Optimization of A Procedure to Improve the Extraction Rate of Biologically Active Compounds in Red Grape Must Using High-Power Ultrasound
Abstract
1. Introduction
2. Materials and Methods
2.1. Grapes
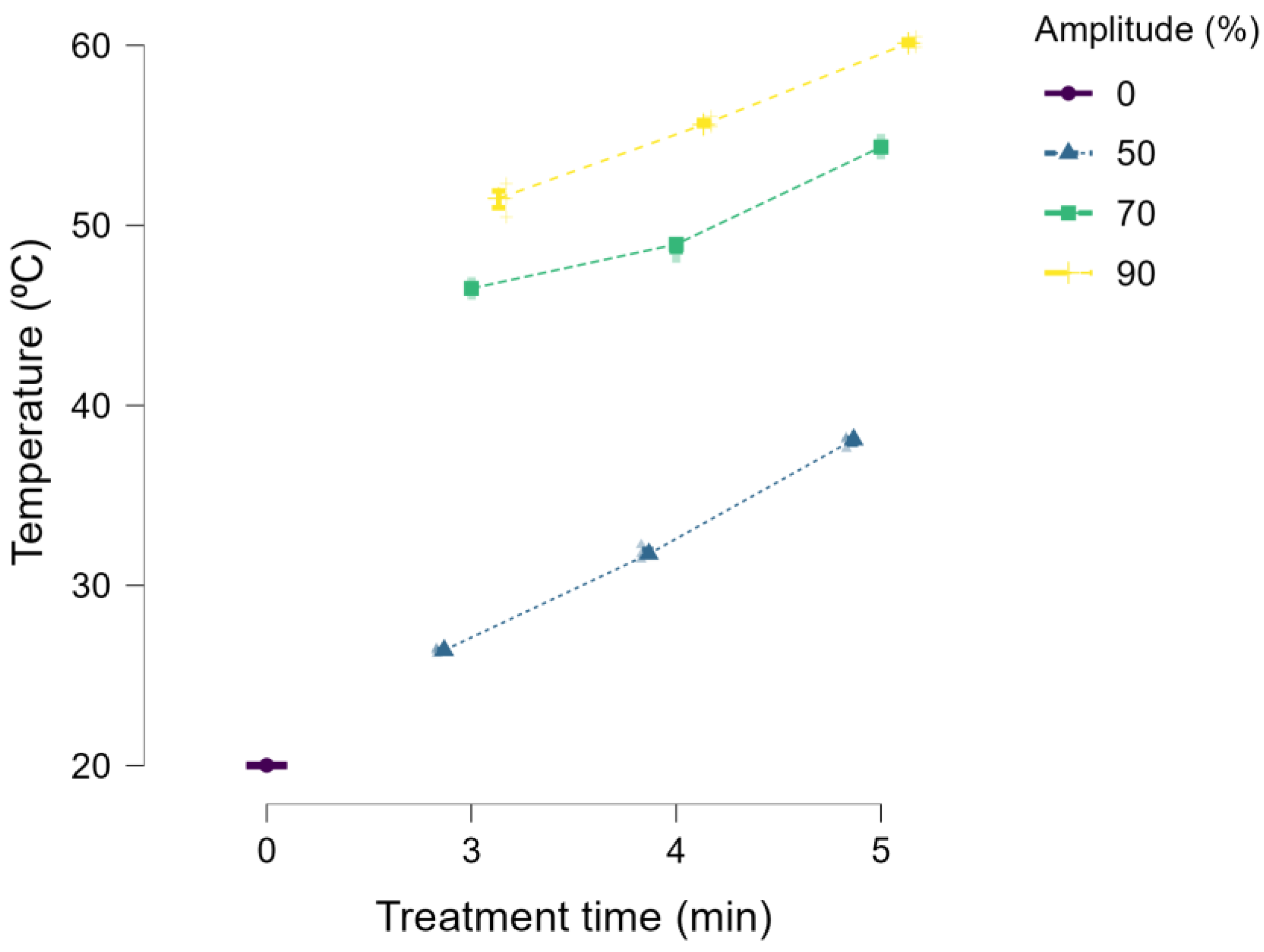
2.2. Ultrasonic Equipment
2.3. Optimization Design
2.4. Sample Preparation
2.5. Total Polyphenolic Content and Anthocyanins Determination
2.6. Antioxidant Activity
2.6.1. FRAP Assay
2.6.2. DPPH Assay
2.7. Total Soluble Content and Acidity
2.8. Statistical Analysis
3. Results and Discussion
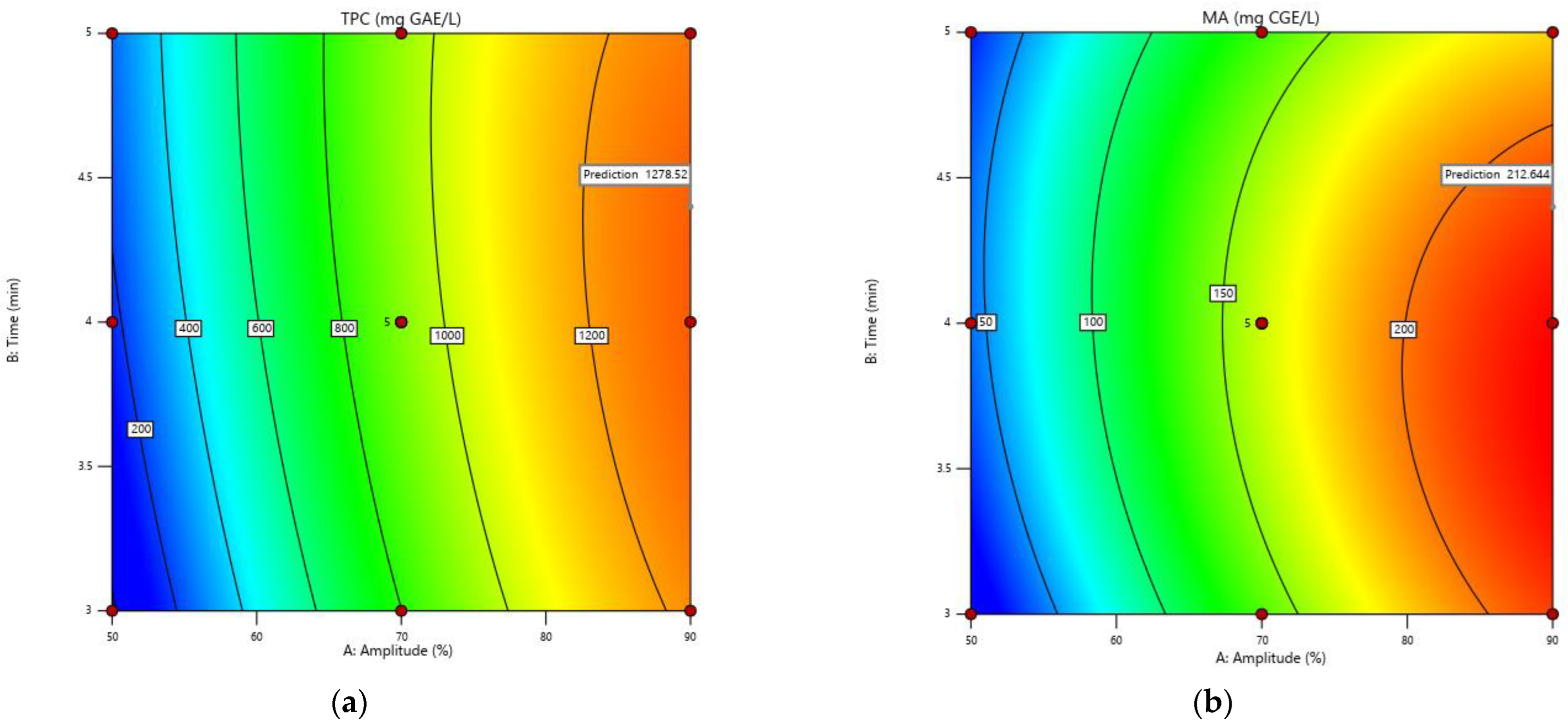
3.1. Total Polyphenolic Content
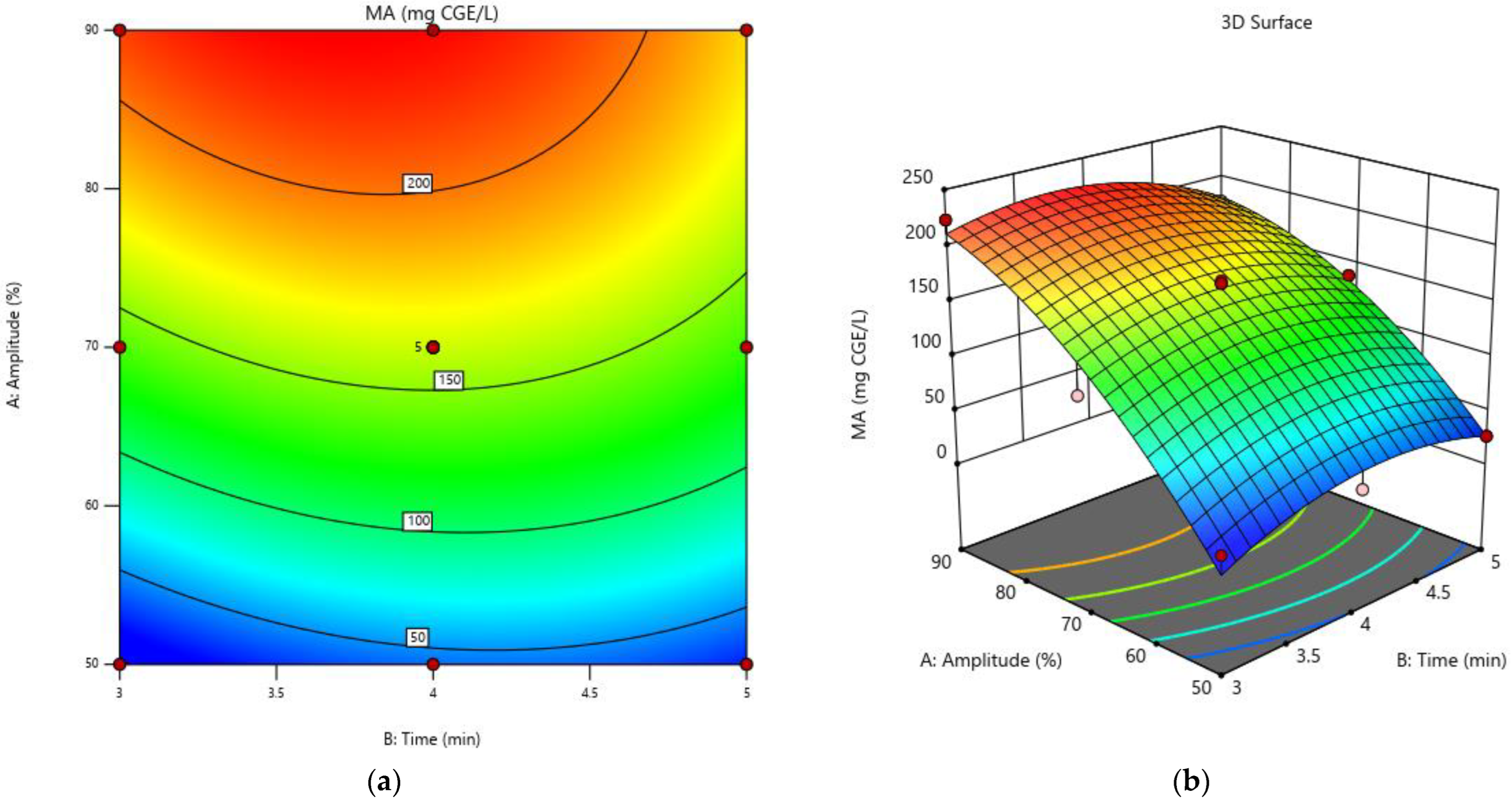
3.2. Monomeric Anthocyanins
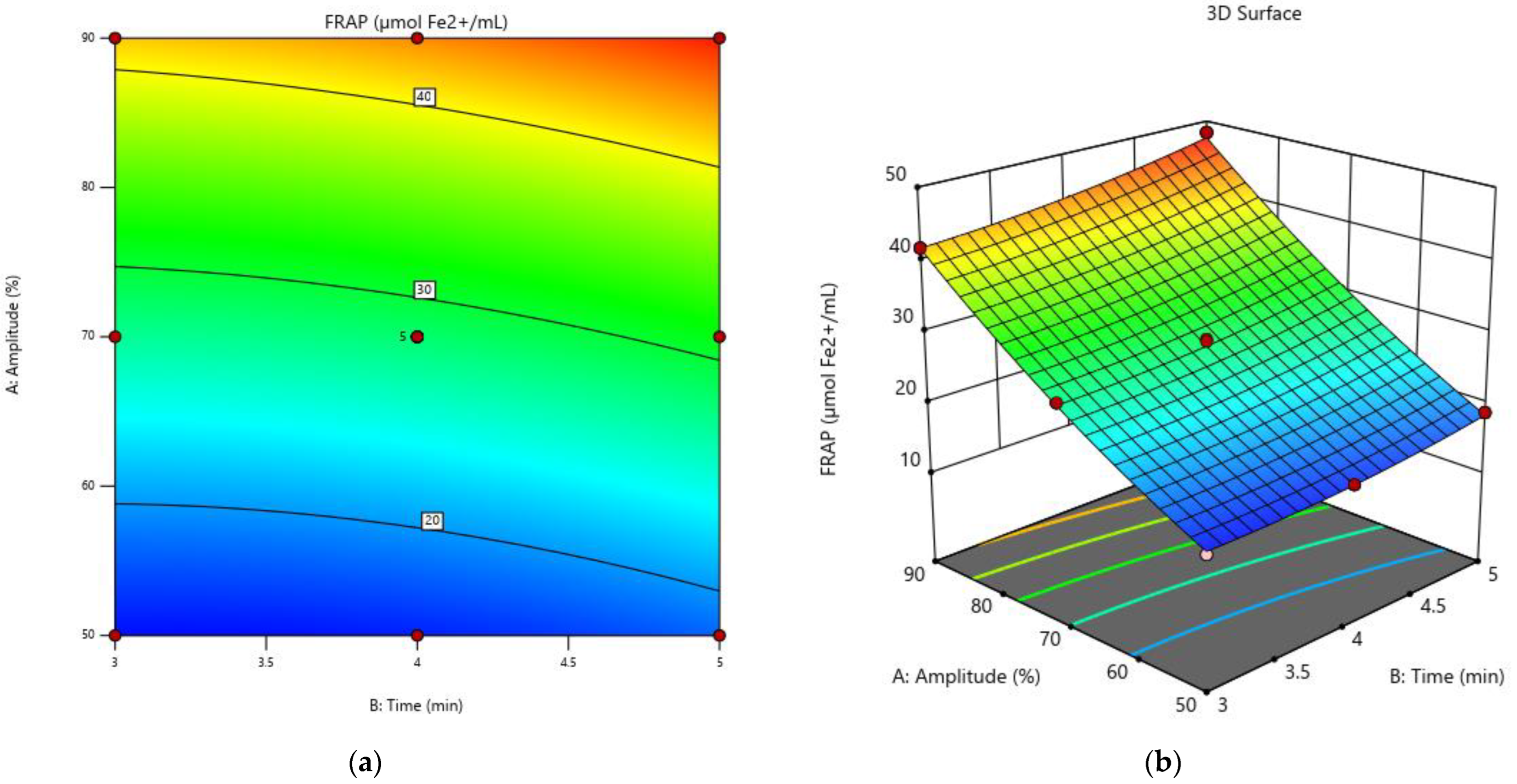
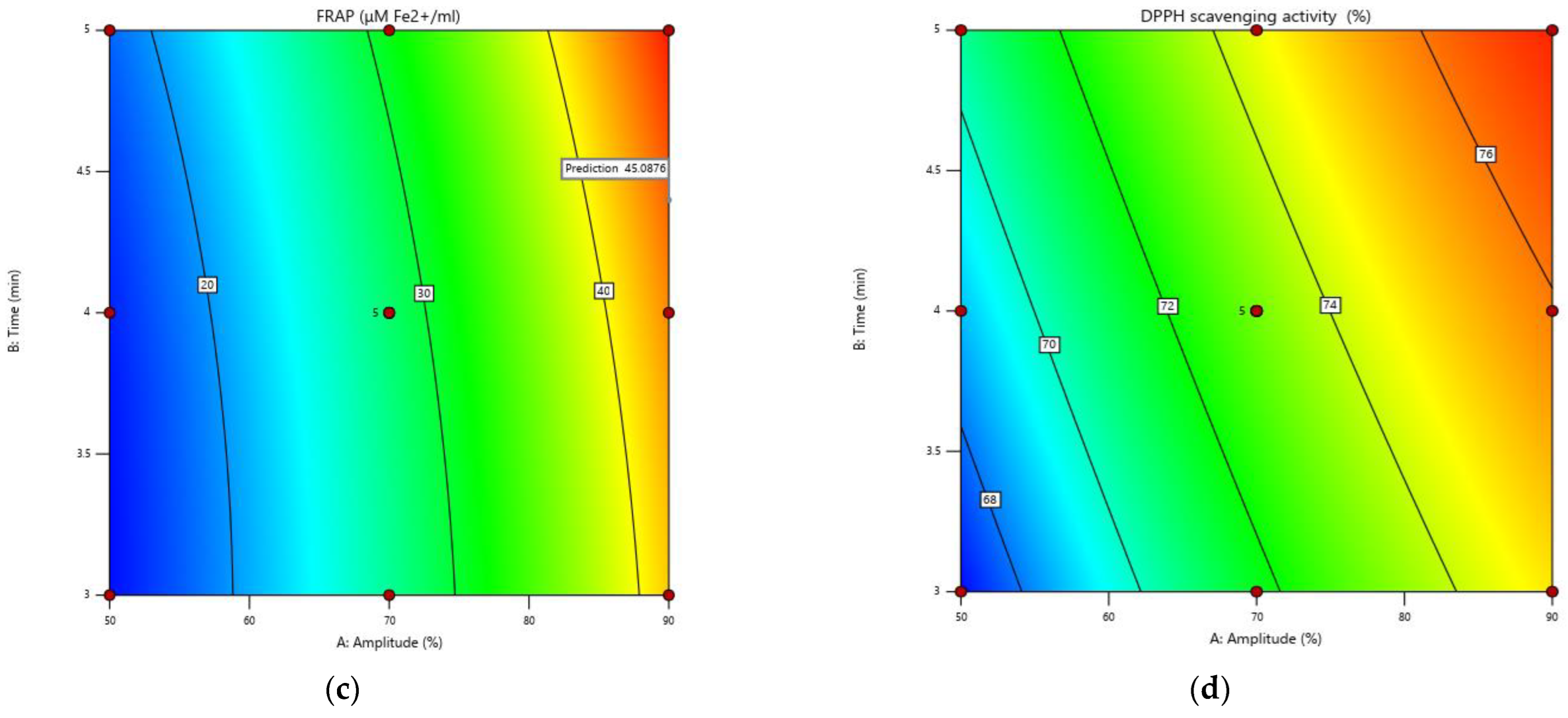
3.3. Antioxidant Activity
3.4. Correlations between Total Phenolic Content, Monomeric Anthocyanins and Antioxidant Activity
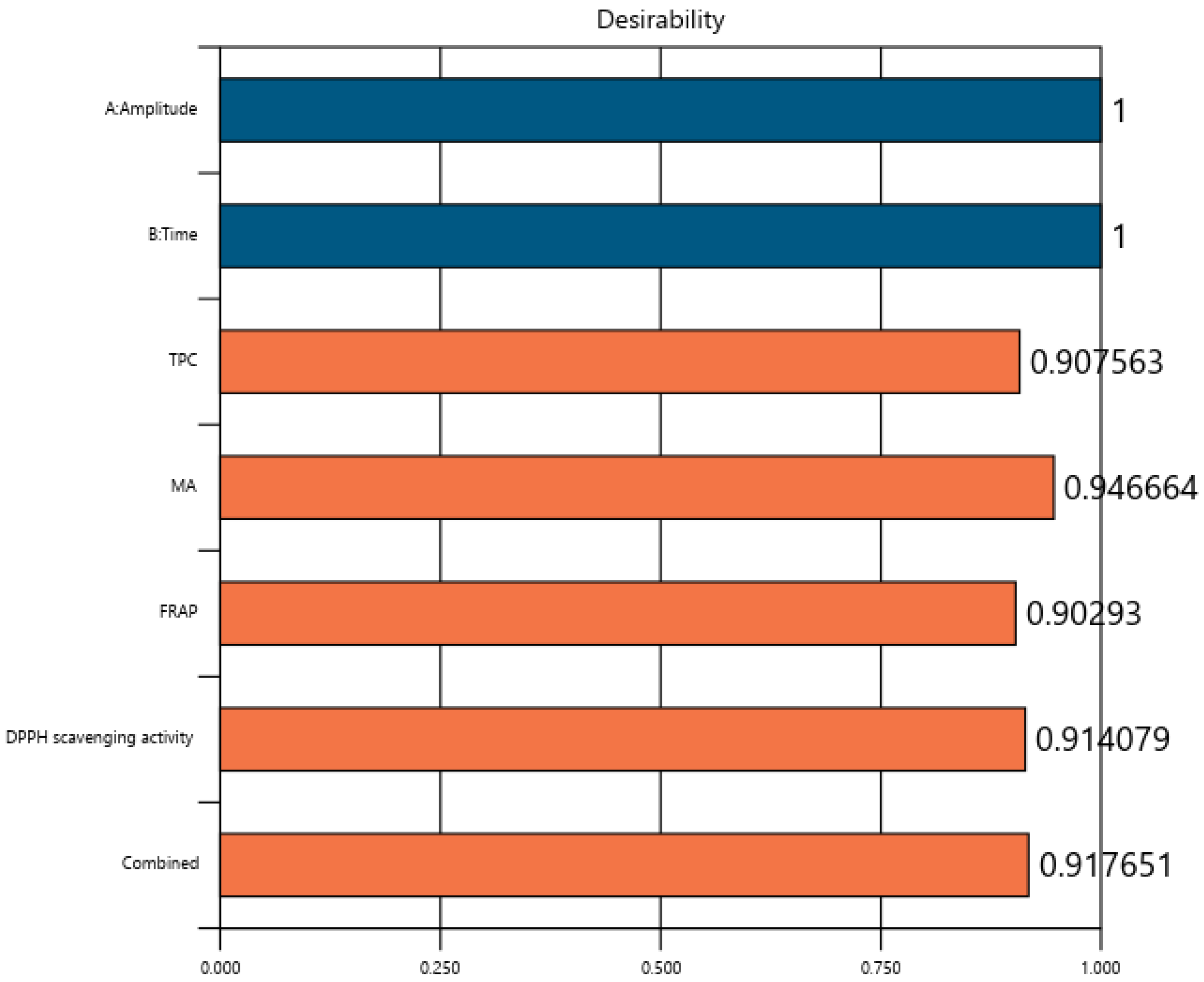
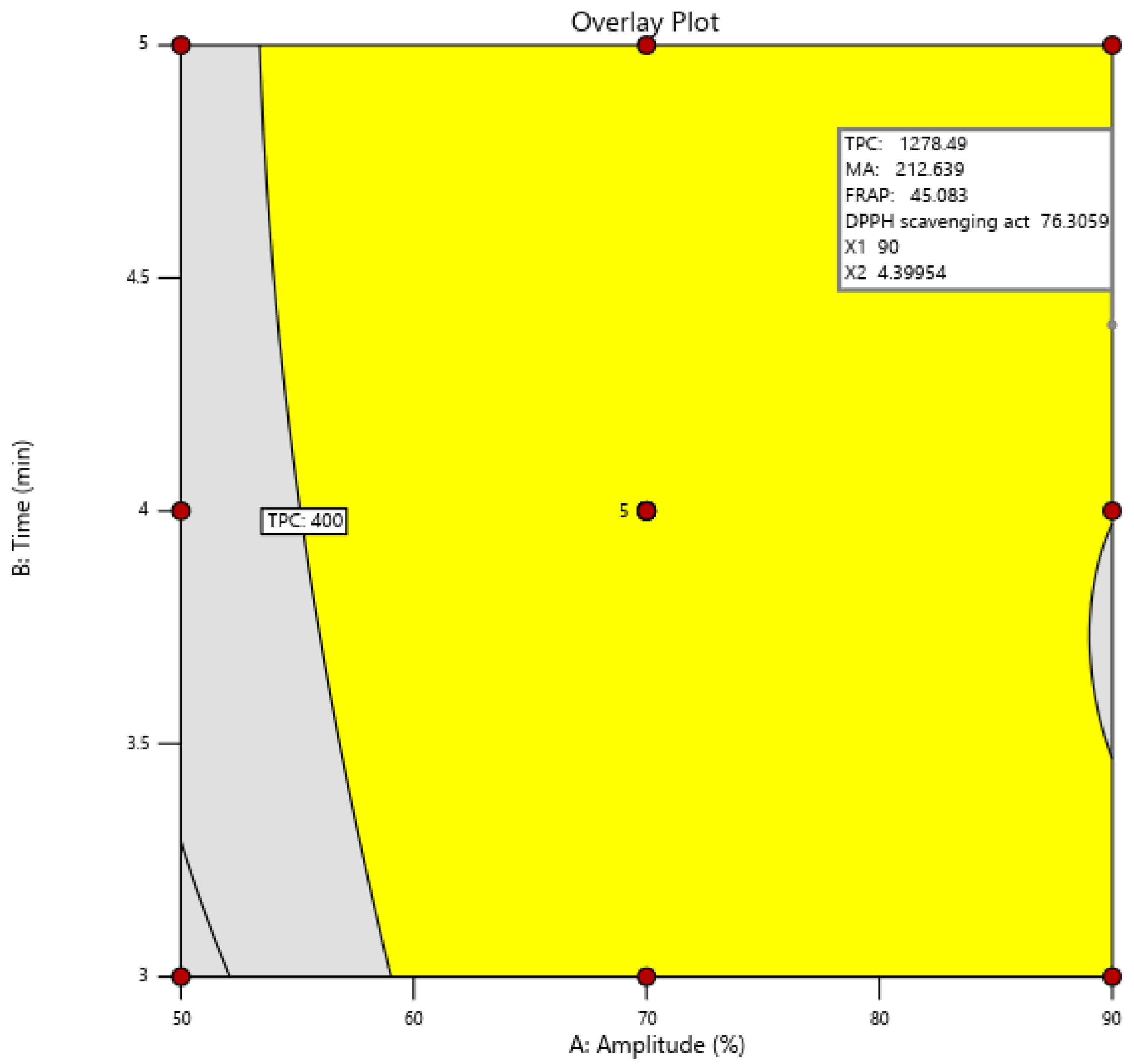
3.5. Optimization of the Experimental Model for Increasing the Extraction Rate of Biologically Active Compounds and the Antioxidant Activity in Grape Must
