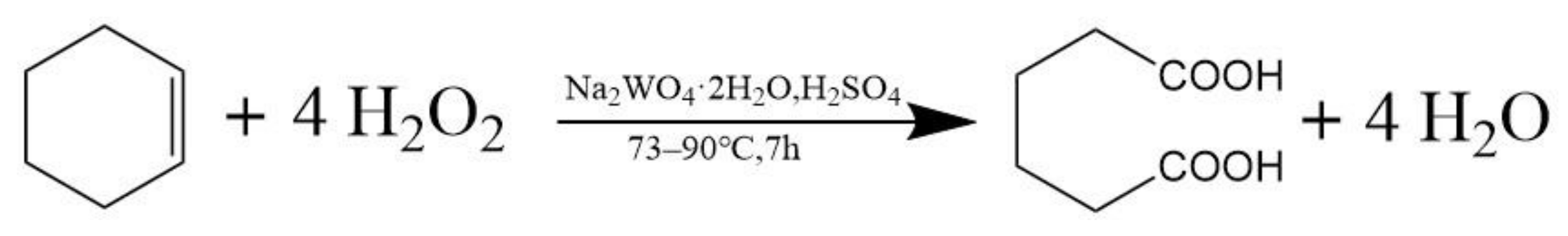1. Introduction
As the most practical dicarboxylic acid, adipic acid has important application values in the organic synthesis of plastics, synthetic resins, food, and other fields. Adipic acid is mainly used as an intermediate in the manufacture of nylon 66, polyurethane, plasticizers, and other materials [
1].
At present, the two main adipic acid synthesis processes are the cyclohexane [
2] and the cyclohexene methods [
3], both of which have low atomic utilization and the serious pollution problem of “the three wastes”. The oxidant used in these two methods is nitric acid, which generates a large amount of NO
x exhaust and acidic wastewater [
2]. The catalyst is cuprum and vanadium, which generates waste residue. Therefore, the research and development of new technologies for the green synthesis of adipic acid has received extensive attention from experts and scholars at home and abroad. Among these new technologies, the hydrogen peroxide (H
2O
2) oxidation method could align with the requirements of green chemistry, with its mild conditions and simple operations, and which can ensure a high conversion rate, high yield, and high selectivity while maintaining clean production [
4,
5,
6]. Jiang et al. [
7] investigated the thermal hazards of adipic acid synthesis achieved by the oxidation of cyclohexene with H
2O
2, combined with reaction calorimetry and mechanism analysis, and found that the process could be divided into two reaction stages, both of which were assessed as a class 5 risk according to the Stoessel criticality diagram method, indicating that the process had a high risk of explosion.
Typically, H
2O
2 is unstable with highly exothermic hazards. The initial decomposition temperature of H
2O
2 ranges from 47 to 81 °C, and the decomposition heat is about 192–1079 J/g [
8,
9]. Wu et al. [
10] suggested that the sulfuric acid (H
2SO
4) at a low concentration could slow down the decomposition rate of H
2O
2, and the rate could be increased with an increase in H
2SO
4 concentration. Jung et al. [
11] found that the addition of C
8H
6O
4 as a stabilizer to a Fenton reagent or Fenton-like reagent could extend the lifetime of H
2O
2 and reduce the rate of H
2O
2 autolysis. Wu et al. [
12] studied the effects of inorganic salts and organic acids on the thermal runaway behavior of hydrogen peroxide, and found that the order of catalytic effect from large to small was Fe
2+ > Fe
3+ > Cu
2+, and the order of risk of thermal runaway from large to small was H
2O
2/HCOOH > H
2O
2 > H
2O
2/CH
3COOH. Zhang et al. [
13] investigated the effects of free manganese ions and manganese on H
2O
2 decomposition in the presence of precipitated lignin under typical pulp bleaching conditions, and found that adding only Ethylene Diamine Tetraacetic Acid (EDTA) was the most effective method for reducing the decomposition of H
2O
2.
Both high temperatures and the catalysts used can accelerate the ineffective decomposition of H2O2 during the oxidation of cyclohexene by H2O2 for the preparation of adipic acid, resulting in the concentration of H2O2 decreasing, and the heat generated by the H2O2 decomposition reaction increasing. Consequently, the thermal hazard of the process is greatly increased. Therefore, it is necessary to select an effective H2O2 stabilizer to inhibit the decomposition reactions of excessive H2O2, so that the H2O2 can fully and effectively participate in the adipic acid synthesis.
In this work, various calorimetric and analytical techniques were carried out to investigate the effects of H
2SO
4 concentration and the addition of EDTA on the thermal hazard of the adipic acid synthesis reaction: (1) Differential scanning calorimetry (DSC) was implemented to obtain the thermal decomposition parameters of H
2O
2 under different catalytic conditions. (2) An automatic reaction calorimeter (EasyMax
TM102) was employed to measure the enthalpy of the H
2O
2 decomposition reaction and the adipic acid synthesis reaction. Meanwhile, high-performance liquid chromatography (HPLC) was used to assess the conversion rate of the substances used in the reaction and the yield of adipic acid. (3) An adiabatic calorimeter (Phi-TEC II) was implemented to simulate the adiabatic decomposition behavior and parameters of H
2O
2 and the reaction products obtained by calorimetric experiments. Finally, the reaction risks of the target reactions were assessed using the ITHI method [
14].
2. Materials and Methods
2.1. Materials
Hydrogen peroxide (30 wt.%) and H2SO4(98 wt.%, AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The cyclohexene and sodium tungstate dehydrate (Na2WO4·2H2O) used in this work were of analytical grade and made by Shanghai Macklin Biochemical (Shanghai, China). Adipic acid (HPLC, Shanghai Aladdin Bio-Chem Technology, Shanghai, China), methanol, (HPLC, Shanghai Aladdin Bio-Chem Technology, Shanghai, China), acetonitrile (HPLC, Shanghai Aladdin Bio-Chem Technology, Shanghai, China), phosphoric acid (AR, Shanghai Macklin Biochemical, Shanghai, China), potassium dihydrogen phosphate (AR, Shanghai Macklin Biochemical, Shanghai, China), sodium dodecyl sulfate (SDS) (AR, Sinopharm Chemical Reagent, Shanghai, China), Ethylene Diamine Tetraacetic Acid (EDTA) (AR, tcichemicals) were used as supplied. All reagents were used without further purification.
2.2. Methods
The reaction equation for the catalytic oxidation of cyclohexene by H
2O
2 to synthesize adipic acid is shown in
Scheme 1.
During the synthesis of adipic acid, a total of four oxidation and two hydrolysis reactions occur. At 73 °C for two hours, cyclohexene is oxidized and hydrolyzed to produce 1,2-cyclohexanediol. The 1,2-cyclohexanediol continues to be oxidized to 2-hydroxycyclohexanone, which is recorded as stage A. At 90 °C for five hours, 2-hydroxycyclohexanone undergoes two steps of oxidation and hydrolysis to produce the final product, adipic acid, and the first three hours of this period are recorded as stage B [
15].
2.2.1. HPLC Experiment
An HPLC device (Agilent-1260, Agilent Technologies Co., Ltd., Santa Clara, CA, USA) was used to characterize the yield of adipic acid and the conversion rate of the raw materials, cyclohexene and H
2O
2 [
16]. The HPLC conditions for the adipic acid testing were as follows: The injection volume was 20 μL. The mobile stage was phosphoric acid-potassium dihydrogen phosphate buffer (50 mmol/L) and methanol. The elution rate was 0.3 mL/min. The column temperature was 30 °C. The chromatographic conditions for the cyclohexene and H
2O
2 tests were as follows: The injection volume was 5 μL. The mobile stage was acetonitrile and ultrapure water. The elution rate was 0.8 L/min. The column temperature was 30 °C and the UV detection wavelength was 209 nm [
17].
2.2.2. Calorimetric Experiments
An EasyMaxTM102 calorimeter (Mettler Toledo Ltd., Greifensee, Switzerland) was used to determine the exothermic characteristics of adipic acid synthesis and the decomposition of H2O2 using a 100 mL atmospheric pressure glass reactor. The reactor temperature (Tr), jacket temperature (Tj), and heat release rates (qr) were all tracked automatically to analyze the reaction enthalpy, adiabatic temperature rises (), and other thermodynamic parameters.
