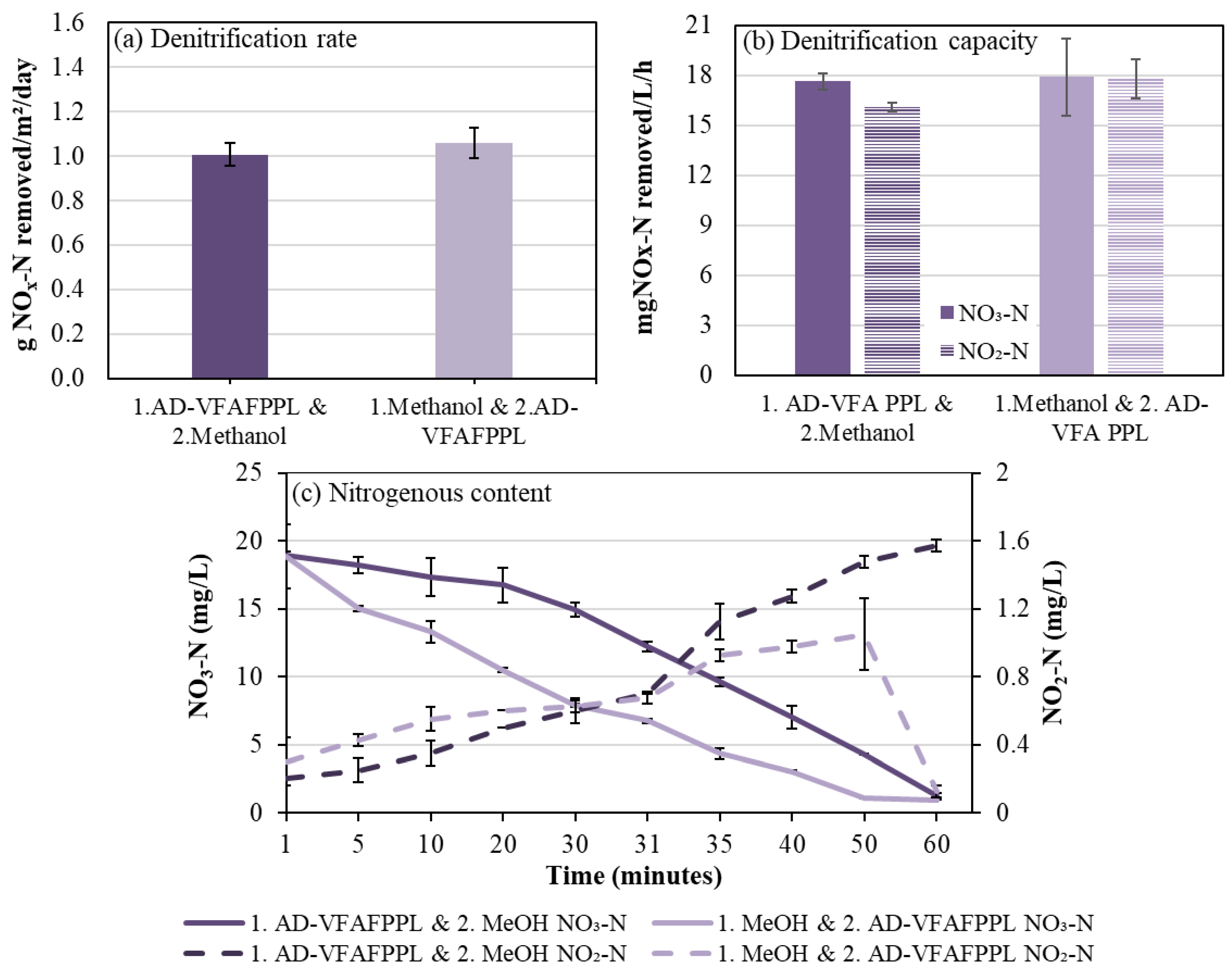
3.3. Effect of Sequential Addition of Co-Fed Carbon Sources
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elefsiniotis, P.; Li, D. The effect of temperature and carbon source on denitrification using volatile fatty acids. Biochem. Eng. J. 2006, 28, 148–155. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Cecconet, D.; Capodaglio, A.G. Sewage Sludge Biorefinery for Circular Economy. Sustainability 2022, 14, 14841. [Google Scholar] [CrossRef]
- Swinarski, M.; Makinia, J.; Czerwionka, K.; Chrzanowska, M.; Drewnowski, J. Comparison of the effects of conventional and alternative external carbon sources on enhancing the denitrification process. Water Environ. Res. 2009, 81, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, E.; Abu-Orf, M.; Bowden, G.; Burton, F.; Pfrang, W.; Stensel, H.; Tchobanoglous, G.; Tsuchihashi, R.; AECOM (Firm). Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2014; ISBN 978-00-7340-118-8. [Google Scholar]
- Akter, J.; Lee, J.; Kim, I. A Fixed-Film Bio-Media Process Used for Biological Nitrogen Removal from Sewage Treatment Plant. Nitrogen 2022, 3, 528–538. [Google Scholar] [CrossRef]
- Odegaard, H.; Rusten, B.; Siljudalen, J. The development of the moving bed biofilm process- from idea to commercial product. Eur. Water Manag. 1999, 2, 36–43. [Google Scholar]
- Wei, Y.; Ji, M.; Li, R.; Qin, F. Organic and nitrogen removal from landfill leachate in aerobic granular sludge sequencing batch reactors. Waste Manag. 2012, 32, 448–455. [Google Scholar] [CrossRef]
- Dhamole, P.B.; D’Souza, S.; Lele, S. A review on alternative carbon sources for biological treatment of nitrate waste. J. Inst. Eng. (India) Ser. E 2015, 96, 63–73. [Google Scholar] [CrossRef]
- MacDonald, D.V. Denitrification by Fluidized Biofilm Reactor. Water Sci. Technol. 1990, 22, 451–461. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, J.; Li, M.; Yan, F.; Gong, C.; Wang, Q. Biological nitrate removal using a food waste-derived carbon source in synthetic wastewater and real sewage. J. Environ. Manag. 2016, 166, 407–413. [Google Scholar] [CrossRef]
- Cappai, G.; Carucci, A.; Onnis, A. Use of industrial wastewaters for the optimization and control of nitrogen removal processes. Water Sci. Technol. 2004, 50, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Nava, Y.; Marañón, E.; Soons, J.; Castrillón, L. Denitrification of high nitrate concentration wastewater using alternative carbon sources. J. Hazard. Mater. 2010, 173, 682–688. [Google Scholar] [CrossRef]
- Rodriguez, L.; Villasenor, J.; Fernández, F. Use of agro-food wastewaters for the optimisation of the denitrification process. Water Sci. Technol. 2007, 55, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wang, J. Various electron donors for biological nitrate removal: A review. Sci. Total Environ. 2021, 794, 148699. [Google Scholar] [CrossRef]
