Assessment of Bioaccumulation of Heavy Metals and Their Ecological Risk in Sea Lettuce (Ulva spp.) along the Coast Alexandria, Egypt: Implications for Sustainable Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Sites and Sampling
2.2. Seaweed Analyses
2.3. Water Analyses
2.4. Sediment Analyses
2.5. Calculation of Pollution Indices
2.5.1. The Geo-Accumulation Index
- Class 0 = Igeo ≤ 0 is uncontaminated;
- Class 1 = 0 < Igeo ≤ 1 is uncontaminated to moderately contaminated;
- Class 2 = 1 < Igeo ≤ 2 is moderately contaminated;
- Class 3 = 2 < Igeo ≤ 3 is moderately to heavily contaminated;
- Class 4 = 3 < Igeo ≤ 4 is heavily contaminated;
- Class 5 = 4 < Igeo ≤ 5 is heavily to extremely contaminated; and
2.5.2. Contamination Factor
2.6. Bioaccumulation Factor (BAF)
2.7. DNA Extraction
2.8. PCR and Gel Electrophoresis
2.9. Quantification of Antioxidant Enzymatic Activities
2.10. Quality Control and Quality Assurance
2.11. Statistical Analyses
3. Results
3.1. Ulva and Pollution
3.1.1. Chemical Analyses of Ulva spp.
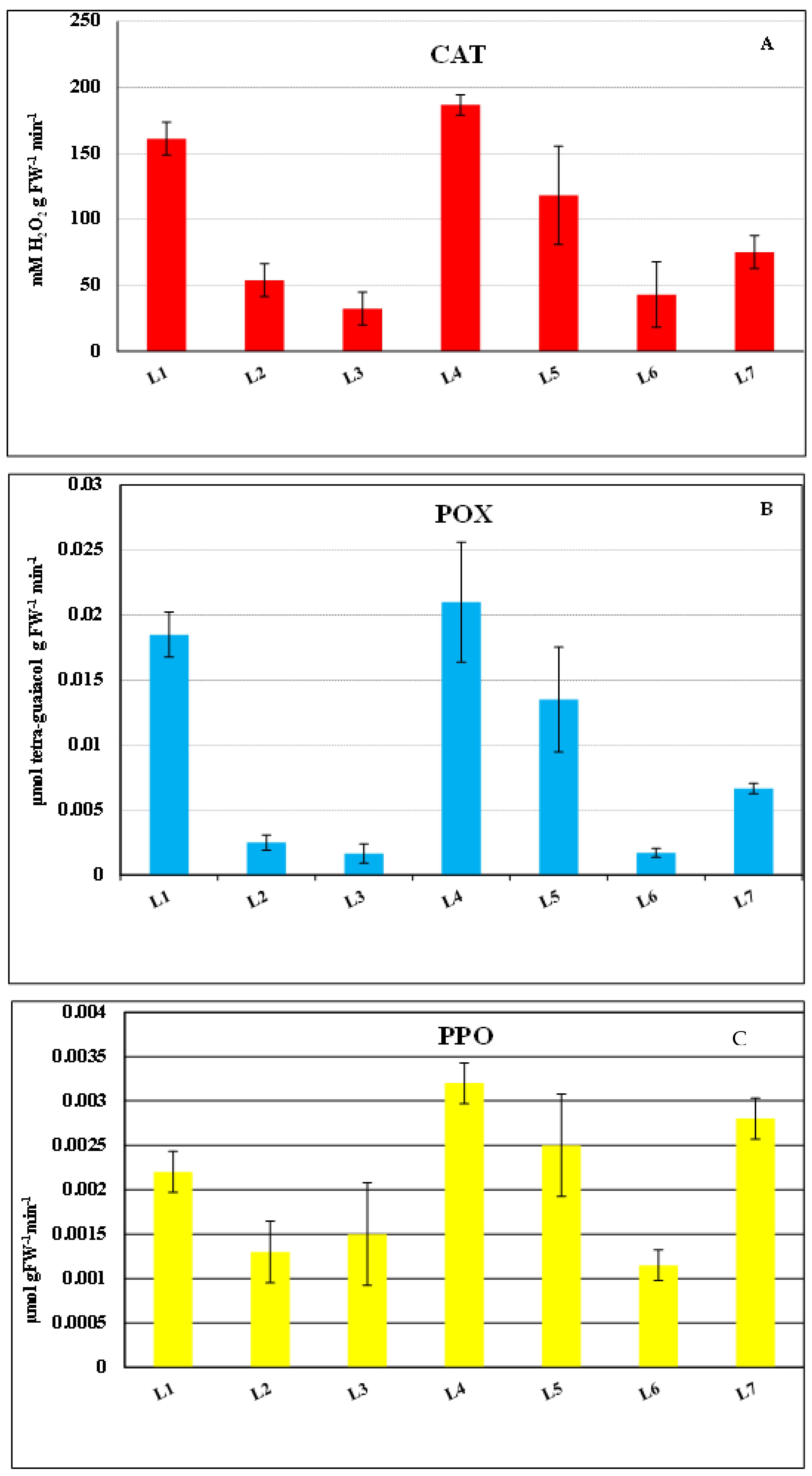
3.1.2. Antioxidant Enzymes Activity of Ulva spp.
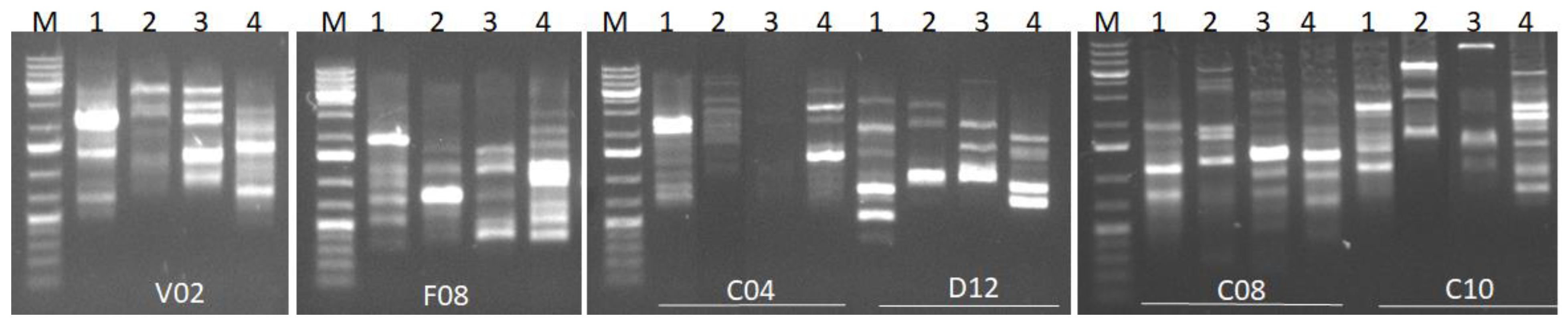
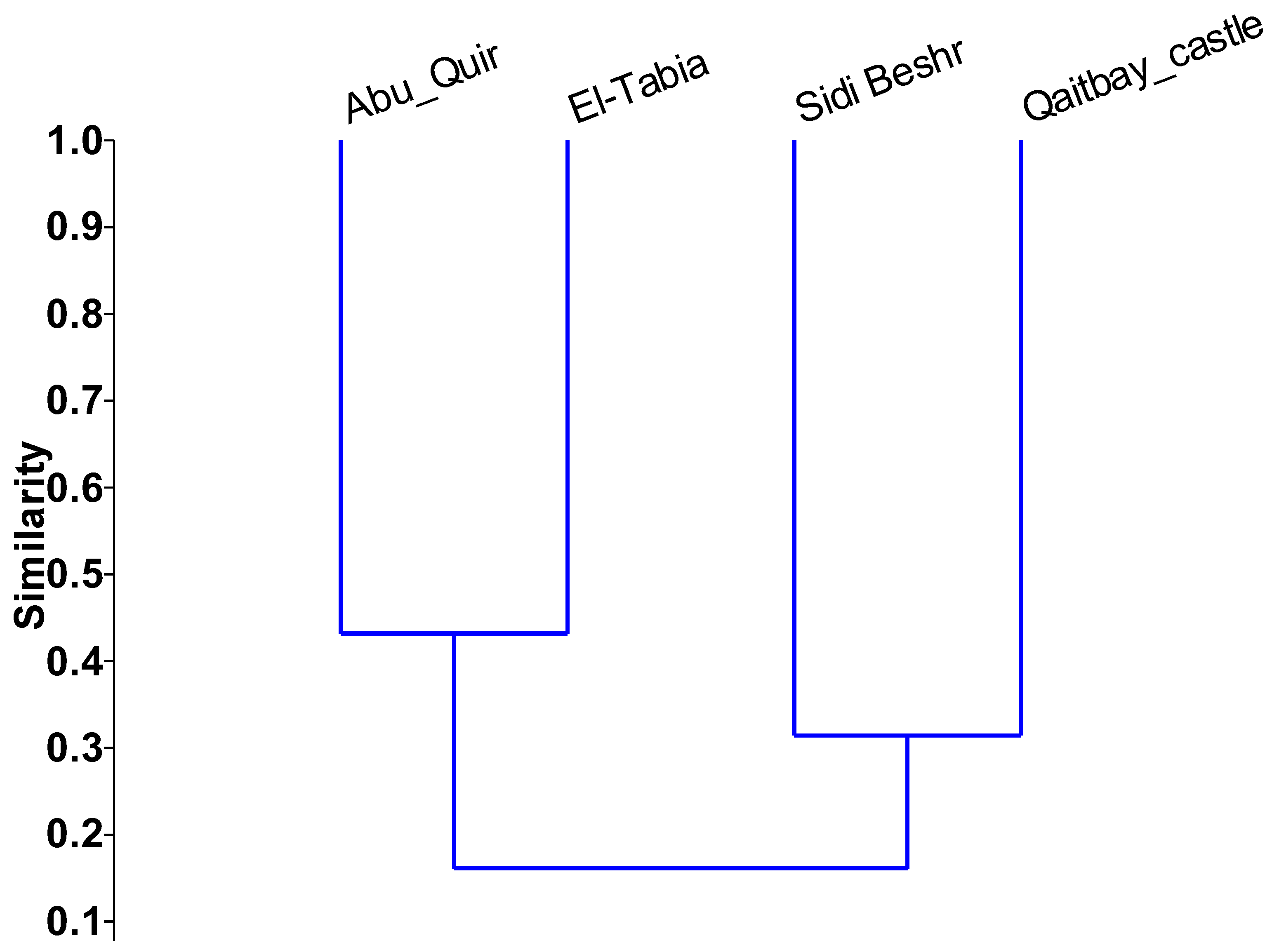
3.1.3. Genetic Diversity of Ulva spp.
3.2. Chemical Characterization of Seawater
3.3. Chemical Properties of Sediments
3.4. Ecological Risk Assessment
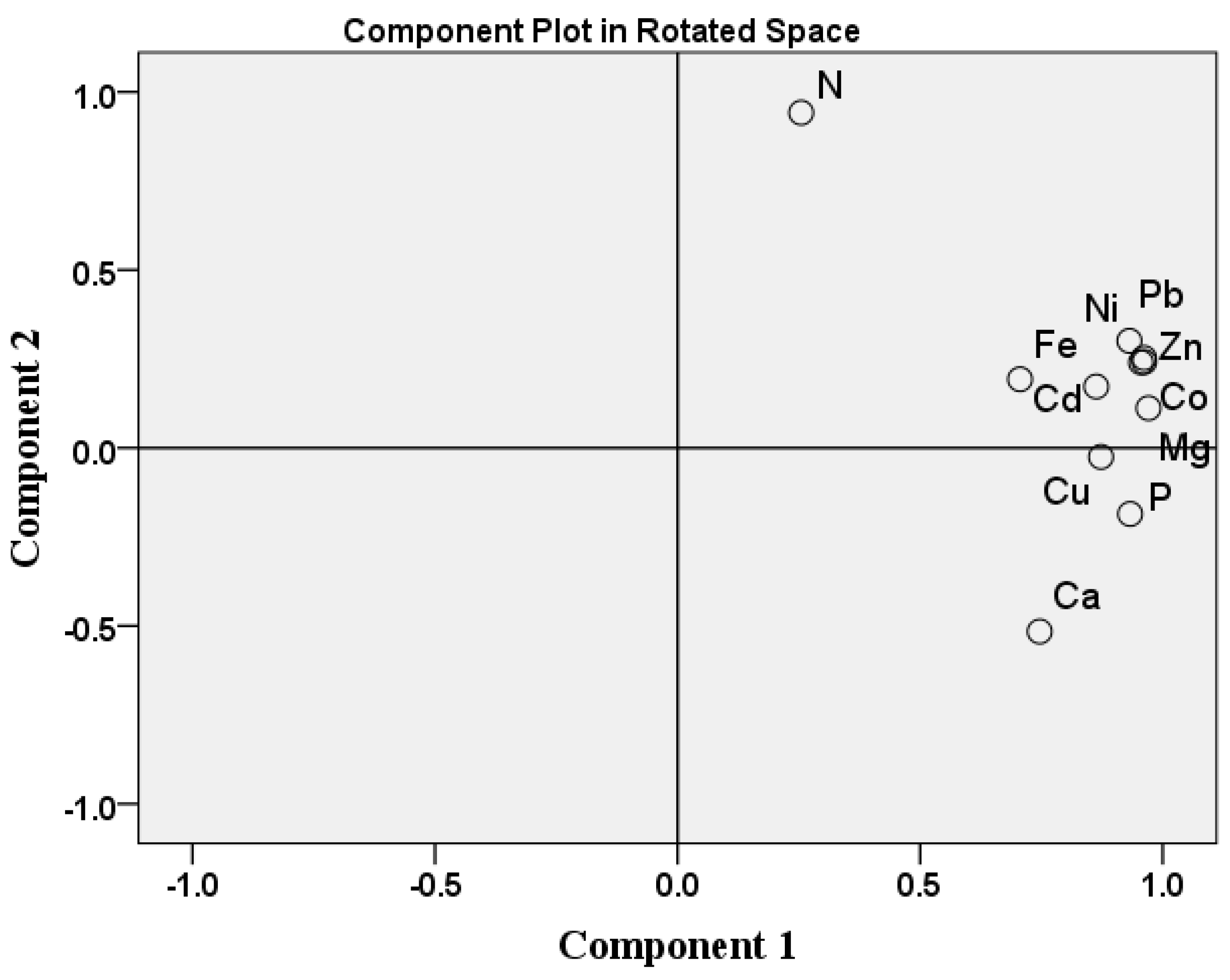
3.5. Principal Component Analysis (PCA) and Correlation
4. Discussion
4.1. Seaweed and Eutrophication in Selected Locations
4.2. Seawater Eutrophication in Selected Locations
4.3. Sediments under Eutrophication in Selected Locations
4.4. Ecological Risk Assessment of Selected Locations
4.5. Suggested Sustainable Management of Eutrophication
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merhaby, D.; Rabodonirina, S.; Net, S.; Ouddane, B.; Halwani, J. Overview of sediments pollution by PAHs and PCBs in Mediterranean basin: Transport, fate, occurrence, and distribution. Mar. Pollut. Bull. 2019, 149, 110646. [Google Scholar] [CrossRef]
- Monier, M.N.; Soliman, A.M.; Al-Halani, A.A. The seasonal assessment of heavy metals pollution in water, sediments, and fish of grey mullet, red seabream, and sardine from the Mediterranean coast, Damietta, North Egypt. Reg. Stud. Mar. Sci. 2023, 57, 102744. [Google Scholar] [CrossRef]
- Alprol, A.E.; Ashour, M.; Mansour, A.T.; Alzahrani, O.M.; Mahmoud, S.F.; Gharib, S.M. Assessment of Water Quality and Phytoplankton Structure of Eight Alexandria Beaches, Southeastern Mediterranean Sea, Egypt. J. Mar. Sci. Eng. 2021, 9, 1328. [Google Scholar] [CrossRef]
- El-Saharty, A.A. Radioactive survey of coastal water and sediments across Alexandria and Rashid coasts. Egypt. J. Aquat. Res. 2013, 39, 21–30. [Google Scholar] [CrossRef]
- Ibrahim, N.; El Afandi, G. Phytoremediation uptake model of heavy metals (Pb, Cd and Zn) in soil using Nerium oleander. Heliyon 2020, 6, e04445. [Google Scholar] [CrossRef]
- Abdel Wahaab, R.; Mahmoud, M.; van Lier, J.B. Toward achieving sustainable management of municipal wastewater sludge in Egypt: The current status and future prospective. Renew. Sustain. Energy Rev. 2020, 127, 109880. [Google Scholar] [CrossRef]
- Zhou, C.; Gaulier, C.; Luo, M.; Guo, W.; Baeyens, W.; Gao, Y. Fine scale measurements in Belgian coastal sediments reveal different mobilization mechanisms for cationic trace metals and oxyanions. Environ. Int. 2020, 145, 106140. [Google Scholar] [CrossRef]
- Li, L.; Shuaijie, W.; Xinqiang, S.; Jiang, M. Ecological risk assessment of heavy metal pollution in the water of China’s coastal shellfish culture areas. Environ. Sci. Pollut. Res. 2020, 27, 18392–18402. [Google Scholar] [CrossRef]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and human health: Current status and future needs. Air Soil Water Res. 2020, 13, 1178622120934441. [Google Scholar] [CrossRef]
- Elbehiry, F.; Elbasiouny, H.; Cappuyns, V.; Brevik, E.C. Available concentrations of some potentially toxic and emerging contaminants in different soil orders in Egypt and assessment of soil pollution. J. Soils Sediments 2021, 21, 3645–3662. [Google Scholar] [CrossRef]
- Elbehiry, F.; Elbasiouny, H.; El-Ramady, H.; Brevik, E.C. Mobility, distribution, and potential risk assessment of selected trace elements in soils of the Nile Delta, Egypt. Environ. Monit. Assess. 2019, 191, 713. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Human health concerns regarding microplastics in the aquatic environment—From marine to food systems. Sci. Total Environ. 2022, 823, 153730. [Google Scholar] [CrossRef]
- Haghshenas, V.; Kafaei, R.; Tahmasebi, R.; Dobaradaran, S.; Hashemi, S.; Sahebi, S.; Sorial, G.A.; Ramavandi, B. Potential of green/brown algae for monitoring of metal(loid)s pollution in the coastal seawater and sediments of the Persian Gulf: Ecological and health risk assessment. Environ. Sci. Pollut. Res. 2020, 27, 7463–7475. [Google Scholar] [CrossRef]
