Vetiver Grass (Chrysopogon zizanoides L.): A Hyper-Accumulator Crop for Bioremediation of Unconventional Water
Abstract
:1. Introduction
2. Phytoremediation
3. Mechanisms of Phytoremediation
4. Vetiver System
4.1. Genetic and Taxonomic Properties
4.2. Morphological Characteristics
4.3. Physiological Characteristics
4.4. Ecologic Properties
5. Vetiver System to Reduce/Eliminate Contaminants from Unconventional Water
6. Traditional and Medicinal Uses of Vetiver Grass
7. Economic Analysis
8. Outlook
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eltarabily, M.G.; Abd-Elaty, I.; Elbeltagi, A.; Zeleňáková, M.; Fathy, I. Investigating Climate Change Effects on Evapotranspiration and Groundwater Recharge of the Nile Delta Aquifer, Egypt. Water 2023, 15, 572. [Google Scholar] [CrossRef]
- Martínez-Sifuentes, A.R.; Trucíos-Caciano, R.; Rodríguez-Moreno, V.M.; Villanueva-Díaz, J.; Estrada-Ávalos, J. The Impact of Climate Change on Evapotranspiration and Flow in a Major Basin in Northern Mexico. Sustainability 2023, 15, 847. [Google Scholar] [CrossRef]
- Lu, Z.-N.; Chen, H.; Hao, Y.; Wang, J.; Song, X.; Mok, T.M. The dynamic relationship between environmental pollution, economic development and public health: Evidence from China. J. Clean. Prod. 2017, 166, 134–147. [Google Scholar]
- Eslamian, S.; Amiri, M.J.; Abedi-Koupai, J.; Karimi, S.S. Reclamation of unconventional water using nano zero-valent iron particles: An application for groundwater. Int. J. Water 2013, 7, 1–13. [Google Scholar]
- Asano, T.; Levine, A.D. Wastewater reclamation, recycling and reuse: Past, present, and future. Water Sci. Technol. 1996, 33, 1–14. [Google Scholar]
- Al Salem, S.S. Environmental considerations for wastewater reuse in agriculture. Water Sci. Technol. 1996, 33, 345–353. [Google Scholar]
- Amiri, M.J.; Eslamian, S.; Arshadi, M.; Khozaei, M. Water recycling and community. In Urban Water Reuse Handbook; Eslamian, S., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 261–273. [Google Scholar]
- Mojiri, A. Effects of municipal wastewater on physical and chemical properties of saline soil. J. Biol. Environ. Sci. 2011, 5, 71–76. [Google Scholar]
- Valdez-Aguilar, L.A.; Reed, D.W. Response of selected greenhouse ornamental plants to alkalinity in irrigation water. J. Plant Nutr. 2007, 30, 441–452. [Google Scholar]
- Amiri, M.J.; Shabani, A.; Javidi, A. Phytoremediation potential of rapeseed in phenanthrene-contaminated soils under different irrigation regimes and pumice levels. Irrig. Drain. 2023, 72, 90–104. [Google Scholar]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of heavy metal-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2020, 249, 71–131. [Google Scholar]
- Shah, V.; Daverey, A. Effects of sophorolipids augmentation on the plant growth and phytoremediation of heavy metal contaminated soil. J. Clean. Prod. 2021, 280, 124406. [Google Scholar]
- Seroja, R.; Effendi, H.; Hariyadi, S. Tofu wastewater treatment using vetiver grass (Vetiveria zizanioides) and zeliac. Appl. Water Sci. 2018, 8, 2. [Google Scholar]
- Fu, W.-Q.; Xu, M.; Zhang, A.-Y.; Sun, K.; Dai, C.-C.; Jia, Y. Remediation of phenanthrene phytotoxicity by the interaction of rice and endophytic fungus P. liquidambaris in practice. Ecotoxicol. Environ. Saf. 2022, 235, 113415. [Google Scholar]
- Sui, X.; Wang, X.; Li, Y.; Ji, H. Remediation of petroleum-contaminated soils with microbial and microbial combined methods: Advances, mechanisms, and challenges. Sustainability 2021, 13, 9267. [Google Scholar]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar]
- Ng, C.C.; Boyce, A.N.; Abas, M.R.; Mahmood, N.Z.; Han, F. Phytoassessment of Vetiver grass enhanced with EDTA soil amendment grown in single and mixed heavy metal-contaminated soil. Environ. Monit. Assess. 2019, 191, 434. [Google Scholar]
- Darajeh, N.; Truong, P.; Rezania, S.; Alizadeh, H.; Leung, D.W. Effectiveness of Vetiver grass versus other plants for phytoremediation of contaminated water. J. Environ. Treat. Tech. 2019, 7, 485–500. [Google Scholar]
- Banerjee, R.; Goswami, P.; Lavania, S.; Mukherjee, A.; Lavania, U.C. Vetiver grass is a potential candidate for phytoremediation of iron ore mine. Ecol. Eng. 2019, 132, 120–136. [Google Scholar]
- Masinire, F.; Adenuga, D.O.; Tichapondwa, S.M.; Chirwa, E.M.N. Phytoremediation of Cr(VI) in wastewater using the vetiver grass (Chrysopogon zizanioides). Miner. Eng. 2021, 172, 107141. [Google Scholar]
- Worku, A.; Tefera, N.; Kloos, H.; Benor, S. Bioremediation of brewery wastewater using hydroponics planted with vetiver grass in Addis Ababa, Ethiopia. Bioresour. Bioprocess. 2018, 5, 39. [Google Scholar]
- Gupta, P.; Roy, S.; Mahindrakar, A.B. Treatment of water using water hyacinth, water lettuce and vetiver grass—A review. System 2012, 49, 50. [Google Scholar]
- Hemalatha, G.; Uma, S.G.; Muthulakshmi, S. Sewage water treatment using vetiver grass. Mater. Today: Proc. 2021, 46, 3795–3798. [Google Scholar]
- Amiri, M.J.; Bahrami, M.; Badkouby, M.; Kalavrouziotis, I.K. Greywater treatment using single and combined adsorbents for landscape irrigation. Environ. Process. 2019, 6, 43–63. [Google Scholar]
- Amiri, M.J.; Arshadi, M.; Giannakopoulos, E.; Kalavrouziotis, I.K. Removal of mercury (II) and lead (II) from aqueous media by using a green adsorbent: Kinetics, thermodynamic, and mechanism studies. J Hazard Toxic Radioact Waste. 2018, 22, 04017026. [Google Scholar]
