Enhanced Photoelectrochemical Water Oxidation on BiVO4 Photoanodes Functionalized by Bimetallic Dicyanamide Molecular Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
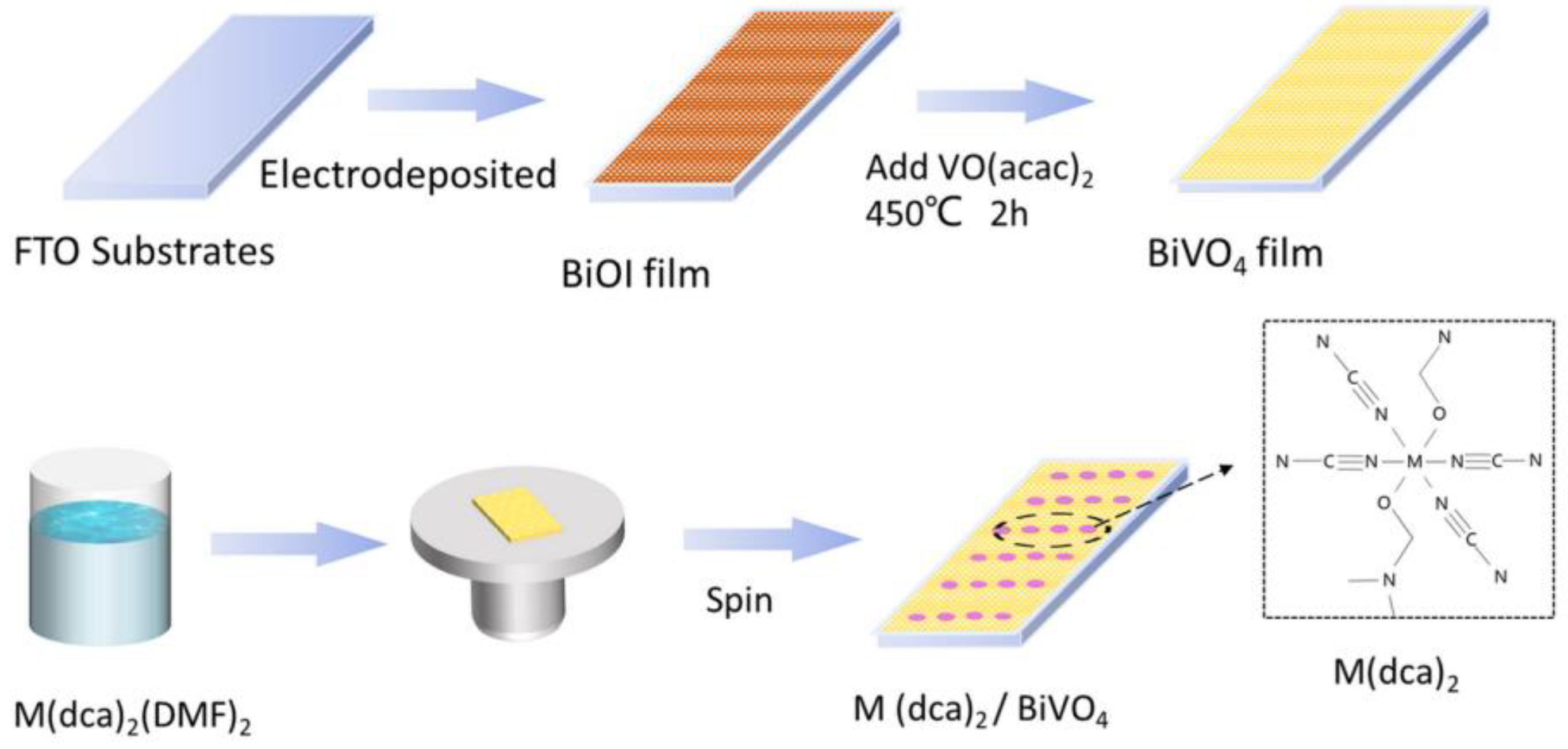
2.2. Fabrication of Bismuth Vanadate Nanoporous Films
2.3. Fabrication of M(dca)2 Catalysts
2.4. Deposition of M(dca)2 on BiVO4 and FTO
2.5. Material Characterization
2.6. Photoelectrochemical Measurements
3. Results
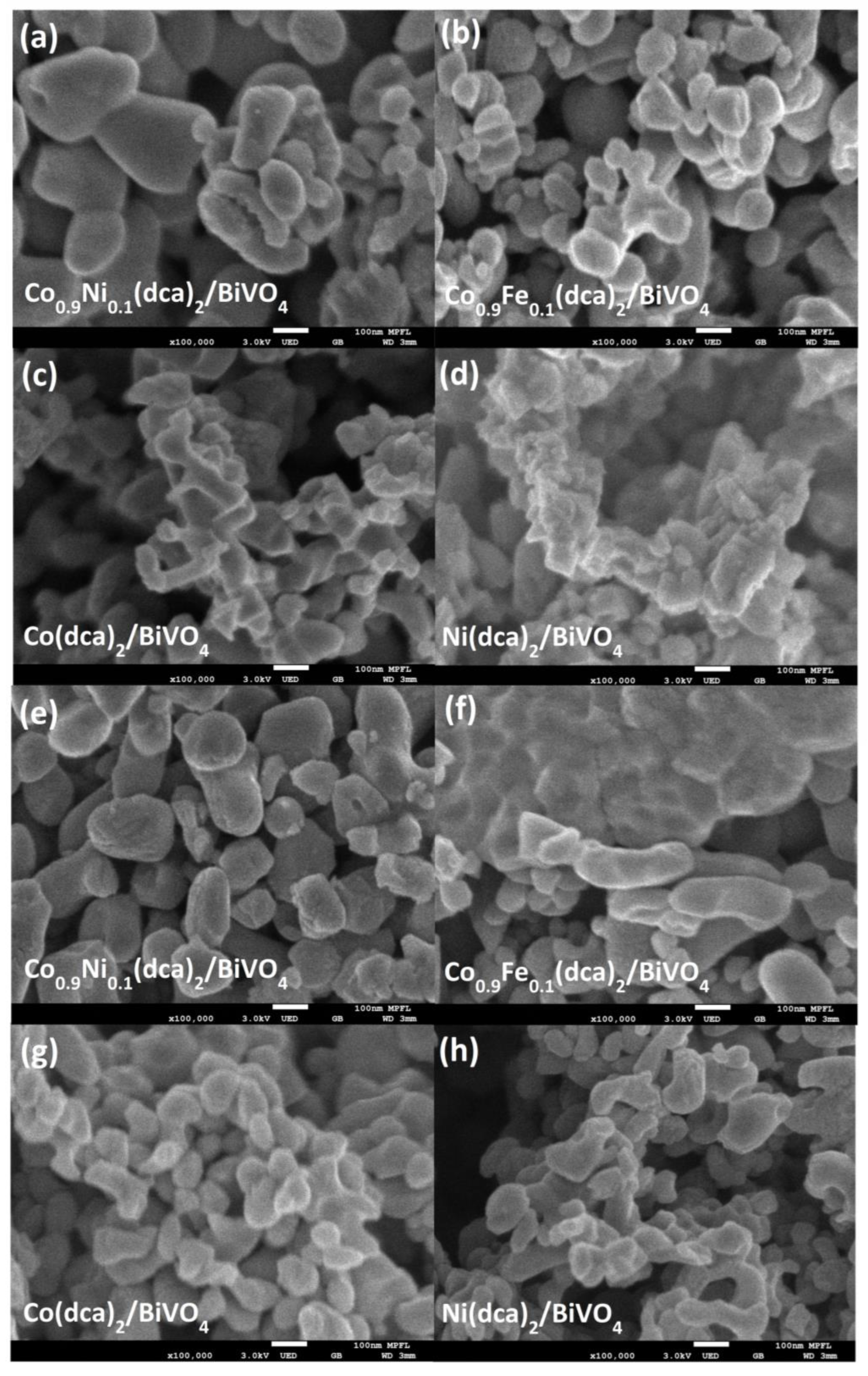
3.1. The Physical Characterization of Samples
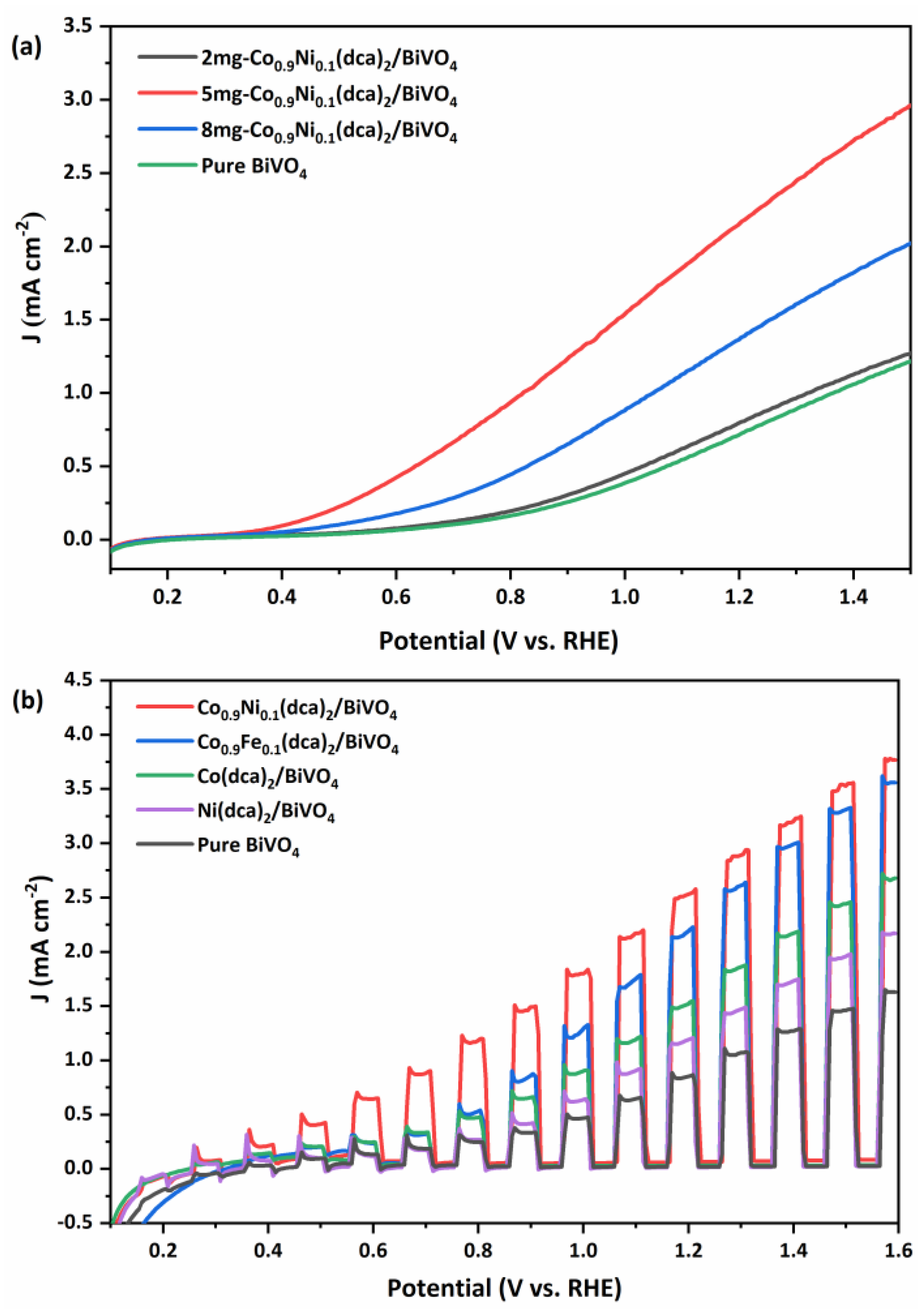
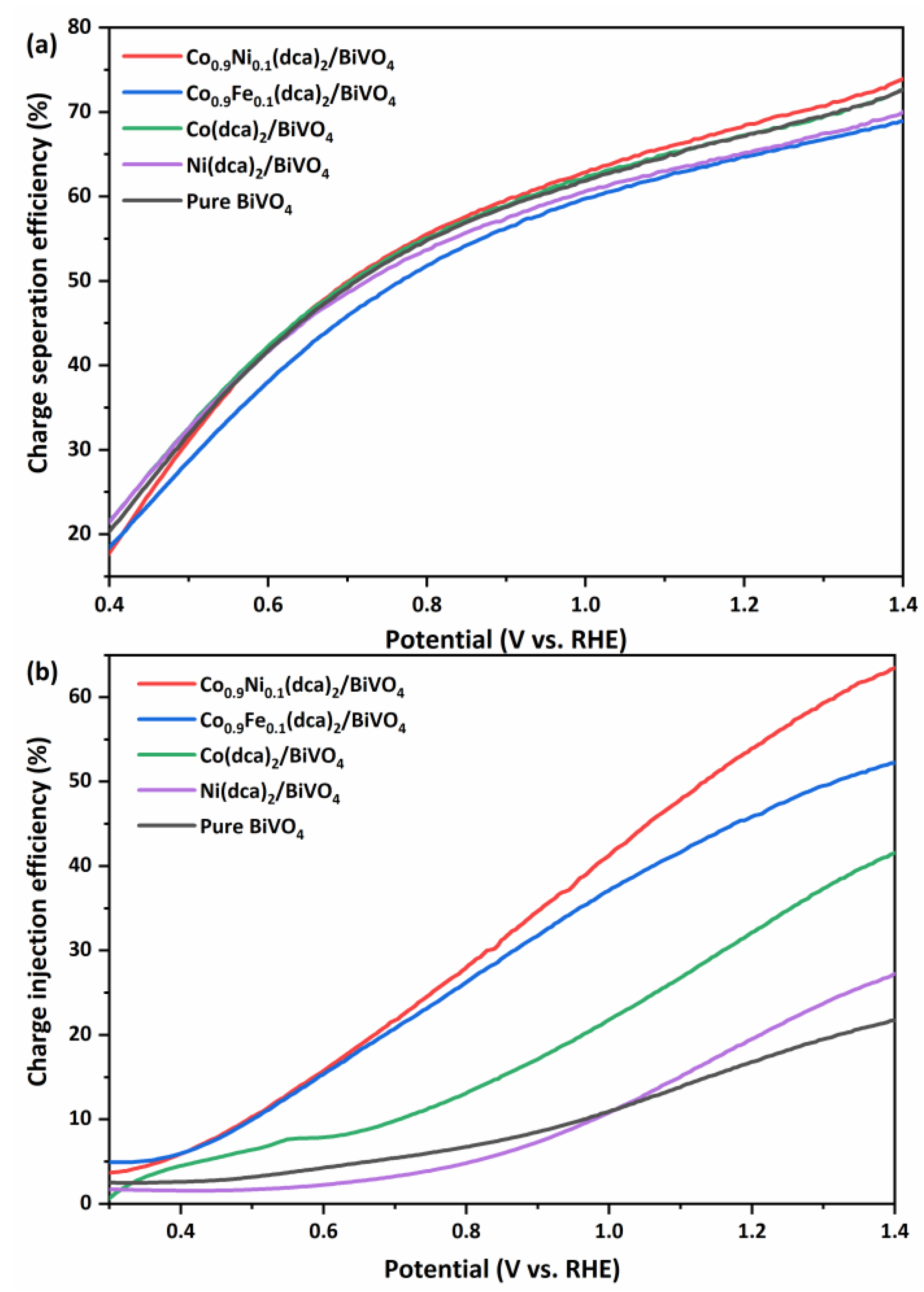
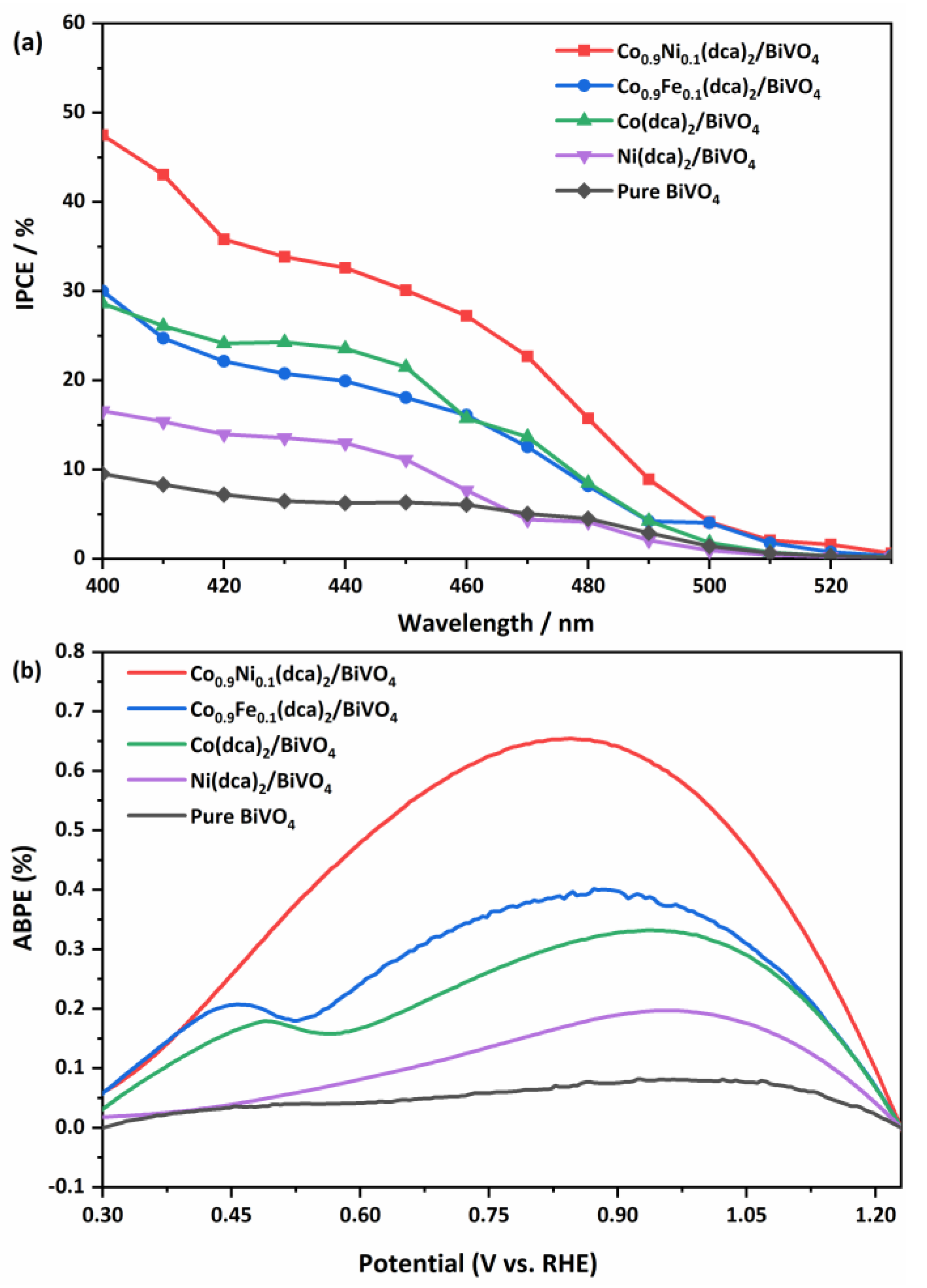
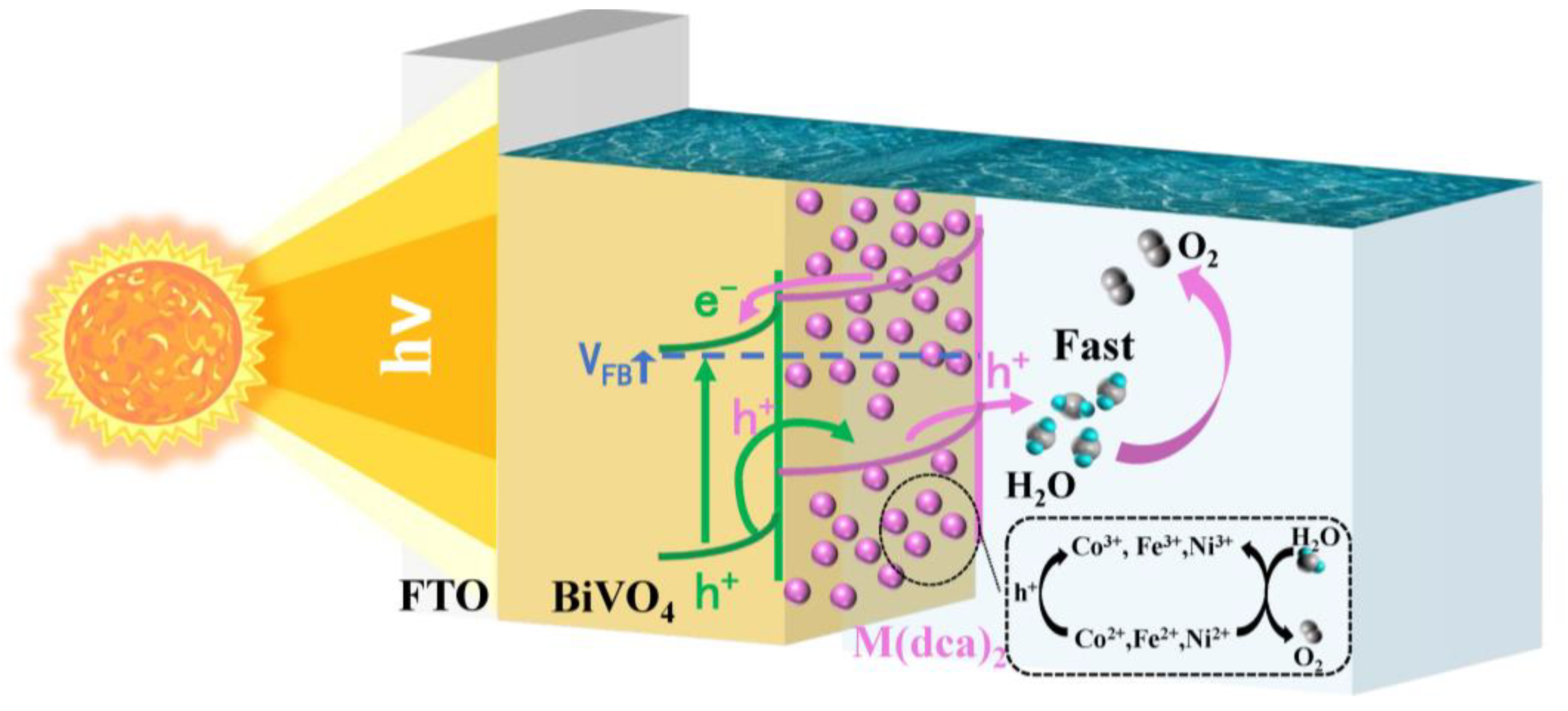
3.2. The PEC Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, Z.; Liu, B.; Zhang, P.; Wang, S.; Jiang, S.; Wang, T.; Gong, J. Performance Prediction of Multiple Photoanodes Systems for Unbiased Photoelectrochemical Water Splitting. ACS Mater. Lett. 2021, 3, 939–946. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Palm, D.W.; Reisner, E. Multifunctional Coatings from Scalable Single Source Precursor Chemistry in Tandem Photoelectrochemical Water Splitting. Adv. Energy Mater. 2015, 5, 201501668. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, F.; Wang, F.; Bai, S.; He, G. Construction of Z-Scheme In2S3-TiO2 for CO2 Reduction under Concentrated Natural Sunlight. Chin. J. Struct. Chem. 2022, 41, 2201034–2201039. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Tan, H.L.; Amal, R.; Ng, Y.H. Alternative strategies in improving the photocatalytic and photoelectrochemical activities of visible light-driven BiVO4: A review. J. Mater. Chem. A 2017, 5, 16498–16521. [Google Scholar] [CrossRef]
- Ye, S.; Ding, C.M.; Liu, M.Y.; Wang, A.Q.; Huang, Q.G.; Li, C. Water Oxidation Catalysts for Artificial Photosynthesis. Adv. Mater. 2019, 31, 1902069. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Wang, D.; Yang, J.; Li, M.; Zhu, J.; Zhou, X.; Han, H.; Li, C. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 2013, 4, 1432. [Google Scholar] [CrossRef]
- Guang, L.; Fei, W.; Xuejun, Z. Hydrothermal synthesis of m-BiVO4 and m-BiVO4/BiOBr with various facets and morphologies and their photocatalytic performance under visible light. J. Alloys Compd 2017, 697, 417–426. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. Elaborately Modified BiVO4 Photoanodes for Solar Water Splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef]