- Li, Y.; Sarpong, L.; Cheng, Y.; Norgbey, E.; Nooni, I.K.; Nasiru, S.; Setordjie, V.E.; Duodu, R.A.B.; Dzakpasu, M. A sediment diagenesis model on sediment oxygen demand in managing eutrophication on Taihu, China. Environ. Sci. Pollut. Res. 2022, 1–15. [Google Scholar] [CrossRef]
- Wei, Y.; Ding, D.; Gu, T.; Xu, Y.; Sun, X.; Qu, K.; Sun, J.; Cui, Z. Ocean acidification and warming significantly affect coastal eutrophication and organic pollution: A case study in the Bohai Sea. Mar. Pollut. Bull. 2023, 186, 114380. [Google Scholar] [CrossRef] [PubMed]
- Sá, A.K.D.S.; Cutrim, M.V.J.; Feitosa, F.A.N.; Flores-Montes, M.J.; Cavalcanti, L.F.; Costa, D.S.; da Cruz, Q.S. Multiple stressors influencing the general eutrophication status of transitional waters of the Brazilian tropical coast: An approach utilizing the pressure, state, and response (PSR) framework. J. Sea Res. 2022, 189, 102282. [Google Scholar] [CrossRef]
- Wu, H.Y.; Fu, S.F.; Hu, W.J.; Chen, F.G.; Cai, X.Q.; Chen, Q.H.; Wu, Y.B. Response of different benthic biotic indices to eutrophication and sediment heavy metal pollution, in Fujian coastal water, East China sea. Chemosphere 2022, 307, 135653. [Google Scholar] [CrossRef]
- Padedda, B.M.; Sechi, N.; Lai, G.G.; Mariani, M.A.; Pulina, S.; Sarria, M.; Satta, C.T.; Virdis, T.; Buscarinu, P.; Lugliè, A. Consequences of eutrophication in the management of water resources in Mediterranean reservoirs: A case study of Lake Cedrino (Sardinia, Italy). Glob. Ecol. Conserv. 2017, 12, 21–35. [Google Scholar] [CrossRef]
- Nakakuni, M.; Loassachan, N.; Ichimi, K.; Nagao, S.; Tada, K. Biophilic elements in core sediments as records of coastal eutrophication in the Seto Inland Sea, Japan. Reg. Stud. Mar. Sci. 2022, 50, 102093. [Google Scholar] [CrossRef]
- Soro, M.-P.; N’goran, K.M.; Ouattara, A.A.; Yao, K.M.; Kouassi, N.L.B.; Diaco, T. Nitrogen and phosphorus spatio-temporal distribution and fluxes intensifying eutrophication in three tropical rivers of Côte d’Ivoire (West Africa). Mar. Pollut. Bull. 2023, 186, 114391. [Google Scholar] [CrossRef]
- Da Le, N.; Nguyen, T.H.; Duong, T.T.; Rochelle-Newall, E.; Hoang, T.T.H.; Vu, T.H.; Pham, T.M.H.; Dinh, L.M.; Phung, T.X.B.; Nguyen, T.D.; et al. Risk of eutrophication in the seawater of the coastal Red River aquaculture zone (Thai Binh province, Vietnam). Reg. Stud. Mar. Sci. 2022, 55, 102587. [Google Scholar] [CrossRef]
- Morsy, A.; Ebeid, M.; Soliman, A.; Abdel Halim, A.; Ali, A.; Fahmy, M. Evaluation of the water quality and the eutrophication risk in Mediterranean sea area: A case study of the Port Said Harbour, Egypt. Environ. Chall. 2022, 7, 100484. [Google Scholar] [CrossRef]
- Abdelsalam, A.H.; Saber, A.A.; El-Kafrawy, S.; Abo-Taleb, H. Long-term evaluation of eutrophication problem using multi-sensor satellite data along El-Max Bay, Alexandria coast and Abu-Qir Bay, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 233–258. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Elbehiry, F.; El-Ramady, H.; Brevik, E.C. Phosphorus Availability and Potential Environmental Risk Assessment in Alkaline Soils. Agriculture 2020, 10, 172. [Google Scholar] [CrossRef]
- Harris, R.J.; Niemand, C.; Pilditch, C.A. Decomposing macroalgae (Ulva spp.) impacts benthic macrofauna and surface sediment erosion. Geo-Mar. Lett. 2020, 40, 281–294. [Google Scholar] [CrossRef]
- Dominguez, H.; Loret, E.P. Ulva lactuca, a source of troubles and potential riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef]
- Fort, A.; Mannion, C.; Fariñas-Franco, J.M.; Sulpice, R. Green tides select for fast expanding Ulva strains. Sci. Total Environ. 2020, 698, 134337. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Zhao, J.; Zhang, X.; Ding, L.; Liu, Y.; Fu, G. Spatiotemporal variation of phosphorus use efficiency across 70 lakes in China: Implications for lake eutrophication management. Ecol. Indic. 2022, 142, 109293. [Google Scholar] [CrossRef]
- Poikane, S.; Kelly, M.G.; Várbíró, G.; Borics, G.; Erős, T.; Hellsten, S.; Kolada, A.; Lukács, B.A.; Solheim, A.L.; López, J.P.; et al. Estimating nutrient thresholds for eutrophication management: Novel insights from understudied lake types. Sci. Total Environ. 2022, 827, 154242. [Google Scholar] [CrossRef]
- Zhao, F.; Zhan, X.; Xu, H.; Zhu, G.; Zou, W.; Zhu, M.; Kang, L.; Guo, Y.; Zhao, X.; Wang, Z.; et al. New insights into eutrophication management: Importance of temperature and water residence time. J. Environ. Sci. 2022, 111, 229–239. [Google Scholar] [CrossRef]
- Lai, C.; Ma, Z.; Liu, Z.; Sun, H.; Yu, Q.; Xia, F.; He, X.; Bao, Q.; Han, Y.; Liu, X.; et al. Alleviating eutrophication by reducing the abundance of Cyanophyta due to dissolved inorganic carbon fertilization: Insights from Erhai Lake, China. J. Environ. Sci. 2023, 131, 68–83. [Google Scholar] [CrossRef]
- Imchen, T.; Singh, K.S. Marine algae colorants: Antioxidant, anti-diabetic properties and applications in food industry. Algal Res. 2023, 69, 102898. [Google Scholar] [CrossRef]