2.2.3. DSC Experiment
DSC is among the most used techniques for thermal analysis [
18]. In this experiment, DSC 250 (TA Instruments, Eden Prairie, MN, USA) was carried out to investigate the exothermic behavior of H
2O
2, the intermediates, and the products in stages A and B under Na
2WO
4·2H
2O, H
2SO
4, and EDTA conditions. The scanning temperature range was 40~180 °C and 40~300 °C. The ramp-up rate was 5 °C/min. Nitrogen was used for protection and the flow rate was 50 mL/min.
2.2.4. Adiabatic Tests
An adiabatic calorimeter (Phi-TEC II, HEL Limited, Hertford, UK) was used to identify the adiabatic decomposition characteristics of the reactants. Samples (~1 g) of the stage A product, the stage B product, and the raw H
2O
2 mixtures from the Calorimetric experiments were loaded into a 10 mL Hastelloy test cell. The temperature ranged from 30 to 300 °C with a step-upward trend of 5 °C. The temperature and pressure data from the adiabatic experiments were tracked to record any exotherms. The detection sensitivity was 0.02 °C/min [
19].
3. Results and Discussion
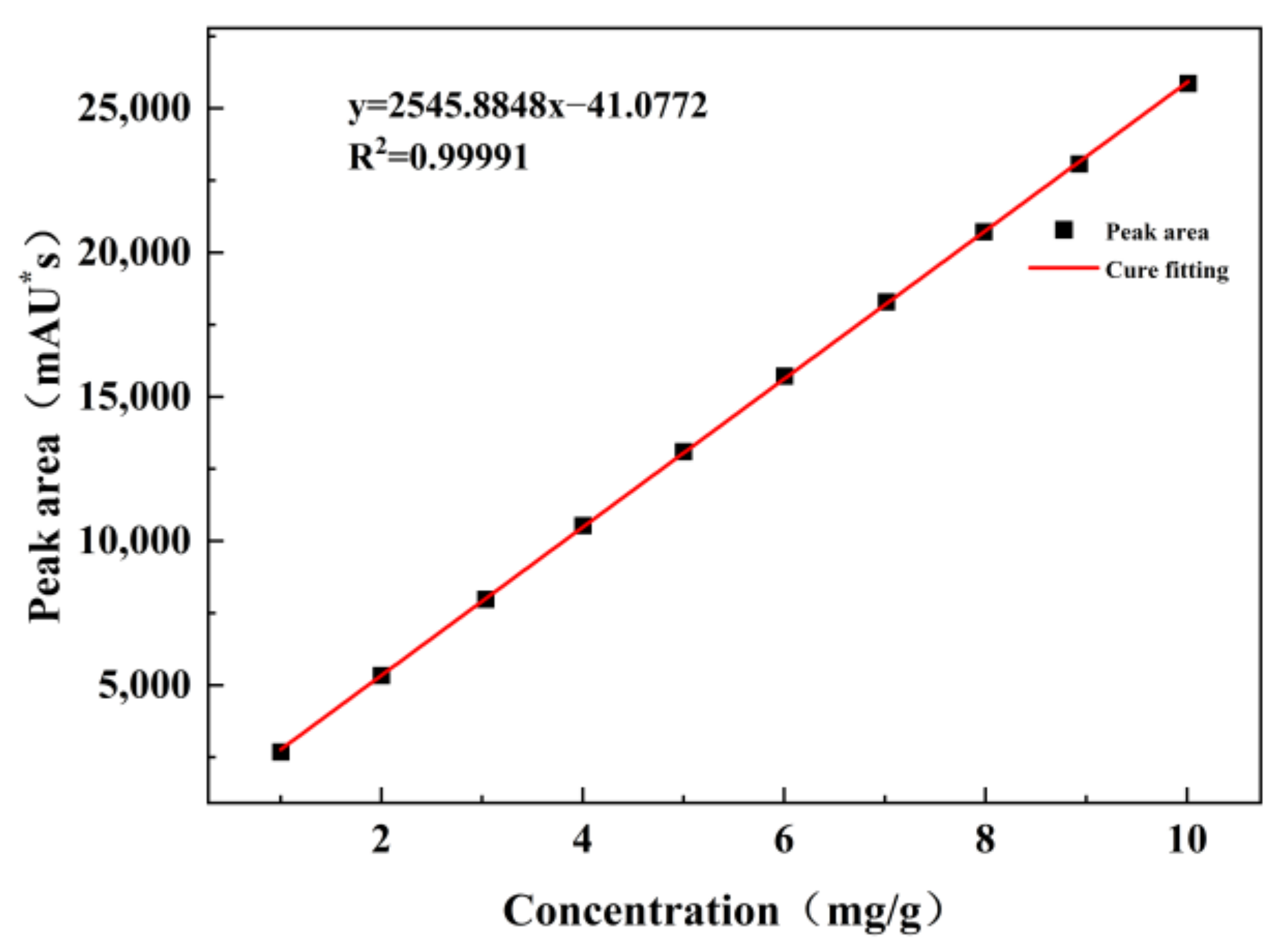
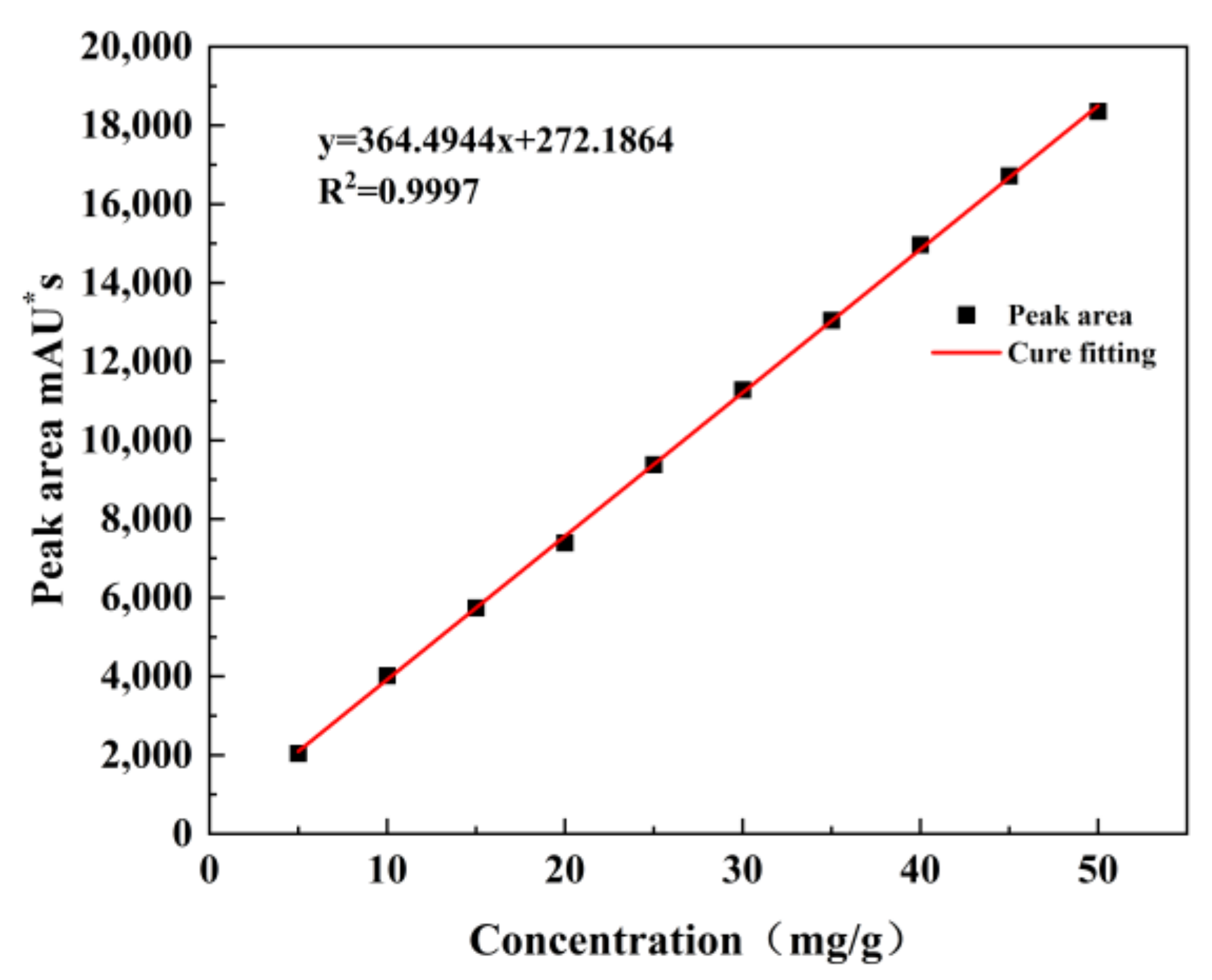
3.1. The Working Curves Obtained from the HPLC Experiments
Three sets of adipic acid, cyclohexene, and H
2O
2 solutions with concentrations ranging from 1 mg/g to 50 mg/g were prepared and used to obtain the working curves using HPLC (mg/g is the ratio of the mass of the substance to the mass of the prepared standard solution). The experimental results are shown in
Figure 1,
Figure 2 and
Figure 3.