- Feng, X.-C.; Bao, X.; Che, L.; Wu, Q.-L. Enhance biological nitrogen and phosphorus removal in wastewater treatment process by adding food waste fermentation liquid as external carbon source. Biochem. Eng. J. 2021, 165, 107811. [Google Scholar] [CrossRef]
- Ngo, T.; Shahsavari, E.; Shah, K.; Surapaneni, A.; Ball, A.S. Improving bioenergy production in anaerobic digestion systems utilising chicken manure via pyrolysed biochar additives: A review. Fuel 2022, 316, 123374. [Google Scholar] [CrossRef]
- Sar, T.; Larsson, K.; Fristedt, R.; Undeland, I.; Taherzadeh, M.J. scale production of protein-rich fungal biomass from potato protein liquor for use as innovative food and feed products. Food Biosci. 2022, 47, 101637. [Google Scholar] [CrossRef]
- Sapmaz, T.; Uwineza, C.; Mahboubi, A.; De Wever, H.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. Towards maximum value creation from potato protein liquor: Volatile fatty acids production from fungal cultivation effluent. Biomass Convers. Biorefin. 2023, 1–14. [Google Scholar] [CrossRef]
- Yin, D.M.; Uwineza, C.; Sapmaz, T.; Mahboubi, A.; De Wever, H.; Qiao, W.; Taherzadeh, M.J. Volatile Fatty Acids (VFA) Production and Recovery from Chicken Manure Using a High-Solid Anaerobic Membrane Bioreactor (AnMBR). Membranes 2022, 12, 1133. [Google Scholar] [CrossRef]
- Elefsiniotis, P.; Wareham, D.G.; Smith, M.O. Use of volatile fatty acids from an acid-phase digester for denitrification. J. Biotechnol. 2004, 114, 289–297. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Mahmoud, A.; Hamza, R.A.; Elbeshbishy, E. Enhancement of denitrification efficiency using municipal and industrial waste fermentation liquids as external carbon sources. Sci. Total Environ. 2022, 816, 151578. [Google Scholar] [CrossRef] [PubMed]
- Jomnonkhaow, U.; Uwineza, C.; Mahboubi, A.; Wainaina, S.; Reungsang, A.; Taherzadeh, M.J. Membrane bioreactor-assisted volatile fatty acids production and in situ recovery from cow manure. Bioresour. Technol. 2021, 321, 124456. [Google Scholar] [CrossRef] [PubMed]
- Sapmaz, T.; Manafi, R.; Mahboubi, A.; Lorick, D.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. Potential of food waste-derived volatile fatty acids as alternative carbon source for denitrifying moving bed biofilm reactors. Bioresour. Technol. 2022, 364, 128046. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Riffat, R.; Bott, C.; Takacs, I.; Stinson, B.; Peric, M.; Neupane, D.; Murthy, S. Denitrification stoichiometry and kinetics of moving bed biofilm reactor. Proc. Water Environ. Fed. 2009, 2009, 153–165. [Google Scholar] [CrossRef]