- Fang, G.; Liu, Z.; Han, C. Enhancing the PEC water splitting performance of BiVO4 co-modifying with NiFeOOH and Co-Pi double layer cocatalysts. Appl. Surf. Sci. 2020, 515, 146095. [Google Scholar] [CrossRef]
- Nagar, A.; Basu, S. Ternary g-C3N4/Ag/BiVO4 nanocomposite: Fabrication and implementation to remove organic pollutants. Environ. Technol. Inno. 2021, 23, 101646. [Google Scholar] [CrossRef]
- Wan, X.; Xu, Y.; Wang, X.; Guan, X.; Fu, Y.; Hu, C.; Hu, H.; Rong, N. Atomic layer deposition assisted surface passivation on bismuth vanadate photoanodes for enhanced solar water oxidation. Appl. Surf. Sci. 2022, 573, 151492. [Google Scholar] [CrossRef]
- Jian, J.; Wang, S.; Ye, Q.; Li, F.; Su, G.; Liu, W.; Qu, C.; Liu, F.; Li, C.; Jia, L.; et al. Activating a Semiconductor–Liquid Junction via Laser-Derived Dual Interfacial Layers for Boosted Photoelectrochemical Water Splitting. Adv. Mater. 2022, 34, 2201140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, X.; Zhang, B.; Bi, Y.J.E.; Science, E. High-performance and stable BiVO4 photoanodes for solar water splitting via phosphorus–oxygen bonded FeNi catalysts. Energy Environ. Sci. 2022, 15, 2867–2873. [Google Scholar] [CrossRef]
- Dashtian, K.; Shahbazi, S.; Tayebi, M.; Masoumi, Z. A review on metal-organic frameworks photoelectrochemistry: A headlight for future applications. Coordin. Chem. Rev. 2021, 445, 214097. [Google Scholar] [CrossRef]