3.2. The Decomposition Mechanism of H2O2 Catalyzed by Na2WO4·2H2O
Typically, in addition to participating in the catalytic oxidation of cyclohexene to adipic acid, H2O2 can also be auto catalyzed for decomposition. This leads to the reduced utilization of H2O2 during the whole process, and the heat released from the decomposition increase the thermal hazard of the reaction. Hence, it is necessary to identify the decomposition mechanism of H2O2 to reduce the thermal hazard of the process and to improve the utilization of H2O2.
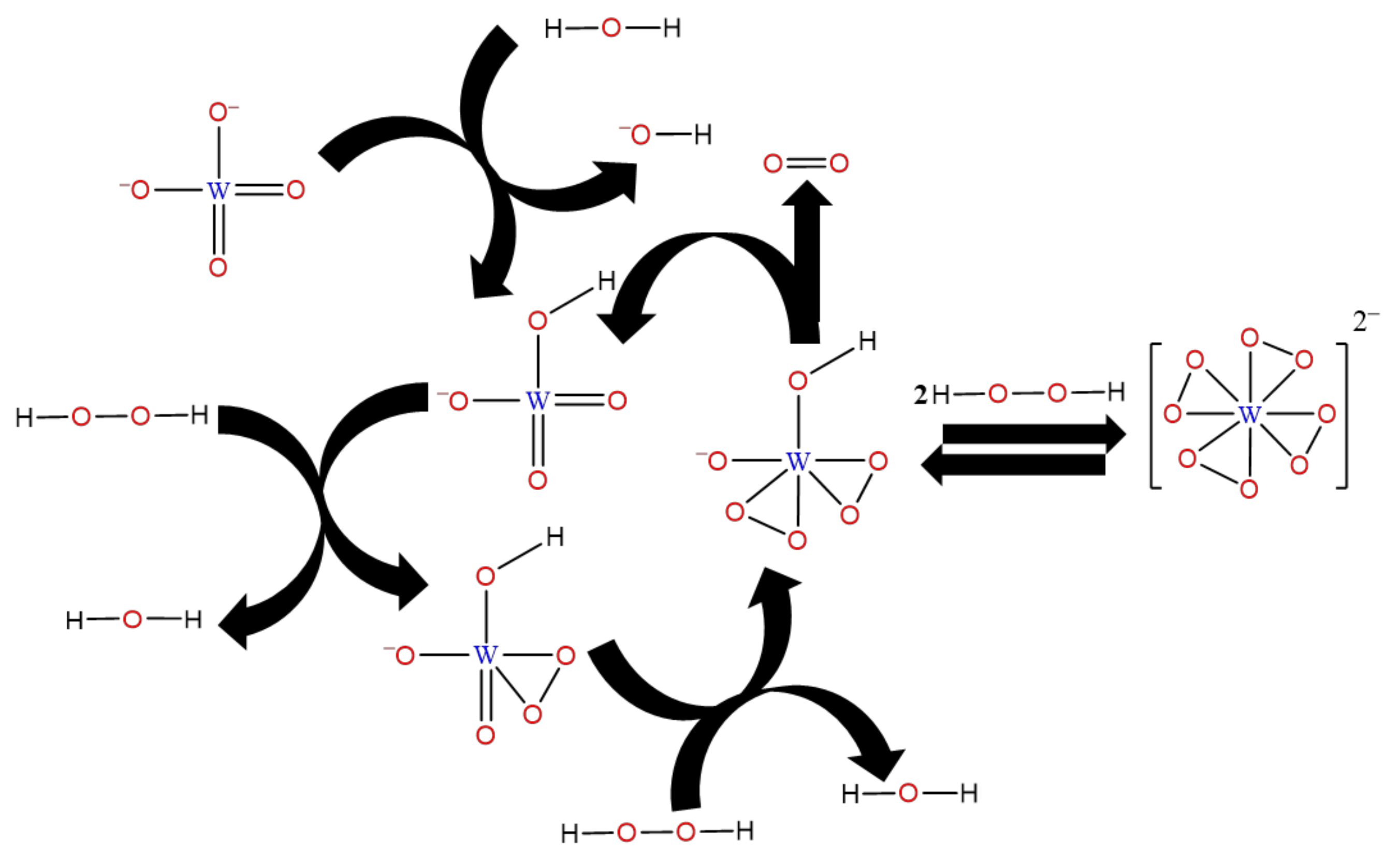
Nardello V et al. [
20] investigated the disproportionation reaction of H
2O
2 catalyzed by Na
2WO
4·2H
2O to form singlet oxygen (
1O
2). The mono-, di-, and tetraperoxotungstate intermediates
(
n = 1, 2, 4) have been characterized using UV and 183W NMR spectroscopies. The diperoxo species was proposed as the precursor of
1O
2. Yoshiro O et al. [
21] carried out the kinetics for the oxidation of dimethyl sulfoxide (DMSO) by H
2O
2 using iodometry. Meanwhile, the major protons were formed at a solution pH of 3.5–5.0 acidity (H
2WO
5 and H
2WO
8) with using Na
2WO
4·2H
2O as the catalyst. The effect of acidity on the rate of oxidation and the rate determining mechanism of peroxotungstic acid were also discussed.
According to Nardello V et al. and Yoshiro O et al., the decomposition of H
2O
2 catalyzed by sodium tungstate was studied because of the presence of H
2O
2, Na
2WO
4·2H
2O, and H
2SO
4 in the oxidative synthesis of adipic acid. In this work, as shown in
Figure 4, a possible decomposition mechanism of H
2O
2 catalyzed by Na
2WO
4·2H
2O was proposed on the basis of existing research. First,
is hydrolyzed to produce
, after which
is gradually oxidized by H
2O
2 to form
and
.
is generated through
, and then
is produced, leading a catalytic decomposition cycle. As the pH of the solution decreases,
and
are in dynamic equilibrium in the presence of 2 mol of H
2O
2.
3.3. Reaction Calorimetry Results and Analysis
3.3.1. The Exothermic Results and Analysis of the Catalytic Decomposition Reaction of H2O2
In reaction condition 1, the molar ratio of H2O2 to Na2WO4·2H2O was 4.4:0.06. In reaction condition 2 the molar ratio of H2O2 to Na2WO4·2H2O was 4.4:0.06 and the H2SO4 concentration was 0.22 mol/L. In reaction condition 3, the molar ratio of H2O2 to Na2WO4·2H2O was 4.4:0.06, the H2SO4 molar concentration was 0.22 mol/L, and the EDTA mass concentration was 18.3 g/L.
The experimental tests were all carried out at room temperature with stirring at 300 rpm, then Tr was heated to 73 °C and held for 120 min. Subsequently, the reactions were maintained for 240 min at 90 °C. Samples were taken at every 60 min intervals and analyzed the conversion of H2O2 by HPLC.
