Prospect Research on the Diversity of Extracellular Mineralization Process Induced by Mineralizing Microorganisms and Its Use as a Treatment for Soil Pollutants
Abstract
1. Introduction
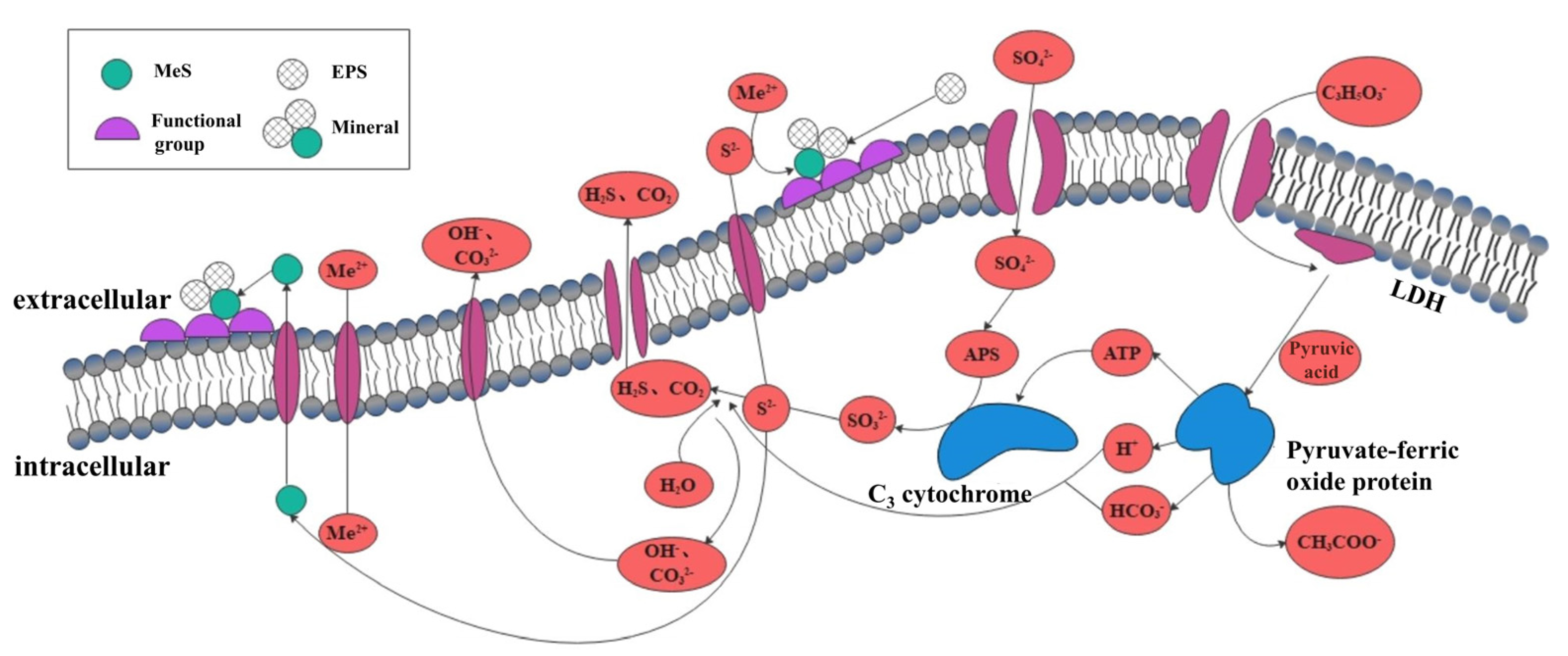
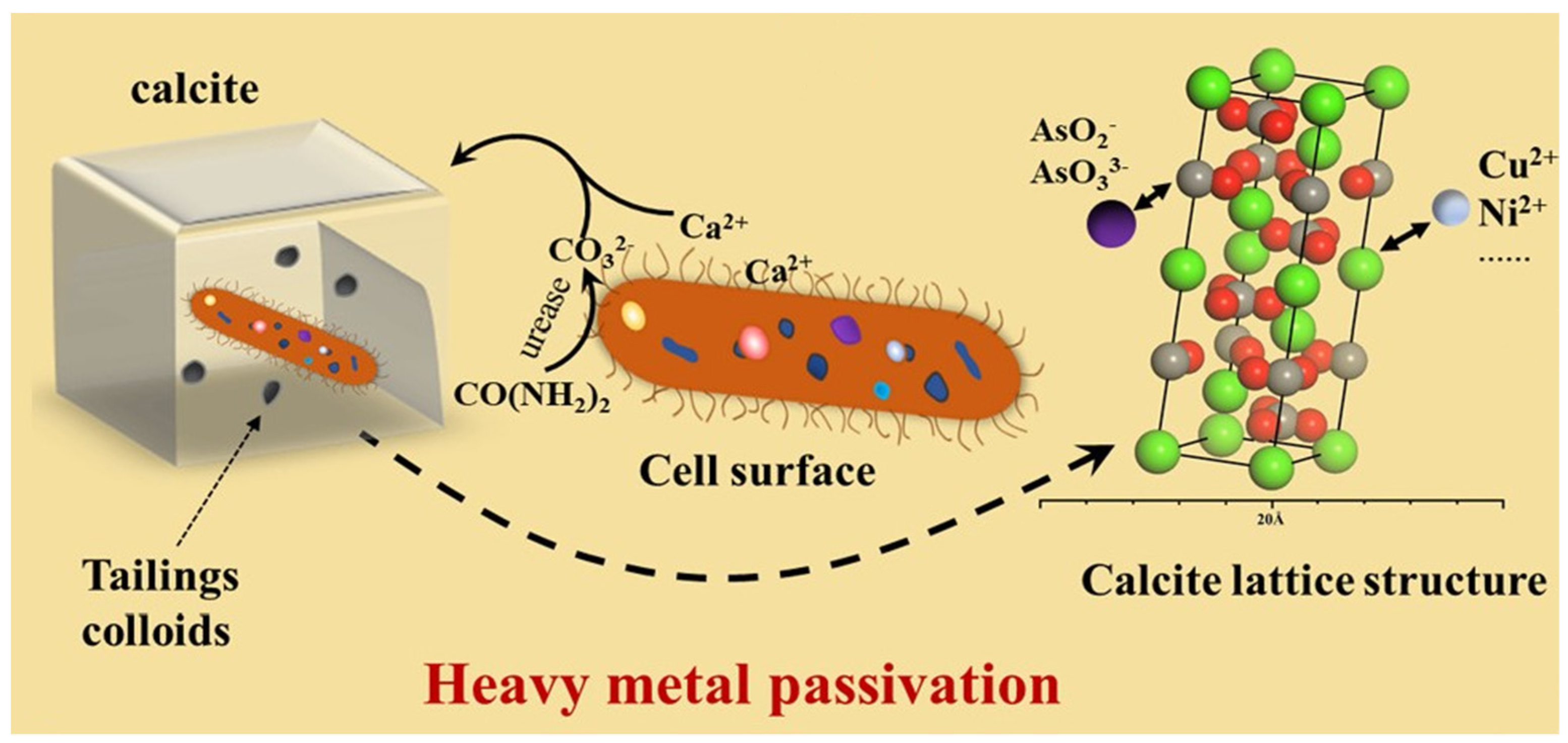
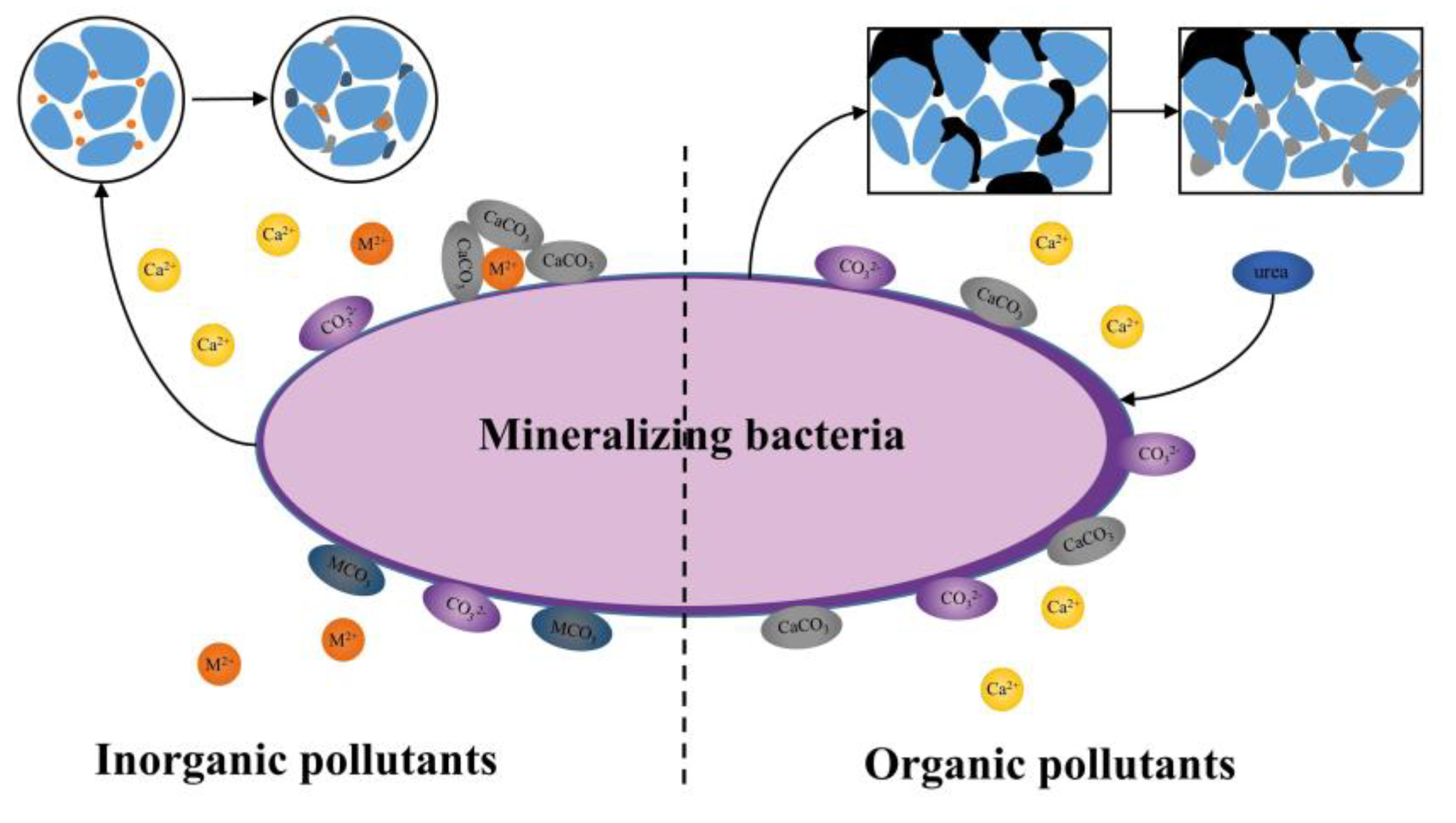
2. Mechanism of Microbial Mineralization to Control Pollutants
3. Soil Mineralization Microorganisms
3.1. Fungi
3.2. Sulfur-Oxidizing Bacteria
3.3. Sulfate-Reducing Bacteria
3.4. Ammonifying Bacteria
3.5. Denitrifying Bacteria
3.6. Urease Bacteria
3.7. Methanogens
4. Influencing Factors of Mineralization in Soil
4.1. Reaction Solution Concentration and Composition
4.2. Temperature
4.3. Properties of Cementing Medium
4.4. Injection Pattern
5. Status of Research on the Microbial Mineralization Control of Inorganic and Organic Pollutants in Soil
5.1. Inorganic Contaminant
5.2. Organic Contaminant
6. Conclusion Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jingzhong, C.; Jie, C.; Xuejian, X.; Xuelei, Z. Soil pollution and its environmental effects. Soil 2003, 35, 298–303. [Google Scholar]
- Guotai, Z. The status quo of soil pollution in china and its prevention and control strategies. J. Chin. Acad. Sci. 2015, 30, 477–483. [Google Scholar] [CrossRef]
- Showkat, A.; Bhat, D.S.; Hassan, T.; Majid, S. Heavy metal toxicity and their harmful effects on living organisms—A review. Int. J. Med. Sci. Diagn. Res. 2019, 3, 106–122. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C. Natural and Human Factors Affect the Distribution of Soil Heavy Metal Pollution: A Review. Water Air Soil Pollut. 2020, 231, 350. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xing, Z.; Junhong, L.; Yunwen, L. Current Status of Soil Pollution and Progress of Soil Reclamation Industry. Environ. Sci. Manag. 2016, 41, 45–48. [Google Scholar]
- Smaranda, C.; Popescu, M.-C.; Bulgariu, D.; Măluţan, T.; Gavrilescu, M. Adsorption of organic pollutants onto a Romanian soil: Column dynamics and transport. Process Saf. Environ. Prot. 2017, 108, 108–120. [Google Scholar] [CrossRef]
- Sushkova, S.; Minkina, T.; Deryabkina, I.; Rajput, V.; Antonenko, E.; Nazarenko, O.; Yadav, B.K.; Hakki, E.; Mohan, D. Environmental pollution of soil with PAHs in energy producing plants zone. Sci. Total Environ. 2019, 655, 232–241. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Jia, J.; Lou, M.; Li, X.; Zhao, S.; Chen, W.; Xiao, B.; Yu, Y. Distribution and Source Apportionment of Polycyclic Aromatic Hydrocarbons in Soils at Different Distances and Depths around Three Power Plants in Bijie, Guizhou Province. Polycycl. Aromat. Compd. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, X.; Ou, Z. [Behaviors of polycylic aromatic hydrocarbons (PAHs) in the soil]. Ying Yong Sheng Tai Xue Bao 2002, 13, 501–504. [Google Scholar]
- Ni, N.; Kong, D.; Wu, W.; He, J.; Shan, Z.; Li, J.; Dou, Y.; Zhang, Y.; Song, Y.; Jiang, X. The Role of Biochar in Reducing the Bioavailability and Migration of Persistent Organic Pollutants in Soil–Plant Systems: A Review. Bull. Environ. Contam. Toxicol. 2020, 104, 157–165. [Google Scholar] [CrossRef]
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; So, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus 2016, 5, 250. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D.; Fu, Q. Bioremediation of Pb-Contaminated Soil Based on Microbially Induced Calcite Precipitation. J. Microbiol. Biotechnol. 2012, 22, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Bio/Technol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Stabnikov, V.; Ivanov, V.; Chu, J. Sealing of sand using spraying and percolating biogrouts for the construction of model aquaculture pond in arid desert. Int. Aquat. Res. 2016, 8, 207–216. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Sulaymon, A.H.; Khaliefa, Q.M. A review of permeable reactive barrier as passive sustainable technology for groundwater remediation. Int. J. Environ. Sci. Technol. 2018, 15, 1123–1138. [Google Scholar] [CrossRef]