3.6. Confirmation of the Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV. World Wine Production Outlook—OIV First Estimates. no. November. International Organisation of Vine and Wine, 2022; p. 1. Available online: https://www.oiv.int/public/medias/8553/en-oiv-2021-world-wine-production-first-estimates-to-update.pdf (accessed on 14 January 2023).
- Ntuli, R.G.; Saltman, Y.; Ponangi, R.; Jeffery, D.W.; Bindon, K.; Wilkinson, K.L. Impact of skin contact time, oak and tannin addition on the chemical composition, color stability and sensory profile of Merlot wines made from flash détente treatment. Food Chem. 2023, 405, 134849. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, T. Effect of ultrasound irradiation on the evolution of color properties and major phenolic compounds in wine during storage. Food Chem. 2017, 234, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, S.; Dai, C.; Sun, L.; Sun, W.; Tang, Y.; Xiong, F.; He, R.; Ma, H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017, 37, 144–149. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, Z.; Yu, P.; Li, T.; Guang, M.; Chen, Z.; Wang, L. Rice peptide nanoparticle as a bifunctional food-grade Pickering stabilizer prepared by ultrasonication: Structural characteristics, antioxidant activity, and emulsifying properties. Food Chem. 2021, 343, 128545. [Google Scholar] [CrossRef]
- Aekthammarat, D.; Tangsucharit, P.; Pannangpetch, P.; Sriwantana, T.; Sibmooh, N. Moringa oleifera leaf extract enhances endothelial nitric oxide production leading to relaxation of resistance artery and lowering of arterial blood pressure. Biomed. Pharmacother. 2020, 130, 110605. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Bin Li, H. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Tian, B.; Harrison, R. Interactions of Vitis vinifera L. cv. Pinot Noir grape anthocyanins with seed proanthocyanidins and their effect on wine color and phenolic composition. LWT 2022, 162, 113428. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Nie, V.; Chen, D.; Ye, J.; Lu, Y.; Dai, Z. Optimization and kinetic modeling of ultrasonic-assisted extraction of fucoxanthin from edible brown algae Sargassum fusiforme using green solvents. Ultrason. Sonochem. 2021, 77, 105671. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Pallarésa, N.; Lorenzo, J.; Munekata, P.; Barba, F. Ultrasound Processing: A Sustainable Alternative. In Sustainable Production Technology in Food, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 155–164. [Google Scholar]
- Körzendörfer, A. Vibrations and ultrasound in food processing—Sources of vibrations, adverse effects, and beneficial applications—An overview. J. Food Eng. 2022, 324, 110875. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, L.; Wang, G.; Zhang, A.; Wang, X.; Jiang, L. Ultrasonication effects on physicochemical and emulsifying properties of Cyperus esculentus seed (tiger nut) proteins. LWT 2021, 142, 110979. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Pagan, J. Optimisation and kinetic study of the ultrasonic-assisted extraction of total saponins from alfalfa (Medicago sativa) and its bioaccessibility using the response surface methodology. Food Chem. 2020, 309, 125786. [Google Scholar] [CrossRef] [PubMed]
- Paniwnyk, L. Applications of ultrasound in processing of liquid foods: A review. Ultrasonic Sonochem. 2017, 38, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Chemat, M.; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrasonics Sonochem. 2010, 18, 813–835. [Google Scholar] [CrossRef]
- Simancas, R.O.; Díaz-Maroto, M.C.; Alañón Pardo, M.E.; Pérez Porras, P.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Pérez-Coello, M.S. Effect of power ultrasound treatment on free and glycosidically-bound volatile compounds and the sensorial profile of red wines. Molecules 2021, 26, 1193. [Google Scholar] [CrossRef]
- Bautista-Ortin, A.B.; Jiménez-Martínez, M.D.; Jurado, R.; Iniesta, J.A.; Terrades, S.; Andrés, A.; Gómez-Plaza, E. Application of high-power ultrasounds during red wine vinification. Int. J. Food Sci. Technol. 2017, 52, 1314–1323. [Google Scholar] [CrossRef]
- Morata, A. Red Wine Technology, 1st ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 210–248. [Google Scholar]
- International Organisation of Vine and Wine. International Code of Oenological Practices, 2022, no. 2022 Issue. Paris, France. Available online: https://www.oiv.int/public/medias/8672/code-2022-en.pdf (accessed on 10 December 2022).