- Kim, S.; Dela Pena, T.A.; Seo, S.; Choi, H.; Park, J.; Lee, J.-H.; Woo, J.; Choi, C.H.; Lee, S. Co-catalytic effects of Bi-based metal-organic framework on BiVO4 photoanodes for photoelectrochemical water oxidation. Appl. Surf. Sci. 2021, 563, 150357. [Google Scholar] [CrossRef]
- Pan, J.B.; Wang, B.H.; Wang, J.B.; Ding, H.Z.; Zhou, W.; Liu, X.; Zhang, J.R.; Shen, S.; Guo, J.K.; Chen, L.; et al. Activity and Stability Boosting of an Oxygen-Vacancy-Rich BiVO4 Photoanode by NiFe-MOFs Thin Layer for Water Oxidation. Angew. Chem. Int. Ed. 2021, 60, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Hernández, S.; Ottone, C.; Proto, S.; Tolod, K.; Díaz de los Bernardos, M.; Solé-Daura, A.; Carbó, J.J.; Godard, C.; Castillón, S.; Russo, N.; et al. Core-substituted naphthalenediimides anchored on BiVO4 for visible light-driven water splitting. Green Chem. 2017, 19, 2448–2462. [Google Scholar] [CrossRef]
- Singh, A.; Karmakar, S.; Basu, S. Role of sputtered WO3 underlayer and NiFeCr-LDH co-catalyst in WO3–BiVO4 heterojunction for enhanced photoelectrochemical water oxidation. Int. J. Hydrogen Energy 2021, 46, 39868–39881. [Google Scholar] [CrossRef]