- Shahri, E.; Sayadi, M.H.; Yousefi, E.; Savabieasfehani, M. Metal Contamination of Oman Sea Seaweed and Its Associated Public Health Risks. Biol. Trace Elem. Res. 2021, 200, 2989–2998. [Google Scholar] [CrossRef]
- Ruangrit, K.; Chaipoot, S.; Phongphisutthinant, R.; Duangjan, K.; Phinyo, K.; Jeerapan, I.; Pekkoh, J.; Srinuanpan, S. A successful biorefinery approach of macroalgal biomass as a promising sustainable source to produce bioactive nutraceutical and biodiesel. Biomass Convers. Biorefin. 2021, 13, 1089–1099. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Lebata-Ramos, M.J.H.L.; Solis, E.F.D. Can Ulva reticulata replace Gracilariopsis heteroclada as natural food for the abalone Haliotis asinina? J. Appl. Phycol. 2021, 33, 1869–1872. [Google Scholar] [CrossRef]
- Agrawal, K.; Bhatt, A.; Bhardwaj, N.; Kumar, B.; Verma, P. Algal Biomass: Potential Renewable Feedstock for Biofuels Production—Part I. In Biofuel Production Technologies: Critical Analysis for Sustainability. Clean Energy Production Technologies; Srivastava, N., Srivastava, M., Mishra, P., Gupta, V., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal biomass valorization for biofuel production and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2797–2851. [Google Scholar] [CrossRef]
- Salehi, B.; Berkay, Y.Y.; Antika, G.; Boyunegmez, T.T.; Mahomoodally, F.M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef]
- Polikovsky, M.; Califano, G.; Dunger, N.; Wichard, T.; Golberg, A. Engineering bacteria-seaweed symbioses for modulating the photosynthate content of Ulva (Chlorophyta): Significant for the feedstock of bioethanol production. Algal Res. 2020, 49, 101945. [Google Scholar] [CrossRef]
- Ashour, M.; Al-Souti, A.S.; Hassan, S.M.; Ammar, G.A.G.; Goda, A.M.A.-S.; El-Shenody, R.; Abomohra, A.E.-F.; El-Haroun, E.; Elshobary, M.E. Commercial Seaweed Liquid Extract as Strawberry Biostimulants and Bioethanol Production. Life 2023, 13, 85. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Rowland, A.P. Chemical Analysis. In Methods in Plant Ecology; Moore, P.D., Chapman, S.B., Eds.; Blackwell: London, UK, 1986; pp. 285–344. [Google Scholar]
- John, M.K. Colorimetric determination of phosphorus in soil and plant materials with ascorbic acid. Soil Sci. 1970, 109, 214–220. [Google Scholar] [CrossRef]
- Beljkaš, B.; Matić, J.; Milovanović, I.; Jovanov, P.; Mišan, A.; Šarić, L. Rapid method for determination of protein content in cereals and oilseeds: Validation, measurement uncertainty and comparison with the Kjeldahl method. Accredit. Qual. Assur. 2010, 15, 555–561. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Waste Water, 22nd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Total nitrogen. In Methods of Soil Analysis, 2nd ed.; Part II Chemical and Microbiological Properties; Agronomy No. 9. American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, 2nd ed.; Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy No. 9. American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor and Francis Group, LLC.: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Brevik, E.C.; Khaledian, Y.; El-Ramady, H. Assessing the complex links between soils and human health: An area of pressing need. Front. Soil Sci. 2021, 1, 731085. [Google Scholar] [CrossRef]
- Muller, G. Heavy-metals in sediment of the Rhine-changes since 1971. Umschau. Wissenschaft. Technik. 1979, 79, 778–783. [Google Scholar]
- Li, X.; Chi, W.; Tian, H.; Zhang, Y.; Zhu, Z. Probabilistic ecological risk assessment of heavy metals in western Laizhou Bay, Shandong Province, China. PLoS ONE 2019, 14, e0213011. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Peng, Z.; Guo, Z.; Wang, Z.; Zhang, R.; Wu, Q.; Gao, H.; Wang, Y.; Shen, Z.; Lek, S.; Xiao, J. Species-specific bioaccumulation and health risk assessment of heavy metal in seaweeds in tropic coasts of South China Sea. Sci. Total Environ. 2022, 832, 155031. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Asso1ciation of enhanced peroxidase activity with induced systemic resistance of cucumber, to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo-Nzymology; Kalyani Publishers: New Delhi, India, 1980; p. 286. [Google Scholar]
- ISO/IEC 17025, 2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization (ISO); The International Electrotechnical Commission (IEC): Geneva, Switzerland, 2017.
- Cardinali, A.; Nason, G.P. Costationarity of Locally Stationary Time Series Using Costat. J. Stat. Softw. 2013, 55, 1–22. Available online: http://www.jstatsoft.org/v55/i01/ (accessed on 9 January 2023). [CrossRef]
- Jaccard, P. New research on floral distribution. Bull. Soc. Vaud. Sci. Nat. 1908, 44, 223–270. [Google Scholar]
- Hammer, O.; Harper, D.A.; Ryan, P.D. Palaeontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Hamdy, R.; Langeneck, J.; Atta, M.M.; Dorgham, M.M.; El-Rashidy, H.H.; Musco, L. Diversity and ecology of crustaceans from shallow rocky habitats along the Mediterranean coast of Egypt. Mar. Biodivers. 2019, 49, 221–233. [Google Scholar] [CrossRef]
