Abstract
To improve the efficiency of chromium (Cr) phytoextraction by Leersia hexandra Swartz, the effects of mowing on Cr accumulation in L. hexandra were investigated using hydroponic experiments. Mowing heights (0, 5, and 10 cm), mowing interval (30, 60, 90 and 120 days), and mowing frequencies (1, 2, and 3 times) were optimized. Mowing at 10 cm above roots significantly increased shoot biomass of L. hexandra (32.9 g/pot). The 90 days mowing interval achieved the highest shoot biomass (62.8 g/pot). The shoot biomass with thrice mowing (67.0 g/pot) was higher than those with one and twice mowing, as well as no mowing (CK). The increases in biomass might ascribe to the changes in endogenous hormone balance by mowing. Proper mowing significantly increased contents of Gibberellin 3 (GA3), 6-Benzylaminopurine (6-BA), 6-Kinetin (6-KT), and trans-Zeatin-riboside (TZR) in leaves, and 3-Indolepropionic acid (IPA) in stems, but decreased Jasmonic acid (JA) in the leaves and stems, thereby enhancing the regeneration of plant. The enhancement of plant regeneration resulted in the increases of biomass and Cr accumulation. Compared to CK, the optimal mowing method (10 cm, 90 days, 3 times) increased shoot biomass and Cr accumulation by 91.4% and 36.0%, respectively. These findings suggested that proper mowing had application potential to promote efficiency of Cr phytoextraction by L. hexandra.
1. Introduction
Chromium (Cr) is widely used in the industry as a plating, pigment, and tanning agent, etc. [1,2]. However, the widespread use of Cr can cause Cr pollution, which is a major environmental and health hazard [3,4]. Therefore, chromium pollution remediation has become a hot research topic in recent years [5,6,7]. Phytoremediation, a green remediation technology, is more efficient and cheaper than traditional physicochemical remediation techniques and does not cause secondary pollution [8,9,10]. The hyperaccumulator is the core vehicle of phytoremediation technology and plays an important role in the remediation of heavy metals (HMs) pollution [11].
Leersia hexandra Swartz is the first chromium hyperaccumulator discovered in China [12]. The stems and leaves of L. hexandra have a high capacity for Cr uptake and tolerance, thus showing great potential for the phytoremediation of Cr contamination [13,14]. However, the phytoremediation efficiency of Cr by L. hexandra is still constrained based on different factors, such as the growth cycle and biomass. Therefore, improving phytoremediation efficiency is the key to promoting the industrial application of Cr pollution remediation by L. hexandra [15].
L. hexandra is a perennial grass plant with a strong regeneration ability and rapid growth rate, which shows that L. hexandra can be mowed multiple times and then regenerated [16]. Moreover, as a Cr hyperaccumulator, the Cr concentration in the stems and leaves of L. hexandra is higher than that of common plants. Mowing the shoots of L. hexandra not only removes a large amount of Cr, but also potentially promotes its own growth and improves the efficiency of Cr phytoextraction [17,18]. Mowing and harvesting can eliminate senescent tissues of plant, maintain the high growth vitality in leaves, stimulate the compensatory growth of plants, and increase the biomass (i.e., overcompensation growth) [19,20,21]. For example, mowing can increase the shoot biomass of lettuce (Lactuca sativa L.) [22]; the biomass of Pteris vittata increased over five consecutive harvests. Previous studies showed that three consecutive mowings did not affecting biomass production and shoot Cr concentration in L. hexandra [23]. With constant Cr concentration, higher biomass means a higher phytoremediation efficiency. Therefore, we hypothesize that mowing may promote the efficiency of Cr phytoextraction by L. hexandra. Compared with the current conventional phytoremediation enhancement measures (such as nutrient amendments, transgenic, endophytic bacteria, etc.), this method has the advantages of simple operation, no additional cost in cost, and no environmental risk.
In fact, proper mowing stimulated plant regeneration, thereby increasing the biomass. On the contrary, unproper mowing inhibited plant growth and decreased biomass [24,25,26]. To promote the performance of L. hexandra in Cr phytoextraction, the mowing method should be optimized.
Plants regeneration is highly regulated by hormones, including heteroauxin (IAA), indolebutyric acid (IBA), jasmonic acid (JA), and gibberellin (GA) [27,28,29]. Different phytohormones play important roles in diverse growth and developmental processes as well as various biotic and abiotic stress responses in plants [30,31]. For instance, GA can promote plant growth by stimulating both cell division and cell elongation, whereas JA can increase plant resistance to ecological stresses [32,33]. However, ABA can inhibit the plant growth. Therefore, it is important to find plant hormones that promote regeneration.
At present, there is a lack of research on the ways in which mowing promotes regeneration to improve phytoremediation by plants. There is only one instance in the literature that reported that mowing increased Pteris vittata frond biomass and thus improved the arsenic (As) phytoextraction effectiveness [34], but did not optimize the mowing method.
