Differentiation of Rare Earth Elements in Coal Combustion Products from the Handan Power Plant, Hebei Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Procedures
3. Results and Discussion
3.1. Characteristics of Coal and Its Products
3.1.1. Physical Characteristics of Coal and Ashes
3.1.2. Mineral Composition
3.2. Content Distribution of REY
3.2.1. REY Content in Raw Coal
3.2.2. Distribution of REY in Coal Combustion Products
3.3. Enrichment of REY
Relative Enrichment Index RE
3.4. Effect of Fly Ash Particle Size on Migration Rules of REY
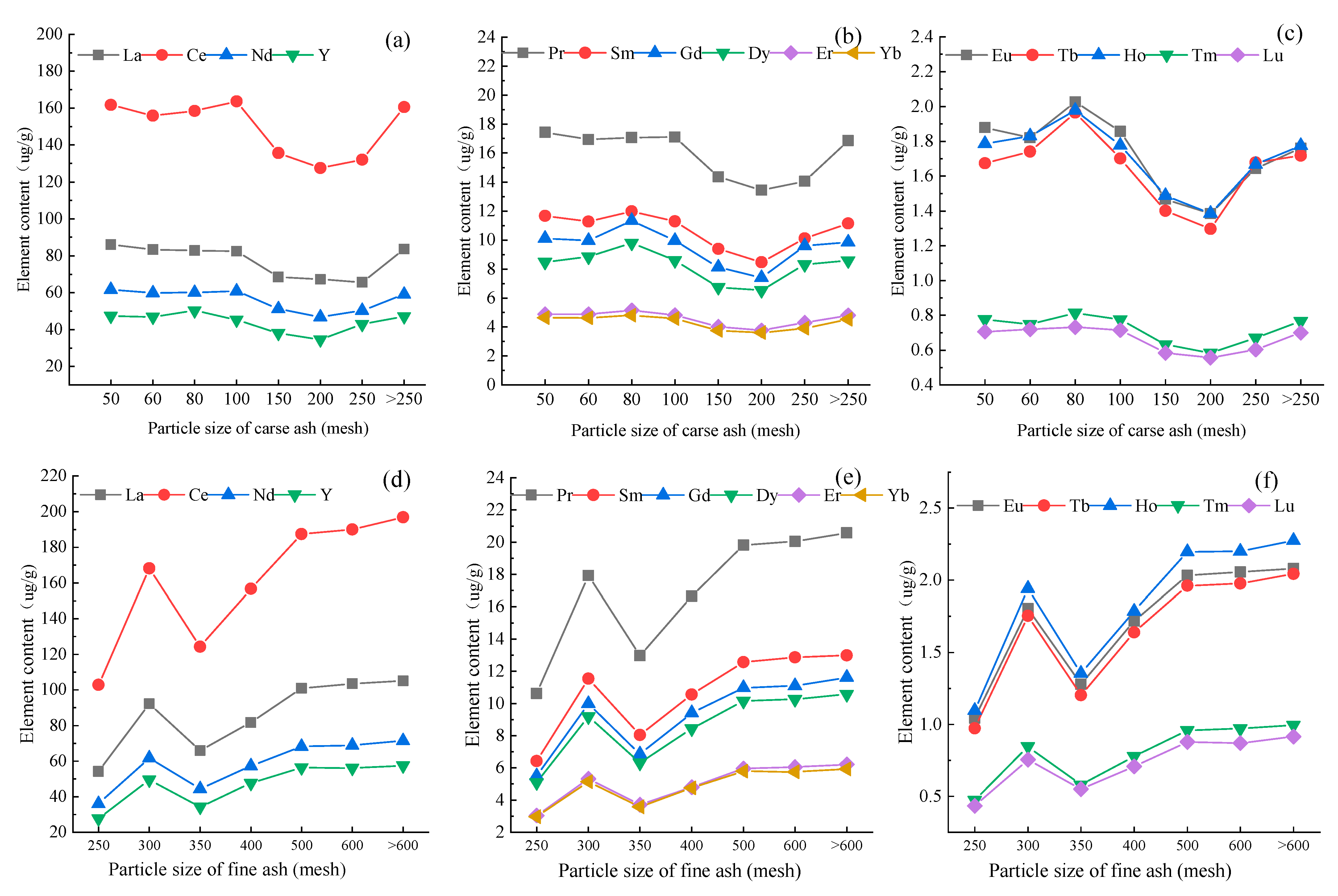
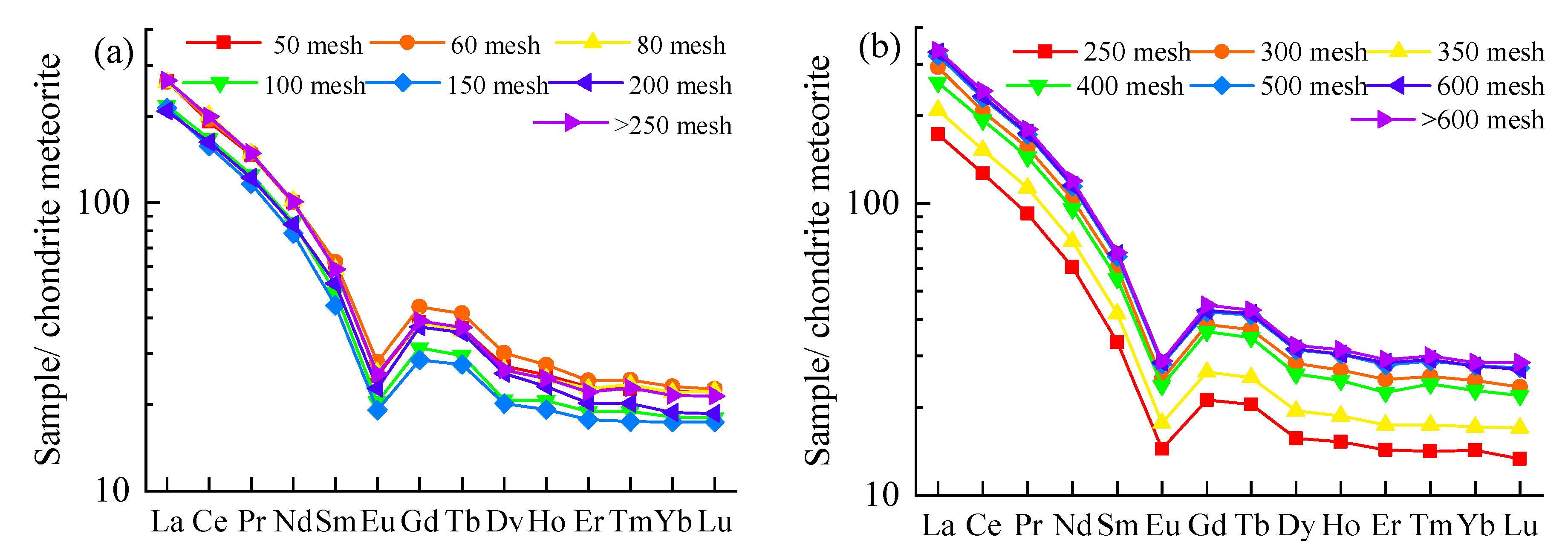
3.4.1. Content Distribution of REY