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Francesco Donsì, F.; Pataro, G.; Daniel, A.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 1–34. [Google Scholar] [CrossRef]
- Jamshaid, S.; Ahmed, D. Optimization of ultrasound-assisted extraction of valuable compounds from fruit of Melia azedarach with glycerol-choline chloride deep eutectic solvent. Sustain. Chem. Pharm. 2022, 29, 100827. [Google Scholar] [CrossRef]
- Anić, M.; Osrečak, M.; Andabaka, Z.; Tomaz, I.; Večenaj, V.; Jelić, D.; Kozina, B.; Kontić, J.K.; Karoglan, M. The effect of leaf removal on canopy microclimate, vine performance and grape phenolic composition of Merlot (Vitis vinifera L.) grapes in the continental part of Croatia. Sci. Hortic. 2021, 285, 110161. [Google Scholar] [CrossRef]
- Ferraretto, P.; Rolle, P.; Celotti, E. Applicazione degli ultrasuoni come tecnica innovativa per ottimizzare l’estrazione dei composti fenolici e favorire la lisi del lievito. Riv. Internet Vitic. Enol. 2011, 9, 1–10. [Google Scholar]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; Donnell, C.P.O. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010, 17, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Natolino, A.; Celotti, E. Ultrasound treatment of red wine: Effect on polyphenols, mathematical modeling, and scale-up considerations. LWT 2022, 154, 112843. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzym. 1999, 299, 152–178. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Kalita, D.; Jayanty, S.S. Comparison of Polyphenol Content and Antioxidant Capacity of Colored Potato Tubers, Pomegranate and Blueberries. J. Food Process Technol. 2014, 5, 358–364. [Google Scholar]
- Commission Regulation (EEC) No 2676/90 of 17 September 1990 Determining Community Methods for the Analysis of Wines (OJ L 272 03.10.1990). Available online: http://data.europa.eu/eli/reg/1990/2676/oj (accessed on 20 October 2022).
- Palma, M.; Barroso, C.G. Ultrasound-assisted extraction and determination of tartaric and malic acids from grapes and winemaking by-products. Anal. Chim. Acta 2002, 458, 119–130. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing; Wiley-VCH: Weinheim, Germany, 2002; pp. 15–43. [Google Scholar]
- Ferraretto, P.; Cacciola, V.; Ferran Batllo, I.; Celotti, E. Ultrasounds application in winemaking: Grape maceration and yeast lysis. Ital. J. Food Sci. 2013, 25, 160–168. [Google Scholar]
- Zhang, Q.; Shen, Y.; Fan, X.H.; Martin, J.F.G. Preliminary study of the effect of ultrasound on physicochemical properties of red wine. CyTA J. Food 2015, 14, 55–64. [Google Scholar] [CrossRef]
- Martin, J.F.G.; Sun, D.W. Ultrasound and electric fields as novel techniques for assisting the wine ageing process: The state-of-the-art research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Ćurko, N.; Tomašević, M.; Jambrak, A.R. The effect of high-power ultrasound on phenolic composition, chromatic characteristics, and aroma compounds of red wines. Croat. J. Food Sci. Technol. 2017, 9, 136–144. [Google Scholar] [CrossRef]
- Luo, H.; Schmid, F.; Grbin, P.R.; Jiranek, V. Viability of common wine spoilage organisms after exposure to high power ultrasonics. Ultrason. Sonochem. 2012, 19, 415–420. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Roman, T.; Tonidandel, L.; Nicolini, G.; Bellantuono, E.; Barp, L.; Larcher, R.; Celotti, E. Evidence of the possible interaction between ultrasound and thiol precursors. Foods 2020, 9, 104. [Google Scholar] [CrossRef]
- Reche, C.; Rosselló, C.; Dalmau, E.; Eim, V.; Simal, S. Quantification of microstructural changes in artichoke by-products by image analysis after high-power ultrasound-assisted extraction of bioactive compounds. LWT 2022, 171, 114127. [Google Scholar] [CrossRef]
- Pérez-Porras, P.; Bautista-Ortín, A.B.; Jurado, R.; Gómez-Plaza, E. Using high-power ultrasounds in red winemaking: Effect of operating conditions on wine physico-chemical and chromatic characteristics. LWT 2021, 138, 110645. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Bi, J.; Yang, Q.; Sun, J.; Chen, J.; Zhang, J. Study on ultrasonic extraction technology and oxidation resistance of total flavonoids from peanut hull. Food Sci. Technol. Res. 2011, 17, 187–198. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, G.A.; Mariam, A.; Chin, J.H. The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf. Pharmacogn. Mag. 2009, 5, 81–85. [Google Scholar]
- Havlikova, L.; Mikova, K. Heat Stability of Anthocyanins. Z. Leb. Forsch. 1985, 181, 427–432. [Google Scholar] [CrossRef]