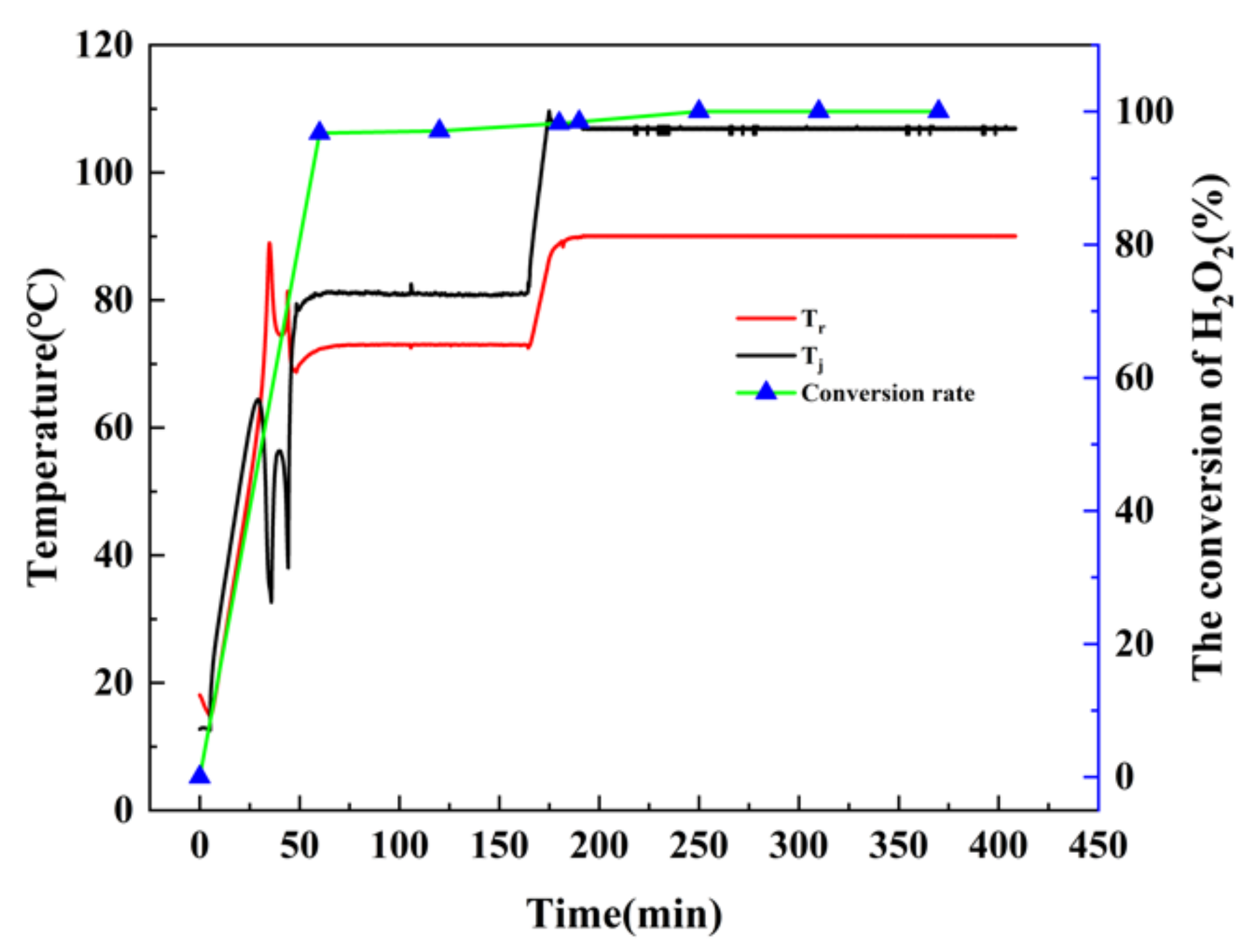
As depicted in
Figure 5, when
Tj was raised to about 60 °C,
Tr increased rapidly, indicating that the reaction was violently exothermic, and a large number of bubbles were also generated during the process. After temperature stabilization, the decomposition of H
2O
2 was basically complete. The maximum temperature difference between
Tr and
Tj during the process was about 59 °C. Thus, if a cooling failure occurred, the reaction temperature could rise sharply, resulting in runaway accidents.
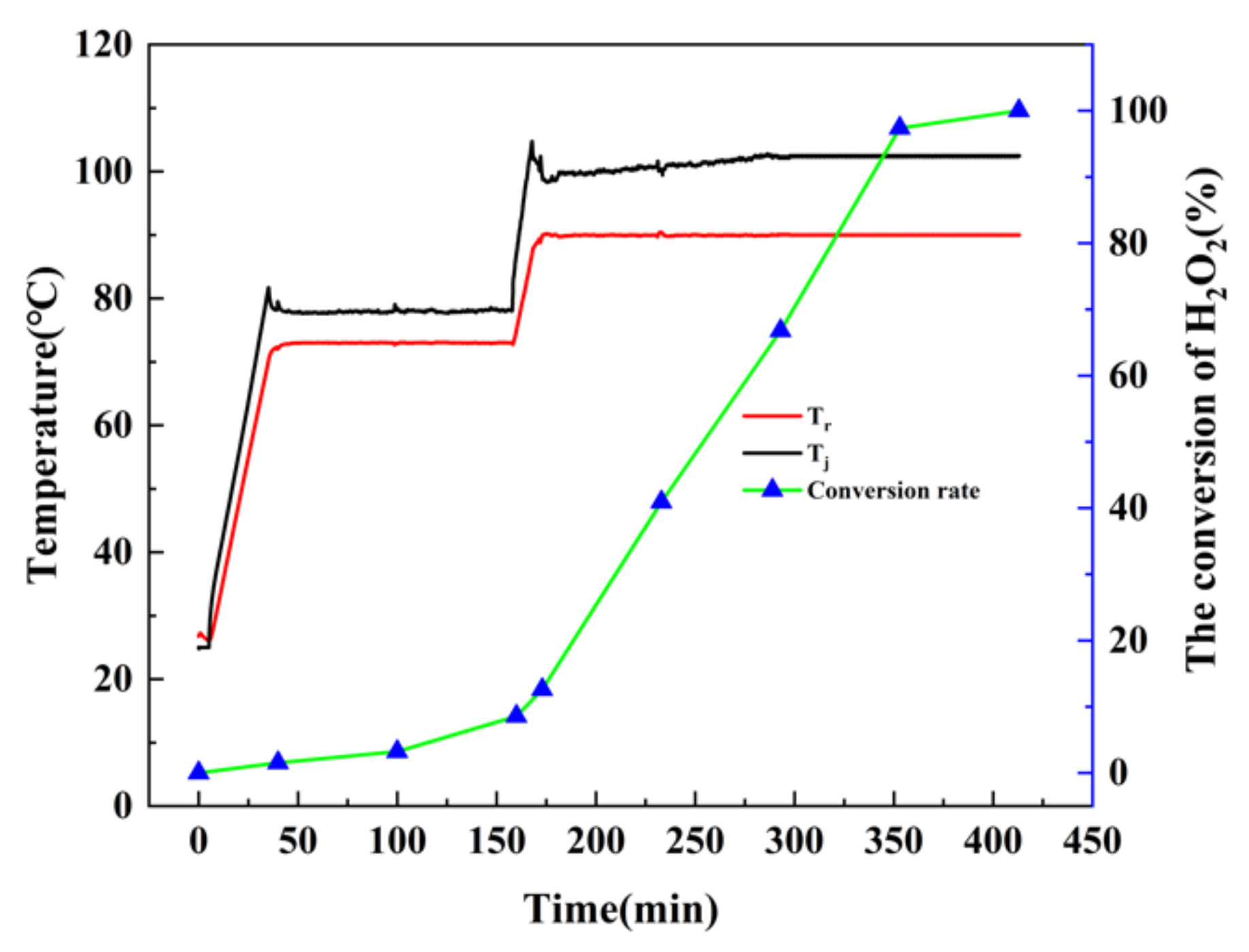
The amount of H
2SO
4 was increased under reaction conditions 2 to reduce the potential of hydrogen (pH) of the solution. Hence, the process of catalytic decomposition could be inhibited under the dynamic equilibrium of
and
in the presence of 2 mol of H
2O
2. The decomposition rate of H
2O
2 slowed down as is shown in
Figure 6. The decomposition rate of H
2O
2 at the isothermal stage of 90 °C is significantly higher than at the isothermal stage of 73 °C. This indicates that the addition of acid effectively reduced the catalytic decomposition of H
2O
2, while the high temperature still led the H
2O
2 to decompose completely; the conversion rate reached 100% after 240 min of reaction time at the 90 °C isothermal stage. Therefore, it is essential to add H
2O
2 stabilizers into H
2O
2 solutions to inhibit the ineffective decomposition of H
2O
2 during the oxidation reaction.