3.4.2. Distribution Patterns of REE in Fly Ash with Particle Sizes
3.4.3. Influence of Occurrence State on Element Differentiation
4. Conclusions
- (1)
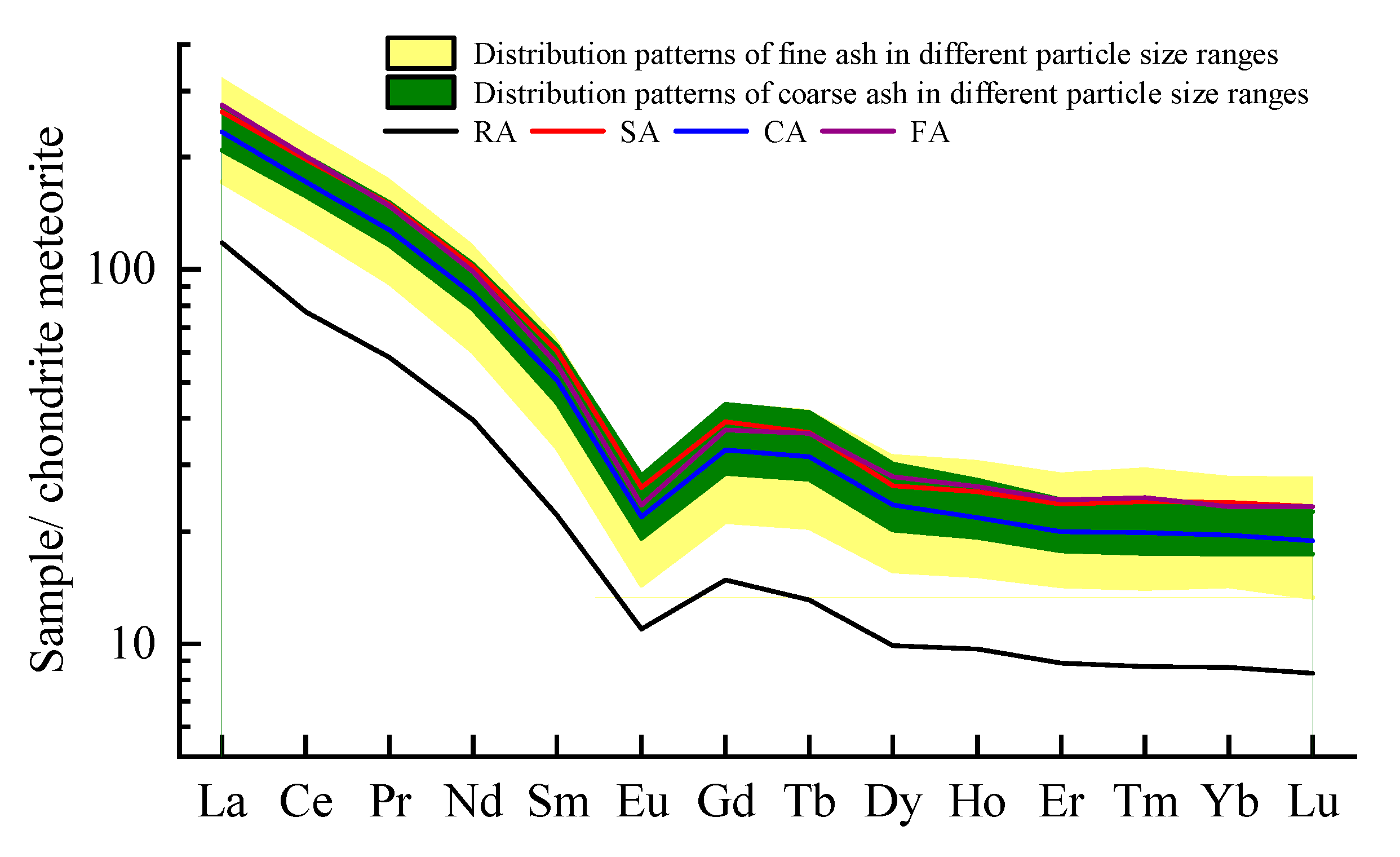
- The REY content in raw coal fed to the Handan Power Plant is higher than the average content in the world coal, and the concentration distribution of REY in raw coal and solid combustion products follows the order of LREY > MREY > HREY. Compared with coarse ash, REY are more easily enriched in slag and fine ash. There is no difference in the distribution patterns of REE in all the samples with different particle sizes, showing “V-shaped” curves with negative Eu.