- Wang, L.; Yu, K.; Li, J.-S.; Tsang, D.C.W.; Poon, C.S.; Yoo, J.-C.; Baek, K.; Ding, S.; Hou, D.; Dai, J.-G. Low-carbon and low-alkalinity stabilization/solidification of high-Pb contaminated soil. Chem. Eng. J. 2018, 351, 418–427. [Google Scholar] [CrossRef]
- Lima, L.R.P.d.A.; Bernardez, L.A.; dos Santos, M.G.; Souza, R.C. Remediation of Clay Soils Contaminated with Potentially Toxic Elements: The Santo Amaro Lead Smelter, Brazil, Case. Soil Sediment Contam. Int. J. 2018, 27, 573–591. [Google Scholar] [CrossRef]
- Mugita, Y.; Nakagami, G.; Minematsu, T.; Kitamura, A.; Sanada, H. Combination of urease inhibitor and antiseptic inhibits urea decomposition-induced ammonia production by Proteus mirabilis. Int. Wound J. 2020, 17, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneyra, M.; Delgado, G.; Ramos-Cormenzana, A.; Delgado, R. Biomineralization of carbonates by Halomonas eurihalina in solid and liquid media with different salinities: Crystal formation sequence. Res. Microbiol. 1998, 149, 277–287. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [PubMed]
- Shan, B.; Hao, R.; Xu, H.; Li, J.; Li, Y.; Xu, X.; Zhang, J. A review on mechanism of biomineralization using microbial-induced precipitation for immobilizing lead ions. Environ. Sci. Pollut. Res. 2021, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guibaud, G.; Bhatia, D.; d’Abzac, P.; Bourven, I.; Bordas, F.; van Hullebusch, E.D.; Lens, P.N.L. Cd(II) and Pb(II) sorption by extracellular polymeric substances (EPS) extracted from anaerobic granular biofilms: Evidence of a pH sorption-edge. J. Taiwan Inst. Chem. Eng. 2012, 43, 444–449. [Google Scholar] [CrossRef]
- Pal, A.; Paul, A.K. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. Indian J. Microbiol. 2008, 48, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Lam, S.; Fortin, D.; Davis, B.S.; Beveridge, T.J. Mineralization of bacterial surfaces. Chem. Geol. 1996, 132, 171–181. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Larson, S.L.; Ballard, J.H.; Knotek-Smith, H.M.; Nie, J.; Hu, N.; Ding, D.; Han, F.X. Microbially Induced Carbonate Precipitation Techniques for the Remediation of Heavy Metal and Trace Element–Polluted Soils and Water. Water Air Soil Pollut. 2021, 232, 268. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, S.; Roh, Y. Effect of divalent cations (Cu, Zn, Pb, Cd, and Sr) on microbially induced calcium carbonate precipitation and mineralogical properties. Front. Microbiol. 2021, 12, 646748. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Biosequestration of heavy metals by microbially induced calcite precipitation of ureolytic bacteria. Rom. Biotechnol. Lett. 2018, 24, 147–153. [Google Scholar] [CrossRef]
- Yi, H.; Zheng, T.; Jia, Z.; Su, T.; Wang, C. Study on the influencing factors and mechanism of calcium carbonate precipitation induced by urease bacteria. J. Cryst. Growth 2021, 564, 126113. [Google Scholar] [CrossRef]
- Zhang, K.-N.; Chen, Y.-G.; Deng, F.-Y.; Tian, Q.-Y. Retention of clay-solidified grouting curtain to Cd2+, Pb2+ and Hg2+ in landfill of municipal solid waste. J. Cent. South Univ. Technol. 2004, 11, 419–422. [Google Scholar] [CrossRef]
- Xue, Q.; Li, J.-s.; Liu, L. Experimental study on anti-seepage grout made of leachate contaminated clay in landfill. Applied. Clay Sci. 2013, 80–81, 438–442. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Zhong, M.; Jing, C.; Guo, B. Research status and development of microbial induced calcium carbonate mineralization technology. PLoS ONE 2022, 17, e271761. [Google Scholar] [CrossRef] [PubMed]
- Veis, A. A Window on Biomineralization. Science 2005, 307, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Seifan, M.; Berenjian, A. Microbially induced calcium carbonate precipitation: A widespread phenomenon in the biological world. Appl. Microbiol. Biotechnol. 2019, 103, 4693–4708. [Google Scholar] [CrossRef]
- Qin, W.; Wang, C.y.; Ma, Y.x.; Shen, M.j.; Li, J.; Jiao, K.; Tay, F.R.; Niu, L.n. Microbe-Mediated Extracellular and Intracellular Mineralization: Environmental, Industrial, and Biotechnological Applications. Adv. Mater. 2020, 32, 1907833. [Google Scholar] [CrossRef]