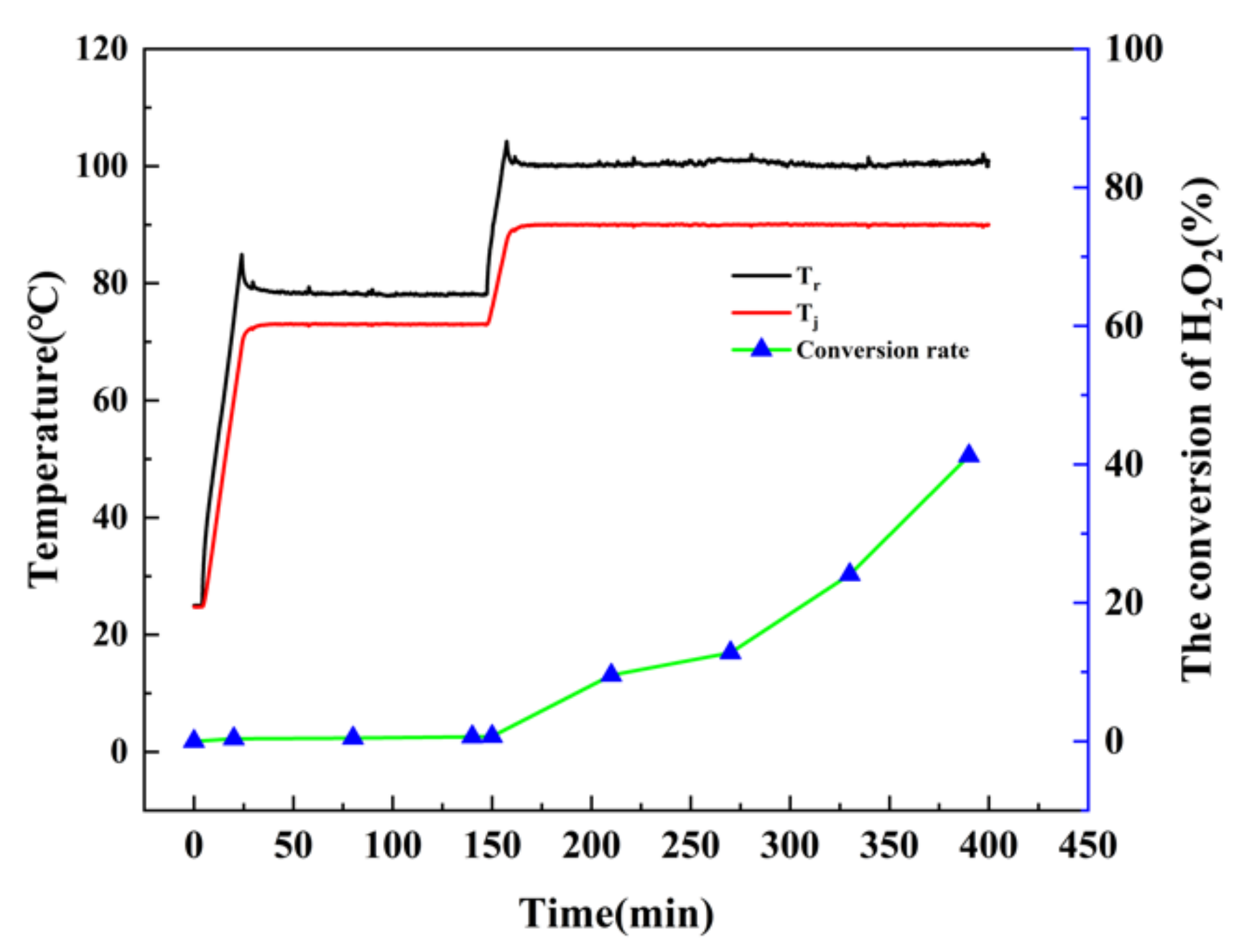
EDTA, which is commonly used as a stabilizer of H
2O
2 in the field of paper bleaching, is rarely used in the chemical industry. In test 3, EDTA was added and the other conditions were kept consistent with those of test 2. As depicted in
Figure 7, after 240 min of reaction time at the 90 °C isothermal stage, only about 41.24% of the H
2O
2 was converted, the results indicate that the addition of EDTA can improve the thermal stability of H
2O
2 Therefore, the application of EDTA in the process of catalytic oxidation of cyclohexene to adipic acid with H
2O
2 was relatively feasible.
3.3.2. Calorimetry and Theoretical Calculation of Catalytic Decomposition of H2O2
Gaussian is a professional calculation software in the field of quantum chemistry, which can be used to calculate thermodynamic parameters such as enthalpy change Δ
H and free energy change Δ
G of chemical reactions, and the calculated values are very close to the experimental values, thus accurately predicting the thermodynamic properties of reactions. Based on the mechanism of the catalytic decomposition of hydrogen peroxide, density functional theory (DFT) calculations of the enthalpy of reaction for the catalytic decomposition of H
2O
2 solution were performed. The standard molar enthalpies of production for each step of the reaction are shown in
Table 1.
gives
after releasing
and
is oxidized to
by 2 mol of hydrogen peroxide as a heat-absorbing reaction. The standard molar enthalpy of production for the entire hydrogen peroxide decomposition reaction is 61.98 kJ/mol.
The heat of decomposition and
of the H
2O
2 decomposition reaction were obtained using test 1. The whole reaction heat was 19.04 kJ and
was 170.77 °C. As shown in
Figure 8, the trend of
qr curve showed two severe peaks of exothermic heat. The maximum heat release rate was about 55.72 W. This indicates that the decomposition rate of H
2O
2 catalyzed by Na
2SO
4·2H
2O was great and acute. The large amount of gas produced could easily trigger an accidental explosion. The mass of the 30 wt.% H
2O
2 used in the experiment was 36.434 g, so the enthalpy of reaction was 59.5 kJ/mol. The theoretical calculation gives a standard molar enthalpy of production of 61.98 kJ/mol. The theoretical value is close to the calculated value.
3.3.3. Adipic Acid Green Synthesis Reaction Results and Analysis
Sodium dodecyl sulfate (SDS) is an anionic surfactant with good emulsification. In the present work, to investigate the effect of H
2SO
4 concentration and the addition of EDTA on the reaction process of adipic acid synthesis, nine experiments were conducted on the synthesis of adipic acid using calorimetric experiments, and the yield of adipic acid was analyzed using HPLC. The molar ratio of the H
2O
2, cyclohexene, and Na
2WO
4·2H
2O used in the tests was all 4.4:1:0.06. Different concentrations of H
2SO
4 (
CH2SO4), SDS(
ωSDS), and EDTA(
ωEDTA) were used in the different tests, as shown in
Table 2.
In experiment 1, the incomplete yield of the entire synthesis reaction, without the addition of SDS, was only 8.67%. Since the process is a liquid–liquid non-homogeneous reaction, both the lack of organic solvents and the ultimate stirring speed could lead to the poor mass transfer of the reaction. The mass transfer could be enhanced by SDS emulsification, and the yields of adipic acid were significantly increased, as depicted in experiments 2 and 3. The comparison of experiments 2, 3, 4, and 5 indicated that the addition of EDTA could reduce the total heat release while increasing the yield of adipic acid. By comparing experiments 4 and 7, it was found that the decrease of H2SO4 concentration also reduced the total heat release and increased the yield of adpic acid. Experiment 7, which had the lowest total heat release and temperature difference, the H2SO4 concentration of 0.12 mol/L, the SDS concentration of 9.15 g/L, and the EDTA concentration of 18.3 g/L, was determined to be the optimum process for adipic acid synthesis after a comprehensive comparison of experiments 1–9.