In the present study, we hypothesize that proper mowing may increase the shoot biomass and thus promote the efficiency of Cr phytoextraction by L. hexandra. The effects of mowing height, interval, and frequency on the biomass production and Cr accumulation in L. hexandra were evaluated. The aim is to establish the optimal mowing method for improvement of Cr phytoextraction by L. hexandra. The 18 phytohormones content in the freshly grown stems and leaves of L. hexandra after last mowing were measured, no mowing as the control. The aim is to explore the key phytohormones for the increase in the freshly grown biomass.
2. Materials and Methods
2.1. Plant Preculture and Treatment
Seedlings of L. hexandra were collected from a paddy field in Guilin, China (110°06′~110°41′ E, 24°18′~24°46′ N). The seedings were washed with deionized water three times. Approximately 20~30 healthy plants of L. hexandra with uniform growth were selected for one pot, and the fresh weight difference between pots was controlled at 4 g. Then, pots of L. hexandra in a round plastic drum made of polyethylene with a specification of 210 mm × 190 mm. Each pot of L. hexandra seedlings was precultured for seven days in a modified Hoagland solution, the same formula as [35]. All plants were grown under controlled environmental conditions with a 14 h (6:00~20:00) photoperiod, a 30 °C/25 °C day/night temperature regime, 70~75% relative humidity, and a light source of white light plus violet light.
2.2. Design of Experiments
2.2.1. Mowing Height Experiment
The 40 μmol/L (representing low chromium pollution environment) and 80 umol/L potassium dichromate solution (K2Cr2O7) were mixed in Hoagland solution to Cr treatmennt on L.hexandra (Figure 1). The nutrient solution with Cr treatments was renewed every three days. After 30 days of Cr treatment, the shoots of the plant were mowed at 0 cm (H0), 5 cm (H5), and 10 cm (H10) above the culture solution with scissors, with no mowing as control. This mowing method is modified from the one described in [23,36]. The root was left intact to allow for the re-growth of shoots. After the re-growth of shoots over the following 30 days, the shoots of L. hexandra were mowed as described above. All mowed shoots were used for biomass and Cr concentration measurement. Each treatment was performed in triplicates and the averages were recorded; the same is true below.
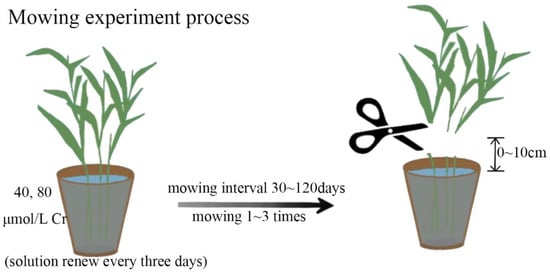
Figure 1.
Experimental design diagram, including mowing height, interval, and frequency experiment.
2.2.2. Mowing Interval Experiment
The Cr treatment of L. hexandra was the same as the mowing height experiment. The shoots of the plant were mowed at 10 cm (optimal mowing height) after 30 (30 d), 60 (60 d), 90 (90 d) and 120 days (120 d) of Cr treatment, with no mowing (CK) as control. The roots are left intact for regrowth. After the re-growth of shoots for following 150, 120, 90 and 60 days, the shoots of L. hexandra were mowed on the 180th day. All mowed shoots were used for biomass and Cr concentration measurement.
2.2.3. Mowing Frequency Experiment
To better evaluate the effect of mowing on Cr remediation efficiency of L. hexandra in different Cr-contaminated environments, the Cr treatment was 60 μmol/L K2Cr2O7 (representing medium Cr-contaminated environment) for mowing frequency experiment. Mowing once (1): the shoots of plant were mowed once at 10 cm (optimal mowing height) on 90th day (optimal mowing interval), and then were mowed at 10 cm after the re-growth of shoots for 90 days. Mowing twice (2): the shoots of plant were mowed at 10 cm on 90th day and 180th day, and then were mowed at 10 cm on 270th day. Mowing thrice (3): the shoots of plant were mowed thrice at 10 cm with 90 days interval, and then were mowed on the 360th day. No mowing was the control. All mowed shoots were used for biomass and Cr concentration measurement. The 18 phytohormones content in the freshly grown stems and leaves of L. hexandra after the last mowing were measured.
2.3. Measurement of Biomass and Cr Concentration
The shoots were washed three times with 10 mM EDTA in an ultrasonic cleaner to remove the adsorbed Cr and were rinsed three times in deionized water. The washed shoots were oven-dried at 70 °C for two days to determine the dry weight (DW). Each dried sample (about 0.2 g) was digested with concentrated nitric acid and perchloric acid (5:3, v/v) in a block heater [37]. The Cr concentrations in digestates were quantified using inductively coupled plasma mass spectrometry (ICP-MS, NexION 2000b, Perkin-Elmer, Waltham, MA, USA). Quality control for Cr analysis was performed by a certified reference material (GBW07603). The recovery of Cr in all cases was 90~108%. The Cr accumulation in shoots of L. hexandra equals shoots in biomass multiplied by Cr concentration in shoots.