- Qiao, S.; Zeng, G.; Wang, X.; Dai, C.; Sheng, M.; Chen, Q.; Xu, F.; Xu, H. Multiple heavy metals immobilization based on microbially induced carbonate precipitation by ureolytic bacteria and the precipitation patterns exploration. Chemosphere 2021, 274, 129661. [Google Scholar] [CrossRef]
- Krajewska, B. Urease-aided calcium carbonate mineralization for engineering applications: A review. J. Adv. Res. 2018, 13, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Soni, R.; Jain, L.; Dash, B.; Goel, R. Endophytic fungi: Recent advances in identification and explorations. Adv. Endophytic Fungal Res. 2019, 90, 267–281. [Google Scholar]
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef]
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef]
- Congeevaram, S.; Dhanarani, S.; Park, J.; Dexilin, M.; Thamaraiselvi, K. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J. Hazard. Mater. 2007, 146, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Fang, C.; Huang, M.; Achal, V. Characterization of fungal-mediated carbonate precipitation in the biomineralization of chromate and lead from an aqueous solution and soil. J. Clean. Prod. 2017, 164, 198–208. [Google Scholar] [CrossRef]
- Kumari, D.; Pan, X.; Achal, V.; Zhang, D.; Al-Misned, F.A.; Golam Mortuza, M. Multiple metal-resistant bacteria and fungi from acidic copper mine tailings of Xinjiang, China. Environ. Earth Sci. 2015, 74, 3113–3121. [Google Scholar] [CrossRef]
- Zhi-Hui, Y.; Stöven, K.; Haneklaus, S.; Singh, B.; Schnug, E. Elemental sulfur oxidation by Thiobacillus spp. and aerobic heterotrophic sulfur-oxidizing bacteria. Pedosphere 2010, 20, 71–79. [Google Scholar]
- Xia, Y.; Lü, C.; Hou, N.; Xin, Y.; Liu, J.; Liu, H.; Xun, L. Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J. 2017, 11, 2754–2766. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef]
- Rawlings, D.E. Characteristics and adaptability of iron-and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Factories 2005, 4, 13. [Google Scholar] [CrossRef]
- Ňancucheo, I.; Johnson, D.B. Selective removal of transition metals from acidic mine waters by novel consortia of acidophilic sulfidogenic bacteria. Microb. Biotechnol. 2012, 5, 34–44. [Google Scholar] [CrossRef]
- Cheng, Y.; Yuan, T.; Deng, Y.; Lin, C.; Zhou, J.; Lei, Z.; Shimizu, K.; Zhang, Z. Use of sulfur-oxidizing bacteria enriched from sewage sludge to biologically remove H2S from biogas at an industrial-scale biogas plant. Bioresour. Technol. Rep. 2018, 3, 43–50. [Google Scholar] [CrossRef]
- Lin, S.; Mackey, H.R.; Hao, T.; Guo, G.; van Loosdrecht, M.C.; Chen, G. Biological sulfur oxidation in wastewater treatment: A review of emerging opportunities. Water Res. 2018, 143, 399–415. [Google Scholar] [CrossRef]
- Pokorna, D.; Zabranska, J. Sulfur-oxidizing bacteria in environmental technology. Biotechnol. Adv. 2015, 33, 1246–1259. [Google Scholar] [CrossRef]
- Barton, L.L.; Fauque, G.D. Chapter 2 Biochemistry, Physiology and Biotechnology of Sulfate-Reducing Bacteria. In Advances in Applied Microbiology; Academic Press: New York, NY, USA, 2009; Volume 68, pp. 41–98. [Google Scholar]
- Bradley, A.; Leavitt, W.; Johnston, D. Revisiting the dissimilatory sulfate reduction pathway. Geobiology 2011, 9, 446–457. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, A. Review on microbial induced calcite precipitation mechanisms leading to bacterial selection for microbial concrete. Constr. Build. Mater. 2019, 225, 67–75. [Google Scholar] [CrossRef]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis—The microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Gallagher, K.L.; Kading, T.J.; Braissant, O.; Dupraz, C.; Visscher, P.T. Inside the alkalinity engine: The role of electron donors in the organomineralization potential of sulfate-reducing bacteria. Geobiology 2012, 10, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Xie, S.B.; Ling, H.; Wang, W.T.; Li, S.Y.; Liu, Y.J. Influence and Mechanism of Cu2+ on Removal of U(VI) by Sulfate Reducing Bacteria. Adv. Mater. Res. 2011, 236–238, 903–908. [Google Scholar] [CrossRef]