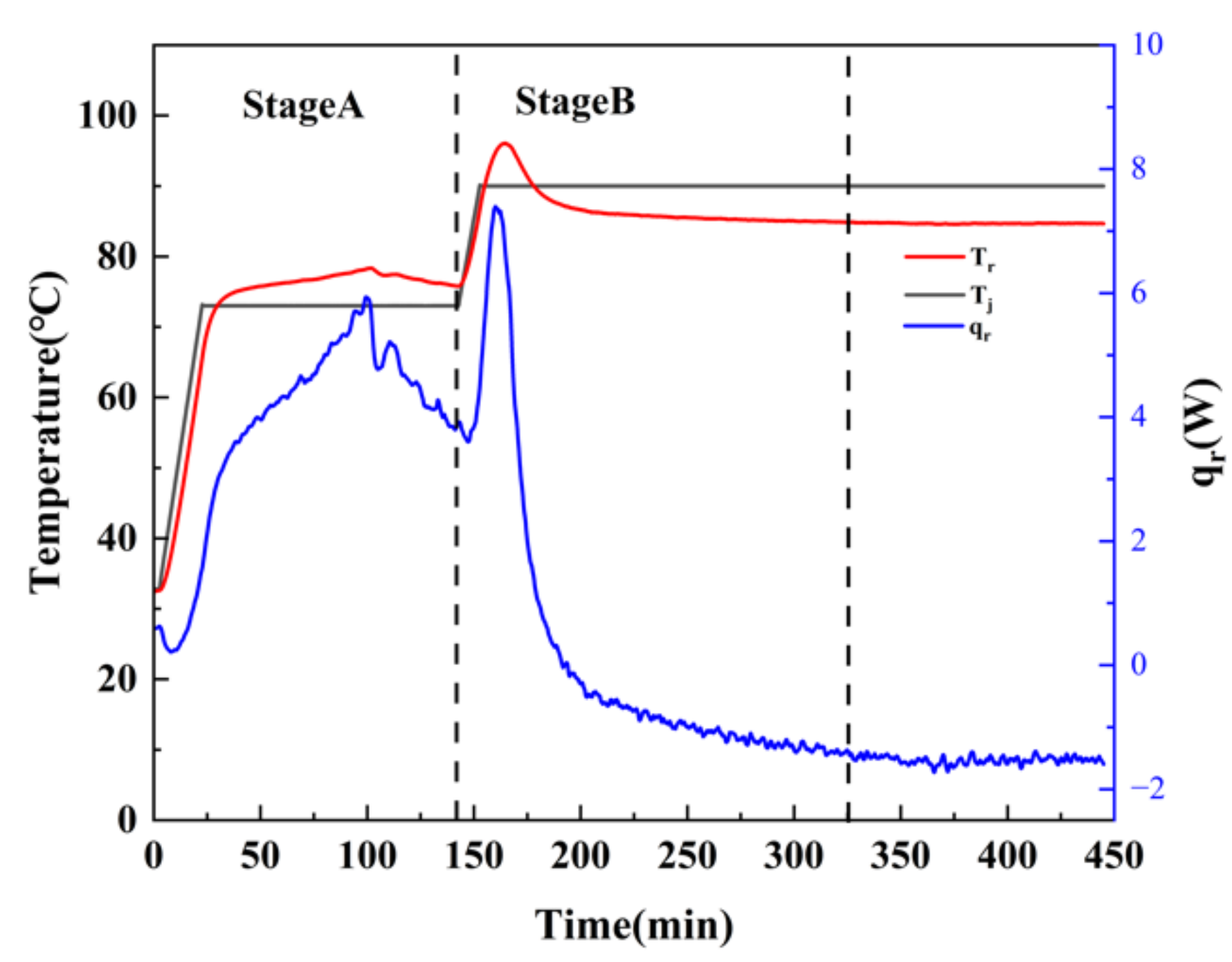
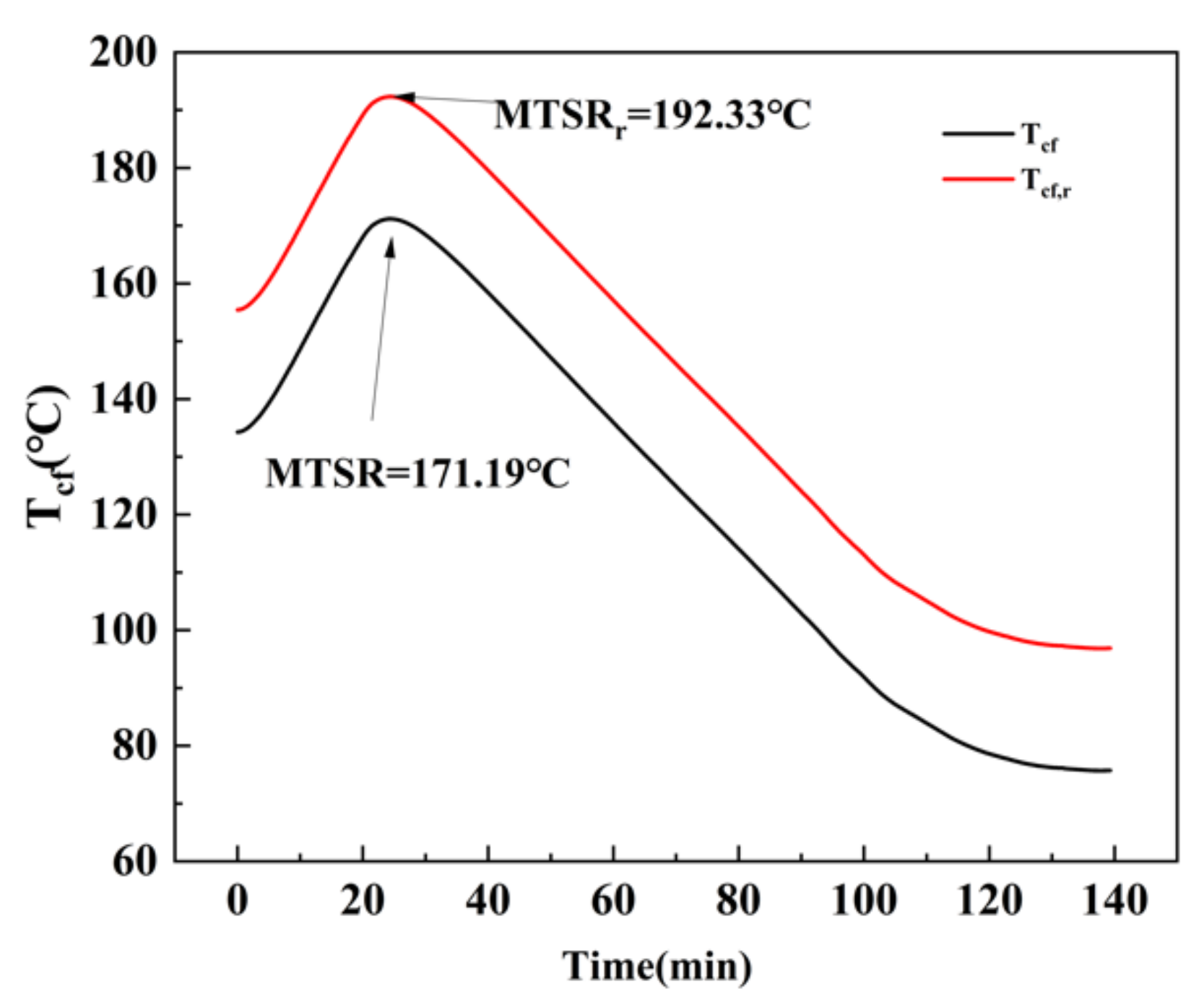
In order to accurately assess the thermal hazard of the adipic acid synthesis process, the entire reaction process was divided into two stages, A and B, as shown in
Figure 9.