2.4. Measurement of Phytohormone Concentration
The solid phase extraction (SPE), and rapid and sensitive liquid chromatography tandem mass spectrometry (LC-MS/MS) were used to analyze and determine 18 phytohormones of L. hexandra [38]. Samples were weighed, ground, and put into a 10 mL centrifuge tube (with plug); 5 mL extracting solution (methanol: water: formic acid, 15:4:1, with 0.5% BHT) was added into the tube. After the mixture had been mixed with the Vortex oscillator and ultrasonic crushed for 30 min, it was placed under −40 °C for 60 min. After that, the mixture was centrifuged (4 °C, 12,000 rpm, 10 min), and the supernatant was moved into new clean centrifuge tubes. Solid phase extraction: (1) activation: wash the extraction column with 3 mL of water and 3 mL of methanol, sequentially. (2) Adsorption: pump the supernatant through the column (the flow rate should be less than 1 mL/min). (3) Washing: wash the column with 3 mL of water and an aqueous solution with 10% methanol. (4) Elution: pump 2 mL of methanol through the column and collect the liquid. The eluent was dried with an evaporation concentrator, then 200 μL of 80% methanol was added, and the solution was whirled and centrifuged (4 °C, 12,000 rpm, 10 min) once again. The supernatant could now be analyzed with LC-MS/MS.
2.5. Statistical Analyses
All data were summarized, and mean and variance were calculated with Excel 2019. All bar charts and box plots were plotted with Origin 2021. All statistical analyses were performed with SPSS 23.0 software. The bar charts data were tested for one-way analysis of variance (ANOVA). The box plots data were tested for independent-samples t-test. The data were tested for normality (Kolmogorov–Smirnov test) and variance homogeneity (Levene’s test) before ANOVA and independent-samples t-test. If the data did not meet normal distribution or variance homogeneity, a logarithmic transformation was carried out. When ANOVA showed significant differences (p < 0.05), a posteriori comparison of means was made using Duncan’s test at the 95% confidence level. When independent-samples t-test showed p < 0.05, there is a significant difference between the two groups’ data.
3. Results
3.1. Effect of Mowing Heights on the Cr Phytoremediation
The effects of different mowing heights on the shoot biomass of L. hexandra are shown in Figure 2a. The biomass of the 10 cm (H10) mowing height was higher than no mowing (CK) at low Cr treatment (40 μmol/L), increasing by 28.2% (p < 0.05). However, there was no significant difference between the 0 cm (H0), 5 cm (H5) mowing height, and CK at low Cr treatment. The shoot biomass with H5 and H10 was significantly higher than CK at high Cr treatment (80 μmol/L), increasing by 20.3 % and 28.9 % (p < 0.05), while the difference in biomass between H0 and CK were not significant.
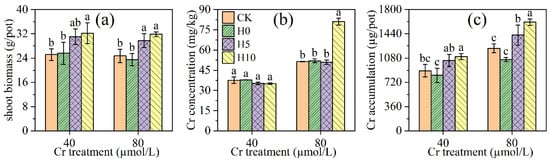
Figure 2.
Effects of different mowing heights on biomass (a), Cr concentration (b), and Cr accumulation (c) in shoot of Leersia hexandra Swartz. CK represents no mowing; H0, H5, and H10 represent mowing at 0 cm (H0), 5 cm (H5), and 10 cm (H10) above culture solution, respectively. One-way ANOVA was performed for each parameter, and the different lowercase letters within the same Cr treatment indicate a significant difference between mowing heights according to Duncan’s test (p< 0.05, n = 3). The same is true in Figure 3 and Figure 4.
Different mowing heights did not influence the Cr concentration in shoots of L. hexandra at low Cr treatment, but the H10 mowing height significantly increased shoot Cr concentration at high Cr treatment (p < 0.05, Figure 2b). The effects of different mowing heights on the Cr accumulation of L. hexandra followed the same trend as the effect on biomass (Figure 2c). The H10 significantly increased Cr accumulation in shoots at low Cr treatment, increasing by 23.4% (p < 0.05). Under high Cr treatment, the Cr accumulation of L. hexandra in H10 and H5 was higher than in the control, with an increase of 31.8% and 16.1%. However, there was no significant difference between H0 and no mowing at all Cr treatments.
3.2. Effect of Mowing Interval on Cr Phytoremediation
The effects of different mowing intervals on the shoot biomass of L. hexandra are shown in Figure 3a. The shoot biomass with 90 days (90 d) and 120 days (120 d) mowing interval were significantly higher than no mowing (CK) at low Cr treatment (40 μmol/L), increasing by 27.1% and 25.6% (p < 0.05), while there was no significant difference in shoot biomass between the mowing interval of 30 days (30 d), 60 days (60 d), and CK. At high Cr treatment (80 μmol/L), the shoot biomass of all mowing intervals was higher than that of CK.
The effect of mowing time on Cr concentration in shoots of L. hexandra is shown in Figure 3b. The highest Cr concentration (120.5 mg/kg) of 90 d was found at low Cr treatment, then followed by 60 d (104.6 mg/kg). However, Cr concentration of 30 d and 120 d was not significantly different from the control at low Cr treatment. At high Cr treatment, there was not a significant difference between all mowing interval treatment groups and CK. This indicates that mowing interval does not affect the Cr concentration in shoots of L. hexandra in a high Cr-contaminated environment.
