Living with Contamination: Insights into an Epigeic Macrofaunal Community in an Area Extremely Polluted by Risk Elements
Abstract
1. Introduction
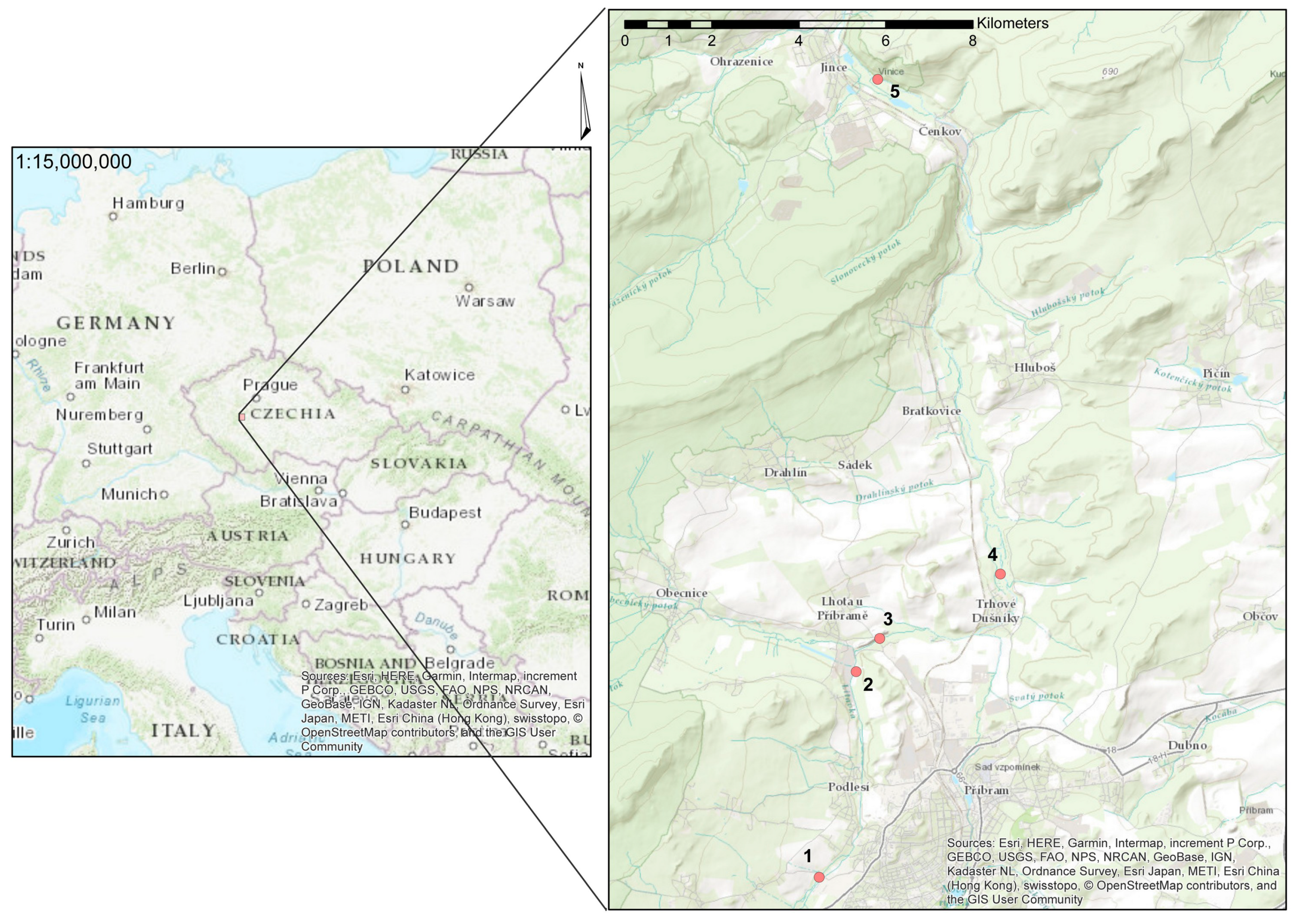
2. Materials and Methods
3. Results
3.1. Risk Elements
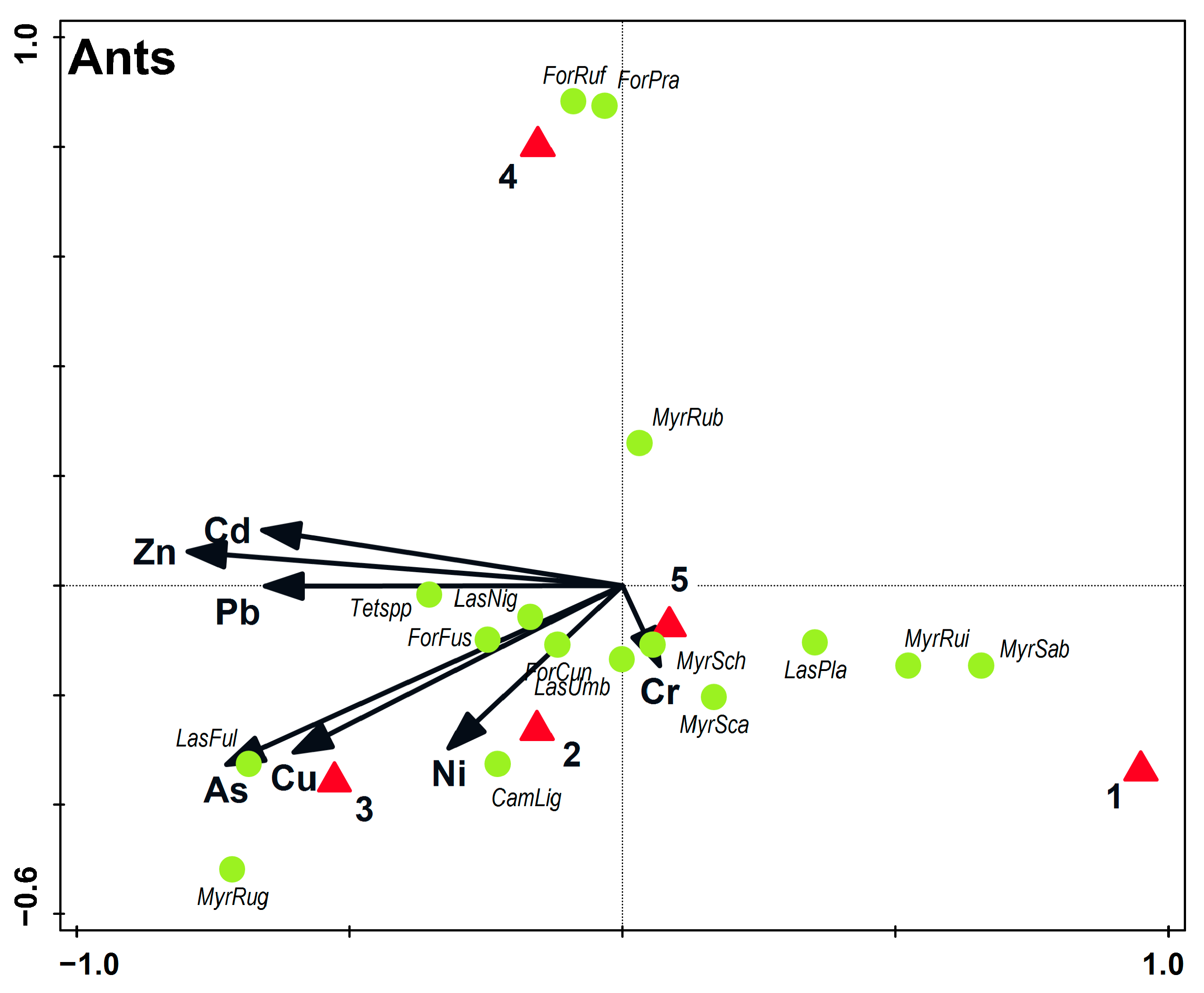
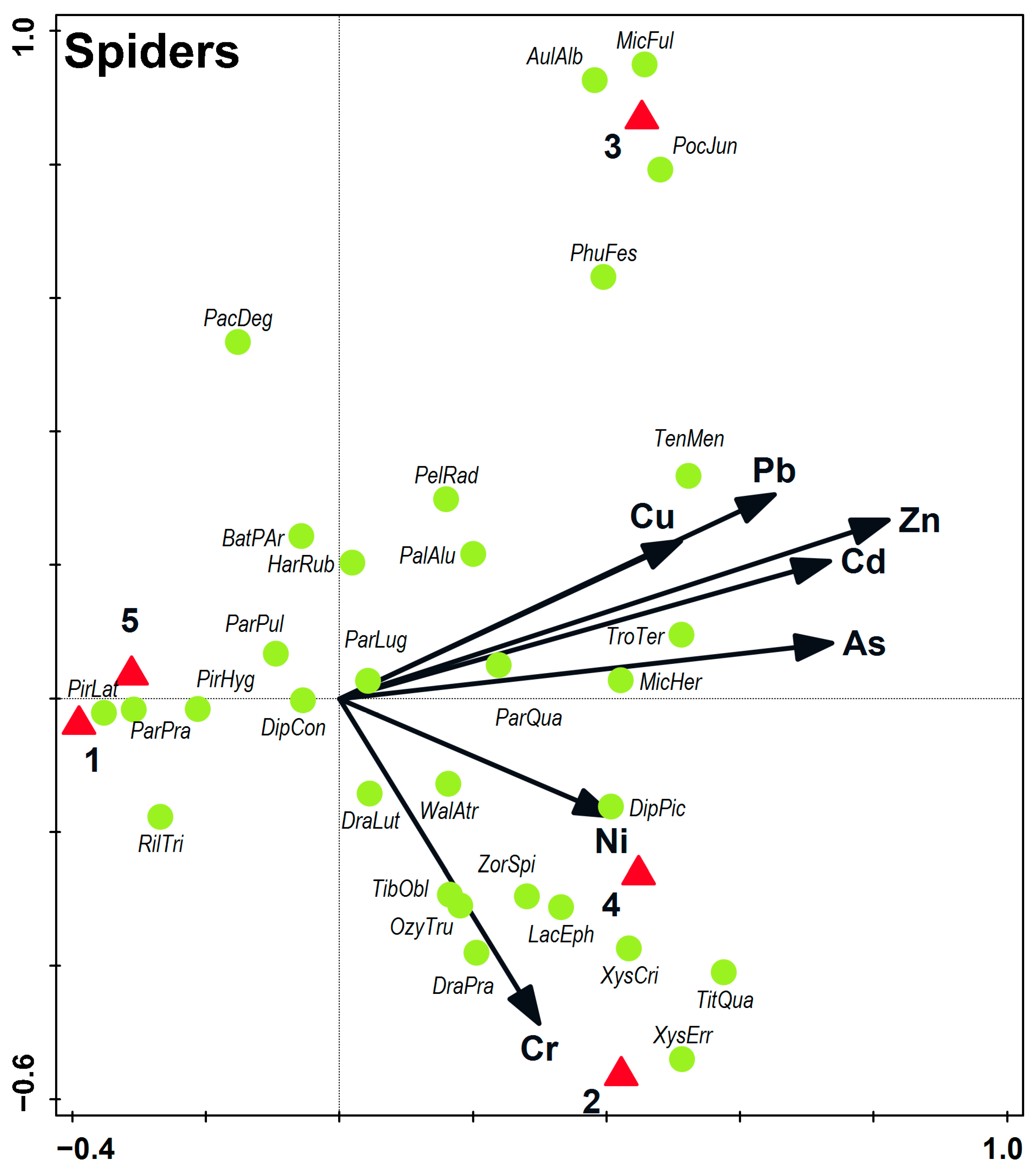
3.2. Soil Macrofauna
4. Discussion
4.1. Ants
4.2. Beetles
4.3. Spiders and Harvestmen
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dazzi, C.; lo Papa, G. A New Definition of Soil to Promote Soil Awareness, Sustainability, Security and Governance. Int. Soil Water Conserv. Res. 2022, 10, 99–108. [Google Scholar] [CrossRef]
- Vink, R.; Behrendt, H.; Salomons, W. Development of the Heavy Metal Pollution Trends in Several European Rivers: An Analysis of Point and Diffuse Sources. Water Sci. Technol. 1999, 39, 215–223. [Google Scholar] [CrossRef]
- Kebonye, N.M.; Eze, P.N.; John, K.; Agyeman, P.C.; Němeček, K.; Borůvka, L. An In-Depth Human Health Risk Assessment of Potentially Toxic Elements in Highly Polluted Riverine Soils, Příbram (Czech Republic). Environ. Geochem. Health 2022, 44, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V.; Navrátil, T.; Mihaljevič, M.; Rohovec, J.; Zuna, M.; Šebek, O.; Strnad, L.; Hojdová, M. Mercury Deposition/Accumulation Rates in the Vicinity of a Lead Smelter as Recorded by a Peat De-posit. Atmos. Environ. 