- Guidone, M.; Thornber, C.; Wysor, B.; O’Kelly, C.J. Molecular and morphological diversity of Narragansett Bay (RI, USA) Ulva (Ulvales, Chlorophyta) populations. J. Phycol. 2013, 49, 979–995. [Google Scholar] [CrossRef]
- Ismail, M.M.; Mohamed, S.E. Differentiation between some Ulva spp. by morphological, genetic and biochemical analyses. Vavilov. J. Genet. Breed. 2017, 21, 360–367. [Google Scholar] [CrossRef]
- Luk, S.Y.; Hoagland, P.; Rheuban, J.E.; Costa, J.E.; Doney, S.C. Modeling the effect of water quality on the recreational shell fishing cultural ecosystem service of Buzzards Bay, Massachusetts. Mar. Pollut. Bull. 2019, 140, 364–373. [Google Scholar] [CrossRef]
- Vea, E.B.; Bendtsen, J.; Richardson, K.; Ryberg, M.; Hauschild, M. Spatially differentiated marine eutrophication method for absolute environmental sustainability assessments. Sci. Total Environ. 2022, 843, 156873. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, B.; Yu, D.; Fan, Y.; An, D.; Pan, S. Monitoring the Spatio-Temporal Distribution of Ulva prolifera in the Yellow Sea (2020–2022) Based on Satellite Remote Sensing. Remote Sens. 2023, 15, 157. [Google Scholar] [CrossRef]
- Nukapothula, S.; Yunus, A.P.; Chen, C. Signals of intense primary production in response to Ulva prolifera bloom in the Yellow Sea during summer 2021. Phys. Chem. Earth Parts A/B/C 2022, 128, 103257. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, M.; Cui, Y.; Tian, L.; Yang, P.; Zhao, L.; Xue, M.; Liu, J. What causes the great green tide disaster in the South Yellow Sea of China in 2021? Ecol. Indic. 2022, 140, 108988. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, W.; Li, F.; Pan, Y.; Wang, C.; Tian, H. Occurrence, partition, and risk of seven heavy metals in sediments, seawater, and organisms from the eastern sea area of Shandong Peninsula, Yellow Sea, China. J. Environ. Manag. 2021, 279, 111771. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.T.; Neto, M.C.L.; Choueri, R.B.; Castro, I.B. Photoprotection and antioxidative metabolism in Ulva lactuca exposed to coastal oceanic acidification scenarios in the presence of Irgarol. Aquat. Toxicol. 2021, 230, 105717. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Wu, J. Enhanced removal of phenolic endocrine disrupting chemicals from coastal waters by intertidal macroalgae. J. Hazard. Mater. 2021, 411, 125105. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, B.; Yao, Q.; Burnett, W.; Charette, M.; Su, R.; Lian, E.; Yu, Z. Nutrient-rich submarine groundwater discharge fuels the largest green tide in the world. Sci. Total Environ. 2021, 770, 144845. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, M.; Tanaka, K.; Yamasaki, T.; Miura, O. Replacement of Ulva ohnoi in the type locality under rapid ocean warming in southwestern Japan. J. Appl. Phycol. 2020, 32, 2489–2494. [Google Scholar] [CrossRef]
- Sun, K.; Sun, J.; Liu, Q.; Lian, Z.; Ren, J.S.; Bai, T.; Wang, Y.; Wei, Z. A numerical study of the Ulva prolifera biomass during the green tides in China-toward a cleaner Porphyra mariculture. Mar. Pollut. Bull. 2020, 161, 111805. [Google Scholar] [CrossRef]
- Wang, K.K.; Tian, Y.; Li, P.F.; Liu, C.Y.; Yang, G.P. Sources of nitric oxide during the outbreak of Ulva prolifera in coastal waters of the Yellow Sea off Qingdao. Mar. Environ. Res. 2020, 162, 105177. [Google Scholar] [CrossRef]
- Duplá, M.V. Eelgrass-associated mesograzers limit the distribution of bloom-forming Ulva via top-down control of its early life stages. Mar. Environ. Res. 2020, 161, 105061. [Google Scholar] [CrossRef]
- Balar, N.; Sharnagat, P.; Kumari, P.; Mantri, V.A. Variation in the proximate composition of edible marine macroalga Ulva rigida collected from different coastal zones of India. J. Food Sci. Technol. 2019, 56, 4749–4755. [Google Scholar] [CrossRef]
- Samanta, P.; Shin, S.; Jang, S.; Song, Y.C.; Oh, S.; Kim, J.K. Stable carbon and nitrogen isotopic characterization and tracing nutrient sources of Ulva blooms around Jeju coastal areas. Environ. Pollut. 2019, 254, 113033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, J.; Wu, J.; Luo, Y. Phycoremediation of coastal waters contaminated with bisphenol A by green tidal algae Ulva prolifera. Sci. Total Environ. 2019, 661, 55–62. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Nafea, E.M.A.; Zyada, M.A. Biomonitoring of heavy metals pollution in Lake Burullus, Northern Delta, Egypt. Afr. J. Environ. Sci. Technol. 2015, 9, 1–7. [Google Scholar]
- Bhagat, C.; Kumar, M.; Mahlknecht, J.; Hdeib, R.; Mohapatra, P.K. Seawater intrusion decreases the metal toxicity but increases the ecological risk and degree of treatment for coastal groundwater: An Indian perspective. Environ. Pollut. 2022, 310, 119771. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Xu, H.; Zhang, X. Spatial and seasonal characteristics of dissolved heavy metals in the surface seawater of the Yellow River Estuary, China. Mar. Pollut. Bull. 2018, 137, 465–473. [Google Scholar] [CrossRef]