The effects of mowing interval on Cr accumulation in shoots of L. hexandra are shown in Figure 3c. The effect of mowing interval on the accumulation of L. hexandra is different from the trend of the effect on biomass. At low Cd treatment, the Cr accumulation of all mowing interval treatments were higher than that of CK, and 90 d was the greatest, with a 57.1% increase over the control (p < 0.05). The Cr accumulation of 60 d and 90 d was higher than CK at high Cr treatment, increasing by 24.3% and 40.2% (p < 0.05), respectively. However, there was no significant difference in Cr accumulation between 30 d, 120 d, and CK at high Cd treatment.
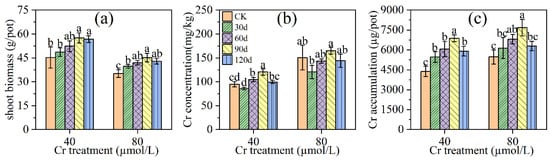
Figure 3.
Effects of different mowing time on biomass (a), Cr concentration (b), and Cr accumulation (c) in shoot of Leersia hexandra Swartz. CK represents no mowing. Finally, 30 d, 60 d, 90 d, 120 d represent a mowing on the 30th, 60th, 90th and 120th day.
3.3. Effect of Mowing Frequency on Cr Phytoremediation
The effects of different mowing frequencies on shoot biomass of L. hexandra are shown in Figure 4a. The shoot biomass of mowing thrice was higher than no mowing (p < 0.05), followed by mowing twice and once increasing by 91.4%, 62.1%, and 48.1%, respectively.
Different mowing frequencies did not influence the Cr concentration in shoots of L. hexandra in Figure 4b.
The effects of different mowing frequencies on the Cr accumulation in shoots of L. hexandra are shown in Figure 4c. The Cr accumulation in L. hexandra’s shoots at mowing once was not significantly different from the control. The Cr accumulation in shoots at mowing twice and mowing thrice were higher than the control, increasing by 28.3% and 36.0% (p < 0.05), respectively.
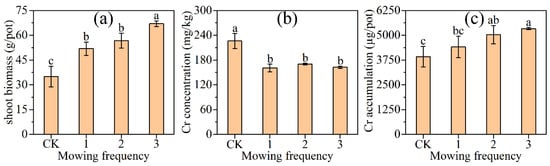
Figure 4.
Effects of different mowing frequency on biomass (a), Cr concentration (b), and Cr accumulation (c) in shoot of Leersia hexandra Swartz. CK represents no mowing; 1, 2, and 3 represent mowed once, twice, and thrice. The different letters indicate significant differences among the values according to the LSD test (p < 0.05, n = 3).
3.4. Changes in Phytohormone Conntent
Based on the MRM method [38], a phytohormone-targeted metabolomes analysis was carried out to compare the 18 phytohormones concentration changes in the freshly grown leaves and stems of L. hexandra after mowing (M) and in the control (CK), as shown in Figure 5 and Figure 6.
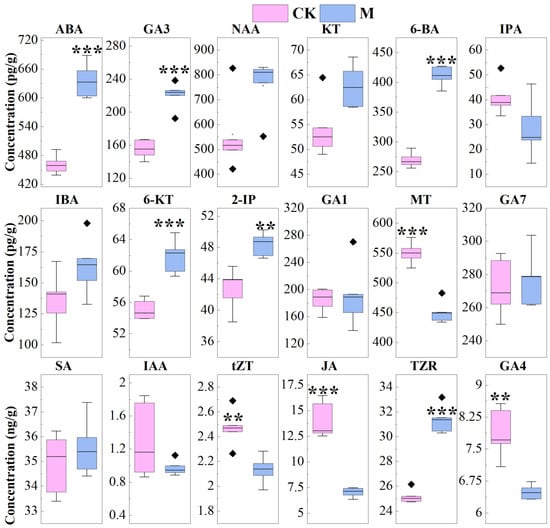
Figure 5.
The contents of 18 phytohormones in leaves of Leersia hexandra Swartz. M represents proper mowing treatment, and CK represents the control (no mowing). Boxes include the 25th to 75th percentiles, horizontal lines within boxes represent median values, horizontal lines outside of boxes represent maximum and minimum values, and black ◇ represent outliers.** p < 0.01, and *** p < 0.001 represents significant differences between two treatments, respectively; the absence of labeling represents no significant difference between two treatments.
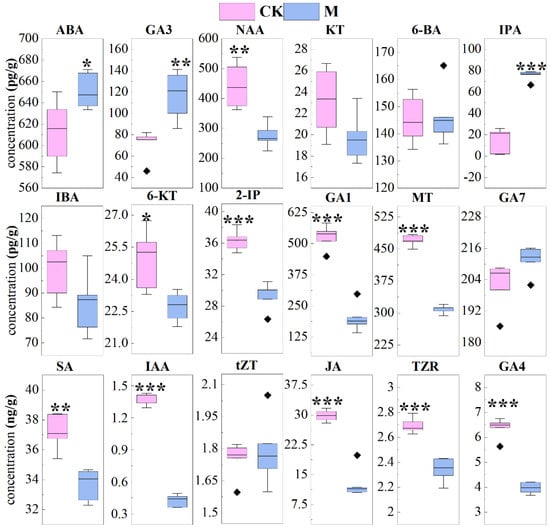
Figure 6.