2008, 42, 5968–5977. [Google Scholar] [CrossRef]
- Paoletti, M.G.; Bressan, M. Soil Invertebrates as Bioindicators of Human Disturbance. Crit. Rev. Plant Sci. 2010, 15, 21–62. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Khan, Z.A.; Jeon, H.; Park, S. SPE Based Soil Processing and Aptasensor Inte-grated Detection System for Rapid on Site Screening of Arsenic Contamination in Soil. Ecotoxicol. Environ. Saf. 2020, 196, 110559. [Google Scholar] [CrossRef]
- Latif, A.; Sheng, D.; Sun, K.; Si, Y.; Azeem, M.; Abbas, A.; Bilal, M. Remediation of Heavy Metals Polluted Environment Using Fe-Based Nanoparticles: Mechanisms, Influencing Factors, and Environmental Implications. Environ. Pollut. 2020, 264, 114728. [Google Scholar] [CrossRef]
- Sterckeman, T.; Douay, F.; Proix, N.; Fourrier, H. Vertical Distribution of Cd, Pb and Zn in Soils near Smelters in the North of France. Environ. Pollut. 2000, 107, 377–389. [Google Scholar] [CrossRef]
- Pulleman, M.; Creamer, R.; Hamer, U.; Helder, J.; Pelosi, C.; Pérès, G.; Rutgers, M. Soil Biodiversity, Biological Indicators and Soil Ecosystem Services—An Overview of European Approaches. Curr. Opin. Environ. Sustain. 2012, 4, 529–538. [Google Scholar] [CrossRef]
- Grześ, I.M. Ant Species Richness and Evenness Increase along a Metal Pollution Gradient in the Bolesław Zinc Smelter Area. Pedobiologia 2009, 53, 65–73. [Google Scholar] [CrossRef]
- Yang, H.; Peng, Y.; Tian, J.; Wang, J.; Hu, J.; Wang, Z. Spiders as Excellent Experimental Models for Investigation of Heavy Metal Impacts on the Environment: A Review. Environ. Earth Sci. 2016, 75, 1059. [Google Scholar] [CrossRef]
- Ghannem, S.; Touaylia, S.; Boumaiza, M. Beetles (Insecta: Coleoptera) as Bioindicators of the Assessment of Environmental Pollution. Hum. Ecol. Risk Assesment 2017, 24, 456–464. [Google Scholar] [CrossRef]
- Mazzei, V.; Longo, G.; Brundo, M.V.; Sinatra, F.; Copat, C.; Oliveri Conti, G.; Ferrante, M. Bioaccumulation of Cadmium and Lead and Its Effects on Hepatopancreas Morphology in Three Terrestrial Isopod Crustacean Species. Ecotoxicol. Environ. Saf. 2014, 110, 269–279. [Google Scholar] [CrossRef]
- Vaněk, A.; Ettler, V.; Grygar, T.; Borůvka, L.; Šebek, O.; Drábek, O. Combined Chemical and Mineralogical Evidence for Heavy Metal Binding in Mining- and Smelting-Affected Alluvial Soils. Pedosphere 2008, 18, 464–478. [Google Scholar] [CrossRef]