- Mustafa, H.M.; Hayder, G. Recent studies on applications of aquatic weed plants in phytoremediation of wastewater: A review article. Ain Shams Eng. J. 2021, 12, 355–365. [Google Scholar]
- Amiri, M.J.; Bahrami, M.; Beigzadeh, B.; Gil, A. A response surface methodology for optimization of 2,4-dichlorophenoxyacetic acid removal from synthetic and drainage water: A comparative study. Environ. Sci. Pollut. Res. 2018, 25, 34277–34293. [Google Scholar]
- Amiri, M.J.; Abedi-Koupai, J.; Eslamian, S. Adsorption of Hg(II) and Pb(II) ions by nanoscale zero-valent iron supported on ostrich bone ash in a fixed-bed column system. Water Sci. Technol. 2017, 76, 671–682. [Google Scholar]
- Zamorska, J.; Kiełb-Sotkiewicz, I. A Biological Method of Treating Surface Water Contaminated with Industrial Waste Leachate. Water 2021, 13, 3644. [Google Scholar] [CrossRef]
- Gholipour, M.; Mehrabanjoubani, P.; Abdolzadeh, A.; Raghimi, M.; Seyedkhademi, S.; Karimi, E.; Sadeghipour, H.R. Facilitated decrease of anions and cations in influent and effluent of sewage treatment plant by vetiver grass (Chrysopogon zizanioides): The uptake of nitrate, nitrite, ammonium, and phosphate. Environ. Sci. Pollut. Res. 2020, 27, 21506–21516. [Google Scholar]
- Raman, J.K.; Gnansounou, E. A review on bioremediation potential of vetiver grass. In Waste Bioremediation. Energy, Environment, and Sustainability; Varjani, S., Gnansounou, E., Gurunathan, B., Pant, D., Zakaria, Z., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Sricoth, T.; Meeinkuirt, W.; Pichtel, J.; Taeprayoon, P.; Saengwilai, P. Synergistic phytoremediation of wastewater by two aquatic plants (Typha angustifolia and Eichhornia crassipes) and potential as biomass fuel. Environ. Sci. Pollut. Res. 2018, 25, 5344–5358. [Google Scholar]
- Malik, A.; Batool, S.; Farooqi, A. Advances in biodegradation and bioremediation of arsenic contamination in the environment. In Biological Approaches to Controlling Pollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 107–120. [Google Scholar]
- Thomas, G.; Andresen, E.; Mattusch, J.; Hubáček, T.; Küpper, H. Deficiency and toxicity of nanomolar copper in low irradiance—A physiological and metalloproteomic study in the aquatic plant Ceratophyllum demersum. Aquat. Toxicol. 2016, 177, 226–236. [Google Scholar]
- Rane, N.R.; Chandanshive, V.V.; Watharkar, A.D.; Khandare, R.V.; Patil, T.S.; Pawar, P.K.; Govindwar, S.P. Phytoremediation of sulfonated Remazol Red dye and textile effluents by Alternanthera philoxeroides: An anatomical, enzymatic and pilot scale study. Water Res. 2015, 83, 271–281. [Google Scholar]
- Kodituwakku, K.; Yatawara, M. Phytoremediation of industrial sewage sludge with Eichhornia crassipes, Salvinia molesta and Pistia stratiotes in batch fed free water flow constructed wetlands. Bull. Environ. Contam. Toxicol. 2020, 104, 627–633. [Google Scholar]
- Bello, A.O.; Tawabini, B.S.; Khalil, A.B.; Boland, C.R.; Saleh, T.A. Phytoremediation of cadmium-, lead-and nickel-contaminated water by Phragmites australis in hydroponic systems. Ecol. Eng. 2018, 120, 126–133. [Google Scholar]
- Qin, H.; Zhang, Z.; Liu, M.; Liu, H.; Wang, Y.; Wen, X.; Zhang, Y.; Yan, S. Site test of phytoremediation of an open pond contaminated with domestic sewage using water hyacinth and water lettuce. Ecol. Eng. 2016, 95, 753–762. [Google Scholar]
- Rahman, M.A.; Hasegawa, H. Aquatic arsenic: Phytoremediation using floating macrophytes. Chemosphere 2011, 83, 633–646. [Google Scholar]
- Saha, P.; Banerjee, A.; Sarkar, S. Phytoremediation potential of Duckweed (Lemna minor L.) on steel wastewater. Int. J. Phytoremediat. 2015, 17, 589–596. [Google Scholar]
- Aydın Temel, F.; Avcı, E.; Ardalı, Y. Full scale horizontal subsurface flow constructed wetlands to treat domestic wastewater by Juncus acutus and Cortaderia selloana. Int. J. Phytoremediat. 2018, 20, 264–273. [Google Scholar]
- Guarino, F.; Ruiz, K.B.; Castiglione, S.; Cicatelli, A.; Biondi, S. The combined effect of Cr(III) and NaCl determines changes in metal uptake, nutrient content, and gene expression in quinoa (Chenopodium quinoa Willd). Ecotoxicol. Environ. Saf. 2020, 193, 110345. [Google Scholar]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar]
- Nisa, W.; Rashid, A.; Aziz, N.B.; Mahmood, T.; Islam, K.; Kazmi, S.K.; Raziq, M. Potential of vetiver (Vetiveria zizanioides L.) grass in removing selected pahs from diesel contaminated soil. Pak. J. Bot. 2015, 47, 291–296. [Google Scholar]
- Xie, Q.E.; Yan, X.L.; Liao, X.Y.; Li, X. The arsenic hyperaccumulator fern Pteris vittata L. Environ. Sci. Technol. 2009, 43, 8488–8495. [Google Scholar]
- Van der Ent, A.; Baker, A.J.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar]
- He, Y.; Chi, J. Phytoremediation of sediments polluted with phenanthrene and pyrene by four submerged aquatic plants. J. Soils Sediments 2016, 16, 309–317. [Google Scholar]
- Dhote, S.; Dixit, S. Water quality improvement through macrophytes—A review. Environ. Monit. Assess. 2009, 152, 149–153. [Google Scholar]
- Rawat, K.; Fulekar, M.H.; Pathak, B. Rhizofiltration: A green technology for remediation of heavy metals. Int. J. Inno Biosci. 2012, 2, 193–199. [Google Scholar]