The contents of 18 phytohormones in stems of Leersia hexandra Swartz. * p < 0.05, ** p < 0.01, and *** p < 0.001 represents significant differences between two treatments, respectively; the absence of labeling represents no significant difference between two treatments.
As shown in Figure 5, the content of ABA, GA3, 6-BA, 6-KT, 2-IP and TZR in M were significantly higher than those in CK. The content of JA, GA4, tZT and MT in M were significantly lower than those in CK. However, the content of NAA, KT, IPA, IBA, GA1, GA7, SA and IAA in M were not significantly different from those in CK.
As shown in Figure 6, only three phytohormones were significantly higher in M than those in CK: ABA, GA3, and IPA. The content of NAA, SA, 6-KT, 2-IP, GA1, GA4, IAA, JA, TZR and MT were significantly lower than those in CK. However, the content of GA7, KT, 6-BA, IBA and tZT in M was not significantly different from those in CK.
4. Discussion
Improving Cr remediation efficiency is the key to promoting the practical application of L. hexandra remediation of Cr-contaminated environments. In this study, the effects of mowing height, interval, and frequency on plant regeneration and phytoremediation efficiency were investigated. The shoots biomass responds directly to the ability of L. hexandra to regenerate. In this study, the results showed that mowing method can affect the biomass accumulation of L. hexandra; the mowing height of 10 cm, the mowing interval of 90 to 120 days, and the thrice mowing frequency were proper under all Cr treatments (Figure 2a, Figure 3a and Figure 4a). The reason is that mowing plays an important role in enhancing plant regeneration and then increasing biomass [22]. Because the average height of L. hexandra cultured in this experiment is less than 15 cm and the experimental period is longer, whether the mowing method range of over 10 cm, over 120 days, and over 3 mowing times has a greater biomass accumulation needs to be further investigated.
The increases in biomass might ascribe to the changes in endogenous hormone balance. The results showed that the contents of ABA, GA3, 6-BA, 2-IP, 6-KT, and TZR in leaves of L. hexandra under the optimal mowing were significantly higher than no mowing, and JA, GA4, tZT and MT were significantly decreased (Figure 5). Further, 6-BA content can improve plant cell growth, inhibiting the degradation of plant chlorophyll and delaying leaf senescence [39]. Similarly, IAA, GA3, and GA4 are also growth-promoting hormones and contribute to the regeneration of plants [40]; 6-KT and TZR can promote cell division and prevent leaf senescence [41]. ABA is considered to be a plant hormone that promotes abscission, inhibits growth, and promotes dormancy [42]. However, it has also been shown that ABA is a key endogenous messenger for plants in response to abiotic stresses [43]. Therefore, the increase in ABA, GA3, 6-BA, 6-KT and TZR content may enhance the leaves regeneration of L. hexandra after mowing. JA can promote leaf senescence, inhibit photosynthesis, and suppress plant growth [44]. The decrease in JA content is beneficial for the regeneration of L. hexandra. MT can prevent chlorophyll degradation and improve plant resistance [45]. The decreased content in MT and GA4 inhibits plant growth. NAA, 2-IP, and tZT can promote the growth of plant roots, not stems and leaves [46,47,48]. Therefore, ABA, GA3, 6-BA, 6-KT, TZR and JA may be the main hormone in leaves regeneration of L. hexandra. The phytohormone changes in the stem of L. hexandra differed significantly from those in the leaves (Figure 6). Compared with no mowing, the ABA, GA3, and IPA content in stems under optimal mowing significantly increased, but the NAA, SA, 6-KT, 2-IP, GA1, GA4, IAA, JA, TZR and MT contents decreased. IPA and SA can enhance plant resistance to adverse environmental conditions [49]. The decrease in NAA, 6-KT, 2-IP, GA1, GA4, IAA, TZR and MT may be detrimental to the regeneration of L. hexandra. However, JA is a hormone that inhibits plant growth, and the decrease in JA may have a positive effect on the regeneration of L. hexandra. Therefore, ABA, GA3, IPA and JA may be the main hormone in stems regeneration of L. hexandra. Plant hormones do not act alone, but in conjunction or in opposition to each other. Thus, the final growth conditions represent a net effect of hormone balance [50,51]. Therefore, proper mowing can promote the regeneration of L. hexandra by altering the hormone content.
In addition to enhanced plant growth, mowing also influenced plant Cr uptake by L. hexandra. In this study, the Cr concentration of H10 was the highest in the environment polluted by high Cr, which indicated that a 10 cm stubble height may provide sufficient carbon and nitrogen nutrition for plant regeneration to uptake more Cr [52,53]. The Cr concentration of 90 d was the highest in the environment polluted by low Cr. Too long or too short mowing intervals can inhibit the large absorption of water and inorganic salts, because Cr cannot compete with K, Mg, and Ca in this process, inhibiting the significant uptake of Cr [54]. The thrice mowing did not influence the Cr uptake by L. hexandra, which is consistent with previous studies [23].