The severity of the reaction runaway is usually determined using
due to the exothermic of the target reaction. Since the yield of adipic acid was 82.78%, the accurate adiabatic temperature rise
should be corrected by the yield
[
22]. The equations for calculating
and
are listed in Equations (1) and (2). The calorimetric experimental data and results are shown in
Table 3, where
is the heat of the oxidation reaction, kJ;
is the total mass of the oxidation system, g; and
is the specific heat capacity of the system after the oxidation reaction J/(K·g).
In Equations (3) and (4),
is the process operating temperature, which depends on the process conditions, where
is the temperature in the kettle during the synthesis process, °C.
is the total mass of the oxidized reaction substance.
is the temperature that can be reached by the oxidation reaction after cooling failure, and the maximum value of
is
MTSR [
23].
should also be corrected by the yield
, which is
, and
is the heat release rate of the reaction, W. The changes in
and
are shown in
Figure 10 and
Figure 11. The stage A
MTSRr was 192.33 °C and the stage B
MTSRr was 161.95 °C.
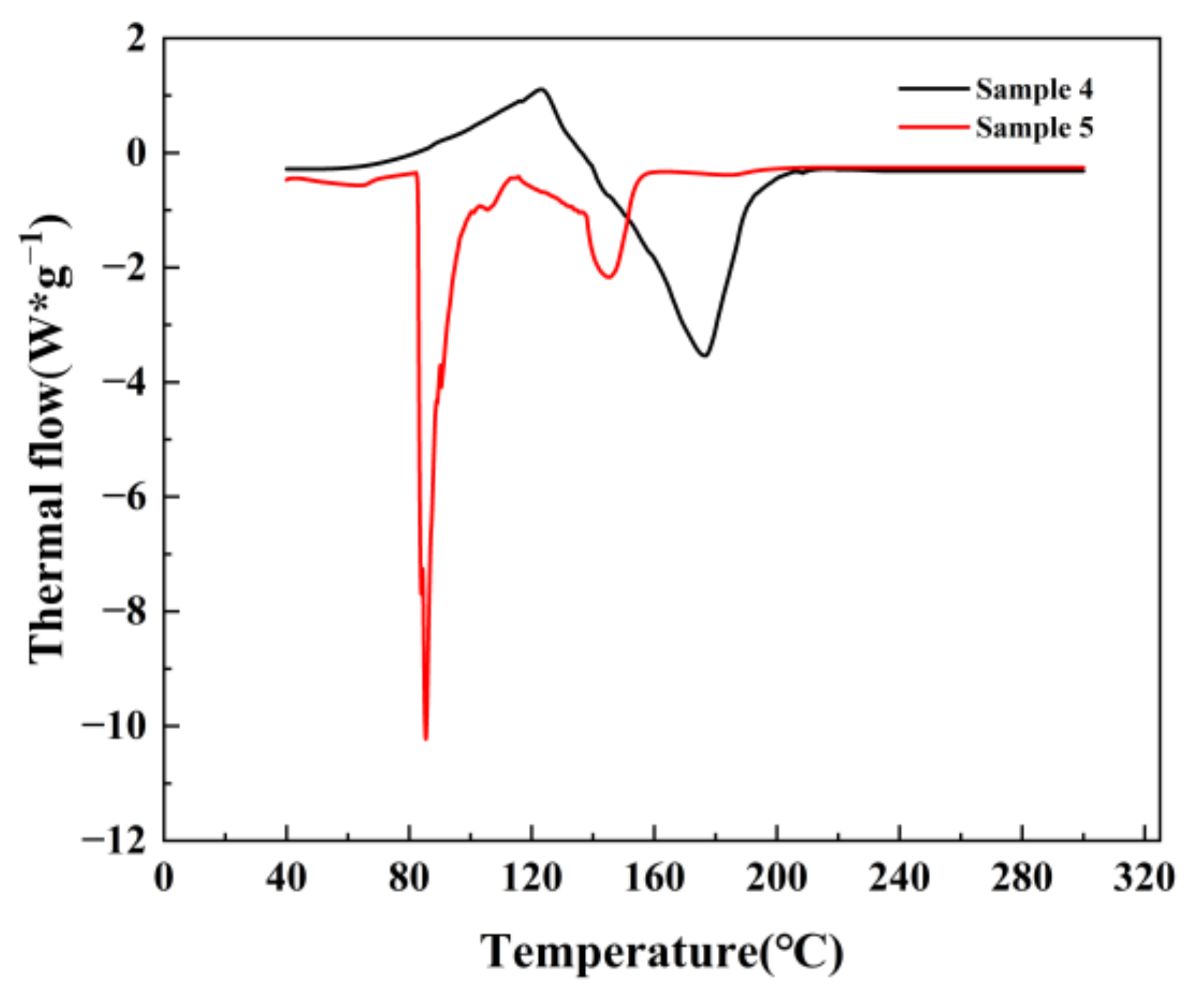
3.4. DSC Experimental Results and Analysis
DSC was used to investigate the effect of H
2SO
4 and EDTA conditions on the catalytic decomposition characteristics of 30 wt.% H
2O
2 and the thermal stability of the products from stages A and B. Sample 1 was a mixture of 30 wt.% H
2O
2 and tungsten Na
2WO
4·2H
2O. Sample 2 was a mixture of 30 wt.% H
2O
2, Na
2WO
4·2H
2O, and H
2SO
4. Sample 3 was a mixture of 30 wt.% H
2O
2, Na
2WO
4·2H
2O, H
2SO4, and EDTA. The proportion of each substance in the mixture followed the process parameters of experiment 7. Samples 4 and 5 were the products from stages A and B. The experimental results are shown in
Figure 12 and
Figure 13.
As indicated in
Table 4, the mixed system became more acidic with the addition of H
2SO
4, and slowed the decomposition rate of H
2O
2. The rate of heat generation and the heat of liberation could be decreased, although the initial decomposition temperature (
) was advanced. The autolytic reaction of H
2O
2 was effectively inhibited by the addition of the H
2O
2 stabilizer, EDTA.
was delayed and the heat released from decomposition was decreased. The product of stage A was exothermic due to the presence of unfinished H
2O
2 and intermediate products. However the product of stage B, was mostly adipic acid, and consequently there was no exothermic heat during the test.