- (2)
- After coal combustion, the levels of REY content are obviously different in coal ash with different particle sizes. In general, the REY content decreases with the decrease in particle size in coarse ash, whereas the REY content increases with the decrease in particle size in fine ash. However, there is an inflection point in the trend at 200 mesh and 300 mesh for coarse ash and fine ash, respectively.
- (3)
- The occurrence state of REY in raw coal and coal ash does not change to an obvious degree (residue state > organic/sulfide-bound state > iron–manganese-oxide-bound state > carbonate-bound state > exchangeable state). The proportion of REY in the residue state is above 93% in the combustion product slag. The residual state is mainly the aluminosilicate combined state. It is inferred that there is a strong correlation between Si, Al, and REY in fly ash, and REY may occur in mullite, which is the main carrier mineral of Si and Al in coal-burning products.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seredin, V.V.; Dai, S.F. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Taggart, R.K.; Hower, J.C.; Dwyer, G.S.; Hsu-Kim, H. Trends in the rare earth element content of US-based coal combustion fly ashes. Environ. Sci. Technol. 2016, 50, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.F.; Graham, I.T.; Ward, C.R. A review of anomalous rare earth elements and yttrium in coal. Int. J. Coal Geol. 2016, 159, 82–95. [Google Scholar] [CrossRef]
- Fiket, Z.; Medunic, G.; Turk, M.F.; Kniewald, G. Rare earth elements in superhigh-organic-sulfur rasa coal ash (Croatia). Int. J. Coal Geol. 2018, 194, 1–10. [Google Scholar] [CrossRef]
- Hower, J.C.; Eble, C.F.; Wang, N.; Dai, S.F. Distribution of rare earth elements and other critical elements in beneficiated Pennsylvania anthracites. Fuel 2021, 304, 121400. [Google Scholar] [CrossRef]
- Karayigit, A.I.; Yerin, M.O.; Oskay, R.G.; Bulut, Y.; Córdoba, P. Enrichment and distribution of elements in the middle Miocene coal seams in the Orhaneli coalfield (NW Turkey). Int. J. Coal Geol. 2021, 247, 103854. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A.; Eble, C.; Ames, P.; Mclaughlin, P. Rare earth elements and yttrium in Pennsylvanian coals and shales in the eastern part of the Illinois Basin. Int. J. Coal Geol. 2020, 231, 103620. [Google Scholar] [CrossRef]
- Jane, W.N.; Arnold, M. Rare earth elements in select Main Karoo Basin (South Africa) coal and coal ash samples. Int. J. Coal Geol. 2018, 196, 82–92. [Google Scholar] [CrossRef]
- Qin, S.J.; Xu, F.; Cui, I.L.; Wang, J.X.; Li, S.Y.; Zhao, Z.S.; Xiao, L.; Guo, Y.X.; Zhao, G.L. Geochemistry characteristics and promising utilization of strategically critical trace elements from coal-related resources. Coal Sci. Technol. 2022, 50, 1–38. [Google Scholar] [CrossRef]
- Mokoena, K.; Mokhahlane, L.S.; Clarke, S. Effects of acid concentration on the recovery of rare earth elements from coal fly ash. Int. J. Coal Geol. 2022, 259, 104037. [Google Scholar] [CrossRef]