The phytoremediation efficiency is determined by HM concentration and biomass; therefore, both parameters should be considered simultaneously [55]. The Cr accumulation in shoots of L. hexandra equals shoots biomass multiplied by Cr concentration in shoots. In the present study, the Cr accumulation of 10 cm mowing height (H10), 90 days (90 d) mowing interval, and thrice (3) mowing frequency was significantly higher than no mowing, which increased by 31.8%, 57.1%, and 36.0%, respectively. The biomass and Cr concentration of H10 and 90 d are the maximum, indicating that the combined effect of them (H10, 90 d) on L. hexandra biomass and Cr uptake can significantly remove Cr from all Cr-polluted environments. The mowing frequency did not influence the Cr uptake. Thus, higher biomass means higher phytoremediation efficiency. In general, using the mowing method of a 10 cm mowing height, 90 days mowing interval, and thrice mowing frequency to improve the biomass and Cr concentration of L. hexandra and enhance its phytoremediation efficiency was proven to be a proper method in this study.
5. Conclusions
Mowing height, interval, and frequency all had significant effects on plant regeneration and Cr accumulation. Proper mowing can alter the phytohormone content and increase biomass in shoots, thereby promoting the regeneration of shoots in L. hexandra. ABA, GA3, 6-BA, 6-KT, TZR, IPA and JA were the main hormone in stems regeneration of L. hexandra. Proper mowing height and interval can improve the Cr uptake of L. hexandra. The mowing frequency did not influence the Cr uptake. In general, using the proper mowing method to improve Cr phytoremediation efficiency of L. hexandra was feasible.
Author Contributions
J.L. and X.J.: conceived the study. S.M. and Z.D.: collected data and prepared the data for analysis. X.J. and S.M.: performed statistical analyses and literature review. S.M. and X.J.: wrote the main manuscript text. D.D., S.C. and G.Y.: improved the draft. All authors contributed to the interpretation of results and critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was sponsored by the Natural Science Foundation of China (52230006), the Key projects of Guangxi Natural Science Foundation (2020GXNSFDA297018), the Special Foundation for “Ten, Hundred and Thousand Talents Project” of Guangxi Province of China (2016207), the Special Funds of Guangxi Distinguished Experts, and the Program for High Level Innovation Team and Outstanding Scholar of Universities in Guangxi (GuiCaiJiaoHan [2018]319).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pushkar, B.; Sevak, P.; Parab, S.; Nilkanth, N. Chromium pollution and its bioremediation mechanisms in bacteria: A review. J. Environ. Manag. 2021, 287, 112279. [Google Scholar] [CrossRef] [PubMed]
- Shanker, A.K. Chromium: Environmental Pollution, Health Effects and Mode of Action. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2019; pp. 624–633. [Google Scholar]
- Sharma, N.; Sodhi, K.K.; Kumar, M.; Singh, D.K. Heavy metal pollution: Insights into chromium eco-toxicity and recent advancement in its remediation. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100388. [Google Scholar] [CrossRef]
- Adhikari, S.; Marcelo-Silva, J.; Beukes, J.P.; van Zyl, P.G.; Coetsee, Y.; Boneschans, R.B.; Siebert, S.J. Contamination of useful plant leaves with chromium and other potentially toxic elements and associated health risks in a polluted mining-smelting region of South Africa. Environ. Adv. 2022, 9, 100301. [Google Scholar] [CrossRef]
- Bao, Z.J.; Feng, H.Y.; Tu, W.Y.; Li, L.J.; Li, Q. Method and mechanism of chromium removal from soil: A systematic review. Environ. Sci. Pollut. Res. 2022, 29, 35501–35517. [Google Scholar] [CrossRef]
- Liu, L.W.; Li, W.; Song, W.P.; Guo, M.X. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Bani Mfarrej, M.F.; Rizwan, M.; Hussain, A.; Shahid, M.J.; Wang, X.; Nafees, M.; Waseem, M.; Alharby, H.F. Microbe-citric acid assisted phytoremediation of chromium by castor bean (Ricinus communis L.). Chemosphere 2022, 296, 134065. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation technology: Hyper-accumulation metals in plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Malaviya, P.; Singh, A.; Anderson, T.A. Aquatic phytoremediation strategies for chromium removal. Rev. Environ. Sci. Bio/Technol. 2020, 19, 897–944. [Google Scholar] [CrossRef]