- Arikibe, J.E.; Prasad, S. Determination and comparison of selected heavy metal concentrations in seawater and sediment samples in the coastal area of Suva, Fiji. Mar. Pollut. Bull. 2020, 157, 111157. [Google Scholar] [CrossRef]
- Nour, H.E.; El-Sorogy, A.S. Heavy metals contamination in seawater, sediments and seashells of the Gulf of Suez, Egypt. Environ. Earth Sci. 2020, 79, 274. [Google Scholar] [CrossRef]
- Liu, J.; Xia, J.; Zhuang, M.; Zhang, J.; Yu, K.; Zhao, S.; Sun, Y.; Tong, Y.; Xia, L.; Qin, Y.; et al. Controlling the source of green tides in the Yellow Sea: NaClO treatment of Ulva attached on Pyropia aquaculture rafts. Aquaculture 2021, 535, 736378. [Google Scholar] [CrossRef]
- Nour, H.E.; El-Sorogy, A.S.; Abd El-Wahab, M.; Mohamaden, M.; Al-Kahtany, K. Contamination and ecological risk assessment of heavy metals pollution from the Shalateen coastal sediments, Red Sea, Egypt. Mar. Pollut. Bull. 2019, 144, 167–172. [Google Scholar] [CrossRef]
- Soliman, N.F.; Nasr, S.M.; Okbah, M.A. Potential ecological risk of heavy metals in sediments from the Mediterranean coast, Egypt. J. Environ. Health Sci. Eng. 2015, 13, 70. [Google Scholar] [CrossRef]
- CCME (Canadian Council of Ministers of the Environment). Protocol for the Derivation of Canadian Sediment Quality Guidelines for the Protection of Aquatic Life; CCME EPC-98E; CCME: Winnipeg, MB, Canada, 1998. [Google Scholar]
- Kanwischer, M.; Asker, N.; Wernersson, A.S.; Wirth, M.A.; Fisch, K.; Dahlgren, E.; Osterholz, H.; Habedank, F.; Naumann, M.; Mannio, J.; et al. Substances of emerging concern in Baltic Sea water: Review on methodological advances for the environmental assessment and proposal for future monitoring. Ambio 2022, 51, 1588–1608. [Google Scholar] [CrossRef]
- El Zokm, G.M.; Ibrahim, M.I.; Mohamed, L.A.; El-Mamoney, M. Critical geochemical insight into Alexandria coast with special reference to diagnostic ratios (TOC/TN & Sr/Ca) and heavy metals ecotoxicological hazards. Egypt. J. Aquat. Res. 2020, 46, 27–33. [Google Scholar] [CrossRef]
- Aitta, A.; El-Ramady, H.; Alshaal, T.; El-Henawy, A.; Shams, M.; Talha, N.; Elbehiry, F.; Brevik, E.C. Seasonal and spatial distribution of soil trace elements around Kitchener drain in the northern Nile Delta, Egypt. Agriculture 2019, 9, 152. [Google Scholar] [CrossRef]
- Bibak, M.; Sattari, M.; Tahmasebi, S.; Kafaei, R.; Sorial, G.A.; Ramavandi, B. Trace and major elements concentration in fish and associated sediment–seawater, northern shores of the Persian Gulf. Biol. Trace Elem. Res. 2021, 199, 2717–2729. [Google Scholar] [CrossRef]
- Njock, P.G.A.; Zhou, A.; Yin, Z.; Shen, S.L. Integrated risk assessment approach for eutrophication in coastal waters: Case of Baltic Sea. J. Clean. Prod. 2023, 387, 135673. [Google Scholar] [CrossRef]
- Ahmed, O.E.; Mahmoud, S.A.; El Nady, M.M. Organic sources in the Egyptian seawater around Alexandria coastal area as integrated from polycyclic aromatic hydrocarbons (PAHs). Egypt. J. Pet. 2017, 26, 819–826. [Google Scholar] [CrossRef]
- El-Naggar, N.A.; Emara, H.I.; Moawad, M.N.; Soliman, Y.A.; El-Sayed, A.A. Detection of polycyclic aromatic hydrocarbons along Alexandria’s coastal water, Egyptian Mediterranean Sea. Egypt. J. Aquat. Res. 2018, 44, 9–14. [Google Scholar] [CrossRef]
- Khaled, A.; Ahdy, H.H.; El Sayed, A.E.; Ahmed, H.O.; Razek, F.A.A.; Fahmy, M.A. Spatial distribution and potential risk assessment of heavy metals in sediment along Alexandria Coast, Mediterranean Sea, Egypt. Egypt. J. Aquat. Res. 2021, 47, 37–43. [Google Scholar] [CrossRef]
- Jadoon, W.A.; Abdel-Dayem, S.M.M.A.; Saqib, Z.; Takeda, K.; Sakugawa, H.; Hussain, M.; Shah, G.M.; Rehman, W.; Syed, J.H. Heavy metals in urban dusts from Alexandria and Kafr El-Sheikh, Egypt: Implications for human health. Environ. Sci. Pollut. Res. 2021, 28, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, F.; Elbasiouny, H.; Ali, R.; Brevik, E.C. Enhanced Immobilization and Phytoremediation of Heavy Metals in Landfill Contaminated Soils. Water Air Soil Pollut. 2020, 231, 204. [Google Scholar] [CrossRef]
- Radziemska, M. Study of applying naturally occurring mineral sorbents of Poland (dolomite halloysite, chalcedonite) for aided phytostabilization of soil polluted with heavy metals. Catena 2018, 163, 123–129. [Google Scholar] [CrossRef]
- Alvarez-Mateos, P.; Ales-Alvarez, F.; Garcia-Martin, J.F. Phytoremediation of highly contaminated mining soils by Jatropha curcas L. and production of catalytic carbons from the generated biomass. J. Environ. Manag. 2019, 231, 886–895. [Google Scholar] [CrossRef]
- Bednářová, Z.; Kalina, J.; Hájek, O.; Sáňka, M.; Komprdová, K. Spatial distribution and risk assessment of metals in agricultural soils. Geoderma 2016, 284, 113–121. [Google Scholar] [CrossRef]
- Jitar, O.; Teodosiu, C.; Oros, A.; Plavan, G.; Nicoara, M. Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. New Biotechnol. 2015, 32, 369–378. [Google Scholar] [CrossRef]