- Dinesh, M.; Kumar, M.V.; Neeraj, P.; Shiv, B. Phytoaccumulation of heavy metals in contaminated soil using Makoy (Solenum nigrum L.) and Spinach (Spinacia oleracea L.) plant. Sciences 2014, 2, 350–354. [Google Scholar]
- Domínguez, M.T.; Madrid, F.; Marañón, T.; Murillo, J.M. Cadmium availability in soil and retention in oak roots: Potential for phytostabilization. Chemosphere 2009, 76, 480–486. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar]
- Yang, S.; Liang, S.; Yi, L.; Xu, B.; Cao, J.; Guo, Y.; Zhou, Y. Heavy metal accumulation and phytostabilization potential of dominant plant species growing on manganese mine tailings. Front. Environ. Sci. Eng. 2014, 8, 394–404. [Google Scholar]
- Zhan, F.; Li, B.; Jiang, M.; Li, T.; He, Y.; Li, Y.; Wang, Y. Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead–zinc mine wasteland. Int. J. Phytoremediat. 2019, 21, 849–856. [Google Scholar]
- Pilon-Smits, E.A.; LeDuc, D.L. Phytoremediation of selenium using transgenic plants. Curr. Opin. Biotechnol. 2009, 20, 207–212. [Google Scholar]
- Khandare, R.V.; Govindwar, S.P. Phytoremediation of textile dyes and effluents: Current scenario and future prospects. Biotechnol. Adv. 2015, 33, 1697–1714. [Google Scholar]
- Mirza, N.; Pervez, A.; Mahmood, Q.; Shah, M.M.; Shafqat, M.N. Ecological restoration of arsenic contaminated soil by Arundo donax L. Ecol. Eng. 2011, 37, 1949–1956. [Google Scholar]
- Rylott, E.L.; Bruce, N.C. Plants disarm soil: Engineering plants for the phytoremediation of explosives. Trends Biotechnol. 2009, 27, 73–81. [Google Scholar]
- Frers, C. El uso de plantas acuaticas para el tratamiento de aguas residuales. Obs. Medioambient. 2008, 11, 301–306. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2009, 7, 1–19. [Google Scholar]
- Darajeh, N.; Idris, A.; Masoumi, H.R.F.; Nourani, A.; Truong, P.; Sairi, N.A. Modeling BOD and COD removal from Palm Oil Mill Secondary Effluent in floating wetland by Chrysopogon zizanioides (L.) using response surface methodology. J. Environ. Manage. 2016, 181, 343–352. [Google Scholar]
- Otunola, B.O.; Aghoghovwia, M.P.; Thwala, M.; Gómez-Arias, A.; Jordaan, R.; Hernandez, J.C.; Ololade, O.O. Influence of Clay Mineral Amendments Characteristics on Heavy Metals Uptake in Vetiver Grass (Chrysopogon zizanioides L. Roberty) and Indian Mustard (Brassica juncea L. Czern). Sustainability 2022, 14, 5856. [Google Scholar] [CrossRef]
- Mahadevan, R. The high price of sweetness: The twin challenges of efficiency and soil erosion in Fiji’s sugar industry. Ecol. Econ. 2008, 66, 468–477. [Google Scholar]
- Panja, S.; Sarkar, D.; Datta, R. Vetiver grass (Chrysopogon zizanioides) is capable of removing insensitive high explosives from munition industry wastewater. Chemosphere 2018, 209, 920–927. [Google Scholar]
- Panja, S.; Sarkar, D.; Datta, R. Removal of antibiotics and nutrients by Vetiver grass (Chrysopogon zizanioides) from secondary wastewater effluent. Int. J. Phytoremediat. 2020, 22, 764–773. [Google Scholar]
- Panja, S.; Sarkar, D.; Datta, R. Removal of tetracycline and ciprofloxacin from wastewater by vetiver grass (Chrysopogon zizanioides (L.) Roberty) as a function of nutrient concentrations. Environ. Sci. Pollut. Res. 2020, 27, 34951–34965. [Google Scholar]
- Darajeh, N.; Idris, A.; Truong, P.; Abdul Aziz, A.; Abu Bakar, R.; Che Man, H. Phytoremediation potential of vetiver system technology for improving the quality of palm oil mill effluent. Adv. Mater. Sci. Eng. 2014, 2014, 683579. [Google Scholar]
- Truong, P.; Van, T.T.; Pinners, E. Vetiver System Applications Technical Reference Manual; The Vetiver Network International (TVNI): San Antonio, TX, USA, 2008; pp. 1–126. [Google Scholar]
- Adams, R.; Turuspekov, M.Z.Y.; Dafforn, M.; Veldkamp, J. DNA fingerprinting reveals clonal nature of Vetiveria zizanioides (L.) Nash, Gramineae and sources of potential new germplasm. Mol. Ecol. 1998, 7, 813–818. [Google Scholar]
- Effendi, H.; Utomo, B.A.; Pratiwi, N.T. Ammonia and orthophosphate removal of tilapia cultivation wastewater with Vetiveria zizanioides. J. King Saud Univ. Sci. 2020, 32, 207–212. [Google Scholar]
- Zhang, X.; Gao, B.; Xia, H. Effect of cadmium on growth, photosynthesis, mineral nutrition and metal accumulation of bana grass and vetiver grass. Ecotoxicol. Environ. Saf. 2014, 106, 102–108. [Google Scholar]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective. Chemosphere 2019, 223, 285–309. [Google Scholar]
- Tangahu, B.V.; Putri, A.P. The degradation of BOD and COD of batik industry wastewater using Egeria densa and Salvinia molesta. J. Sains Teknol. Lingkung. (JSTL) 2017, 9, 82–91. [Google Scholar]
- Alam, A.R.; Hoque, S. Phytoremediation of industrial wastewater by culturing aquatic macrophytes, Trapa natans L. and Salvinia cucullata Roxb. Jahangirnagar Univ. J. Biol. Sci. (JUJBS) 2017, 6, 19–27. [Google Scholar]
- Chandanshive, V.V.; Rane, N.R.; Tamboli, A.S.; Gholave, A.R.; Khandare, R.V.; Govindwar, S.P. Co-plantation of aquatic macrophytes Typha angustifolia and Paspalum scrobiculatum for effective treatment of textile industry effluent. J. Hazard. Mater. 2017, 338, 47–56. [Google Scholar]
- Sa’at, S.K.M.; Zaman, N.Q. Phytoremediation potential of palm oil mill effluent by constructed wetland treatment. Eng. Herit. J. 2017, 1, 49–54. [Google Scholar]