- Elefsiniotis, P.; Wareham, D.G. Utilization patterns of volatile fatty acids in the denitrification reaction. Enzym. Microb. Technol. 2007, 41, 92–97. [Google Scholar] [CrossRef]
- Sapmaz, T.; Mahboubi, A.; Taher, M.N.; Beler-Baykal, B.; Karagunduz, A.; Taherzadeh, M.J.; Koseoglu-Imer, D.Y. Waste-derived volatile fatty acid production and ammonium removal from it by ion exchange process with natural zeolite. Bioengineered 2022, 13, 14751–14769. [Google Scholar] [CrossRef]
- Barber, W.P.; Stuckey, D.C. Nitrogen removal in a modified anaerobic baffled reactor (ABR): 1, denitrification. Water Res. 2000, 34, 2413–2422. [Google Scholar] [CrossRef]
- Du, S.; Ya, T.; Zhang, M.; Zhu, M.; Li, N.; Liu, S.; Wang, X. Distinct microbial communities and their networks in an anammox coupled with sulfur autotrophic/mixotrophic denitrification system. Environ. Pollut. 2020, 262, 114190. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Shin, S.G.; Hwang, S.; Lee, C. Continuous fermentation of food waste leachate for the production of volatile fatty acids and potential as a denitrification carbon source. Bioresour. Technol. 2016, 207, 440–445. [Google Scholar] [CrossRef]
- Liu, F.; Tian, Y.; Ding, Y.; Li, Z. The use of fermentation liquid of wastewater primary sedimentation sludge as supplemental carbon source for denitrification based on enhanced anaerobic fermentation. Bioresour. Technol. 2016, 219, 6–13. [Google Scholar] [CrossRef]
- Bill, K.A.; Bott, C.B.; Murthy, S.N. Evaluation of alternative electron donors for denitrifying moving bed biofilm reactors (MBBRs). Water Sci. Technol. 2009, 60, 2647–2657. [Google Scholar] [CrossRef]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Albina, P.; Durban, N.; Bertron, A.; Albrecht, A.; Robinet, J.-C.; Erable, B. Influence of hydrogen electron donor, alkaline pH, and high nitrate concentrations on microbial denitrification: A review. Int. J. Mol. Sci. 2019, 20, 5163. [Google Scholar] [CrossRef]
- Ji, J.; Peng, Y.; Wang, B.; Wang, S. Achievement of high nitrite accumulation via endogenous partial denitrification (EPD). Bioresour. Technol. 2017, 224, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Rusten, B.; Wien, A.; Skjefstad, J. Spent aircraft deicing fluid as external carbon source for denitrification of municipal wastewater: From waste problem to beneficial use. In Proceedings of the 51st Purdue Industrial Waste Conference, West Lafayette, IN, USA, 1 May 1997; pp. 359–370. [Google Scholar]
- Aspegren, H.; Nyberg, U.; Andersson, B.; Gotthardsson, S.; la Cour Jansen, J. Post denitrification in a moving bed biofilm reactor process. Water Sci. Technol. 1998, 38, 31–38. [Google Scholar] [CrossRef]
- Schulz, H. Oxidation of fatty acids in eukaryotes. In New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 2002; Volume 36, pp. 127–150. [Google Scholar]