- Trevizani, T.H.; Figueira, R.C.L.; Ribeiro, A.P.; Theophilo, C.Y.S.; Majer, A.P.; Petti, M.A.V.; Corbisier, T.N.; Montone, R.C. Bioaccumulation of heavy metals in marine organisms and sediments from Admiralty Bay, King George Island, Antarctica. Mar. Pollut. Bull. 2016, 106, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerdà, A. The effect of soil on human health: An overview. Eur. J. Soil Sci. 2018, 69, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.G.; Gao, Y.P.; Chen, F.; Huang, H.H.; Yu, S.H.; Jordan, R.W.; Jiang, S.J. Risk assessment of heavy metal and pesticide mixtures in aquatic biota using the DGT technique in sediments. Water Res. 2022, 224, 119108. [Google Scholar] [CrossRef]
- Sun, J.; Zhai, N.; Miao, J.; Mu, H.; Li, W. How do heterogeneous environmental regulations affect the sustainable development of marine green economy? Empirical evidence from China’s coastal areas. Ocean Coast. Manag. 2023, 232, 106448. [Google Scholar] [CrossRef]
- Ji, M.; Li, B.; Majdi, A.; Alkhalifah, T.; Alturise, F.; Ali, H.E. Application of nano remediation of mine polluted in acid mine drainage water using machine learning model. Chemosphere 2023, 311, 136926. [Google Scholar] [CrossRef]
- Bonanno, G.; Veneziano, V.; Piccione, V. The alga Ulva lactuca (Ulvaceae, Chlorophyta) as a bioindicator of trace element contamination along the coast of Sicily, Italy. Sci. Total Environ. 2020, 699, 134329. [Google Scholar] [CrossRef]

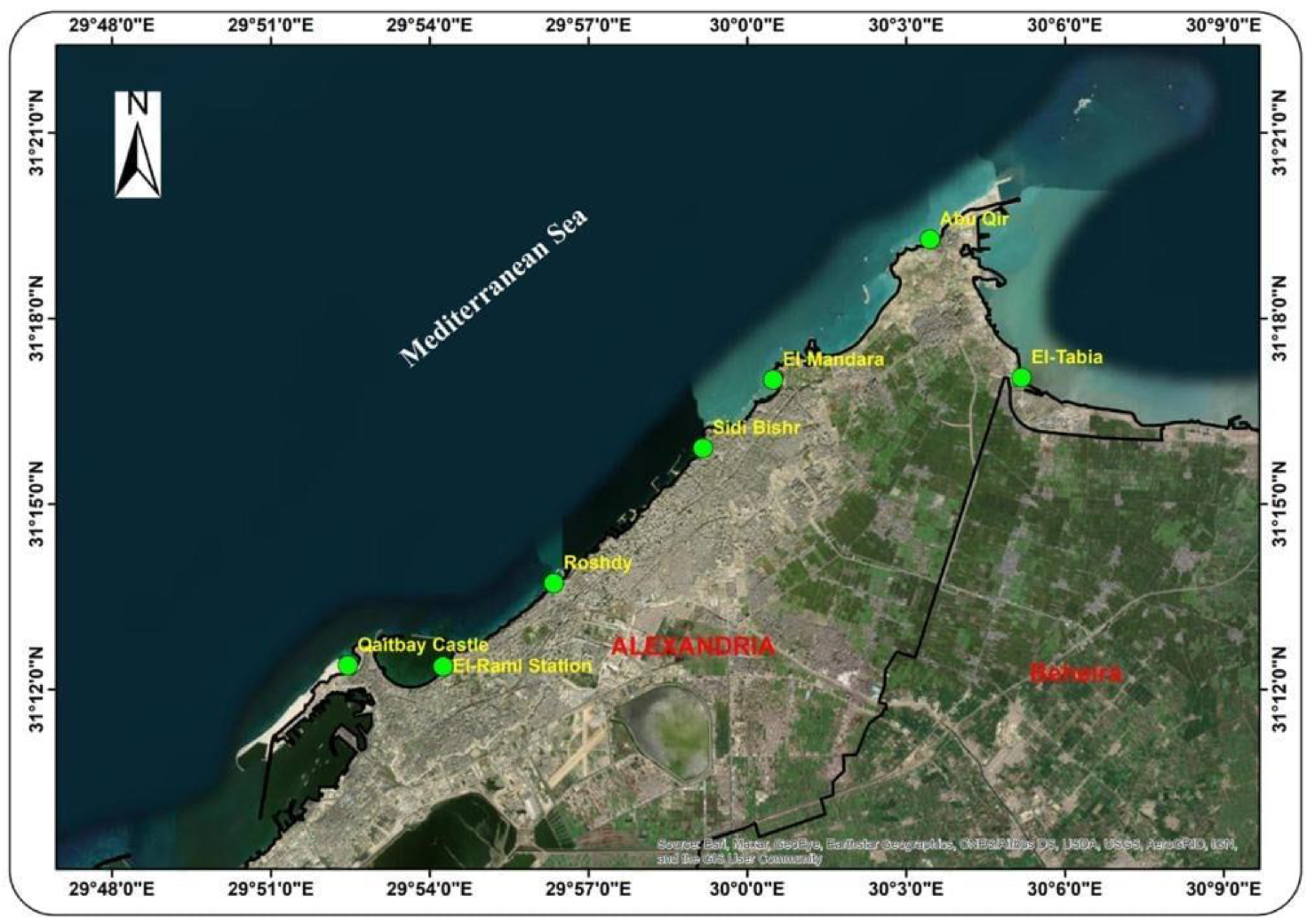


| Location | Code | Description of Location |
|---|---|---|
| El-Tabia | L1 | The main drain for this station is the El-Amia, where industrial wastes from El-Tabia Pumping Station (average 1.5–2.0 million m3 day−1) are discharged to Abu-Qir Bay, which is a semi-circular basin. |
| Abu Qir | L2 | This location is a beach at the north of the town and near the Abu Qir port. There are no wastes flowing to the beach. |
| El-Mandara | L3 | This location is a private beach with no wastes flowing to it. |
| Sidi Bishr | L4 | This location is a very crowded beach with no wastes flowing to it. An attempt to protect the beach using cement blocks can be seen in Figure 1. |
| Roshdy | L5 | This location is a rocky shoreline with no wastes flowing to it. |
| El-Raml Station | L6 | This location is a rocky shoreline with no wastes flowing to it. |
| Qaitbay Castle | L7 | This location is a rocky shoreline with no wastes flowing to it. |
| Site | N | P | K | Mg | Ca | Fe | Cu | Cd | Co | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 1090 e | 5810 a | 7670 h | 15,440 c | 6670 c | 4437 a | 26.7 a | 4.6 d | 20.9 a | 24.6 d | 31.3 b | 19.5 a |
| L2 | 890 g | 3100 e | 7702 g | 14,000 e | 8000 b | 1563 c | 10.2 b | 5.8 cd | 18.4 c | 23.9 e | 30.7 c | 14.3 g |
| L3 | 3360 a | 4610 c | 13,130 c | 17,820 a | 5330 d | 1004 d | 10.8 b | 5.3 b | 20.4 a | 33.8 a | 35.8 a | 16.3 b |
| L4 | 1940 d | 2960 g | 10,260 d | 15,660 c | 12,000 a | 533 h | 9.6 c | 5.2 cd | 17.5 d | 29.9 b | 28.6 e | 14.2 h |
| L5 | 2740 c | 4210 d | 13,280 b | 15,680 c | 5330 d | 739 g | 13.3 b | 12.8 a | 19.3 b | 29 b | 30.5 bc | 15.1 d |
| L6 | 860 g | 4570 c | 8130 e | 14,800 d | 400 e | 3940 b | 11 b | 5.8 c | 19.3 b | 27.2 c | 30.1 c | 15.3 c |
| L7 | 3230 b | 5220 b | 20,820 a | 16,180 b | 33,330 f | 966 e | 7.7 d | 5.5 c | 18.4 c | 26.8 c | 29.7 d | 14.8 e |
| Primer Name | Nucleotide Sequence | Total Bands | Monomorphic Bands | Polymorphic Bands | Polymorphism (%) |
|---|---|---|---|---|---|
| OPC-04 | CCGCATCTAC | 9 | 0 | 9 | 100 |
| OPC-08 | TGGACCGGTG | 11 | 0 | 11 | 100 |
| OPC-10 | TGTCTGGGTG | 10 | 0 | 10 | 100 |
| OPD-12 | CACCGTATCC | 12 | 0 | 12 | 100 |
| OPF-08 | GGGATATCGG | 10 | 1 | 9 | 90 |
| OPV-02 | AGTCACTCCC | 9 | 0 | 9 | 100 |
| Site | N | P | K | Mg | Ca | Fe | Cu | Cd | Co | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 0.182 e | 0.227 a | 455 e | 371 d | 176 g | 9.86 c | 0.263 b | 0.433 c | 2.20 b | 3.30 c | 3.69 b | 1.46 d |
| L2 | 0.226 d | 0.000 c | 870 bc | 372 cd | 310 d | 3.88 e | 0.227 b | 0.507 a | 2.38 a | 3.81 a | 4.11 a | 1.42 e |
| L3 | 3.361 a | 0.000 c | 856 d | 375 bc | 192 f | 5.57 d | 0.227 b | 0.480 b | 1.63 d | 3.75 a | 4.11 a | 1.68 b |
| L4 | 0.241 c | 0.000 c | 1031 a | 384 a | 860 b | 12.78 b | 0.250 b | 0.497 ab | 1.85 c | 3.71 a | 4.29 a | 1.69 b |
| L5 | 0.388 b | 0.013 b | 891 b | 373 bcd | 900 a | 23.67 a | 0.260 b | 0.463 bc | 1.75 cd | 3.37 bc | 3.87 b | 1.52 c |
| L6 | 0.211 d | 0.013 b | 885 bc | 376 b | 664 c | 3.46 g | 0.220 b | 0.443 c | 1.62 d | 3.23 c | 3.79 b | 1.35 f |
| L7 | 0.246 c | 0.000 c | 870 c | 376 b | 216 e | 3.70 f | 0.503 a | 0.507 a | 1.45 e | 3.44 b | 3.85 b | 2.25 a |
| Site | N | P | Mg | Ca | Fe | Cu | Cd | Co | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 5950 b | 1585 b | 8402 e | 180 c | 1578 d | 9.2 b | 4.0 a | 14.6 a | 23.9 a | 27.2 a | 117.7 c |
| L2 | 6700 ab | 755 f | 4223 g | 080 e | 9656 h | 1.7 d | 3.4 d | 13 b | 19.5 d | 20.2 e | 78.1 e |