- Jackman, R.L.; Yada, R.Y.; Tung, M.A.; Speers, R.A. Anthocyanins as food colorants—A review. J. Food Biochem. 1987, 11, 201–247. [Google Scholar] [CrossRef]
- Tao, Y.; Han, Y.; Liu, W.; Peng, L.; Wang, Y.; Kadam, S.; Show, P.L.; Ye, X. Parametric and phenomenological studies about ultrasound-enhanced biosorption of phenolics from fruit pomace extract by waste yeast. Ultrason. Sonochem. 2019, 52, 193–204. [Google Scholar] [CrossRef]
- Martínez-Ramos, T.; Benedito-Fort, J.; Watson, N.J.; Ruiz-López, I.I.; Che-Galicia, G.; Corona-Jiménez, E. Effect of solvent composition and its interaction with ultrasonic energy on the ultrasound-assisted extraction of phenolic compounds from Mango peels (Mangifera indica L.). Food Bioprod. Process. 2020, 122, 41–54. [Google Scholar] [CrossRef]
- Zainol, M.K.; Mohd Subri, I.; Zamri, A.I.; Mohd Zin, Z.; Fisal, A.; Mamat, H. Antioxidative properties and proximate analysis of spent coffee ground (SCG) extracted using ultrasonic-methanol assisted technique as a potential functional food ingredient. Food Res. 2020, 4, 636–644. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Constantin, O.E.; Istrati, D.I. Extraction, Quantification and Characterization Techniques for Anthocyanin Compounds in Various Food Matrices—A Review. Horticulturae 2022, 8, 1084. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016, 212, 596–603. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Optimisation of extraction of anthocyanins from black currants with aqueous ethanol. J. Food Sci. 2006, 68, 240–248. [Google Scholar] [CrossRef]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Li, L.; Zou, L.; Zhang, L.; Luo, Z. Natural deep eutectic solvent enhanced pulse-ultrasonication assisted extraction as a multi-stability protective and efficient green strategy to extract anthocyanin from blueberry pomace. LWT 2021, 144, 111220. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, G.; Khan, M.A.; Yan, Z.; Beta, T. Ultrasonic-assisted enzymatic extraction and identification of anthocyanin components from mulberry wine residues. Food Chem. 2020, 323, 126714. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Q.; Belwal, T.; Li, L.; Aalim, H.; Wu, Q.; Duan, Z.; Zhang, X.; Luo, Z. Ultrasonic impact on viscosity and extraction efficiency of polyethylene glycol: A greener approach for anthocyanins recovery from purple sweet potato. Food Chem. 2019, 283, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.J.R.; Alberto, M.R.; Nadra, M.C.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Vasyliev, G.S.; Vorobyova, V.I.; Linyucheva, O.V. Evaluation of reducing ability and antioxidant activity of fruit pomace extracts by spectrophotometric and electrochemical methods. J. Anal. Methods Chem. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef]
- Dani, C.; Oliboni, L.S.; Vanderlinde, R.; Pra, D.; Dias, J.F.; Yoneama, M.L.; Bonatto, D.; Salvador, M.; Henriques, J.A.P. Antioxidant activity and phenolic and mineral content of rose grape juice. J. Med. Food 2009, 12, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Aprile, A.; Luvis, A.I.; De Bellis, L.; Miceli, A. Antioxidant activity and polyphenols characterization of four monovarietal grape pomaces from salento (Apulia, italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Banc, R.; Socaciu, C.; Miere, D.; Filip, L.; Cozma, A.; Stanciu, O.; Loghin, F. Benefits of Wine Polyphenols on Human Health: A Review. Bull. UASVM Food Sci. Technol. 2014, 71, 79–87. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pachón, M.S.; Villaño, D.; García-Parrilla, M.C.; Troncoso, A.M. Antioxidant activity of wines and relation with their polyphenolic composition. Anal. Chim. Acta 2004, 513, 113–118. [Google Scholar] [CrossRef]
- Gismondi, A.; Marco, G.; di Canuti, L.; Canini, A. Antiradical activity of phenolic metabolites extracted from grapes of white and red Vitis vinifera L. cultivars. Vitis 2017, 56, 19–26. [Google Scholar]
- Rivero-Pérez, M.D.; Muñiz, P.; González-Sanjosé, M.L. Antioxidant Profile of Red Wines Evaluated by Total Antioxidant Capacity, Scavenger Activity, and Biomarkers of Oxidative Stress Methodologies. J. Agric. Food Chem. 2007, 55, 5476–5483. [Google Scholar] [CrossRef]
- Banc, R.; Loghin, F.; Miere, D.; Ranga, F.; Socaciu, C. Phenolic composition and antioxidant activity of red, rosé and white wines originating from Romanian grape cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 716–734. [Google Scholar] [CrossRef]


| Independent Variables | Dependent Variables | |||
|---|---|---|---|---|
| Probe Diameter (mm) | Frequency (kHz) | Amplitude (A) % | Treatment Time (t) min | |
| 13 | 20 | 50 | 3 | TPC |