- Li, X.; Dai, L.; Zhang, C.; Zeng, G.; Liu, Y.; Zhou, C.; Xu, W.; Wu, Y.; Tang, X.; Liu, W.; et al. Enhanced biological stabilization of heavy metals in sediment using immobilized sulfate reducing bacteria beads with inner cohesive nutrient. J. Hazard. Mater. 2017, 324, 340–347. [Google Scholar] [CrossRef]
- Qian, Z.; Tianwei, H.; Mackey, H.R.; van Loosdrecht, M.C.M.; Guanghao, C. Recent advances in dissimilatory sulfate reduction: From metabolic study to application. Water Res. 2019, 150, 162–181. [Google Scholar] [CrossRef]
- Lin, C.Y.; Turchyn, A.V.; Steiner, Z.; Bots, P.; Lampronti, G.I.; Tosca, N.J. The role of microbial sulfate reduction in calcium carbonate polymorph selection. Geochim. Et Cosmochim. Acta 2018, 237, 184–204. [Google Scholar] [CrossRef]
- Xu, Y.-N.; Chen, Y. Advances in heavy metal removal by sulfate-reducing bacteria. Water Sci. Technol. 2020, 81, 1797–1827. [Google Scholar] [CrossRef]
- Azabou, S.; Mechichi, T.; Sayadi, S. Zinc precipitation by heavy-metal tolerant sulfate-reducing bacteria enriched on phosphogypsum as a sulfate source. Miner. Eng. 2007, 20, 173–178. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, G.; Liu, S.; Fu, Z.; Chen, J.; Ma, C. Bioremoval of arsenic and antimony from wastewater by a mixed culture of sulfate-reducing bacteria using lactate and ethanol as carbon sources. Int. Biodeterior. Biodegrad. 2018, 126, 152–159. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, L.; Zhang, Z.; Chen, G.-H.; Jiang, F. Realizing high-rate sulfur reduction under sulfate-rich conditions in a biological sulfide production system to treat metal-laden wastewater deficient in organic matter. Water Res. 2018, 131, 239–245. [Google Scholar] [CrossRef]
- Wen, Q.; Qin, Y.; Zheng, J.; Wei, Q.; Zhang, Y.; Jiang, Y. Research progress on the fixation of heavy metals in acid mine wastewater by acid salt reducing bacteria. Chem. Ind. Eng. 2022, 41, 5578–5587. [Google Scholar] [CrossRef]
- Takeuchi, J. Habitat segregation of a functional gene encoding nitrate ammonification in estuarine sediments. Geomicrobiol. J. 2006, 23, 75–87. [Google Scholar] [CrossRef]
- Kucharski, J.; Bacmaga, M.; Wyszkowska, J. Effect of herbicides on the course of ammonification in soil. J. Elem. 2009, 14, 477–487. [Google Scholar] [CrossRef]
- Stein, L.Y. Insights into the physiology of ammonia-oxidizing microorganisms. Curr. Opin. Chem. Biol. 2019, 49, 9–15. [Google Scholar] [CrossRef]
- Xia, F.; Wang, J.-G.; Zhu, T.; Zou, B.; Rhee, S.-K.; Quan, Z.-X. Ubiquity and Diversity of Complete Ammonia Oxidizers (Comammox). Appl. Environ. Microbiol. 2018, 84, e01390-01318. [Google Scholar] [CrossRef]
- Liu, T.-t.; Yang, H. Different nutrient levels, rather than seasonal changes, significantly affected the spatiotemporal dynamic changes of ammonia-oxidizing microorganisms in Lake Taihu. World J. Microbiol. Biotechnol. 2021, 37, 91. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Wang, Z.; Su, J.; Ali, A.; Zhang, R.; Yang, W.; Xu, L.; Zhao, T. Microbially induced calcium precipitation based simultaneous removal of fluoride, nitrate, and calcium by Pseudomonas sp. WZ39: Mechanisms and nucleation pathways. J. Hazard. Mater. 2021, 416, 125914. [Google Scholar] [CrossRef]
- van Paassen, L.A.; Daza, C.M.; Staal, M.; Sorokin, D.Y.; van der Zon, W.; van Loosdrecht, M.C.M. Potential soil reinforcement by biological denitrification. Ecological Engineering 2010, 36, 168–175. [Google Scholar] [CrossRef]
- Kavazanjian, E., Jr.; Karatas, I. Microbiological improvement of the physical properties of soil. In Proceedings of the 6th International Conference on Case Histories in Geotechnical Engineering, Arlington, TX, USA, 11–16 August 2008. [Google Scholar]
- Chen, H.; Tu, Z.; Wu, S.; Yu, G.; Du, C.; Wang, H.; Yang, E.; Zhou, L.; Deng, B.; Wang, D.; et al. Recent advances in partial denitrification-anaerobic ammonium oxidation process for mainstream municipal wastewater treatment. Chemosphere 2021, 278, 130436. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, T.; Zhang, H.; Zeng, M.; Liu, F.; Bai, S.; Shi, J.; Qiu, X.; Yang, X. Nitrogen removal characteristics of enhanced in situ indigenous aerobic denitrification bacteria for micro-polluted reservoir source water. Bioresour. Technol. 2016, 201, 195–207. [Google Scholar] [CrossRef]