- He, Q.; Song, J.; Zhang, W.; Gao, S.; Wang, H.; Yu, J. Enhanced simultaneous nitrification, denitrification and phosphorus removal through mixed carbon source by aerobic granular sludge. J. Hazard. Mater. 2020, 382, 121043. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Carbon Sources | |||||
|---|---|---|---|---|---|---|
| AD-VFACKM | sVFACKM | AD-VFAPPL | sVFAPPL | Methanol | Ethanol | |
| pH | 6.32 ± 0.02 | 2.99 ± 0.02 | 7.73 ± 0.02 | 2.97 ± 0.02 | - | - |
| sCOD (g/L) | 14.5 ± 0.71 | 9 ± 0.00 | 12.5 ± 0.00 | 8.75 ± 0.00 | 1185 | 1650 |
| NH4+-N (mg/L) | 2540.0 ± 28.28 | - | 485.0 ± 7.07 | - | - | - |
| PO4-P (mg/L) | 1747.0 ± 15.56 | - | 33.0 ± 0.28 | - | - | - |
| NO3-N (mg/L) | 20.8 ± 0.57 | - | 16.05 ± 0.21 | - | - | - |
| NO2-N (mg/L) | 0.20 ± 0.00 | - | 0.31 ± 0.00 | - | - | - |
| Acetic acid (g/L) | 4.36 ± 0.02 | 4.4 ± 0.10 | 4.15 ± 0.09 | 4.1 ± 0.10 | - | - |
| Propionic acid (g/L) | 0.74 ± 0.01 | 0.7 ± 0.10 | 0.50 ± 0.19 | 0.5 ± 0.10 | - | - |
| Iso-butyric acid (g/L) | 0.26 ± 0.04 | 0.3 ± 0.10 | 0.13 ± 0.06 | 0.1 ± 0.10 | - | - |
| Butyric acid (g/L) | 1.03 ± 0.09 | 1 ± 0.10 | 0.66 ± 0.05 | 0.7 ± 0.10 | - | - |
| Isovaleric acid (g/L) | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.00 ± 0.00 | 0.0 ± 0.00 | - | - |
| Valeric acid (g/L) | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.01 | - | - |
| Caproic acid (g/L) | 0.49 ± 0.01 | 0.5 ± 0.10 | 0.52 ± 0.01 | 0.5 ± 0.10 | - | - |
| tVFA (g/L) | 6.92 ± 0.08 | 7 ± 0.10 | 5.98 ± 0.07 | 6 ± 0.10 | - | - |
| Carbon Source Used | Carbon Sources (CS) | ||||
|---|---|---|---|---|---|
| (Methanol) | (AD-VFAPPL) | (sVFAPPL) | (AD-VFACKM) | (sVFACKM) | |
| pH, 0 min | 7.05 ± 0.11 | 6.94 ± 0.03 | 7.03 ± 0.06 | 7.10 ± 0.14 | 7.16 ± 0.25 |
| pH, 60 min | 7.66 ± 0.11 | 7.71 ± 0.15 | 7.37 ± 0.41 | 7.40 ± 0.28 | 7.43 ± 0.49 |
| T (°C), 0 min | 15.02 ± 1.00 | 15.70 ± 0.57 | 15.85 ± 0.78 | 15.70 ± 0.99 | 15.45 ± 1.77 |
| T (°C), 60 min | 17.52 ± 1.00 | 17.70 ± 0.57 | 17.55 ± 0.78 | 18.00 ± 0.99 | 18.15 ± 1.77 |
| NH4-N, 0 min | 8.3 ± 1.3 | 11.8 ± 0.3 | 7.6 ± 0.6 | 19.0 ± 0.8 | 8.1 ± 0.6 |
| NH4-N, 60 min | 8.8 ± 0.0 | 12.3 ± 1.8 | 7.7 ± 1.2 | 17.4 ± 3.0 | 8.5 ± 0.7 |
| PO4-P, 0 min | 3.4 ± 1.7 | 3.1 ± 3.2 | 2.1 ± 1.9 | 4.3 ± 1.2 | 3.0 ± 0.4 |
| PO4-P, 60 min | 2.1 ± 1.3 | 3.3 ± 1.9 | 2.2 ± 1.2 | 5.7 ± 2.4 | 3.3 ± 0.2 |
| COD of CS (g/L) | 1185.0 | 12.5 | 8.8 | 15.5 | 11.0 |
| Volume of CS added (mL) | 0.201 | 19.04 | 27.20 | 16.41 | 21.64 |
| Carbon Source Used | Carbon Sources (CS) | ||||
|---|---|---|---|---|---|
| (Ethanol) | (Ethanol + Methanol) | (Ethanol + sVFAPPL) | (Methanol + sVFAPPL) | (Methanol + AD-VFAPPL) | |
| pH, 0 min | 7.7 ± 0.02 | 7.44 ± 0.00 | 6.99 ± 0.21 | 6.98 ± 0.01 | 7.06 ± 0.03 |