- Navrátil, T.; Rohovec, J.; Žák, K. Floodplain Sediments of the 2002 Catastrophic Flood at the Vltava (Moldau) River and Its Tributaries: Mineralogy, Chemical Composition, and Post-Sedimentary Evolution. Environ. Geol. 2008, 56, 399–412. [Google Scholar] [CrossRef]
- Boruvka, L.; Vacha, R. Litavka River Alluvium as a Model Area Heavily Polluted with Potentially Risk Elements. In Phytoremediation of Metal-Contaminated Soils; Springer: Dordrecht, The Netherlands, 2006; pp. 267–298. [Google Scholar]
- Příbram Official Webpage Přírodní Podmínky Příbrami-Město Příbram. Available online: https://pribram.eu/zivot-ve-meste/zivotni-prostredi/prirodni-podminky-pribrami.html (accessed on 6 June 2022).
- Vrubel, J.; Tratinová, M.; Trojáček, P. Contamination of Agricultural Soil by Lead, Cadmium and Arsenic in the Area of Agriculture Farm Sádek Located in Lhota near Příbram. In Plant, Soil and Environment; Institute of Agricultural and Food Information: Paris, Frace, 1996. [Google Scholar]
- Mukhtorova, D.; Hlava, J.; Száková, J.; Kubík, Š.; Vrabec, V.; Tlustoš, P. Risk Element Accumulation in Coleoptera and Hymenoptera (Formicidae) Living in an Extremely Contaminated Area—A Pre-liminary Study. Environ. Monit. Assess. 2019, 191, 432. [Google Scholar] [CrossRef]