- Schroeder, A.; Souza, D.H.; Fernandes, M.; Rodrigues, E.B.; Trevisan, V.; Skoronski, E. Application of glycerol as carbon source for continuous drinking water denitrification using microorganism from natural biomass. J. Environ. Manag. 2020, 256, 109964. [Google Scholar] [CrossRef]
- McCarty, G.W.; Bremner, J.M. Availability of organic carbon for denitrification of nitrate in subsoils. Biol. Fertil. Soils 1992, 14, 219–222. [Google Scholar] [CrossRef]
- Jalilvand, N.; Akhgar, A.; Alikhani, H.A.; Rahmani, H.A.; Rejali, F. Removal of heavy metals zinc, lead, and cadmium by biomineralization of urease-producing bacteria isolated from Iranian mine calcareous soils. J. Soil Sci. Plant Nutr. 2020, 20, 206–219. [Google Scholar] [CrossRef]
- Cheng, C.; Han, H.; Wang, Y.; He, L.; Sheng, X. Metal-immobilizing and urease-producing bacteria increase the biomass and reduce metal accumulation in potato tubers under field conditions. Ecotoxicol. Environ. Saf. 2020, 203, 111017. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Ike, M.; Fujita, M. Dissimilatory arsenate reduction by a facultative anaerobe, Bacillus sp. strain SF-1. J. Biosci. Bioeng. 2003, 96, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Garg, A. Level of Cd in different types of soil of Rohtak district and its bioremediation. J. Environ. Chem. Eng. 2016, 4, 3797–3802. [Google Scholar]
- Li, M.; Cheng, X.; Guo, H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Henze, J.; Randall, D.G. Microbial induced calcium carbonate precipitation at elevated pH values (>11) using Sporosarcina pasteurii. J. Environ. Chem. Eng. 2018, 6, 5008–5013. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- He, Z.; Xu, Y.; Yang, X.; Shi, J.; Wang, X.; Jin, Z.; Zhang, D.; Pan, X. Passivation of heavy metals in copper–nickel tailings by in-situ bio-mineralization: A pilot trial and mechanistic analysis. Sci. Total Environ. 2022, 838, 156504. [Google Scholar] [CrossRef]
- Mekonnen, E.; Kebede, A.; Nigussie, A.; Kebede, G.; Tafesse, M. Isolation and Characterization of Urease-Producing Soil Bacteria. Int. J. Microbiol. 2021, 2021, 8888641. [Google Scholar] [CrossRef] [PubMed]
- Elmi, F.; Etemadifar, Z.; Emtiazi, G. Biosynthesis of Calcite Nanocrystal by a Novel Polyextremophile Bhargavaea cecembensis-Related Strain Isolated from Sandy Soil. Microb. Ecol. 2022, 85, 698–707. [Google Scholar] [CrossRef]
- Phang, I.R.K.; Chan, Y.S.; Wong, K.S.; Lau, S.Y. Isolation and characterization of urease-producing bacteria from tropical peat. Biocatal. Agric. Biotechnol. 2018, 13, 168–175. [Google Scholar] [CrossRef]
- Kenward, P.A.; Goldstein, R.H.; González, L.A.; Roberts, J.A. Precipitation of low-temperature dolomite from an anaerobic microbial consortium: The role of methanogenic Archaea. Geobiology 2009, 7, 556–565. [Google Scholar] [CrossRef]
- Whitman, W.B.; Bowen, T.L.; Boone, D.R. The methanogenic bacteria. Prokaryotes 2006, 3, 165–207. [Google Scholar]
- Roberts, J.A.; Bennett, P.C.; González, L.A.; Macpherson, G.L.; Milliken, K.L. Microbial precipitation of dolomite in methanogenic groundwater. Geology 2004, 32, 277–280. [Google Scholar] [CrossRef]
- Liu, Y.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Castanier, S.; Le Metayer-Levrel, G.; Perthuisot, J.-P. Bacterial Roles in the Precipitation of Carbonate Minerals. Microb. Sediments 2013, 32, 32–39. [Google Scholar]
- Visscher, P.T.; Stolz, J.F. Microbial mats as bioreactors: Populations, processes, and products. In Geobiology: Objectives, Concepts, Perspectives; Noffke, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 87–100. [Google Scholar]
- Cooke, A.J.; Rowe, R.K.; Rittmann, B.E.; Fleming, I.R. Modeling biochemically driven mineral precipitation in anaerobic biofilms. Water Sci. Technol. 1999, 39, 57–64. [Google Scholar] [CrossRef]