- Wang, M.; Yu, H.; Wang, P.; Chi, Z.; Zhang, Z.; Dong, B.; Dong, H.; Yu, K.; Yu, H. Promoted photocatalytic degradation and detoxication performance for norfloxacin on Z-scheme phosphate-doped BiVO4/graphene quantum dots/P-doped g-C3N4. Sep. Purif. Technol. 2021, 274, 118692. [Google Scholar] [CrossRef]
- Zhong, M.; Hisatomi, T.; Kuang, Y.; Zhao, J.; Liu, M.; Iwase, A.; Jia, Q.; Nishiyama, H.; Minegishi, T.; Nakabayashi, M.; et al. Surface Modification of CoOx Loaded BiVO4 Photoanodes with Ultrathin p-Type NiO Layers for Improved Solar Water Oxidation. J. Am. Chem. Soc. 2015, 137, 5053–5060. [Google Scholar] [CrossRef]
- Guo, Y.H.; Li, Q.G.; Gao, X.M.; Gao, K.L.; Liu, L.B. Facile synthesis of ZnO/BiVO4 photocatalyst with enhanced photocatalytic performance. Optoelectron. Adv. Mat. 2020, 14, 84–91. [Google Scholar]
- Wang, D.; Marquard, S.L.; Troian-Gautier, L.; Sheridan, M.V.; Sherman, B.D.; Wang, Y.; Eberhart, M.S.; Farnum, B.H.; Dares, C.J.; Meyer, T.J. Interfacial Deposition of Ru(II) Bipyridine-Dicarboxylate Complexes by Ligand Substitution for Applications in Water Oxidation Catalysis. J. Am. Chem. Soc. 2018, 140, 719–726. [Google Scholar] [CrossRef]