- Li, J.T.; Gurajala, H.K.; Wu, L.H.; van der Ent, A.; Qiu, R.L.; Baker, A.J.M.; Tang, Y.T.; Yang, X.E.; Shu, W.S. Hyperaccumulator Plants from China: A Synthesis of the Current State of Knowledge. Environ. Sci. Technol. 2018, 52, 11980–11994. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, J.; Huang, H.T.; Chen, J.; Zhu, Y.N.; Wang, D.Q. Chromium accumulation by the hyperaccumulator plant Leersia hexandra Swartz. Chemosphere 2007, 67, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.H.; You, S.H.; Wu, Q.X.; Chen, S.M.; Zhou, K.N. Cr(VI) removal and detoxification in constructed wetlands planted with Leersia hexandra Swartz. Ecol. Eng. 2014, 71, 36–40. [Google Scholar] [CrossRef]
- Wang, C.; Tan, H.; Li, H.; Xie, Y.; Liu, H.; Xu, F.; Xu, H. Mechanism study of Chromium influenced soil remediated by an uptake-detoxification system using hyperaccumulator, resistant microbe consortium, and nano iron complex. Environ. Pollut. 2020, 257, 113558. [Google Scholar] [CrossRef]
- Yang, W.; Dai, H.P.; Skuza, L.; Wei, S.H. The front-heavy and back-light nitrogen application mode to increase stem and leaf biomass significantly improved cadmium accumulation in Solanum nigrum L. J. Hazard. Mater. 2020, 393, 122482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Wang, D.; Zhu, Y.; Hu, C.; Sun, J. Bioaccumulation and Chemical Form of Chromium in Leersia hexandra Swartz. Bull. Environ. Contam. Toxicol. 2009, 82, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Dai, H.; Skuza, L.; Wei, S. Cadmium removal potential of hyperaccumulator Solanum nigrum L. under two planting modes in three years continuous phytoremediation. Environ. Pollut. 2022, 307, 119493. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, Q.; Koval, P.V. Flowering stage characteristics of cadmium hyperaccumulator Solanum nigrum L. and their significance to phytoremediation. Sci. Total Environ. 2006, 369, 441–446. [Google Scholar] [CrossRef]
- Lebon, A.; Mailleret, L.; Dumont, Y.; Grognard, F. Direct and apparent compensation in plant–herbivore interactions. Ecol. Model. 2014, 290, 192–203. [Google Scholar] [CrossRef]
- Gunnar, A.; James, D.M.S.; Vegard, M.; Jan, M.; Atle, M. Experimental Effects of Herbivore Density on Aboveground Plant Biomass in an Alpine Grassland Ecosystem. Arct. Antarct. Alp. Res. 2014, 46, 535–541. [Google Scholar]
- Belsky, A.J.; Carson, W.P.; Jensen, C.L.; Fox, G.A. Overcompensation by plants: Herbivore optimization or red herring? Evol. Ecol. 1993, 7, 109–121. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Song, J.; Liu, X.; Jiao, X. Effects of different mowing treatments and stubble heights on the compensatory growth and quality of lettuce (Lactuca sativa L.). J. Hortic. Sci. Biotechnol. 2018, 93, 537–544. [Google Scholar] [CrossRef]
- Liu, J.; Duan, C.; Zhang, X.; Zhu, Y.; Lu, X. Potential of Leersia hexandra Swartz for phytoextraction of Cr from soil. J. Hazard. Mater. 2011, 188, 85–91. [Google Scholar] [CrossRef]
- Achichi, I.; Slimani, A.; Ghamri, A.N.; Semmar, M.F. Effect of mowing frequencies on morphology and biomass production of natural populations of Sulla coronaria (L.) in the mountainous region of northeast Algeria. Fourrages 2021, 248, 57–62. [Google Scholar]
- Zhao, C.; Zhong, R.; Zhou, D.; Zheng, C. Effects of mowing time and interval on dry matter yield and chemical composition of Leymus chinensis. Soils Crops 2019, 8, 212–219. [Google Scholar]
- Bunnell, B.T.; Mccarty, L.B.; Bridges, W.C. ‘TifEagle’ Bermudagrass Response to Growth Factors and Mowing Height when Grown at Various Hours of Sunlight. Crop Sci. 2005, 45, 575–581. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, M.; Zhang, S.; Wang, Z.; Meng, M.; Sun, F.; Zhang, C.; Xi, Y. MicroRNA and regulation of auxin and cytokinin signalling during post-mowing regeneration of winter wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2020, 155, 769–779. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Shang, G.-D.; Wang, F.-X.; Gao, J.; Wan, M.-C.; Xu, Z.-G.; Wang, J.-W. Dynamic chromatin state profiling reveals regulatory roles of auxin and cytokinin in shoot regeneration. Dev. Cell 2022, 57, 526–542.e527. [Google Scholar] [CrossRef]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A Jasmonate Signaling Network Activates Root Stem Cells and Promotes Regeneration. Cell 2019, 177, 942–956.e914. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Hayashi, K.-i.; Arai, K.; Aoi, Y.; Tanaka, Y.; Hira, H.; Guo, R.; Hu, Y.; Ge, C.; Zhao, Y.; Kasahara, H.; et al. The main oxidative inactivation pathway of the plant hormone auxin. Nat. Commun. 2021, 12, 6752. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, J.; Yu, G.; Lei, L.; Jiang, X. Moderate Mn accumulation enhances growth and alters leaf hormone contents in the hyperaccumulator Celosia argentea Linn. Environ. Exp. Bot. 2021, 191, 104603. [Google Scholar] [CrossRef]