| 70 | 4 | MA | ||
| 90 | 5 | FRAP | ||
| DPPH | ||||
| Std Order | Run Order | Factors | Responses | ||||
|---|---|---|---|---|---|---|---|
| A (%) | B (min) | TPC (µg GAE/mL) | MA (mg CGE/L) | FRAP (µmol Fe2+/mL) | DPPH Scavenging Activity (%) | ||
| 2 | 1 | 90 | 3 | 1396.06 ± 5.23 | 223.46 ± 2.32 | 41.78 ± 2.25 | 74.5 ± 0.2 |
| 9 | 2 | 70 | 4 | 947.92 ± 3.79 | 167.05 ± 1.46 | 27.52 ± 1.21 | 73.3 ± 0.6 |
| 1 | 3 | 50 | 3 | 124.5 ± 1.01 | 20.67 ± 0.72 | 15.02 ± 0.83 | 66.8 ± 0.5 |
| 6 | 4 | 90 | 4 | 1177.85 ± 4.87 | 214.97 ± 2.36 | 42.96 ± 1.98 | 75.9 ± 0.6 |
| 11 | 5 | 70 | 4 | 948.05 ± 4.56 | 169.7 ± 1.55 | 28.85 ± 1.09 | 73.2 ± 0.3 |
| 5 | 6 | 50 | 4 | 136.4 ± 1.08 | 25.49 ± 0.65 | 16.09 ± 0.78 | 68.2 ± 0.2 |
| 7 | 7 | 70 | 3 | 482.31 ± 3.82 | 108.37 ± 1.02 | 27.17 ± 0.94 | 72.1 ± 0.5 |
| 4 | 8 | 90 | 5 | 1172.1 ± 3.75 | 176.29 ± 2.45 | 48.32 ± 2.23 | 77.2 ± 1.0 |
| 12 | 9 | 70 | 4 | 949.23 ± 3.98 | 166.6 ± 1.47 | 28.02 ± 0.74 | 73.2 ± 0.9 |
| 10 | 10 | 70 | 4 | 951.19 ± 4.56 | 168.15 ± 1.62 | 29.15 ± 1.16 | 73.3 ± 0.8 |
| 3 | 11 | 50 | 5 | 148.3 ± 2.04 | 26.22 ± 1.23 | 18.68 ± 0.43 | 71.1 ± 0.6 |
| 13 | 12 | 70 | 4 | 950.24 ± 4.21 | 167.24 ± 1.74 | 27.97 ± 0.54 | 73.4 ± 0.5 |
| 8 | 13 | 70 | 5 | 1126.11 ± 2.01 | 137.74 ± 1.03 | 29.97 ± 0.81 | 73.5 ± 0.7 |
| Sample | TSS (° Brix) | TA (g Tartaric Acid/L) | TPC (µg GAE/mL) | MA (mg CGE/L) | FRAP (µmol Fe2+/mL) | DPPH Scavenging Activity (%) |
|---|---|---|---|---|---|---|
| C | 29.9 ± 0.1 | 3.4 ± 0.03 | 114.42 ± 2.67 | 16.06 ± 0.9 | 13.43 ± 1.02 | 65.0 ± 0.4 |
| Source | F-Value | p-Value | R2 | Adjusted R2 | Coefficients |
|---|---|---|---|---|---|
| Model | 13.40 | 0.0018 | 0.9054 | 0.8378 | |
| Intercept | 921.98 | ||||
| A | 60.33 | 0.0001 | 556.13 | ||
| B | 1.07 | 0.3361 | 73.94 | ||
| AB | 0.4989 | 0.5028 | 61.94 | ||
| A2 | 3.47 | 0.1049 | −196.5 | ||
| B2 | 0.2193 | 0.6538 | −49.42 | ||
| Lack of fit | 36229.38 | <0.0001 |
| Source | F-Value | p-Value | R2 | Adjusted R2 | Coefficients |
|---|---|---|---|---|---|
| Model | 44.35 | <0.0001 | 0.9694 | 0.9475 | |
| Intercept | 162.77 | ||||
| A | 190.42 | <0.0001 | 90.39 | ||
| B | 0.0972 | 0.7644 | 2.04 | ||
| AB | 2.70 | 0.1444 | –13.18 | ||
| A2 | 9.71 | 0.0169 | –30.08 | ||
| B2 | 7.97 | 0.0257 | –27.25 | ||
| Lack of fit | 396.80 | <0.0001 |
| Source | F-Value | p-Value | R2 | Adjusted R2 | Coefficients | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FRAP | DPPH | FRAP | DPPH | FRAP | DPPH | FRAP | DPPH | FRAP | DPPH | |
| Model | 330.16 | 58.81 | <0.0001 | <0.0001 | 0.9958 | 0.9767 | 0.9928 | 0.9601 | ||
| Intercept | 28.14 | 73.17 | ||||||||
| A | 1587.16 | 246.65 | <0.0001 | <0.0001 | 13.88 | 3.58 | ||||
| B | 33.68 | 37.65 | 0.0004 | 0.0005 | 2.17 | 1.40 | ||||
| AB | 2.85 | 2.05 | 0.1354 | 0.1954 | 0.7200 | –0.4000 | ||||
| A2 | 12.04 | 6.08 | 0.0104 | 0.0431 | 1.78 | –0.8293 | ||||
| B2 | 2.59 | 0.0556 | 0.1515 | 0.8203 | 0.8266 | –0.0793 | ||||
| Lack of fit | 2.40 | 102.78 | 0.2085 | 0.0003 | ||||||
| Response Data | TPC (µg GAE/mL) | MA (mg CGE/L) | FRAP (µmol Fe2+/mL) | DPPH Scavenging Activity (%) |
|---|---|---|---|---|
| Observed | 1176.16 | 210.56 | 46.55 | 76.5 |
| Predicted | 1278.52 | 212.64 | 45.09 | 76.31 |
| Error (%) | –8.70 | –0.99 | 3.14 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, A.; Padureanu, V.; Lupu, M.I.; Canja, C.M.; Badarau, C.; Padureanu, C.; Alexa, E.; Poiana, M.-A. Optimization of A Procedure to Improve the Extraction Rate of Biologically Active Compounds in Red Grape Must Using High-Power Ultrasound. Sustainability 2023, 15, 6697. https://doi.org/10.3390/su15086697
Maier A, Padureanu V, Lupu MI, Canja CM, Badarau C, Padureanu C, Alexa E, Poiana M-A. Optimization of A Procedure to Improve the Extraction Rate of Biologically Active Compounds in Red Grape Must Using High-Power Ultrasound. Sustainability. 2023; 15(8):6697. https://doi.org/10.3390/su15086697
Chicago/Turabian StyleMaier, Alina, Vasile Padureanu, Mirabela Ioana Lupu, Cristina Maria Canja, Carmen Badarau, Cristina Padureanu, Ersilia Alexa, and Mariana-Atena Poiana. 2023. "Optimization of A Procedure to Improve the Extraction Rate of Biologically Active Compounds in Red Grape Must Using High-Power Ultrasound" Sustainability 15, no. 8: 6697. https://doi.org/10.3390/su15086697
APA StyleMaier, A., Padureanu, V., Lupu, M. I., Canja, C. M., Badarau, C., Padureanu, C., Alexa, E., & Poiana, M.-A. (2023). Optimization of A Procedure to Improve the Extraction Rate of Biologically Active Compounds in Red Grape Must Using High-Power Ultrasound. Sustainability, 15(8), 6697. https://doi.org/10.3390/su15086697