- Paulo, L.M.; Stams, A.J.M.; Sousa, D.Z. Methanogens, sulphate and heavy metals: A complex system. Rev. Environ. Sci. Bio/Technol. 2015, 14, 537–553. [Google Scholar] [CrossRef]
- Ferris, F.G.; Fyfe, W.S.; Beveridge, T.J. Bacteria as nucleation sites for authigenic minerals in a metal-contaminated lake sediment. Chem. Geol. 1987, 63, 225–232. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.; Braissant, O.; Decho, A.; Norman, R.; Visscher, P. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Al-Salloum, Y.; Abbas, H.; Sheikh, Q.I.; Hadi, S.; Alsayed, S.; Almusallam, T. Effect of some biotic factors on microbially-induced calcite precipitation in cement mortar. Saudi J. Biol. Sci. 2017, 24, 286–294. [Google Scholar] [CrossRef]
- Mortensen, B.; Haber, M.; DeJong, J.; Caslake, L.; Nelson, D. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 2011, 111, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Improvement of microbial-induced calcium carbonate precipitation technology for sand solidification. J. Mater. Civ. Eng. 2018, 30, 04018301. [Google Scholar] [CrossRef]
- Wen, K.; Li, Y.; Amini, F.; Li, L. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate. Acta Geotech. 2020, 15, 17–27. [Google Scholar] [CrossRef]
- Chunxiang, Q.; Jianyun, W.; Ruixing, W.; Liang, C. Corrosion protection of cement-based building materials by surface deposition of CaCO3 by Bacillus pasteurii. Mater. Sci. Eng. C 2009, 29, 1273–1280. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K.; ASCE, M.; Santamarina, C.; ASCE, A. Factors Affecting Efficiency of Microbially Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2012, 138, 992–1001. [Google Scholar] [CrossRef]
- Qabany, A.A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. In Proceedings of the Bio- and Chemo-Mechanical Processes in Geotechnical Engineering: Géotechnique Symposium in Print 2013; ICE Publishing: London, UK, 2014; pp. 107–115. [Google Scholar]
- van Wijngaarden, W.K.; Vermolen, F.J.; van Meurs, G.A.M.; Vuik, C. Modelling Biogrout: A New Ground Improvement Method Based on Microbial-Induced Carbonate Precipitation. Transp. Porous Media 2011, 87, 397–420. [Google Scholar] [CrossRef]
- Canakci, H.; Sidik, W.; Halil Kilic, I. Effect of bacterial calcium carbonate precipitation on compressibility and shear strength of organic soil. Soils Found. 2015, 55, 1211–1221. [Google Scholar] [CrossRef]
- Rowshanbakht, K.; Khamehchiyan, M.; Sajedi, R.H.; Nikudel, M.R. Effect of injected bacterial suspension volume and relative density on carbonate precipitation resulting from microbial treatment. Ecol. Eng. 2016, 89, 49–55. [Google Scholar] [CrossRef]
- Okwadha, G.D.O.; Li, J. Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef]
- Lee, L.M.; Ng, W.S.; Tan, C.K.; Hii, S.L. Bio-mediated soil improvement under various concentrations of cementation reagent. Appl. Mech. Mater. 2012, 204-208, 326–329. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Improvements in engineering properties of soils through microbial-induced calcite precipitation. KSCE J. Civ. Eng. 2013, 17, 718–728. [Google Scholar] [CrossRef]
- Lai, H.-J.; Cui, M.-J.; Wu, S.-F.; Yang, Y.; Chu, J. Retarding effect of concentration of cementation solution on biocementation of soil. Acta Geotech. 2021, 16, 1457–1472. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.X.; Cheng, X.H. Influences of calcium sources on microbially induced carbonate precipitation in porous media. Mater. Res. Innov. 2014, 18, S2–S79. [Google Scholar] [CrossRef]
- Akoğuz, H.Ç.S.; Barış, Ö. The effects of different sources of calcium in improvement of soils by microbially induced calcite precipitation (MICP). Sigma J. Eng. Nat. Sci. 2020, 37, 953–965. [Google Scholar]
- Abdeh Keykha, H.; Asadi, A.; Zareian, M. Environmental Factors Affecting the Compressive Strength of Microbiologically Induced Calcite Precipitation-Treated Soil. Geomicrobiol. J. 2017, 34, 889–894. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Soga, K.; DeJong, J.T.; Kabla, A.J. Micro-scale investigations of temperature-dependent Microbial-Induced Carbonate Precipitation (MICP) in the temperature range 4–50 {\deg} C. arXiv 2022, arXiv:2202.09815. [Google Scholar]