- Amare, E.; Kebede, F.; Mulat, W. Wastewater treatment by Lemna minor and Azolla filiculoides in tropical semi-arid regions of Ethiopia. Ecol. Eng. 2018, 120, 464–473. [Google Scholar]
- Basiglini, E.; Pintore, M.; Forni, C. Effects of treated industrial wastewaters and temperatures on growth and enzymatic activities of duckweed (Lemna minor L.). Ecotoxicol. Environ. Saf. 2018, 153, 54–59. [Google Scholar]
- Lu, B.; Xu, Z.; Li, J.; Chai, X. Removal of water nutrients by different aquatic plant species: An alternative way to remediate polluted rural rivers. Ecol. Eng. 2018, 110, 18–26. [Google Scholar]
- Marzec, M.; Jóźwiakowski, K.; Dębska, A.; Gizińska-Górna, M.; Pytka-Woszczyło, A.; Kowalczyk-Juśko, A.; Listosz, A. The efficiency and reliability of pollutant removal in a hybrid constructed wetland with common reed, manna grass, and Virginia mallow. Water 2018, 10, 1445. [Google Scholar]
- Abbasi, S.A.; Ponni, T.-A.G.; Tauseef, S. Potential of joyweed Alternanthera sessilis for rapid treatment of domestic sewage in SHEFROL® bioreactor. Int. J. Phytoremediat. 2019, 21, 160–169. [Google Scholar]
- Abd Rasid, N.; Naim, M.; Man, H.C.; Bakar, N.A.; Mokhtar, M. Evaluation of surface water treated with lotus plant; Nelumbo nucifera. J. Environ. Chem. Eng. 2019, 7, 103048. [Google Scholar]
- Sudiarto, S.I.A.; Renggaman, A.; Choi, H.L. Floating aquatic plants for total nitrogen and phosphorus removal from treated swine wastewater and their biomass characteristics. J. Environ. Manage. 2019, 231, 763–769. [Google Scholar]
- Ayache, L.; Boudehane, A. Wastewater treatment by floating macrophytes (Salvinia natans) under algerian semi-Arid climate. Eur. J. Eng. Nat. Sci. (EJENS) 2019, 3, 103–110. [Google Scholar]
- Rai, P.K. Heavy metals/metalloids remediation from wastewater using free floating macrophytes of a natural wetland. Environ. Technol. Innov. 2019, 15, 100393. [Google Scholar]
- Saleh, H.M.; Moussa, H.R.; Mahmoud, H.H.; El-Saied, F.A.; Dawoud, M.; Wahed, R.S.A. Potential of the submerged plant Myriophyllum spicatum for treatment of aquatic environments contaminated with stable or radioactive cobalt and cesium. Prog. Nucl. Energy 2020, 118, 103147. [Google Scholar]
- Abdolhoseini, M.; Heidarpour, M.; Abedi-Koupai, J. The feasibility study of water salinity reduction by Atriplex lentiformis plant in a zeolite substrate. Iran. J. Soil Water Res. (IJSR) 2021, 51, 2901–2912. [Google Scholar]
- Abedi-Koupaei, J.; Dorafshan, M.; Gohari, A. Investigation of Bioremediation of Quinoa plant for Desalination of unconventional water. JWSS-Isfahan Univ. Technol. 2022, 26, 329–342. [Google Scholar]
- Leguizamo, M.A.O.; Gómez, W.D.F.; Sarmiento, M.C.G. Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands—A review. Chemosphere 2017, 168, 1230–1247. [Google Scholar]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. Remediation of acid mine drainage-impacted water. Curr. Pollut. Rep. 2015, 1, 131–141. [Google Scholar]
- Cull, R.; Hunter, H.; Hunter, M.; Truong, P. Application of vetiver grass technology in off-site pollution control II. Tolerance to herbicides under selected wetland conditions. In Proceedings of the Second International Vetiver Conference, Bangkok, Thailand, 18–22 January 2000. [Google Scholar]
- Brandt, R.; Merkl, N.; Schultze-Kraft, R.; Infante, C.; Broll, G. Potential of vetiver (Vetiveria zizanioides (L.) Nash) for phytoremediation of petroleum hydrocarbon-contaminated soils in Venezuela. Int. J. Phytoremediat. 2006, 8, 273–284. [Google Scholar]
- Maiti, S.; Kumar, A. Energy plantations, medicinal and aromatic plants on contaminated soil. In Bioremediation and Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 29–47. [Google Scholar]
- Kiiskila, J.D.; Sarkar, D.; Panja, S.; Sahi, S.V.; Datta, R. Remediation of acid mine drainage-impacted water by vetiver grass (Chrysopogon zizanioides): A multiscale long-term study. Ecol. Eng. 2019, 129, 97–108. [Google Scholar]
- Chandanshive, V.V.; Kadam, S.K.; Khandare, R.V.; Kurade, M.B.; Jeon, B.-H.; Jadhav, J.P.; Govindwar, S.P. In situ phytoremediation of dyes from textile wastewater using garden ornamental plants, effect on soil quality and plant growth. Chemosphere 2018, 210, 968–976. [Google Scholar]
- Datta, R.; Das, P.; Smith, S.; Punamiya, P.; Ramanathan, D.M.; Reddy, R.; Sarkar, D. Phytoremediation potential of vetiver grass [Chrysopogon zizanioides (L.)] for tetracycline. Int. J. Phytoremediat. 2013, 15, 343–351. [Google Scholar]
- RoyChowdhury, A.; Mukherjee, P.; Panja, S.; Datta, R.; Christodoulatos, C.; Sarkar, D. Evidence for phytoremediation and phytoexcretion of NTO from industrial wastewater by vetiver grass. Molecules 2020, 26, 74. [Google Scholar]
- Truong, P.; Hart, B. Vetiver System for Wastewater Treatment; Pacific Rim Vetiver Network Technical Bulletin No. 2001/2; Office of the Royal Development Projects Board: Bangkok, Thailand, 2001; p. 26. [Google Scholar]
- Liao, X.; Luo, S.; Wu, Y.; Wang, Z. Studies on the abilities of Vetiveria zizanioides and Cyperus alternifolius for pig farm wastewater treatment. Int. Conf. Vetiver Exhib. 2003, 3, 174–181. [Google Scholar]
- Jayashree, S.; Rathinamala, J.; Lakshmanaperumalsamy, P. Determination of heavy metal removal efficiency of Chrysopogon zizanioides (Vetiver) using textile wastewater contaminated soil. J. Environ. Sci. Technol. 2011, 4, 543–551. [Google Scholar]