- Nováková, M.; Šašek, V.; Dobrev, P.I.; Valentová, O.; Burketová, L. Plant hormones in defense response of Brassica napus to Sclerotinia sclerotiorum–Reassessing the role of salicylic acid in the interaction with a necrotroph. Plant Physiol. Biochem. 2014, 80, 308–317. [Google Scholar] [CrossRef]
- Lessl, J.T.; Ma, L.Q. Sparingly-Soluble Phosphate Rock Induced Significant Plant Growth and Arsenic Uptake by Pteris vittata from Three Contaminated Soils. Environ. Sci. Technol. 2013, 47, 5311–5318. [Google Scholar] [CrossRef]
- Liu, J.; Yu, G.; Jiang, P.P.; Zhang, X.F.; Meng, D.J.; Chen, Z.; Baker, A.J.M.; Qiu, R.L. Interaction of Mn and Cd during their uptake inCelosia argenteadiffers between hydroponic and soil systems. Plant Soil 2020, 450, 323–336. [Google Scholar] [CrossRef]
- Yang, Z.; Minggagud, H.; Baoyin, T.; Li, F.Y. Plant production decreases whereas nutrients concentration increases in response to the decrease of mowing stubble height. J. Environ. Manag. 2020, 253, 109745. [Google Scholar] [CrossRef] [PubMed]
- Skrzydlewska, E.; Balcerzak, M.; Vanhaecke, F. Determination of chromium, cadmium and lead in food-packaging materials by axial inductively coupled plasma time-of-flight mass spectrometry. Anal. Chim. Acta 2003, 479, 191–202. [Google Scholar] [CrossRef]
- Sibel, Y.; Şükran, O.; Özge, K.; Nur, Ö.A. Determination of Major Phytohormones in Fourteen Different Seaweeds Utilizing SPE-LC-MS/MS. J. Chromatogr. Sci. 2019, 58, 98–108. [Google Scholar]
- Fang, S.; Hu, W.; Wang, S.; Chen, B.; Zhou, Z. Exogenous application of 6-BA and GA3 collaboratively improves cottonseed yield and seed quality via altering production of carbohydrates in the embryo. Arch. Agron. Soil Sci. 2021, 67, 329–341. [Google Scholar] [CrossRef]
- Singh, T.; Sharma, M.K.; Tyagi, J.P.; Singh, S. Effect of gibberellic acid (GA3) on yield, floral and morphological traits in rice (Oryza sativa). Indian J. Agric. Sci. 2011, 79, 831–834. [Google Scholar]
- Ahmed, N.; Tetlow, I.J.; Nawaz, S.; Iqbal, A.; Mubin, M.; Rehman, M.S.N.U.; Butt, A.; Lightfoot, D.A.; Maekawa, M. Effect of high temperature on grain filling period, yield, amylose content and activity of starch biosynthesis enzymes in endosperm of basmati rice. J. Sci. Food Agric. 2014, 95, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.E.; LeNoble, M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002, 53, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef]
- Dathe, W.; Preiss, A.; Schade, W.; Sembdner, G.; Schreiber, K. Endogenous plant hormones of the broad bean, Vicia faba L. (-)-jasmonic acid, a plant growth inhibitor in pericarp. Planta 1981, 153, 530–535. [Google Scholar] [CrossRef]
- Janave, M.T.; Sharma, A. Inhibition of chlorophyll degradation in stay-green langra mango (Mangifera indica L.) fruits. Crop Sci. 2006, 45, 575–581. [Google Scholar]
- Rajan, S.S. 1-Naphthaleneacetic acid. Acta Crystallogr. 2010, 34, 998–1000. [Google Scholar] [CrossRef]
- Jaakola, L.; Tolvanen, A.; Laine, K.; Hohtola, A. Effect of N6-isopentenyladenine concentration on growth initiation in vitro and rooting of bilberry and lingonberry microshoots. Plant Cell Tissue Organ Cult. 2001, 66, 73–77. [Google Scholar] [CrossRef]
- Cooper, J.B. Morphogenetic Rescue of Rhizobium meliloti Nodulation Mutants by trans-Zeatin Secretion. Plant Cell 1994, 6, 215–225. [Google Scholar] [CrossRef]
- Dogo, M.; Toyoda, H.; Matsuda, K.; Bingo, M.; Ouchi, S. Control of Bacterial Wilt of Tomato in Hydroponic Culture by 3-Indolepropionic Acid and Its Detoxification in Tomato Plants. Jpn. J. Phytopathol. 2009, 63, 406–408. [Google Scholar] [CrossRef]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- Davies, P.J. The Plant Hormones: Their Nature, Occurrence, and Functions. In Plant Hormones: Biosynthesis, Signal Transduction, Action! Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 1–15. [Google Scholar]
- Buckeridge, M.S. Chapter 3-The diversity of plant carbohydrate hydrolysis in nature and technology. In Polysaccharide-Degrading Biocatalysts; Goldbeck, R., Poletto, P., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 55–74. [Google Scholar]
- Wang, Y.J.; Wang, K.J.; Dong, S.T.; Hu, C.H.; Zhang, J.W. Effects of Stubble Height and Clipping Stage on Regrowth Performances of Zea mexicana. Sci. Agric. Sin. 2005, 38, 1555–1561. [Google Scholar]
- Najeeb, U.; Jilani, G.; Ali, S.; Sarwar, M.; Xu, L.; Zhou, W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard. Mater. 2011, 186, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Saifullah; Meers, E.; Qadir, M.; Caritat, P.D.; Tack, F.; Laing, G.D.; Zia, M.H. EDTA-assisted Pb phytoextraction. Chemosphere 2009, 74, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).