| L3 | 5900 b | 1204 c | 8880 d | 200 b | 1360 e | 1.2 d | 3.8 c | 13.6 b | 20.7 c | 21.3 c | 100.2 d |
| L4 | 7050 a | 338 g | 9148 c | 180 c | 1000 g | 6.3 c | 2.2 g | 9.5 d | 16.4 e | 15.0 h | 94.1 d |
| L5 | 5780 c | 1951 a | 10,844 b | 120 d | 1698 c | 6.3 c | 3.7 e | 12.4 bc | 19.4 cd | 20.9 d | 121.2 b |
| L6 | 7250 a | 847 e | 11,546 a | 200 b | 2045 a | 13.5 a | 4.8 b | 14.6 a | 22.5 b | 25.0 b | 149.5 a |
| L7 | 5660 c | 994 d | 8814 d | 1280 a | 1799 b | 5.4 c | 3.9 cd | 12.1 c | 19.0 d | 16.3 g | 120.5 b |
| Site | Cu | Cd | Co | Fe | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|
| Contamination Factor (CF) | |||||||
| L1 | 0.37 b ± 0.01 | 9.21 a ± 0.1 | 1.50 a ± 0.04 | 0.05 c ± 0.001 | 0.87 a ± 0.02 | 0.93 a ± 0.02 | 1.68 c ± 0.01 |
| L2 | 0.07 d ± 0.1 | 7.29 d ± 0.1 | 1.30 b ± 0.03 | 0.03 d ± 0.001 | 0.73 d ± 0.01 | 0.70 d ± 0.01 | 1.12 f ± 0.01 |
| L3 | 0.05 d ± 0.1 | 7.55 c ± 0.1 | 1.34 b ± 0.03 | 0.05 c ± 0.001 | 0.75 c ± 0.01 | 0.76 c ± 0.01 | 1.43 d ± 0.02 |
| L4 | 0.25 c ± 0.03 | 4.84 e ± 0.1 | 1.00 d ± 0.01 | 0.03 d ± 0.001 | 0.61 e ± 0.03 | 0.52 f ± 0.01 | 1.34 e ± 0.01 |
| L5 | 0.25 c ± 0.04 | 6.93 e ± 0.2 | 1.29 b ± 0.1 | 0.06 b ± 0.001 | 0.73 d ± 0.01 | 0.71 d ± 0.02 | 1.73 b ± 0.01 |
| L6 | 0.54 a ± 0.03 | 8.36 b ± 0.1 | 1.46 a ± 0.01 | 0.07 a ± 0.002 | 0.83 b ± 0.01 | 0.87 b ± 0.03 | 2.14 a ± 0.02 |
| L7 | 0.22 c ± 0.03 | 7.39 d ± 0.1 | 1.25 c ± 0.02 | 0.06 b ± 0.001 | 0.72 d ± 0.01 | 0.56 e ± 0.02 | 1.72 b ± 0.01 |
| Geo-accumulation index (Igeo) | |||||||
| L1 | 0.34 b ± 0.01 | 8.66 a ± 0.08 | 1.40 a ± 0.04 | 0.05 b ± 0.001 | 0.82 a ± 0.02 | 0.88 a ± 0.01 | 1.58 c ± 0.01 |
| L2 | 0.06 d ± 0.02 | 6.85 d ± 0.1 | 1.22 bc ± 0.03 | 0.03 d ± 0.001 | 0.68 d ± 0.01 | 0.65 d ± 0.01 | 1.05 f ± 0.01 |
| L3 | 0.04 d ± 0.01 | 7.10 c ± 0.1 | 1.26 b ± 0.02 | 0.04 c ± 0.001 | 0.71 d ± 0.01 | 0.71 c ± 0.02 | 1.34 d ± 0.02 |
| L4 | 0.24 c ± 0.03 | 4.55 f ± 0.1 | 0.94 e ± 0.01 | 0.03 d ± 0.001 | 0.57 e ± 0.1 | 0.49 f ± 0.02 | 1.26 e ± 0.01 |
| L5 | 0.24 c ± 0.04 | 6.52 e ± 0.1 | 1.22 c ± 0.1 | 0.05 b ± 0.001 | 0.69 d ± 0.01 | 0.67 d ± 0.02 | 1.63 b ± 0.01 |
| L6 | 0.51 a ± 0.02 | 7.86 b ± 0.1 | 1.37 a ± 0.1 | 0.06 a ± 0.001 | 0.78 b ± 0.01 | 0.82 b ± 0.03 | 2.01 a ± 0.02 |
| L7 | 0.20 c ± 0.03 | 6.94 d ± 0.1 | 1.18 d ± 0.03 | 0.06 a ± 0.001 | 0.68 d ± 0.02 | 0.53 e ± 0.02 | 1.62 b ± 0.01 |
| Sites | Cu | Cd | Co | Fe | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|
| Bioaccumulation factor (BAF)seawater | |||||||
| L1 | 105 a ± 20.4 | 11.4 bc ± 0.5 | 9.16 c ± 0.5 | 44.99 b ± 0.5 | 7.45 d ± 0.3 | 8.46 a ± 0.3 | 133 a ± 1.5 |
| L2 | 46.17 b ± 9.5 | 10.05 d ± 0.4 | 7.72 d ± 0.2 | 40.33 c ± 0.2 | 6.11 e ± 0.4 | 7.41 c ± 0.2 | 100 c ± 2.2 |
| L3 | 47.67 b ± 1.5 | 12.36 b ± 0.2 | 12.53 b ± 1.1 | 18.03 e ± 0.2 | 8.97 a ± 0.4 | 8.59 a ± 0.1 | 97.1 d ± 0.5 |
| L4 | 38.74 b ± 2.6 | 10.11 d ± 0.1 | 9.47 c ± 0.5 | 4.18 f ± 0.1 | 7.85 c ± 0.2 | 6.75 d ± 0.5 | 84.5 e ± 0.7 |
| L5 | 51.10 b ± 2.7 | 26.10 a ± 1.1 | 11.07 b ± 0.4 | 3.12 e ± 0.1 | 8.7 ab ± 0.2 | 7.94 b ± 0.1 | 99.5 c ± 0.8 |
| L6 | 50.14 b ± 6.2 | 11.71 b ± 0.7 | 11.94 ab ± 0.6 | 113.9 a ± 3.5 | 8.4 b ± 0.1 | 8.03 b ± 0.2 | 113 b ± 0.5 |
| L7 | 15.26 c ± 1.0 | 10.57 cd ± 0.5 | 12.68 b ± 0.5 | 26.15 d ± 0.2 | 7.64 cd ± 0.3 | 7.65 bd ± 0.1 | 66.1 f ± 0.2 |
| Bioaccumulation factor (BAF)sediment | |||||||
| L1 | 2.92 b ± 0.1 | 1.08 f ± 0.1 | 1.35 d ± 0.03 | 0.28 a ± 0.001 | 1.04 f ± 0.03 | 1.16 e ± 0.05 | 1.66 b ± 0.01 |
| L2 | 6.20 ab ± 1.6 | 1.40 d ± 0.05 | 1.42 c ± 0.06 | 0.16 a ± 0.001 | 1.19 e ± 0.08 | 1.51 d ± 0.02 | 1.84 a ± 0.02 |
| L3 | 14.86 a ± 4.5 | 1.57 c ± 0.01 | 1.53 b ± 0.05 | 0.07 a ± 0.001 | 1.66 b ± 0.03 | 1.61 c ± 0.03 | 1.63 c ± 0.02 |
| L4 | 1.55 b ± 0.2 | 2.07 b ± 0.01 | 1.76 a ± 0.02 | 0.05 a ± 0.001 | 1.76 a ± 0.02 | 1.91 a ± 0.04 | 1.52 d ± 0.01 |
| L5 | 2.14 b ± 0.4 | 3.49 a ± 0.06 | 1.49 b ± 0.01 | 0.04 a ± 0.001 | 1.48 c ± 0.03 | 1.49 d ± 0.03 | 1.25 e ± 0.01 |
| L6 | 0.81 b ± 0.1 | 1.24 e ± 0.02 | 1.33 d ± 0.02 | 0.19 a ± 0.001 | 1.22 e ± 0.02 | 1.20 e ± 0.05 | 1.03 f ± 0.01 |
| L7 | 1.44 b ± 0.3 | 1.45 d ± 0.01 | 1.48 bc ± 0.03 | 0.05 a ± 0.001 | 1.36 d ± 0.02 | 1.80 b ± 0.06 | 1.23 e ± 0.03 |
| Cu | Cd | Co | Fe | Ni | Pb | Zn | Mg | Ca | N | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | 1 | ||||||||||
| Cd | 0.68 ** | 1 | |||||||||
| Co | 0.78 ** | 0.88 ** | 1 | ||||||||
| Fe | 0.79 ** | 0.46 ** | 0.70 ** | 1 | |||||||
| Ni | 0.72 ** | 0.88 ** | 0.99 ** | 0.62 ** | 1 | ||||||
| Pb | 0.78 ** | 0.87 ** | 0.99 ** | 0.67 ** | 0.99 ** | 1 | |||||
| Zn | 0.85 ** | 0.84 ** | 0.98 ** | 0.76 ** | 0.96 ** | 0.97 ** | 1 | ||||
| Mg | 0.80 ** | 0.85 ** | 0.96 ** | 0.63 ** | 0.96 ** | 0.96 ** | 0.97 ** | 1 | |||
| Ca | 0.59 ** | 0.56 ** | 0.61 ** | 0.27 * | 0.59 ** | 0.61 ** | 0.59 ** | 0.69 ** | 1 | ||
| N | 0.19 | 0.38 ** | 0.47 ** | 0.28 * | 0.54 ** | 0.47 ** | 0.49 ** | 0.37 ** | −0.22 | 1 | |
| P | 0.80 ** | 0.77 ** | 0.85 ** | 0.65 ** | 0.81 ** | 0.85 ** | 0.84 ** | 0.89 ** | 0.70 ** | 0.032 | 1 |