- Aneez, E.; Mohammed, A.; Jawahar, N.; Sekarbabu, H. A preliminary attempt to reduce total dissolved solids in ground water using different plant parts. Int. J. Pharma Bio Sci. (IJPBS) 2011, 2, 414–422. [Google Scholar]
- Keshtkar, A.R.; Ahmadi, M.; Naseri, H.; Atashi, H.; Hamidifar, H.; Razavi, S.; Yazdanpanah, A.; Karimpour Reihan, M.; Moazami, N. Application of a vetiver system for unconventional water treatment. Desalin. Water Treat. 2016, 57, 25474–25483. [Google Scholar]
- Pongthornpruek, S. Treatment of Piggery Wastewater by Three Grass Species Growing in a Constructed Wetland. Appl. Environ. Sci. 2017, 39, 75–83. [Google Scholar]
- Hasan, S.N.M.S.; Kusin, F.M.; Lee, A.L.S.; Ukang, T.A.; Yusuff, F.M.; Ibrahim, Z.Z. Performance of vetiver grass (Vetiveria zizanioides) for phytoremediation of contaminated water. MATEC Web Conf. 2017, 103, 06003. [Google Scholar] [CrossRef]
- Maharjan, A.; Pradhanang, S. Potential of Vetiver grass for wastewater treatment. Environ. Ecol. Res. 2017, 5, 489–494. [Google Scholar]
- Astuti, J.T.; Sriwuryandari, L.; Sembiring, T. Application of vetiver (Vetiveria zizanioides) on phytoremediation of carwash wastewater. Pertanika J. Trop. Agric. Sci. 2018, 41, 1463–1477. [Google Scholar]
- Angassa, K.; Leta, S.; Mulat, W.; Kloos, H.; Meers, E. Organic matter and nutrient removal performance of horizontal subsurface flow constructed wetlands planted with Phragmite karka and Vetiveria zizanioide for treating municipal wastewater. Environ. Process. 2018, 5, 115–130. [Google Scholar]
- Kusin, F.; Hasan, S.; Nordin, N.; Mohamat-Yusuff, F.; Ibrahim, Z. Floating Vetiver island (FVI) and implication for treatment system design of polluted running water. Appl. Ecol. Environ. Res. 2019, 17, 497–510. [Google Scholar]
- Deva, M.A.; Manderia, S.; Singh, S.; Sheikh, M.Y. Phytoremedial treatment of domestic wastewater at GWALIOR (MP) by chrysopogon zizanioides (Vetiver grass). Adv. Innov. Res. 2019, 6, 78–81. [Google Scholar]
- Itam, M.O.; Nnamani, C.V.; Oku, E.E. African Vetiver grass cleans abattoir effluent. Agric. Nat. Resour. 2019, 53, 260–266. [Google Scholar]
- Hemamalini, C.; Niveditha, K.; Ramyashree, H.; Sumithra, T.M. Waste water treatment by phytoremediation technique. Bull. Pure Appl. Sci.-Chem. 2019, 38, 128–137. [Google Scholar]
- Abedi-Koupai, J.; Jamalian, M.; Dorafshan, M. Improving isfahan landfill leachate quality by phytoremediation using vetiver and phragmites plants in green space irrigation. J. Water Wastewater 2020, 31, 101–111. [Google Scholar]
- Davamani, V.; Parameshwari, C.I.; Arulmani, S.; John, J.E.; Poornima, R. Hydroponic phytoremediation of paperboard mill wastewater by using vetiver (Chrysopogon zizanioides). J. Environ. Chem. Eng. 2021, 9, 105528. [Google Scholar]
- Abedi-Koupai, J.; Hakimian, M.H.; Motamedi, A.; Ghods Motahari, A. Performance of Vetiver system in complementary municipal wastewater treatment. Water Irrig. Manag. 2021, 11, 275–290. [Google Scholar]
- Goren, A.Y.; Yucel, A.; Sofuoglu, S.C.; Sofuoglu, A. Phytoremediation of olive mill wastewater with Vetiveria zizanioides (L.) Nash and Cyperus alternifolius L. Environ. Technol. Innov. 2021, 24, 102071. [Google Scholar]
- Dhanya, G.; Gopal, V.V.; Jaya, D. An appraisal on the stress amelioration of effluent treated Vetiver plants amended with ascorbic acid in constructed wetlands. J. Stress physiol. Biochem. 2022, 18, 88–100. [Google Scholar]
- Aregu, M.B. Industrial wastewater treatment efficiency of mixed substrate (Pumice and scoria) in horizontal subsurface flow constructed wetland: Comparative experimental study design. Air Soil Water Res. 2022, 15, 11786221211063888. [Google Scholar]
- Abedi-Koupai, J.; Ghods Motahari, A.; Najafi, N. Improvement of saline effluent quality using phytoremediation. Water Wastewater Sci. Eng. 2022, 7, 53–62. [Google Scholar]
- Nugroho, A.P.; Butar, E.S.B.; Priantoro, E.A.; Sriwuryandari, L.; Pratiwi, Z.B.; Sembiring, T. Phytoremediation of electroplating wastewater by vetiver grass (Chrysopogon zizanoides L.). Sci. Rep. 2021, 11, 14482. [Google Scholar]
- Suelee, A.L.; Hasan, S.N.M.S.; Kusin, F.M.; Yusuff, F.M.; Ibrahim, Z.Z. Phytoremediation potential of vetiver grass (Vetiveria zizanioides) for treatment of metal-contaminated water. Water Air Soil Pollut. 2017, 228, 158. [Google Scholar]
- Alsghayer, R.; Salmiaton, A.; Mohammad, T.; Idris, A.; Ishak, C.F. Removal efficiencies of constructed wetland planted with Phragmites and Vetiver in treating synthetic wastewater contaminated with high concentration of PAHs. Sustainability 2020, 12, 3357. [Google Scholar]
- Mirzaee, M.M.; ZakeriNia, M.; Farasati, M. The effects of phytoremediation of treated urban wastewater on the discharge of surface and subsurface drippers (Case study: Gorgan wastewater treatment plant in northern Iran). Clean. Eng. Technol. 2021, 4, 100210. [Google Scholar]
- Mirzaee, M.M.; Zakerinia, M.; Farasati, M. Performance evaluation of vetiver and pampas plants in reducing the hazardous ions of treated municipal wastewater for agricultural irrigation water use. Water Pract. Technol. 2022, 17, 1002–1018. [Google Scholar]
- Grover, M.; Behl, T.; Virmani, T.; Bhatia, S.; Al-Harrasi, A.; Aleya, L. Chrysopogon zizanioides—A review on its pharmacognosy, chemical composition and pharmacological activities. Environ. Sci. Pollut. Res. 2021, 28, 44667–44692. [Google Scholar]