- Pan, J.H.; Nie, T.C.; Hassas, B.V.; Rezaee, M.; Wen, Z.P.; Zhou, C.C. Recovery of rare earth elements from coal fly ash by integrated physical separation and acid leaching. Chemosphere 2020, 248, 126112. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, S.; Zou, J.; French, D.; Graham, I.T. Rare earth elements and yttrium in coal ash from the Luzhou power plant in Sichuan, Southwest China: Concentration, characterization and optimized extraction. Int. J. Coal Geol. 2019, 203, 1–14. [Google Scholar] [CrossRef]
- Smith, R.C.; Taggart, R.K.; Hower, J.C.; Wiesner, M.R.; Hsu-Kim, H. Selective recovery of rare earth elements from coal fly ash leachates using liquid membrane processes. Environ. Sci. Technol. 2019, 53, 4490–4499. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Chen, B.R.; Qian, Q.F.; Yang, Y.N.; Yang, S.J. Distribution of trace elements concentration in 110 coal mine samples in China. Nucl. Technol. 1985, 43–44. [Google Scholar]
- Sun, J.X.; Jervis, R.E. The trace elements in coal and their characteristics of distribution during coal combustion. Sci. China Ser. A 1986, 1287–1294. [Google Scholar]
- Ratafia-Brown, J.A. Overview of trace element partitioning in flames and furnaces of utility coal-fired boilers. Fuel Process. Technol. 1994, 39, 139–157. [Google Scholar] [CrossRef]
- Wu, G.Q.; Wang, T.; Zhang, Y.S.; Wang, J.W.; Pan, W.P. Study on the Enrichment of Rare Earth Elements Between Coals and Their By-products at Coal-fired Power Plants. Proc. CSEE 2020, 40, 1963–1972. [Google Scholar] [CrossRef]
- Yao, D.X.; Zhi, X.C.; Wang, X. Geochemical feature and laws of concentration and dispersion of rare earth elements between coals and their fly and bottom ash. Geochimica 2003, 5, 491–500, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S.F.; Sun, Y.Z.; Chekryzhov, I.Y. Coal deposits as promising sources of rare metals for alternative power and energy-efficient technologies. Appl. Geochem. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Dai, S.F.; Zhao, L.; Peng, S.P.; Chou, C.L.; Wang, X.B.; Zhang, Y.; Li, D.; Sun, Y.Y. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2010, 81, 320–332. [Google Scholar] [CrossRef]
- Hower, J.C.; Dai, S.F.; Seredin, V.V.; Zhao, L.; Kostova, I.L.; Silva, L.F.O.; Mardon, S.M.; Gurdal, G. A note on the occurrence of yttrium and rare earth elements in coal combustion products. Coal Combust. Gasif. Prod. 2013, 5, 39–47. [Google Scholar]
- Lanzerstorfer, C. Pre-processing of coal combustion fly ash by classification for enrichment of rare earth elements. Energy Reports 2018, 4, 660–663. [Google Scholar] [CrossRef]
- Zhang, W.; Groppo, J.; Honaker, R. Ash Beneficiation for REE Recovery. In Proceedings of the 2015 World of Coal Ash (WOCA) Conference, Nasvhille, TN, USA, 5–7 May 2015. [Google Scholar]
- Pan, J.H.; Zhou, C.C.; Liu, C.; Tang, M.C.; Cao, S.S.; Hu, T.T.; Ji, W.S.; Luo, Y.L.; Wen, M.Z.; Zhang, N. Modes of Occurrence of Rare Earth Elements in Coal Fly Ash: A Case Study. Energy Fuels 2018, 32, 9738–9743. [Google Scholar] [CrossRef]