- Smetana, A. Drabčíkovití-Staphylinidae I, Staphylininae (Řád: Brouci-Coleoptera); Československá Akademie Věd: Prague, Czech Republic, 1958. [Google Scholar]
- Hůrka, K. Brouci České a Slovenské Republiky (Beetles of the Czech Republic and Slovakia); Kabourek: Zlín, Czech Republic, 2005. [Google Scholar]
- Růžička, J. Icones Insectorum Europae Centralis. Agyrtidae, Silphidae. Folia Heyrovskyana Ser. B 2003, 3, 9–14. [Google Scholar]
- Seifert, B. The Ants of Central and North Europe; Lutra Verlag: Boxberg, Germany, 2018. [Google Scholar]
- Czechowski, W.; Radchenko, A.; Czechowska, W. The Ants (Hymenoptera, Formicidae) of Poland; Museum and Institute of Zoology PAS: Warsaw, Poland, 2002. [Google Scholar]
- Nentwig, W.; Blick, T.; Gloor, D.; Jäger, P.; Kropf, C. Tackling Taxonomic Redundancy in Spiders: The Infraspecific Spider Taxa Described by Embrik Strand (Arachnida: Araneae). Arachnol. Mitt. Arachnol. Lett. 2019, 58, 29–51. [Google Scholar] [CrossRef]
- Naturhistorisches Museum Bern World Spider Catalog. Available online: https://wsc.nmbe.ch (accessed on 3 July 2022).