- Singh, K.K.; Maheshwari, J.K. Traditional phytotherapy amongst the tribals of Varanasi district, Uttar Pradesh. J. Ecol. Taxon. Bot. 1983, 4, 829–838. [Google Scholar]
- Jain, V.; Jain, S.K. Compendium of Indian Folk Medicine and Ethnobotany; Deep Publications: New Delhi, India, 2016. [Google Scholar]
- Duke, J.A. Handbook of Medicinal Herbs; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Chomchalow, N. The Utilization of Vetiver as Medicinal and Aromatic Plants: With Special Reference to Thailand; Office of the Royal Development Projects Board: Bangkok, Thailand, 2001. [Google Scholar]
- Pareek, A.R.C.H.A.N.A.; Kumar, A.S.H.W.A.N.I. Ethnobotanical and pharmaceutical uses of Vetiveria zizanioides (Linn) Nash: A medicinal plant of Rajasthan. Int. J. Life Sci. Pharm. Sci. 2013, 50, 12–18. [Google Scholar]
- Kim, K.; Riley, S.; Fischer, E.; Khan, S. Greening Roadway Infrastructure with Vetiver Grass to Support Transportation Resilience. CivilEng 2022, 3, 147–164. [Google Scholar] [CrossRef]
- Dudai, N.; Tsion, I.; Shamir, S.Z.; Nitzan, N.; Chaimovitsh, D.; Shachter, A.; Haim, A. Agronomic and economic evaluation of Vetiver grass (Vetiveria zizanioides L.) as means for phytoremediation of diesel polluted soils in Israel. J. Environ. Manage. 2018, 211, 247–255. [Google Scholar]
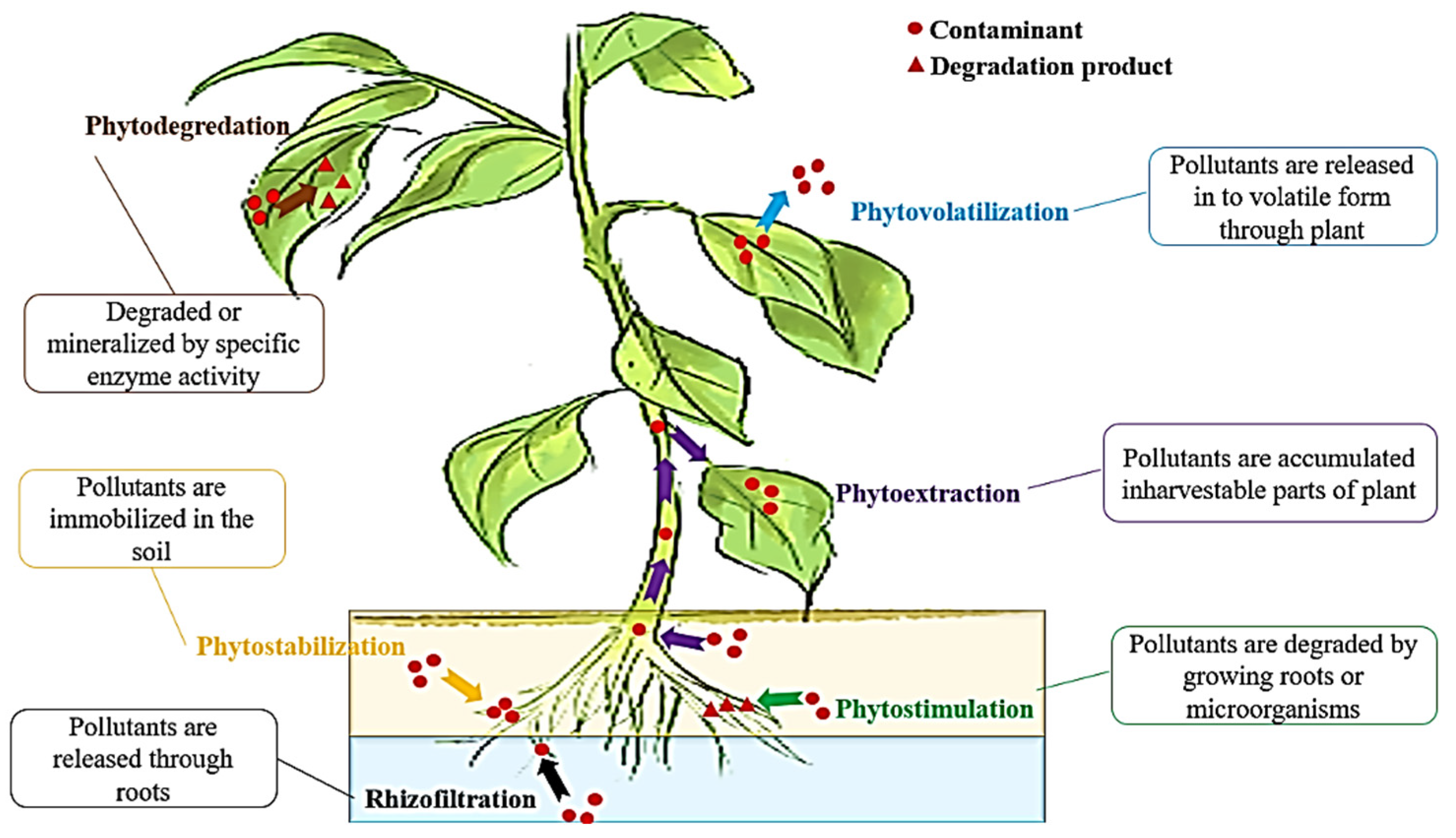

| Processes | Other Names | Mechanism | Pollutants | Applicability | Benefits | References |
|---|---|---|---|---|---|---|
| Phytoextraction 1 | Phytoaccumulation, phytoabsorption, phytosequestration | Hyper-accumulation. | Pb, Cd, Zn, Ni, Cu, radionuclides, pentachlorophenol, and aliphatic compounds. | Polluted soil/sites, water, and wastewaters. | Instant abundant biomass, decreased soil erosion, cost-effectiveness, wide range of applications. | [46,47,48] |
| Rhizofiltration 2 | Phytofiltration | Rhizosphere accumulation. | Pb, Cd, Zn, Ni, Cu, radionuclides (Cs, Sr, U), hydrophobic organics, and radionuclides. | Polluted water and wastewaters. | Purification of polluted surface water, industrial wastewaters, as well as agricultural runoff. | [49,50,51] |
| Phytostabilization 3 | Phytoimmobilization | Precipitation, complexation, and metal valence decrease. | Pb, Cd, Zn, As, Cu, Cr, Se, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyl (PCBs), dioxins, furans, pentachlorophenol, Dichlorodiphenyltrichloroethane (DDT), and dieldrin. | Polluted soil/sediments and sludge. | Ecologic efficiency, polluted medium stabilization without polluted biomass disposal, decreased soil erosion, and applicability in field and mine contaminated area. | [52,53,54] |
| Phytovolatilization 4 | Phytoevaporation | Leaves-based volatilization/evaporative. | Chlorinated solvents, such as carbon tetrachloride, trichloroethylene, methylene chloride, and tetrachloroethylene. | Polluted wastewaters, soil, sediments, and sludges. | Environment cleaner without leading to plant harvesting and biomass disposal. | [55,56,57] |
| Phytodegradation 5 | Phytotransformation | Plant-tissue degradation. | DDT, PAHs, bisphenol A, and organo-phosphorus compounds. | Polluted soil, sediments, sludge, groundwater, surface water, and wastewaters. | Rhizosphere biodegraded recalcitrant contaminants. | [58,59,60] |
| Phytostimulation 6 | Rhizodegradation | Rhizosphere degradation. | Atrazine, ammunition wastes, petroleum hydrocarbon, Polychlorinated biphenyl (PCBs), PAHs, Trichloroethylene (TCE), and diesel fuel. | Polluted soil, sediments, sludge, groundwater, and wastewaters. | Organic acid release; rhizosphere-resulted increase of biodegradation; more consumption of metabolic compounds by micro-organisms in rhizosphere. | [54,61,62] |
| Index | Scientific Classification |
|---|---|
| Kingdom | Plantae |
| Order | Poales |
| Family | Graminae |
| Subfamily | Panicoideae; Tribe-Andropogoneae; Subtribe-Sorghinae |
| Genus | Chrysopogon |
| Species | Zizanioides |
| General name | Vetiver grass |
| NO. | Plants | Type of Unconventional Water | Residence Time | Initial Concentration | Removal Efficiency | References |
|---|---|---|---|---|---|---|
| 1 | Egeria densa (Brazilian waterweed) | Industrial wastewater | 17 days/laboratory scale reactor within batch systems | BOD (104.5 mg/L), COD (426.4 mg/L) | BOD (93%), COD (95%) | [75] |
| 2 | Salvinia. cucullata | Industrial wastewater (textile industries) | 45 days/batch cultures | COD (71.6–122.7 mg/L), NH3-N (5.32–8.4 mg/L), DO (1.55–1.99 mg/L), BOD (160–188 mg/L), Nitrate (1.4–1.96 mg/L), TP (160–240 mg/L) | COD (31.04%), NH3-N (5.26%), DO (100%), BOD (43.02%), Nitrate (20%), TP (81.25%) | [76] |
| 3 | Typha angustifolia L. | Textile wastewater | 7 days/constructed wetlands | COD (1328 mg/L), BOD (1140 mg/L), Colour (1035 Unit), TDS (9562 mg/L), Cd (0.07 mg/L), Cr (2.91 mg/L), As (2.12 mg/L), Pb (0.42 mg/L), TSS (7280 mg/L) | COD (65%), BOD (68%), Colour (62%), TDS (45%), Cd (28%), Cr (59%), As (60%), Pb (45%), TSS (35%) | [77] |
| 4 | Ipomeo aquatica (Water spinach) | Palm oil mill effluent | 25 days/bucket treatment system | COD (1500 mg/L), NH3–N (4–80 mg/L), TSS (5000 mg/L) | COD (80%), NH3–N (82.7%), TSS (90%) | [78] |
| 5 | Lemna minor (Lesser duckweed) | Mixture of textile, distillery, and institutional wastewater | 28 days | COD (34133.3 mg/L), EC (5.58 dS/m), BOD (15493.3 mg/L), pH (7.16); TDS (2641 mg/L) | COD (92%), EC (68%), BOD (92%), pH (8–9), TDS (68%) | [79] |