- Yang, Z. Geochemical Characteristics and Migration Patterns of Hazardous Elements in Coal Combustion Products of Handan Power Plants. M.D. Thesis, Hebei University of Engineering, Handan, China, 2019. [Google Scholar]
- Dai, S.F.; Wang, X.B.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O'Keefe, M.K.J.; Huang, W.H.; Li, T.; Liu, H.D.; Xue, W.F.; et al. Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit, Inner Mongolia, China: New data and genetic implications. Int. J. Coal Geol. 2012, 90-91, 72–99. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate hazardous metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Brown, P.; Jones, T.; BéRUBé, K. The internal microstructure and fibrous mineralogy of fly ash from coal-burning power stations. Environ. Pollut. 2011, 159, 3324–3333. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S.F. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]
- Yuan, B. CFB Zhungeer Coal Power Plant Ash Metallurgical Grade Alumina Extraction Process Research. M.D. Thesis, Jilin University, Changchun, China, 2008. [Google Scholar]
- Zhao, Y.C. Partition Mechanism and Interaction of Minerals and Trace Element during Coal Combustion. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2008. [Google Scholar]
- Dai, S.F.; Zhao, L.; Hower, J.C.; Johnston, M.N.; Song, W.J.; Wang, P.P.; Zhang, S.F. Petrology, mineralogy, and chemistry of size-fractioned fly ash from the Jungar power plant, Inner Mongolia, China, with emphasis on the distribution of rare earth elements. Energy Fuels 2014, 28, 1502–1514. [Google Scholar] [CrossRef]
- Styszko-Grochowiak, K.; Gołaś, J.; Jankowski, H.; Koziński, S. Characterization of the coal fly ash for the purpose of improvement of industrial on-line measurement of unburned carbon content. Fuel 2004, 83, 1847–1853. [Google Scholar] [CrossRef]
- Dai, S.F.; Jiang, Y.F.; Ward, C.R.; Gu, L.D.; Seredin, V.V.; Liu, H.D.; Zhou, D.; Wang, X.B.; Sun, Y.Z.; Zou, J.H.; et al. Mineralogical and geochemical compositions of the coal in the Guanbanwusu Mine, Inner Mongolia, China: Further evidence for the existence of an Al (Ga and REE) ore deposit in the Jungar Coalfield. Int. J. Coal Geol. 2012, 98, 10–40. [Google Scholar] [CrossRef]
- Wang, Q.C.; Shao, Q.C.; Kang, S.L.; Zhou, Z.H.; Wang, Z.G.; Zou, S.T.; Fu, D.M. Removal of 9 kinds of trace elements in burning coal in the layer-burning boilers. Environ. Sci. 1996, 17, 18–20+91. [Google Scholar]
- Meij, R.; te Winkel, B.H. Trace elements in world steam coal and their behaviour in Dutch coal-fired power stations: A review. Int. J. Coal Geol. 2009, 77, 289–293. [Google Scholar] [CrossRef]