- Smilauer, P.; Leps, J. Multivariate Analysis of Ecological Data Using CANOCO 5, 2nd ed; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781107694408. [Google Scholar]
- Hejda, R.; Farkač, J.; Chobot, K. Červený Seznam Ohrožených Druhů České Republiky Bezo-bratlí Red List of Threatened Species in the Czech Republic Invertebrates; Agentura Ochrany Přírody a Krajiny ČR: Praha, Czech Republic, 2017; ISBN 8086064964. [Google Scholar]
- Anonymous. Public Notice No. 153/2016 about the Conditions for the Protection of the Agricultural Soil Quality. In Legal Code of The Czech Republic; The Parliament of the Czech Republic: Prague, Czech Republic, 2016; pp. 2692–2699. [Google Scholar]
- Hůrka, K. Carabidae of the Czech and Slovak Republics; Kabourek: Zlín, Czech Republic, 1996; ISBN 9788090146624. [Google Scholar]
- Underwood, E.C.; Fisher, B.L. The Role of Ants in Conservation Monitoring: If, When, and How. Biol. Conserv. 2006, 132, 166–182. [Google Scholar] [CrossRef]
- Gramigni, E.; Calusi, S.; Gelli, N.; Giuntini, L.; Massi, M.; Delfino, G.; Chelazzi, G.; Baracchi, D.; Frizzi, F.; Santini, G. Ants as Bioaccumulators of Metals from Soils: Body Content and Tissue-Specific Distribution of Metals in the Ant Crematogaster Scutellaris. Eur. J. Soil Biol. 2013, 58, 24–31. [Google Scholar] [CrossRef]
- Ribas, C.R.; Solar, R.R.C.; Campos, R.B.F.; Schmidt, F.A.; Valentim, C.L.; Schoereder, J.H. Can Ants Be Used as Indicators of Environmental Impacts Caused by Arsenic? J. Insect Conserv. 2012, 16, 413–421. [Google Scholar] [CrossRef]
- Kavehei, A.; Gore, D.B.; Wilson, S.P.; Hosseini, M.; Hose, G.C. Assessment of Legacy Mine Metal Contamination Using Ants as Indicators of Contamination. Environ. Pollut. 2021, 274, 116537. [Google Scholar] [CrossRef]
- Okrutniak, M.; Grześ, I.M. Accumulation of Metals in Lasius Niger: Implications for Using Ants as Bioindicators. Environ. Pollut. 2021, 268, 115824. [Google Scholar] [CrossRef]
- Skaldina, O.; Peräniemi, S.; Sorvari, J. Ants and Their Nests as Indicators for Industrial Heavy Metal Contamination. Environ. Pollut. 2018, 240, 574–581. [Google Scholar] [CrossRef]
- Eeva, T.; Sorvari, J.; Koivunen, V. Effects of Heavy Metal Pollution on Red Wood Ant (Formica s. Str.) Populations. Environ. Pollut. 2004, 132, 533–539. [Google Scholar] [CrossRef]
- Migliorini, M.; Pigino, G.; Bianchi, N.; Bernini, F.; Leonzio, C. The Effects of Heavy Metal Con-tamination on the Soil Arthropod Community of a Shooting Range. Environ. Pollut. 2004, 129, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Belskaya, E.; Gilev, A.; Trubina, M.; Belskii, E. Diversity of Ants (Hymenoptera, Formicidae) along a Heavy Metal Pollution Gradient: Evidence of a Hump-Shaped Effect. Ecol. Indic. 2019, 106, 105447. [Google Scholar] [CrossRef]
- Vohralík, V.; Werner, P. Mravenci (Hymenoptera: Formicidae) Dolního Pojizeří. Ants (Hymenop-tera: Formicidae) of the Dolní Pojizeří Region (Czech Republic). Klapalekiana 2020, 56, 271–291. [Google Scholar]