- Bae, S.; Jang, J.E.; Lee, H.W.; Ryu, J. Tailored Assembly of Molecular Water Oxidation Catalysts on Photoelectrodes for Artificial Photosynthesis. Eur. J. Inorg. Chem. 2019, 2019, 2040–2057. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Wu, H.L.; Liu, W.Q.; Jiang, X.; Li, Z.J.; Chen, B.; Tung, C.H.; Wu, L.Z. Improved Photoelectrocatalytic Performance for Water Oxidation by Earth-Abundant Cobalt Molecular Porphyrin Complex-Integrated BiVO4 Photoanode. ACS Appl. Mater. Interfaces 2016, 8, 18577–18583. [Google Scholar] [CrossRef]
- Pintado, S.; Goberna-Ferron, S.; Escudero-Adan, E.C.; Galan-Mascaros, J.R. Fast and persistent electrocatalytic water oxidation by Co-Fe Prussian blue coordination polymers. J. Am. Chem. Soc. 2013, 135, 13270–13273. [Google Scholar] [CrossRef]
- Ressnig, D.; Shalom, M.; Patscheider, J.; Moré, R.; Evangelisti, F.; Antonietti, M.; Patzke, G.R. Photochemical and electrocatalytic water oxidation activity of cobalt carbodiimide. J. Mater. Chem. A 2015, 3, 5072–5082. [Google Scholar] [CrossRef]
- Nune, S.V.K.; Basaran, A.T.; Ülker, E.; Mishra, R.; Karadas, F. Metal Dicyanamides as Efficient and Robust Water-Oxidation Catalysts. ChemCatChem 2017, 9, 300–307. [Google Scholar] [CrossRef]
- Ghobadi, T.G.U.; Ghobadi, A.; Soydan, M.C.; Vishlaghi, M.B.; Kaya, S.; Karadas, F.; Ozbay, E. Strong Light-Matter Interactions in Au Plasmonic Nanoantennas Coupled with Prussian Blue Catalyst on BiVO4 for Photoelectrochemical Water Splitting. ChemSusChem 2020, 13, 2577–2588. [Google Scholar] [CrossRef]