- Wang, J.X.; Yang, Z.; Qin, S.J.; Panchal, B.; Sun, Y.Z.; Niu, H.Y. Distribution characteristics and migration patterns of hazardous trace elements in coal combustion products of power plants. Fuel 2019, 258, 116062. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.Q. Tetrad effect of REE in apatites from pegmatite No.3, Altay, Xinjiang and its implications. Mineral. Petrol. 2001, 4, 323–334. [Google Scholar] [CrossRef]
- Yang, X.W.; Jia, Z.L.; Wang, J.R.; Zhang, Y.K. The tetrad effect and magmatic evolution in Guobaoshan granite of Beishan area, Gansu Province. Gansu Geol. 2017, 26, 25–31. [Google Scholar]
- Ouyang, Z.H.; Zeng, H.C.; Lu, X.H.; Yu, Q.M.; Hu, T.L.; Lu, N.X.; Yao, B. The investigation of the environment of heavy metals in fine particles in coal combustion. J. Combust. Sci. Technol. 1996, 2, 111–120. [Google Scholar]
- Zhao, Z.H. Calculation methods of geochemical parameters of some common rare earth elements and their geochemical significance. Earth Environ. 1985, S1, 11–14. [Google Scholar]
- Zhao, Z.G.; Tang, X.Y.; Li, B.F. Geochemistry of rare earth elements of coal in Huaibei Coalfield. Geochimica. 2000, 29, 578–583. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Palmer, C.A.; Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol. 2018, 185, 138–160. [Google Scholar] [CrossRef]
- Gong, B.; Chong, T.; Zhuo, X.; Zhao, Y.; Zhang, J. Mineral changes and trace element releases during extraction of alumina from high aluminum fly ash in Inner Mongolia, China. Int. J. Coal Geol. 2016, 166, 96–107. [Google Scholar] [CrossRef]
- Esknazy, G.M. Rare earth elements in a sampled coal from the Pirin deposit, Bulgaria. Int. J. Coal Geol. 1987, 7, 301–314. [Google Scholar] [CrossRef]
- Liu, B.J.; Wang, J.Y.; He, H.T.; Mishra, V.; Li, Y.H.; Wang, J.X.; Zhao, C.L. Geochemistry of Carboniferous coals from the Laoyaogou mine, Ningwu coalfield, Shanxi Province, northern China: Emphasis on the enrichment of valuable elements. Fuel 2020, 279, 118414. [Google Scholar] [CrossRef]
- Pan, J.H. Study on the Enrichment, Extraction, and Mechanism of Occurrence of Rare Earth Elements in Coal Fly Ash. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, 2021. [Google Scholar]
- Spears, D.A. Role of clay minerals in UK coal combustion. Appl. Clay Sci. 2000, 16, 87–95. [Google Scholar] [CrossRef]
- Wang, J.X.; Fu, Z.H.; Hu, Y.F.; Yang, Z.; Ma, J.J.; Sun, Y.Z. Geochemical characteristics of REY, Li, Ga trace elements in the No. 9 coal seam of the Daheng mine, Ningwu coalfield, Shanxi Province, China. China Geol. 2021, 4, 266–273. [Google Scholar]
- Haberl, J.; Koralewska, R.; Schlumberger, S.; Schuster, M. Quantification of main and trace metal components in the fly ash of waste-to-energy plants located in Germany and Switzerland: An overview and comparison of concentration fluctuations within and between several plants with particular focus on valuable metals. Waste Manag. 2018, 75, 361–371. [Google Scholar] [CrossRef]
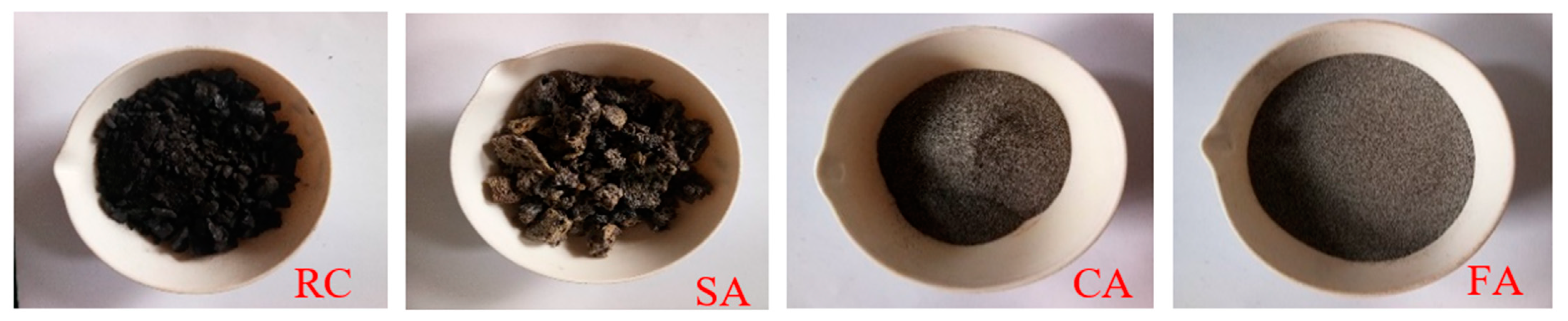
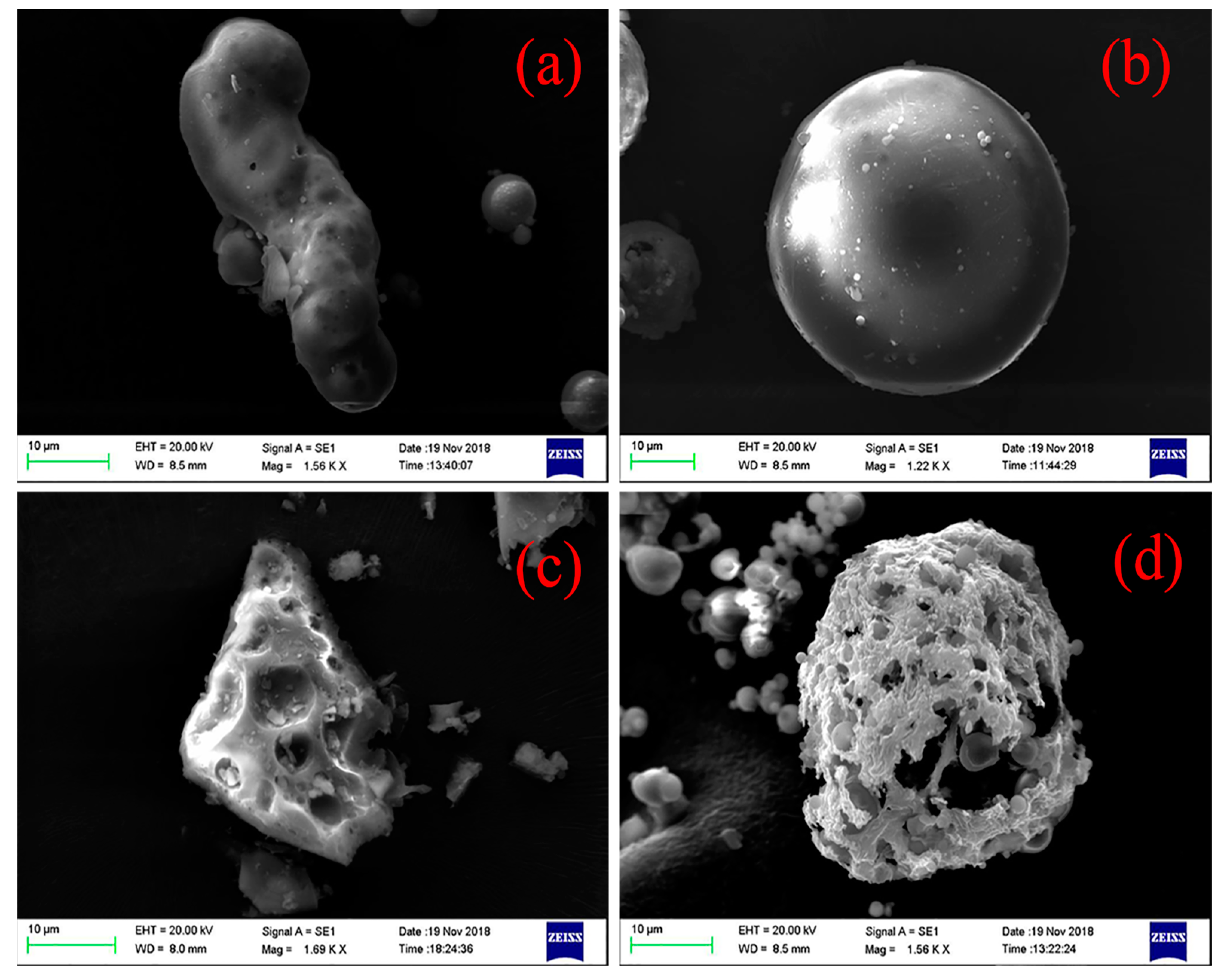


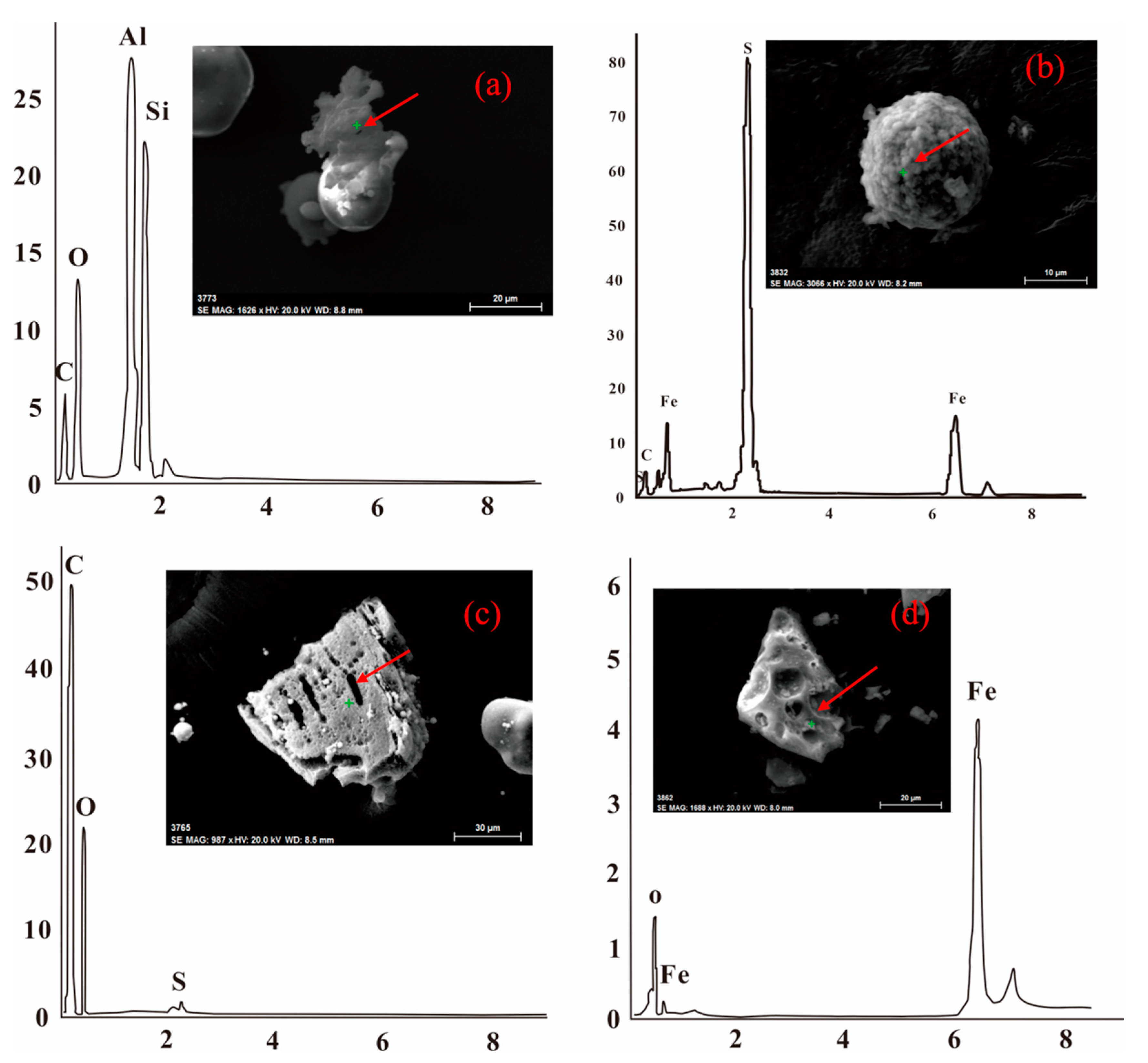

| Major Elements | RC | SA | CA | FA |
|---|---|---|---|---|
| SiO2 | 48.43 | 51.80 | 43.63 | 48.03 |