- Cheng, L.; Shahin, M.; Cord-Ruwisch, R.; Addis, M.; Hartanto, T.; Elms, C. Soil stabilisation by microbial-induced calcite precipitation (MICP): Investigation into some physical and environmental aspects. In Proceedings of the 7th International Congress on Environmental Geotechnics, Melbourne, Australia, 10–14 November 2014; pp. 1105–1112. [Google Scholar]
- Peng, J.; Liu, Z. Influence of temperature on microbially induced calcium carbonate precipitation for soil treatment. PLoS ONE 2019, 14, e0218396. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tang, X.; Chu, J.; He, J. Microbially Induced Calcite Precipitation for Seepage Control in Sandy Soil. Geomicrobiol. J. 2019, 36, 366–375. [Google Scholar] [CrossRef]
- Yasuhara, H.; Neupane, D.; Hayashi, K.; Okamura, M. Experiments and predictions of physical properties of sand cemented by enzymatically-induced carbonate precipitation. Soils Found. 2012, 52, 539–549. [Google Scholar] [CrossRef]
- Hataf, N.; Baharifard, A. Reducing Soil Permeability Using Microbial Induced Carbonate Precipitation (MICP) Method: A Case Study of Shiraz Landfill Soil. Geomicrobiol. J. 2020, 37, 147–158. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Upscaling Effects of Soil Improvement by Microbially Induced Calcite Precipitation by Surface Percolation. Geomicrobiol. J. 2014, 31, 396–406. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Significant indicators for biomineralisation in sand of varying grain sizes. Constr. Build. Mater. 2016, 104, 198–207. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Li, C.; Li, M.; Amini, F.; Zhang, H. Factors affecting improvement of engineering properties of MICP-treated soil catalyzed by bacteria and urease. J. Mater. Civ. Eng. 2014, 26, 04014094. [Google Scholar] [CrossRef]
- Khodadadi, T.H.; Kavazanjian, E.; Bilsel, H. Mineralogy of calcium carbonate in MICP-treated soil using soaking and injection treatment methods. Geotech. Front. 2017, 2017, 195–201. [Google Scholar]
- Jisheng, Z.; Wei, L.I.U.; Dawei, G.; Yingzheng, Z.; Liang, C.; Jinhai, Z. Influence of different bacterial grouting strategies on MICP one-phase injection method. J. Hohai Univ. (Nat. Sci.) 2020, 48, 222–230. [Google Scholar]
- Cheng, L.; Shahin, M.A.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2019, 14, 615–626. [Google Scholar] [CrossRef]
- de Alencar, F.L.S.; Navoni, J.A.; do Amaral, V.S. The use of bacterial bioremediation of metals in aquatic environments in the twenty-first century: A systematic review. Environ. Sci. Pollut. Res. 2017, 24, 16545–16559. [Google Scholar] [CrossRef]
- Eltarahony, M.; Zaki, S.; Abd-El-Haleem, D. Aerobic and anaerobic removal of lead and mercury via calcium carbonate precipitation mediated by statistically optimized nitrate reductases. Sci. Rep. 2020, 10, 4029. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Zhan, L.; Huang, M.; Zhang, Q.; Achal, V. The large-scale process of microbial carbonate precipitation for nickel remediation from an industrial soil. Environ. Pollut. 2016, 219, 149–155. [Google Scholar] [CrossRef]
- Chu, J.; Ivanov, V.; Stabnikov, V.; Li, B. Microbial method for construction of an aquaculture pond in sand. In Proceedings of the Bio- and Chemo-Mechanical Processes in Geotechnical Engineering: Géotechnique Symposium in Print 2013; ICE Publishing: London, UK, 2014; pp. 215–219. [Google Scholar]
- Pan, F.; Ma, J.; Wang, Y.; Zhang, Y.; Chen, L.; Edmunds, W.M. Simulation of the migration and transformation of petroleum pollutants in the soils of the Loess plateau: A case study in the Maling oil field of northwestern China. Environ. Monit. Assess. 2013, 185, 8023–8034. [Google Scholar] [CrossRef] [PubMed]
- Jasu, A.; Lahiri, D.; Nag, M.; Ray, R.R. Chapter 17—Fungi in bioremediation of soil organic pollutants. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Sharma, V.K., Shah, M.P., Parmar, S., Kumar, A., Eds.; Academic Press: New York, NY, USA, 2021; pp. 381–405. [Google Scholar]