- Vohralík, V.; Werner, P.; Amcha, P. Ants (Hymenoptera: Formicidae) of the Dolní Povltaví Region (Czech Republic). Klapalekiana 2018, 54, 253–274. [Google Scholar]
- Heikens, A.; Peijnenburg, W.J.G.M.; Hendriks, A.J. Bioaccumulation of Heavy Metals in Terres-trial Invertebrates. Environ. Pollut. 2001, 113, 385–393. [Google Scholar] [CrossRef]
- Simon, E.; Harangi, S.; Baranyai, E.; Braun, M.; Fábián, I.; Mizser, S.; Nagy, L.; Tóthmérész, B. Distribution of Toxic Elements between Biotic and Abiotic Components of Terrestrial Ecosystem along an Urbanization Gradient: Soil, Leaf Litter and Ground Beetles. Ecol. Indic. 2016, 60, 258–264. [Google Scholar] [CrossRef]
- Skalski, T.; Kędzior, R.; Kolbe, D.; Skalsk, T.; Knutelski, S. Different Responses of Epigeic Beetles to Heavy Metal Contamina-Tion Depending on Functional Traits at the Family Level. Balt. J. Coleopterol. 2015, 15, 81–90. [Google Scholar]
- Lock, K.; Desender, K.; Janssen, C.R. Effects of Metal Contamination on the Activity and Diver-sity of Carabid Beetles in an Ancient Pb-Zn Mining Area at Plombières (Belgium). Entomol. Exp. Appl. 2001, 99, 355–360. [Google Scholar] [CrossRef]
- Baranová, B.; Demková, L.; Arvay, J. Surface-Dwelling Soil Macrofauna and Ground Beetles (Coleoptera: Carabidae) of Metal Post-Mining Spoil Heaps–Community Composition and Potential Risk Element Bioaccumulation. Chem. Ecol. 2021, 37, 530–551. [Google Scholar] [CrossRef]
- Dallinger, R.; Rainbow, P.S. Ecotoxicology of Metals in Invertebrates; SETAC: Sheffield, UK, 1991. [Google Scholar]
- Smith DiCarlo, L.A.; DeBano, S.J. Spider Community Responses to Grassland Restoration: Balancing Trade-Offs between Abundance and Diversity. Restor. Ecol. 2019, 27, 210–219. [Google Scholar] [CrossRef]
- Marc, P.; Canard, A.; Ysnel, F. Spiders (Araneae) Useful for Pest Limitation and Bioindication. Agric. Ecosyst. Environ. 1999, 74, 229–273. [Google Scholar] [CrossRef]
- Jung, M.P.; Kim, S.T.; Kim, H.; Lee, J.H. Species Diversity and Community Structure of Ground-Dwelling Spiders in Unpolluted and Moderately Heavy Metal-Polluted Habitats. Water Air Soil Pollut. 2008, 195, 15–22. [Google Scholar] [CrossRef]
- Vorobeichik, E.; Nesterkov, A.; Ermakov, A.; Zolotarev, M.; Grebennikov, M. Diversity and Abundance of Soil Macroinvertebrates along a Contamination Gradient in the Central Urals, Russia. Biodivers. Data J. 2022, 10, 76968. [Google Scholar] [CrossRef]
- Żmudzki, S.; Laskowski, R. Biodiversity and Structure of Spider Communities along a Metal Pollution Gradient. Ecotoxicology 2012, 21, 1523–1532. [Google Scholar] [CrossRef]
- Koponen, S. Ground-living Spiders (Araneae) at Polluted Sites in the Subarctic. Arachnol. Mitt. 2011, 40, 80–84. [Google Scholar] [CrossRef]
- Koponen, S. On the Biogeography and Faunistics of European Spiders: Latitude, Altitude and Insularity Par Seppo Koponen. Bull. Soc. Neuchâtel. Sci. Nat. 1993, 116, 141–152. [Google Scholar]