| pH, 60 min | 7.38 ± 0.38 | 7.72 ± 0.00 | 7.33 ± 0.01 | 7.51 ± 0.03 | 7.71 ± 0.01 |
| T (°C), 0 min | 14.6 ± 0.7 | 14.8 ± 0.0 | 14.8 ± 0.78 | 18.3 ± 0.26 | 18.7 ± 0.07 |
| T (°C), 60 min | 17.4 ± 0.7 | 17.9 ± 0.00 | 18.45 ± 0.8 | 19.7 ± 0.07 | 19.5 ± 0.07 |
| NH4-N, 0 min | 7.1 ± 1.8 | 9.5 ± 0.1 | 7.7 ± 1.2 | 7.8 ± 0.4 | 9.5 ± 0.7 |
| NH4-N, 60 min | 8.5 ± 2.1 | 10.0 ± 1.3 | 8.1 ± 1.0 | 7.0 ± 0.0 | 9.5 ± 0.7 |
| PO4-P, 0 min | 2.5 ± 0.3 | 2.4 ± 0.3 | 3.6 ± 0.2 | 1.7 ± 0.1 | 2.0 ± 0.1 |
| PO4-P, 60 min | 2.6 ± 0.2 | 2.3 ± 0.2 | 2.8 ± 0.3 | 1.2 ± 0.0 | 1.8 ± 0.0 |
| COD of CS (g/L) | 1650 | 1650∣1185 | 1650∣8.75 | 1185∣8.75 | 1185∣12.5 |
| Volume of CS added (µL) | 144 | 72∣100.5 | 72∣13,600 | 100.5∣13,600 | 100.5∣9520 |
| Parameters | Carbon Sources (CS) Used | |
|---|---|---|
| 1. AD-VFAPPL (0–30 min) + 2. Methanol (30–60 min) | 1. Methanol (0–30 min) + 2. AD-VFAPPL (30–60 min) | |
| pH, 0 min | 7.25 ± 0.03 | 7.29 ± 0.01 |
| pH, 60 min | 7.82 ± 0.06 | 7.82 ± 0.01 |
| T (°C), 0 min | 18.3 ± 0.71 | 18.15 ± 0.21 |
| T (°C), 60 min | 19.70 ± 0.14 | 19.85 ± 0.07 |
| NH4-N (mg/L), 0 min | 11.0 ± 0.0 | 11.5 ± 0.7 |
| NH4-N (mg/L), 60 min | 11.0 ± 1.4 | 11.0 ± 1.4 |
| PO4-P (mg/L), 0 min | 2.5 ± 0.0 | 2.5 ± 0.1 |
| PO4-P (mg/L), 60 min | 2.3 ± 0.1 | 2.2 ± 0.1 |
| COD of CS (g/L) | 12.5∣1185 | 1185∣12.5 |
| Volume of CS added (mL) | 9.52∣0.1005 | 0.1005∣9.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapmaz, T.; Manafi, R.; Mahboubi, A.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. The Effect of Sequential and Simultaneous Supplementation of Waste-Derived Volatile Fatty Acids and Methanol as Alternative Carbon Source Blend for Wastewater Denitrification. Sustainability 2023, 15, 6849. https://doi.org/10.3390/su15086849
Sapmaz T, Manafi R, Mahboubi A, Koseoglu-Imer DY, Taherzadeh MJ. The Effect of Sequential and Simultaneous Supplementation of Waste-Derived Volatile Fatty Acids and Methanol as Alternative Carbon Source Blend for Wastewater Denitrification. Sustainability. 2023; 15(8):6849. https://doi.org/10.3390/su15086849
Chicago/Turabian StyleSapmaz, Tugba, Reza Manafi, Amir Mahboubi, Derya Y. Koseoglu-Imer, and Mohammad J. Taherzadeh. 2023. "The Effect of Sequential and Simultaneous Supplementation of Waste-Derived Volatile Fatty Acids and Methanol as Alternative Carbon Source Blend for Wastewater Denitrification" Sustainability 15, no. 8: 6849. https://doi.org/10.3390/su15086849
APA StyleSapmaz, T., Manafi, R., Mahboubi, A., Koseoglu-Imer, D. Y., & Taherzadeh, M. J. (2023). The Effect of Sequential and Simultaneous Supplementation of Waste-Derived Volatile Fatty Acids and Methanol as Alternative Carbon Source Blend for Wastewater Denitrification. Sustainability, 15(8), 6849. https://doi.org/10.3390/su15086849