| 6 | Lemna minor (Lesser duckweed) | Treated industrial wastewater | 7 days (summer and winter) | N (12.2 mg/L in summer), P (2.9 mg/L in summer; 4.1 mg/L in winter) | N (56% in summer), P (76% summer; 66% winter) | [80] |
| 7 | Pistia stratiotes | Polluted rural river water | 6 months/PVC water tanks | TN (14.18–19.9 mg/L), COD (61–72 mg/L), TP (1.07–1.79 mg/L), NH4+-N (9.94–15.17 mg/L) | TN (77%), COD (61.70%), TP (88%), NH4+-N (93%) | [81] |
| 8 | Common reed (Phragmites australis), Manna Grass (Glyceria grandis), and Virginia Mallow (Sida hermaphrodita) | Secondary domestic wastewater | 5 years)/hybrid constructed wetland systems | BOD (284 mg/L), TSS (143 mg/L), TN (84.9 mg/L), COD (588 mg/L), TP (13.6 mg/L) | BOD (95%), TSS (95%), TN (94%), COD (95%), TP (95%) | [82] |
| 9 | Alternanthera (Joyweed) | Domestic wastewater | 10 days/level of sheet flow root (Shefrol bioreactor) | BOD (1400–1950 mg/L), COD (2900–3400 mg/L), TKN (63–91 mg/L), TP (37–59 mg/L), suspended solids (221–263 mg/L) (93%), Cu (3.9 mg/L) | BOD (87%), COD (78.9–83.9%), TKN (45%), TP (36%), suspended solids (SS) (93%), Cu (43%) | [83] |
| 10 | Nelumbo nucifera | Contaminated surface water | 30 days/batch type | turbidity (80.7 NTU), BOD (95 mg/L), COD (78.4 mg/L) | turbidity (88.3%), BOD (97.1%), COD (55%) | [84] |
| 11 | Limnobium laevigatum | Swine wastewater (10% effluent) | 3 months/batch system | TN (151.67 mg/L), TP (82.77 mg/L) | TN (48.80%), TP (28.20%) | [85] |
| 12 | Salvinia natans | Raw domestic wastewater | 8 months/tanks | TKN (102.4 mg/L), NH4-N (64.4 mg/L), BOD5 (311.1 mg/L), COD (981.7 mg/L), NO2-N (0.128 mg/L), PO4 (10.95 mg/L) | TKN (85.2%), NH4-N (79%), BOD5 (96.9%), COD (95%), NO2-N (40%), PO4 (37%) | [86] |
| 13 | Eichhornia crassipes (Water hyacinth) | Eichhornia crassipes (Water hyacinth) | 15 days | pH = 6.7–7.2; initial concentration: not found | Cr (66%), Zn (79%), Ni (67%), Fe (83%), Cu (63%), Cd (76%) | [87] |
| 14 | Myriophyllum spicatum (Eurasian watermilfoil) | Wastewater contaminated with constant/ radioactive Cobalt (Co) and Cesium (Cs) | 20 days | Cs and Co (20, 50, 100 and 150 mg/L) | Cs (60%), Co (90%) | [88] |
| 15 | Vertiveria zizaniodes | Fish pond wastewater | Six weeks/ aquaculture system | NH3 (0.0034 mg/L), NO2 (0.05 mg/L), NO3 (0.13 mg/L), NH4 (0.49 mg/L), PO4 (0.04 mg/L) | NH3 (65.16%), NO2 (27.51%), NO3 (25.5%), NH4 (30.17%), PO4 (42.75%) | [72] |
| 16 | Atriplex Lentiformis | Well drainage | 28 days | EC (14.7 dS/m), Ca (252 mg/L), Mg (157.9 mg/L), Na (3355 mg/L), Cl (4041 mg/L) | EC (11.80%), Ca (8.75%), Mg (5.9%), Na (13.7%), Cl (12.7%) | [89] |
| 17 | Chenopodium quinoa Willd. | Well drainage | 30 days | EC (2 dS/m), Ca (200 mg/L), Mg (72 mg/L), Na (285 mg/L), Cl (532 mg/L) | EC (9.35%), Ca (10%), Mg (7.62%), Na (5.6%), Cl (7.01%) | [90] |
| NO. | Type of Unconventional Water | Residence Time | Initial Concentration | Removal Efficiency | References |
|---|---|---|---|---|---|
| 1 | Domestic effluent | 4 days | TH (60%), P (10 mg/L), N (100 mg/L), EC (928 µS/cm), pH (7.26) | TH (60%), P (90%), N (94%), EC (50%), pH (17.63%) | [100] |
| 2 | Pig farm wastewater | 4 days | COD (825.63 mg/L), BOD (509.89 mg/L), NH3-N (134.43 mg/L), TP(24.31 mg/L) | COD (64%), BOD (68%), NH3-N (20%), TP (18%), TN (75%), TP (58%) | [101] |
| 3 | Textile wastewater | 60 days | N (8.76 mg/L), P(4.8 mg/L), K (3.4 mg/L), pH (8.6), EC (1.45 dS/m) | N (85.61%), P(79%), K (94.7%), pH (9.3%), EC (73%) | [102] |
| 4 | Groundwater | 5 min | TDS (1400 ppm) | TDS (55.93%) | [103] |
| 5 | Saline groundwater/ Mine wastewater | 30 days | TDS (11.2 mg/L), EC (27.27 mmhos/cm), TH (6243 mg/L), SO4 (98.5 meq/L), Cl (326 meq/L), Na (247 meq/L), K (0.514 meq/L), Mg (42 meq/L), Ca (66 meq/L) / TDS (20.6 mg/L), EC (46.30 mmhos/cm), TH (9884 mg/L), SO4 (94.9 meq/L), Cl (586 meq/L), Na (397 meq/L), K (1.12 meq/L), Mg (36.7 meq/L), Ca (247 meq/L) | TDS (33%), EC (28%), TH (45.1%), SO4 (70.86%), Cl (48.77%), Na (59.10%), K (58.36%), Mg (23.80%), Ca (51.47%) / /TDS (31.5%), EC (28.3%), TH (46.1%), SO4 (63.9%), Cl (47.6%), Na (52.4%), K (19.6%), Mg (43.6%), Ca (46.6%) | [104] |
| 6 | Piggery effluent | 5 days | BOD (854.77 mg/L), COD (1690.44 mg/L), TN (104.38 mg/L), TP (67.19), EC (3591.94 dS/m) | BOD (%74), COD (%70), TN (%87), TP (%83), EC (78.41%) | [105] |
| 7 | Synthetic wastewater | 7 days | Pb (9.94 ppm), Mn (10.01 ppm), Cu (9.96 ppm), Fe (10.5 ppm), Zn (10.2 ppm) | Pb (50%), Mn (33%), Cu (25%), Fe (96%), Zn (75%) | [106] |
| 8 | Bagmati river | 30 days | BOD5 (7.11 mg/L), Cl (123.54 mg/L), NO3 (3.3 mg/L), PO4 −3 (4.3 mg/L), TH (139.33 mg/L), alkalinity (153.34 mg/L) | BOD5 (71.03%), Cl (42.9%), NO3 (93.93%), PO4 −3 (88.04%), TH (46.04%), alkalinity (22.2%). | [107] |
| 9 | Tofu wastewater | 15 days | TSS (552 mg/L), pH (3.9), BOD (580 mg/L), COD (5759 mg/L) | TSS (75.28%), pH (7.8%), BOD (76%), COD (71.78%) | [15] |
| 10 | Carwash wastewater | 70 days | BOD (398 mg/L), COD (812 mg/L), P (12.10 mg/L), N (16.11 mg/L), Pb (0.13 mg/L), Zn (0.29 mg/L), NO3 (1.27 mg/L), NO2 (3.76 mg/L), NH3 (11.08 mg/L) | BOD (64.8%), COD (65.3%), P (69%), N (57.9%), Pb (61.5%), Zn (82.8%), NO3 (69.3%), NO2 (59.3%), NH3 (56.1%) | [108] |