- Hula, V.; Košulič, O.; Šťastná, P. Spiders (Araneae) of Selected Sinkholes of Moravský Kras Protected Landscape Area (Czech Republic). Acta Univ. Agric. Silvic. Mendel. Brun. 2021, 40, 57–70. [Google Scholar] [CrossRef]
- Vymazalová, P.; Košulič, O. Epigeic Spiders from Oak-Hornbeam Woodland in the Děvín National Nature Reserve (Czech Republic). Arachnol. Mitt. Arachnol. Lett. 2020, 60, 55–62. [Google Scholar] [CrossRef]
- Zolotarev, M.P.; Nesterkov, A.V. Arachnids (Aranei, Opiliones) in Meadows: Response to Pollution with Emissions from the Middle Ural Copper Smelter. Russ. J. Ecol. 2015, 46, 48–56. [Google Scholar] [CrossRef]

| As | Cd | Cu | Ni | Pb | Zn | Cr | |
|---|---|---|---|---|---|---|---|
| 1 * | 55.4 ± 22.2 a | 2.7 ± 12 a | 44.3 ± 17.1 a | 21.6 ± 3.5 ab | 1350.7 ± 493.2 c | 455.7 ± 193.5 a | 40.5 ± 5.0 |
| 2 * | 663.0 ± 14.1 b | 19.5 ± 0.5 abc | 97.1 ± 2.6 bc | 37.8 ± 0.9 c | 2169.8 ± 178.5 b | 3304.8 ± 65.9 b | 58.8 ± 0.8 |
| 3 * | 628.6 ± 191.7 b | 43.9 ± 26.3 c | 127.2 ± 35.0 c | 29.8 ± 10.4 cb | 3898.3 ± 329.1 d | 6309.8 ± 1724.6 c | 60.5 ± 55.1 |
| 4 * | 226.2 ± 128.3 a | 28.4 ± 12.9 bc | 61.9 ± 30.7 ab | 22.9 ± 8.3 ab | 2682.2 ± 460.3 b | 3866.4 ± 1146.2 b | 40.4 ± 5.7 |
| 5 | 18.5 ± 9.5 a | 1.6 ± 1.3 ab | 16.4 ± 3.2 a | 14.6 ± 2.4 a | 241.4 ± 145.5 a | 273.7 ± 177.8 a | 30.1 ± 1.9 |
| p | >0.001 | >0.001 | >0.001 | >0.001 | >0.001 | >0.001 | 0.356 |
| F | 43.28 | 9.42 | 17.75 | 8.39 | 62.29 | 38.44 | 1.168 |
| Arthropods | Number of Species at Each Location | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Spiders (Aranae) | 11 | 18 | 20 | 19 | 16 |
| Harvestmen (Opiliones) | 3 | 2 | 1 | 2 | 3 |
| Ants (Formicidae) | 6 | 10 | 10 | 10 | 10 |
| Beetles (Coleoptera) | 14 | 15 | 13 | 13 | 13 |
| Total | 35 | 47 | 47 | 48 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlava, J.; Vachová, P.; Száková, J.; Vrabec, V.; Kubík, Š.; Tlustoš, P.; Langrová, I.; Kulma, M. Living with Contamination: Insights into an Epigeic Macrofaunal Community in an Area Extremely Polluted by Risk Elements. Sustainability 2023, 15, 4243. https://doi.org/10.3390/su15054243
Hlava J, Vachová P, Száková J, Vrabec V, Kubík Š, Tlustoš P, Langrová I, Kulma M. Living with Contamination: Insights into an Epigeic Macrofaunal Community in an Area Extremely Polluted by Risk Elements. Sustainability. 2023; 15(5):4243. https://doi.org/10.3390/su15054243
Chicago/Turabian StyleHlava, Jakub, Pavla Vachová, Jiřina Száková, Vladimír Vrabec, Štěpán Kubík, Pavel Tlustoš, Iva Langrová, and Martin Kulma. 2023. "Living with Contamination: Insights into an Epigeic Macrofaunal Community in an Area Extremely Polluted by Risk Elements" Sustainability 15, no. 5: 4243. https://doi.org/10.3390/su15054243
APA StyleHlava, J., Vachová, P., Száková, J., Vrabec, V., Kubík, Š., Tlustoš, P., Langrová, I., & Kulma, M. (2023). Living with Contamination: Insights into an Epigeic Macrofaunal Community in an Area Extremely Polluted by Risk Elements. Sustainability, 15(5), 4243. https://doi.org/10.3390/su15054243