| Al2O3 | 38.60 | 32.11 | 31.56 | 34.25 |
| Fe2O3 | 4.29 | 8.40 | 13.30 | 7.45 |
| K2O | 1.28 | 1.22 | 1.48 | 1.43 |
| NaO2 | 0.62 | 0.30 | 1.07 | 0.79 |
| P2O5 | 0.73 | 0.25 | 0.27 | 0.55 |
| CaO | 2.77 | 3.26 | 4.09 | 3.47 |
| MgO | 0.87 | 0.78 | 0.75 | 0.94 |
| TiO2 | 1.19 | 1.07 | 2.10 | 1.74 |
| MnO | 0.04 | 0.10 | 0.11 | 0.06 |
| ZrO2 | 0.08 | 0.09 | 0.28 | 0.17 |
| SrO | 0.10 | 0.09 | 0.27 | 0.21 |
| Other elements | 1.00 | 0.53 | 1.09 | 0.91 |
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | |
| RC | 37.25 | 62.68 | 6.68 | 23.70 | 4.24 | 0.79 | 3.84 | 0.62 | 3.22 | 18.03 |
| WC | 11.00 | 23.00 | 3.50 | 12.00 | 2.00 | 0.47 | 2.70 | 0.32 | 2.10 | 8.40 |
| CC | 3.39 | 2.73 | 1.91 | 1.97 | 2.12 | 1.69 | 1.42 | 1.95 | 1.53 | 2.15 |
| Ho | Er | Tm | Yb | Lu | ∑LREY | ∑MREY | ∑HREY | ∑REY | ||
| RC | 0.70 | 1.89 | 0.29 | 1.80 | 0.27 | 134.55 | 26.50 | 4.95 | 166.00 | |
| WC | 0.54 | 0.93 | 0.31 | 1.00 | 0.20 | 51.50 | 13.99 | 2.98 | 68.47 | |
| CC | 1.30 | 2.03 | 0.94 | 1.80 | 1.37 | 2.61 | 1.89 | 1.66 | 6.16 |
| RC | SA | CA | FA | WCA | C/S | F/S | |
|---|---|---|---|---|---|---|---|
| La | 37.25 | 83.38 | 73.41 | 86.66 | 69 | 0.88 | 1.04 |
| Ce | 62.68 | 159.95 | 139.71 | 163.30 | 130 | 0.87 | 1.02 |
| Pr | 6.68 | 17.19 | 14.65 | 17.06 | 20 | 0.85 | 0.99 |
| Nd | 23.70 | 60.70 | 51.42 | 58.91 | 67 | 0.85 | 0.97 |
| Sm | 4.24 | 11.64 | 9.75 | 10.75 | 13 | 0.84 | 0.92 |
| Eu | 0.79 | 1.89 | 1.58 | 1.70 | 2.5 | 0.83 | 0.90 |
| Gd | 3.84 | 10.16 | 8.56 | 9.64 | 16 | 0.84 | 0.95 |
| Tb | 0.62 | 1.74 | 1.50 | 1.73 | 2.1 | 0.86 | 0.99 |
| Dy | 3.22 | 8.60 | 7.64 | 9.10 | 14 | 0.89 | 1.06 |
| Y | 18.03 | 49.56 | 40.45 | 47.87 | 51 | 0.82 | 0.97 |
| Ho | 0.70 | 1.84 | 1.57 | 1.90 | 4 | 0.85 | 1.03 |
| Er | 1.89 | 5.04 | 4.25 | 5.15 | 5.5 | 0.84 | 1.02 |
| Tm | 0.29 | 0.80 | 0.66 | 0.82 | 2 | 0.82 | 1.03 |
| Yb | 1.80 | 4.97 | 4.07 | 4.84 | 6.2 | 0.82 | 0.98 |
| Lu | 0.27 | 0.75 | 0.61 | 0.75 | 1.2 | 0.81 | 0.99 |
| LREY | 134.55 | 332.86 | 288.94 | 336.68 | 0.87 | 1.17 | |
| MREY | 26.5 | 71.95 | 59.73 | 70.04 | 0.83 | 1.17 | |
| HYEY | 4.95 | 13.4 | 11.16 | 13.46 | 0.83 | 1.21 | |
| REY | 166.00 | 418.21 | 359.83 | 420.17 | 0.86 | 1.00 | |
| LaN/LuN | 1.36 | 1.11 | 1.21 | 1.16 |
| RE ≦ 0.1 | 0.1 < RE < 0.85 | 0.85 ≦ RE < 1 |
|---|---|---|
| Rarely remain in solid combustion products. | Partly remain in solid combustion products. | Mainly remain in solid combustion products. |
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | 0.76 | 0.87 | 0.88 | 0.87 | 0.93 | 0.81 | 0.90 | 0.95 | 0.91 | 0.93 | 0.89 | 0.91 | 0.94 | 0.94 | 0.94 |
| CA | 0.67 | 0.76 | 0.75 | 0.74 | 0.78 | 0.68 | 0.76 | 0.82 | 0.81 | 0.76 | 0.76 | 0.76 | 0.77 | 0.77 | 0.75 |
| FA | 0.79 | 0.89 | 0.87 | 0.85 | 0.86 | 0.73 | 0.85 | 0.94 | 0.96 | 0.90 | 0.92 | 0.93 | 0.97 | 0.92 | 0.93 |
| Particle Size/Mesh | Element Content | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | REY | LaN/LuN | |