| 11 | Sewage effluent | 6 days | BOD (233.8 mg/L), TSS (346.8 mg/L), TP (12.2 mg/L) | BOD (92%), TSS (92%), TP (87%) | [109] |
| 12 | Polluted river water | 42 days | COD (41 mg/L), NO3 (2.6 mg/L), PO4 (1.86 mg/L), TSS (5.20 mg/L) | COD (77%), NO3 (73%), PO4 (35%), TSS (26%) | [110] |
| 13 | Domestic wastewater | 60 days | pH (8.36), EC (0.015 dS/m), TDS (1754 mg/L), TH (2010.33 mg/L), NO3 (10.44 mg/L), Cl (65.82 mg/L), PO4 −3 (8.65 mg/L), K (39.4 mg/L) | pH (8.73%), EC (40.88%), TDS (30.84%), TH (33.46%), NO3 (44.25%), Cl (25.84%), PO4 −3 (50.63%), K (12.16%). | [111] |
| 14 | Abattoir wastewater | 6 days | N (131 mg/L), P (56.3 mg/L), Mg (1.06 mg/L), Fe (1.30 mg/L), BOD (206 mg/L), COD (204 mg/L) | N (52%), P (70%), Mg (88%), Fe (99.2%), BOD (84%), COD (86%) | [112] |
| 15 | Synthetic wastewater | 3 days | TDS (1463.20 mg/L), Zn (0.97 mg/L), Pb (0.63 mg/L), Cu (1.59 mg/L), DO (7.53 mg/L), BOD (2.26 mg/L), | TDS (74.91%), Zn (13.40%), Pb (34.92%), Cu (23.89%), DO (79.46%), BOD (78.10%) | [113] |
| 16 | Synthetic wastewater | 52 days | Cr (5 ppm), Cr (10 ppm), Cr (30 ppm), Cr (70 ppm) | Cr (5 ppm) (87%), Cr (10 ppm) (51%), Cr (30 ppm) (28%), Cr (70 ppm) (5.11%) | [22] |
| 17 | Fish pond wastewater | 6 weeks | NH3 (0.0034 mg/L), NO2 (0.05 mg/L), NO3 (0.13 mg/L), NH4 (0.48 mg/L), PO4 (0.04 mg/L) | NH3 (65.16%), NO2 (27.51%), NO3 (25.5%), NH4 (30.17%), PO4 (42.75%). | [72] |
| 18 | Effluent sewage | 18 days | Na (55.4 mg/L), K (21.9 mg/L), Mg (49 mg/L), HCO3 (260 mg/L), Ca (378.8 mg/L), Cl (167.1 mg/L), SO4 (137.5 mg/L) | Na (9%), K (29%), Mg (10%), HCO3 (4%), Ca (25%), Cl (25%), SO4 (9%) | [32] |
| 19 | Landfill leachate | 21 days | BOD (1153 mg/L), COD (2895 mg/L), PO4 (3.2 mg/L), NO3 (121 mg/L) | BOD (60%), COD (68%), PO4 (82%), NO3 (83%) | [114] |
| 20 | Wastewater effluent | 30 days | NO3 (29 mg/L), PO4 −3 (10.5 mg/L), COD (62 mg/L) | NO3 (40%), PO4 −3 (60%), COD (40%) | [67] |
| 21 | Paper board mill effluent (treated) | 10 days | TDS (1000 mg/L), TSS (200 mg/L), BOD (44 mg/L), COD (256 mg/L), TN (25 mg/L), TP (8.50 mg/L), Cd (0.42 mg/L), pH (8.18), EC (1.98 dS/m) | TDS (59.94%), TSS (74.58%), BOD (72.3%), COD (56.25%), TN (70%), TP (42.94%), Cd (80.95), pH (4.3%), EC (37.37%) | [115] |
| 22 | Municipal wastewater | 14 days | NH4 (55 mg/L), NO3 (18 mg/L), K (20.5 mg/L), PO4 (5.70 mg/L), BOD (103 mg/L), COD (262 mg/L) | NH4 (91%), NO3 (66%), K (97%), PO4 (89%), BOD (42%), COD (55%) | [116] |
| 23 | Olive mill wastewater (15%) | 67 days | TN (26.6 mg/L), Phenolic compounds (219 mg/L) | TN (23.7%), Phenolic compounds (92.1%) | [117] |
| 24 | Automobile service station effluent (50%) | 15 days | TDS (6240 mg/L), Cl (184.9 mg/L), Ca (121.6 mg/L), Mg (75.50 mg/L), Na (437.50 mg/L), K (79.8 mg/L), Fe (10.60 mg/L), SO4 (172.60 mg/L), BOD (11.62 mg/L), COD (740 mg/L) | TDS (91.73%), Cl (49.67%), Ca (60.48%), Mg (61.48%), Na (60.78%), K (58.41%), Fe (67.08%), SO4 (63.38%), BOD (69.02%), COD (72.16%) | [118] |
| 25 | Industrial wastewater | 9 days | BOD5 (1641 mg/L), COD (6953.33 mg/L), SO4 (1072.82 mg/L), Cl (1919 mg/L), TDS (5877.30 mg/L), EC (8550 µS/cm), Salinity (0.69%) | BOD5 (96.24%), COD (97.9%), SO4 (91.81%), Cl (80.16%), TDS (90.89%), EC (88.27%), Salinity (79.71%) | [119] |
| 26 | Well drainage | 14 days | EC (10.01 dS/m), Na (61.65 mg/L), Ca (32 mg/L), Mg (1.40 mg/L), NO3 (146.11 mg/L), PO4 (43.17 mg/L) | EC (15.88%), Na (14.36%), Ca (41.67%), Mg (57.14%), NO3 (44%), PO4 (44.51%) | [120] |
| 27 | Electroplating wastewater | 28 days | Cr (50.77 mg/L), Ni (24.73 mg/L) | Cr (61.10%), Ni (95.65%) | [121] |
| 28 | Synthetic water | 10 days | Cu (1.94 mg/L), Fe (0.84 mg/L), Mn (2.77 mg/L), Pb (0.67 mg/L), Zn (1.02 mg/L) | Cu (48.96%), Fe (90.47%), Mn (29.24%), Pb (53.74%), Zn (25.49%) | [122] |
| 29 | Synthetic wastewater | 72 days | Phenanthrene (194.24 mg/L), Pyrene (123.82 mg/L), Benzo (101.11 mg/L) | Phenanthrene (67%), Pyrene (66%), Benzo (73%) | [123] |
| 30 | Treated wastewater | 3 days | TH (502.75 mg/L), TDS (966.40 mg/L), SAR (0.41) | TH (20.19%), TDS (12.58%), SAR (34.14%) | [124] |
| 31 | Municipal wastewater | 15 days | EC (1.51 dS/m), Ca (67.33 mg/L), Mg (81.09 mg/L), Na (8.49 mg/L), K (14.61 mg/L), Cl (130.16 mg/L) | EC (15.67%), Ca (71.82%), Mg (10%), Na (38.32%), K (84.60%), Cl (72.60%) | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorafshan, M.M.; Abedi-Koupai, J.; Eslamian, S.; Amiri, M.J. Vetiver Grass (Chrysopogon zizanoides L.): A Hyper-Accumulator Crop for Bioremediation of Unconventional Water. Sustainability 2023, 15, 3529. https://doi.org/10.3390/su15043529
Dorafshan MM, Abedi-Koupai J, Eslamian S, Amiri MJ. Vetiver Grass (Chrysopogon zizanoides L.): A Hyper-Accumulator Crop for Bioremediation of Unconventional Water. Sustainability. 2023; 15(4):3529. https://doi.org/10.3390/su15043529
Chicago/Turabian StyleDorafshan, Mohammad Mahdi, Jahangir Abedi-Koupai, Saeid Eslamian, and Mohammad Javad Amiri. 2023. "Vetiver Grass (Chrysopogon zizanoides L.): A Hyper-Accumulator Crop for Bioremediation of Unconventional Water" Sustainability 15, no. 4: 3529. https://doi.org/10.3390/su15043529
APA StyleDorafshan, M. M., Abedi-Koupai, J., Eslamian, S., & Amiri, M. J. (2023). Vetiver Grass (Chrysopogon zizanoides L.): A Hyper-Accumulator Crop for Bioremediation of Unconventional Water. Sustainability, 15(4), 3529. https://doi.org/10.3390/su15043529