- Cao, L.-M.; Lu, D.; Zhong, D.-C.; Lu, T.-B. Prussian blue analogues and their derived nanomaterials for electrocatalytic water splitting. Coordin. Chem. Rev. 2020, 407, 213156. [Google Scholar] [CrossRef]
- Shang, Y.; Niu, F.; Shen, S. Photocatalytic water oxidation over BiVO4 with interface energetics engineered by Co and Ni-metallated dicyanamides. Chinese J. Catal. 2018, 39, 502–509. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, T.; Chen, Y.; Fu, Y.; Su, J.; Guo, L. Hybrid nanostructured Copper(II) phthalocyanine/TiO2 films with efficient photoelectrochemical performance. Chem. Eng. J. 2020, 382, 122783. [Google Scholar] [CrossRef]
- Wen, Z.B.; Xu, S.X.; Zhu, Y.; Liu, G.Q.; Gao, H.; Sun, L.C.; Li, F. Aqueous CO2 Reduction on Si Photocathodes Functionalized by Cobalt Molecular Catalysts/Carbon Nanotubes. Angew. Chem. Int. Edit. 2022, 61, 202201086. [Google Scholar] [CrossRef]
- Liu, C.; Luo, H.; Xu, Y.; Zhang, Z.; Liang, Q.; Wang, W.; Chen, Z. Synergistic cocatalytic effect of ultra-thin metal-organic framework and Mo-dopant for efficient photoelectrochemical water oxidation on BiVO4 photoanode. Chem. Eng. J. 2020, 384, 123333. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Lu, D.; Wang, X.; Liu, G.; Fu, Y.; Hu, C.; Rong, N.; Wang, H.; Cheng, Z. Enhanced Photoelectrochemical Water Oxidation on BiVO4 Photoanodes Functionalized by Bimetallic Dicyanamide Molecular Catalysts. Sustainability 2023, 15, 3129. https://doi.org/10.3390/su15043129
Wan X, Lu D, Wang X, Liu G, Fu Y, Hu C, Rong N, Wang H, Cheng Z. Enhanced Photoelectrochemical Water Oxidation on BiVO4 Photoanodes Functionalized by Bimetallic Dicyanamide Molecular Catalysts. Sustainability. 2023; 15(4):3129. https://doi.org/10.3390/su15043129
Chicago/Turabian StyleWan, Xiaokang, Dashun Lu, Xianyun Wang, Gezhong Liu, Yanming Fu, Chao Hu, Nai Rong, Haitao Wang, and Zude Cheng. 2023. "Enhanced Photoelectrochemical Water Oxidation on BiVO4 Photoanodes Functionalized by Bimetallic Dicyanamide Molecular Catalysts" Sustainability 15, no. 4: 3129. https://doi.org/10.3390/su15043129
APA StyleWan, X., Lu, D., Wang, X., Liu, G., Fu, Y., Hu, C., Rong, N., Wang, H., & Cheng, Z. (2023). Enhanced Photoelectrochemical Water Oxidation on BiVO4 Photoanodes Functionalized by Bimetallic Dicyanamide Molecular Catalysts. Sustainability, 15(4), 3129. https://doi.org/10.3390/su15043129