| CA 50 | 86.0 | 162 | 17.4 | 61.7 | 11.7 | 1.88 | 10.1 | 1.67 | 8.48 | 47.3 | 1.79 | 4.89 | 0.78 | 4.65 | 0.71 | 421 | 1.22 |
| CA 60 | 83.3 | 156 | 16.9 | 59.9 | 11.3 | 1.82 | 9.96 | 1.74 | 8.85 | 46.9 | 1.83 | 4.89 | 0.75 | 4.62 | 0.72 | 409 | 1.16 |
| CA 80 | 82.8 | 158 | 17.1 | 60.1 | 12.0 | 2.03 | 11.3 | 1.96 | 9.81 | 50.3 | 1.98 | 5.15 | 0.81 | 4.80 | 0.73 | 419 | 1.13 |
| CA 100 | 82.5 | 164 | 17.1 | 60.9 | 11.3 | 1.86 | 9.97 | 1.70 | 8.60 | 45.3 | 1.78 | 4.83 | 0.78 | 4.58 | 0.72 | 416 | 1.15 |
| CA 150 | 68.5 | 136 | 14.4 | 51.2 | 9.39 | 1.47 | 8.13 | 1.40 | 6.74 | 38.0 | 1.49 | 4.02 | 0.63 | 3.76 | 0.58 | 345 | 1.17 |
| CA 200 | 67.2 | 128 | 13.4 | 46.8 | 8.48 | 1.38 | 7.40 | 1.30 | 6.54 | 34.6 | 1.39 | 3.77 | 0.58 | 3.61 | 0.56 | 325 | 1.21 |
| CA 250 | 65.7 | 132 | 14.1 | 50.3 | 10.1 | 1.64 | 9.61 | 1.68 | 8.32 | 43.0 | 1.67 | 4.31 | 0.67 | 3.90 | 0.60 | 348 | 1.09 |
| CA > 250 | 83.6 | 161 | 16.9 | 60.0 | 11.1 | 1.76 | 9.85 | 1.71 | 8.59 | 47.1 | 1.77 | 4.81 | 0.77 | 4.53 | 0.70 | 414 | 1.19 |
| FA 250 | 54.2 | 103 | 10.6 | 36.1 | 6.42 | 1.04 | 5.49 | 0.97 | 5.09 | 27.7 | 1.10 | 3.05 | 0.47 | 2.97 | 0.43 | 259 | 1.25 |
| FA 300 | 92.2 | 168 | 17.9 | 61.7 | 11.5 | 1.80 | 9.97 | 1.75 | 9.19 | 49.5 | 1.94 | 5.31 | 0.85 | 5.15 | 0.76 | 438 | 1.22 |
| FA 350 | 65.9 | 124 | 13.0 | 44.3 | 8.04 | 1.28 | 6.85 | 1.20 | 6.31 | 34.3 | 1.35 | 3.71 | 0.58 | 3.57 | 0.55 | 315 | 1.20 |
| FA 400 | 81.7 | 157 | 16.6 | 57.3 | 10.6 | 1.72 | 9.42 | 1.64 | 8.45 | 47.6 | 1.79 | 4.80 | 0.80 | 4.75 | 0.71 | 405 | 1.15 |
| FA 500 | 101 | 187 | 19.8 | 68.2 | 12.6 | 2.03 | 11.0 | 1.96 | 10.2 | 56.4 | 2.20 | 5.96 | 0.96 | 5.79 | 0.88 | 486 | 1.15 |
| FA 600 | 104 | 190 | 20.0 | 68.9 | 12.9 | 2.06 | 11.1 | 1.98 | 10.3 | 56.1 | 2.20 | 6.06 | 0.97 | 5.76 | 0.87 | 493 | 1.19 |
| FA > 600 | 105 | 197 | 20.6 | 71.4 | 13.0 | 2.08 | 11.6 | 2.04 | 10.6 | 57.4 | 2.28 | 6.21 | 1.00 | 5.93 | 0.92 | 507 | 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Ma, J.; Wang, J.; Niu, H.; Yang, Z.; Hao, H.; Panchal, B. Differentiation of Rare Earth Elements in Coal Combustion Products from the Handan Power Plant, Hebei Province, China. Sustainability 2023, 15, 3420. https://doi.org/10.3390/su15043420
Hu Y, Ma J, Wang J, Niu H, Yang Z, Hao H, Panchal B. Differentiation of Rare Earth Elements in Coal Combustion Products from the Handan Power Plant, Hebei Province, China. Sustainability. 2023; 15(4):3420. https://doi.org/10.3390/su15043420
Chicago/Turabian StyleHu, Yafan, Juanjuan Ma, Jinxi Wang, Hongya Niu, Zhen Yang, Huidi Hao, and Balaji Panchal. 2023. "Differentiation of Rare Earth Elements in Coal Combustion Products from the Handan Power Plant, Hebei Province, China" Sustainability 15, no. 4: 3420. https://doi.org/10.3390/su15043420
APA StyleHu, Y., Ma, J., Wang, J., Niu, H., Yang, Z., Hao, H., & Panchal, B. (2023). Differentiation of Rare Earth Elements in Coal Combustion Products from the Handan Power Plant, Hebei Province, China. Sustainability, 15(4), 3420. https://doi.org/10.3390/su15043420