- Wang, J.; Sandoval, K.; Ding, Y.; Stoeckel, D.; Minard-Smith, A.; Andersen, G.; Dubinsky, E.A.; Atlas, R.; Gardinali, P. Biodegradation of dispersed Macondo crude oil by indigenous Gulf of Mexico microbial communities. Sci. Total Environ. 2016, 557–558, 453–468. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Lukk, T.; Gupta, V.K. Role of Fungi in Bioremediation of Soil Contaminated with Persistent Organic Compounds. In Industrially Important Fungi for Sustainable Development: Volume 1: Biodiversity and Ecological Perspectives; Abdel-Azeem, A.M., Yadav, A.N., Yadav, N., Usmani, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 461–478. [Google Scholar]
- Li, C.; Wengang, X.; Guifen, P. ⟪Technical Code for Sanitary Landfill of Domestic Waste⟫ Study on the Preparation of National Standards—Taking the Seepage Control System as an Example. Resour. Conserv. Environ. Prot. 2014, 8, 95–99+103. [Google Scholar] [CrossRef]
- Ghorbanzadeh, N.; Abduolrahimi, S.; Forghani, A.; Farhangi, M.B. Bioremediation of cadmium in a sandy and a clay soil by microbially induced calcium carbonate precipitation after one week incubation. Arid. Land Res. Manag. 2020, 34, 319–335. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization based remediation of As(III) contaminated soil by Sporosarcina ginsengisoli. J. Hazard. Mater. 2012, 201–202, 178–184. [Google Scholar] [CrossRef]
- Mwandira, W.; Nakashima, K.; Kawasaki, S. Bioremediation of lead-contaminated mine waste by Pararhodobacter sp. based on the microbially induced calcium carbonate precipitation technique and its effects on strength of coarse and fine grained sand. Ecol. Eng. 2017, 109, 57–64. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D. Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol. Eng. 2011, 37, 1601–1605. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D. Bioremediation of strontium (Sr) contaminated aquifer quartz sand based on carbonate precipitation induced by Sr resistant Halomonas sp. Chemosphere 2012, 89, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Lara-Moreno, A.; Morillo, E.; Merchán, F.; Villaverde, J. A comprehensive feasibility study of effectiveness and environmental impact of PAH bioremediation using an indigenous microbial degrader consortium and a novel strain Stenotrophomonas maltophilia CPHE1 isolated from an industrial polluted soil. J. Environ. Manag. 2021, 289, 112512. [Google Scholar] [CrossRef]
- Qian, Z.; Haijun, L.; Jixiang, L.; Xiong, Z. Permeability and deformation characteristics of cement solidified kaolin contaminated by leachate. J. Dalian Univ. Technol. 2016, 56, 510–517. [Google Scholar]
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially Induced Calcium Carbonate Precipitation on Surface or in the Bulk of Soil. Geomicrobiol. J. 2012, 29, 544–549. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Liu, B.; Chen, J.; Jing, C.; Zhong, M.; Shan, Q. Prospect Research on the Diversity of Extracellular Mineralization Process Induced by Mineralizing Microorganisms and Its Use as a Treatment for Soil Pollutants. Sustainability 2023, 15, 4858. https://doi.org/10.3390/su15064858
Guo B, Liu B, Chen J, Jing C, Zhong M, Shan Q. Prospect Research on the Diversity of Extracellular Mineralization Process Induced by Mineralizing Microorganisms and Its Use as a Treatment for Soil Pollutants. Sustainability. 2023; 15(6):4858. https://doi.org/10.3390/su15064858
Chicago/Turabian StyleGuo, Baoyou, Baolei Liu, Jun Chen, Chuan Jing, Ming Zhong, and Qi Shan. 2023. "Prospect Research on the Diversity of Extracellular Mineralization Process Induced by Mineralizing Microorganisms and Its Use as a Treatment for Soil Pollutants" Sustainability 15, no. 6: 4858. https://doi.org/10.3390/su15064858
APA StyleGuo, B., Liu, B., Chen, J., Jing, C., Zhong, M., & Shan, Q. (2023). Prospect Research on the Diversity of Extracellular Mineralization Process Induced by Mineralizing Microorganisms and Its Use as a Treatment for Soil Pollutants. Sustainability, 15(6), 4858. https://doi.org/10.3390/su15064858