| Country | The Coastal Region (Sea or Ocean) | Brief Description of Study | Dominant Species of Ulva | Reference |
|---|---|---|---|---|
| Egypt | Alexandria (Mediterranean Sea) | Evaluation of pollution in an area affected by green tides | U. lactuca, U. compressa, U. fasciata, U. linzea | Current study |
| China | Yellow Sea | Monitoring distribution of U. prolifera during 2020–2022 using remote sensing | Ulva prolifera | Wang et al. [67] |
| China | Yellow Sea | Largest U. prolifera bloom coverage in this sea was observed during June/July 2021 | Ulva prolifera | Nukapothula et al. [68] |
| China | South Yellow Sea | Green tide reached its largest scale in history during 2021 | Ulva prolifera | Zheng et al. [69] |
| China | Jiangsu Province (Yellow Sea) | Mitigation of green tides | Ulva prolifera | Liu et al. [70] |
| Brazil | Itanhaém, Sao Paulo (South Atlantic Ocean) | U. lactuca exposed to laboratory coastal oceanic acidification | Ulva lactuca | Sousa et al. [71] |
| China | Yantai City (Yellow Sea) | Pollution due to phenolic endocrine-disrupting compounds, EDCs | Ulva pertusa, U. prolifera | Zhang et al. [72] |
| China | Subei Shoal (Jiangsu Province, Yellow Sea) | Pollution due to nutrient-rich submarine groundwater discharge | Ulva prolifera | Zhao et al. [73] |
| Japan | Tosa Bay, Kochi (Pacific Ocean) | Mitigation of green tides | Ulva ohnoi, U. reticulata | Hiraoka et al. [74] |
| China | Subei Shoal (Yellow Sea) | Mitigation of green tides | Ulva prolifera | Sun et al. [75] |
| China | Qingdao (Yellow Sea) | Pollution by nitric oxide (NO) | Ulva prolifera | Wang et al. [76] |
| USA | Elkhorn Slough, Monterey bay, California | Mitigation of green tides | U. intestinalis, U. lactuca | Duplá [77] |
| India | Gujarat and others | Mitigation of green tides | Ulva rigida | Balar et al. [78] |
| South Korea | Jeju coast (Bangdu Bay) | Polluted with high nitrate concentration (NO3−) due to sewage discharge | Ulva spp. | Samanta et al. [79] |
| China | Rushan city (Yellow Sea) | Polluted with bisphenol A (BPA) | Ulva prolifera | Zhang et al. [80] |
| Kabata- Pendias [48] | Wang et al. [84] | Arikibe and Prasad [85] | Nour and El-Sorogy [86] | Liu et al. [87] | Current Study | |
|---|---|---|---|---|---|---|
| Seawater | ||||||
| Cd | 0.0001 | 0.17–1.55 | 0.15–0.25 | 0.013–0.13 | 0.08–0.73 | 0.433–0.507 |
| Co | 0.00001 | - | - | - | - | 1.45–2.38 |
| Cu | 0.0002 | 0.04–31.0 | 0.88–10.29 | 0.43–0.62 | 0.83–5.38 | 0.227–0.503 |
| Fe | 0.001 | - | - | 1.97–2.45 | - | 3.70–23.67 |
| Ni | 0.0005 | - | 0.23–0.80 | 0.003–0.008 | - | 3.30–3.81 |
| Pb | 0.00003 | 0.42–7.25 | 0.88–1.76 | 0.08–1.80 | 0.52–3.60 | 3.69–4.11 |
| Zn | 0.00003 | 1.97–42.2 | 0.08–1.45 | 0.22–0.25 | 2.22–40.7 | 1.42–2.25 |
| Sediments | ||||||
| Arikibe and Prasad [85] | Nour and El-Sorogy [86] | Liu et al. [87] | Nour et al. [88] | Soliman et al. [89] | Current study | |
| Cd | 5.49–9.16 | 0.55 | 0.08–0.23 | 0.53 | 0.04–0.47 | 2.2–4.8 |
| Co | - | - | - | - | 0.43–26.39 | 9.5–14.6 |
| Cu | 78.43–490.18 | 5.10 | 13.5–26.0 | 9.43 | 0.46–26.26 | 17–13.5 |
| Fe | - | 2384 | - | 8451 | 243–38,045 | 1000–2045 |
| Ni | 17.24–28.74 | 2.87 | - | 17.5 | 1.65–60.25 | 16–23 |
| Pb | 116.96–233.92 | 17.3 | 11.0–20.8 | 11.4 | 3.34–53.67 | 15–27 |
| Zn | 16.00–68.78 | 22.4 | 17.6–34.1 | 44.2 | 2.05–62.21 | 78–149 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mahrouk, M.E.; Dewir, Y.H.; Hafez, Y.M.; El-Banna, A.; Moghanm, F.S.; El-Ramady, H.; Mahmood, Q.; Elbehiry, F.; Brevik, E.C. Assessment of Bioaccumulation of Heavy Metals and Their Ecological Risk in Sea Lettuce (Ulva spp.) along the Coast Alexandria, Egypt: Implications for Sustainable Management. Sustainability 2023, 15, 4404. https://doi.org/10.3390/su15054404
El-Mahrouk ME, Dewir YH, Hafez YM, El-Banna A, Moghanm FS, El-Ramady H, Mahmood Q, Elbehiry F, Brevik EC. Assessment of Bioaccumulation of Heavy Metals and Their Ecological Risk in Sea Lettuce (Ulva spp.) along the Coast Alexandria, Egypt: Implications for Sustainable Management. Sustainability. 2023; 15(5):4404. https://doi.org/10.3390/su15054404
Chicago/Turabian StyleEl-Mahrouk, Mohammed E., Yaser H. Dewir, Yaser M. Hafez, Antar El-Banna, Farahat S. Moghanm, Hassan El-Ramady, Qaisar Mahmood, Fathy Elbehiry, and Eric C. Brevik. 2023. "Assessment of Bioaccumulation of Heavy Metals and Their Ecological Risk in Sea Lettuce (Ulva spp.) along the Coast Alexandria, Egypt: Implications for Sustainable Management" Sustainability 15, no. 5: 4404. https://doi.org/10.3390/su15054404
APA StyleEl-Mahrouk, M. E., Dewir, Y. H., Hafez, Y. M., El-Banna, A., Moghanm, F. S., El-Ramady, H., Mahmood, Q., Elbehiry, F., & Brevik, E. C. (2023). Assessment of Bioaccumulation of Heavy Metals and Their Ecological Risk in Sea Lettuce (Ulva spp.) along the Coast Alexandria, Egypt: Implications for Sustainable Management. Sustainability, 15(5), 4404. https://doi.org/10.3390/su15054404