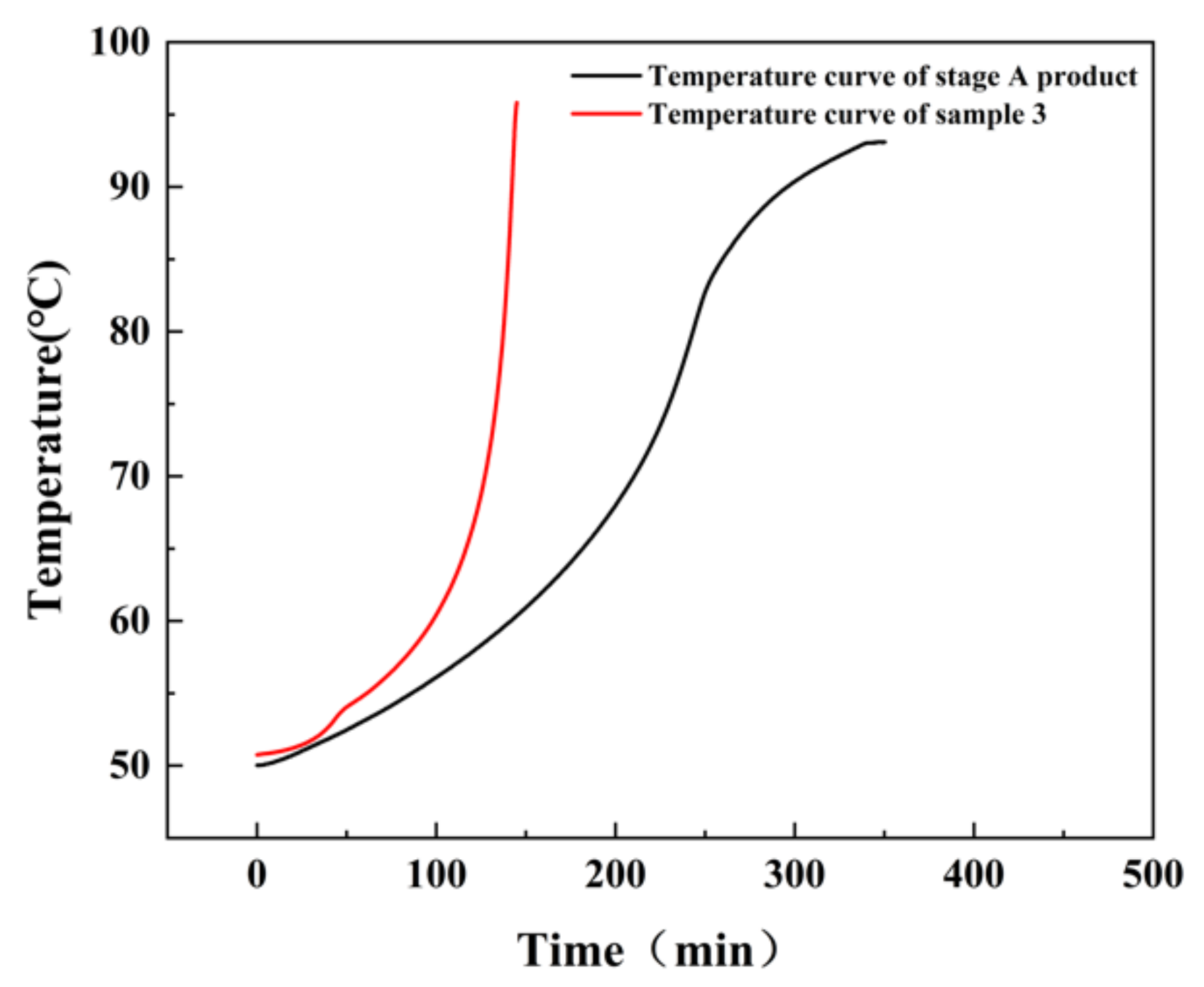
3.5. Adiabatic Tests Results and Analysis
In this work, the adiabatic tests of the stage A and B products and sample 3 were executed using Phi-TEC II, as depicted in
Figure 14. No exothermic heat was detected in the stage B products. The characteristic parameters of the adiabatic decomposition reaction of the stage A products and sample 3 are shown in
Table 5.
In order to assess the thermal hazard of the adipic acid green synthesis process,
and
were calculated for the stage A products to access the possibility and reaction risk of the target process.
is the time required to reach the maximum rate under adiabatic conditions [
24], calculated using Equation (5), and
is the temperature at which
equals 24 h [
25].
Here, Tf is the maximum temperature obtained by self-heating under adiabatic conditions, K; R is the gas constant, 8.314 J/(mol·K); E is the apparent activation energy, kJ/mol; A is pre-exponential, s−1; and n is the number of reaction stages.
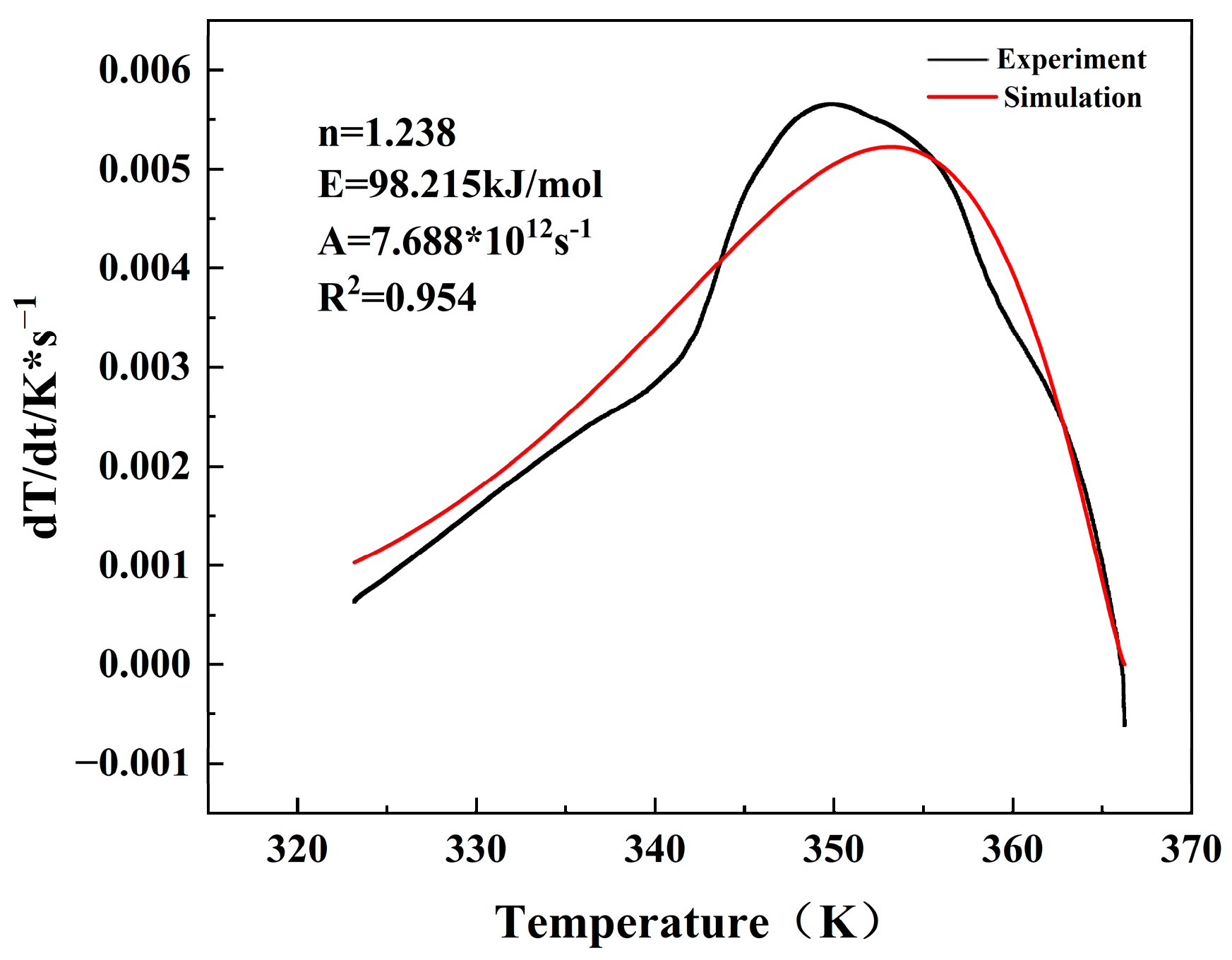
According to Equation (6), the thermodynamic parameters were obtained by non-linear fitting. The correlation coefficient (R
2) was 0.954 and the fit was essentially the same as the experimental data.
E was 98.215 kJ/mol,
A was 7.688 × 10
12 s
−1, and
n was 1.238, as depicted in
Figure 15. As indicated in
Figure 16, the curve of
vs. Temperature was fitted from the adiabatic kinetic data, and
was 34.8 min and
was 27.95 °C.
3.6. ITHI Method for Assessing the Thermal Hazards of Adipic Acid Synthesis Reactions
The ITHI method, an intrinsic control index method that considers the combination of substance hazard and reaction heat hazard, was chosen to provide a comprehensive and accurate assessment of the hazards of adipic acid synthesis [
12]. The two stages, A and B, of the adipic acid synthesis process were assessed, respectively.
As depicted in
Table 6,
Table 7 and
Table 8, the material coefficients (MF) of stages A and B were 1.25 and 1.188.
The probability of runaway (P) in stages A and B were 10 and 4, respectively, and the severity of runaway (S) is the sum of (IH,rx,IΔTad,rx)max and (IH,dec,IΔTad,dec)max. The accident consequence severity S in stage A was 5, and the post-accident severity S in stage B was 3.
The runaway consequence severity (RI) is the product of S and P. The ITHI value is the product of MF and RI. Thus, the ITHI of stages A and B were 62.5 and 13.42, respectively. The risk level of stage A was IV, since stage A was carried out under the reflux period. Consequently, the cooling of reflux should be increased to remove more of the heat generated from stage A and to more steadily control the temperature. The risk level of Stage B was I, which indicated the risk was low and no action should be taken.