Abstract
Agricultural practices such as wastewater irrigation and manure application may contaminate soils with antibiotics and, consequently, lead to human health risk. The co-application of three waste-derived materials, sewage sludge (SL), Chinese medicinal herbal residues (CMHR) and biochar (BC), as a soil amendment was proposed recently for minimizing the antibiotic amount in crop tissues. The fate of six antibiotics—amoxicillin, tetracycline, sulfamethazine, norfloxacin, erythromycin and chloramphenicol—were investigated in a greenhouse soil-plant system with a fruit crop species: tomato. The pots were mixed with 5%, 10% or 20% SL-BC and SL-CMHR-BC and irrigated with wastewater with 3 μg/L or 30 μg/L antibiotics. The pot containing 20% SL-CMHR-BC captured the lowest antibiotic concentration in soils and tomato tissues. Norfloxacin was the most abundant antibiotic in the fruits, followed by tetracycline. The pot containing 20% SL-CMHR-BC significantly lowered the bioconcentration factor of the fruit, while its effects on the translocation factor were more varied. Current and some previous data were used to assess the human health risk of consuming carrot, lettuce and tomato. The estimated daily intake suggested a negligible risk to human health in general compared with the acceptable daily intake, except for CAP. A concentration of 20% SL-CMHR-BC helps minimize the human exposure risk to antibiotics contamination in edible crops.
1. Introduction
Antibiotics are widely used in treating human and animal infections, as well as animal feeds for growth promotion [1]. It is suggested that the demand for antibiotics will continue to increase globally [2]. The use of antibiotics undoubtedly brings benefits. However, their extensive use has also created problems, including in agriculture. Wastewater irrigation and manure application are two common agricultural practices nowadays. The former practice reduces freshwater demand and supplies nutrients for growing crops in an economic way [3]. Manure application is the reuse of animal waste as organic fertilizers, which is a more sustainable alternative to chemical fertilizers. However, these two practices are also the two main sources that lead to the entry of antibiotics into the soils [4]. The antibiotics are present in both wastewater and manure due to the excretion of urine and feces from humans and animals.
The accumulation of antibiotics in plants has been widely observed [5]. Once introduced into the soils, antibiotics will be taken up by crops through root absorption and accumulate in the crop tissues. Some main contributors to the level of contamination in crop tissues are the physicochemical properties of antibiotics, the crop species, the concentration of antibiotics in the soil environment and the soil texture [6]. In addition to the potential negative effect on crop production, such as growth inhibition [7,8] and affecting fruit quality [9], another major issue caused by the presence of antibiotics in plant tissues is human health risk. Humans are exposed to antibiotics through the consumption of crops that are grown in antibiotic-contaminated soils. It is suggested that the consumption of plant-derived food is the main contributor to the daily intake of antibiotics [10]. The cooking process usually can only partially eliminate antibiotic residues in food; depending on the cooking methods and antibiotic types, it may even have no significant effect on reduction [11]. Therefore, minimizing the antibiotic residues in raw food materials is important to reduce exposure levels.
A possible solution to antibiotic pollution in soils is the use of alternative soil amendment that can substitute manure and promote the degradation of antibiotics in soils. The co-application of three waste-derived materials—sewage sludge (SL), Chinese medicinal herbal residues (CMHR) and biochar (BC) (SL-CMHR-BC)—is proposed as a potential amendment. The recycling of these materials also promotes sustainable waste management. SL, sometimes called biosolids, is derived from sewage treatment processes. The use of SL has been widely explored, with applications such as acting as a CO2 adsorbent and producing energy [12]. Being a good source of nutrients, it is also used as a soil amendment [13]. However, the application of SL itself is associated with the introduction of antibiotics and other contaminants to soils [14]. Therefore, the co-application of SL and BC (SL-BC) has been proposed and investigated in current years. BC is obtained from the pyrolysis of biomass. It is widely used on its own as an organic soil amendment, to ameliorate soil quality and enhance nutrient availability [15]. It was observed in several studies that SL-BC is a viable approach to minimize the environmental risk of the use of SL [16,17]. BC has been proven to reduce the bioavailability of antibiotics [18] and reduce the antibiotic concentration in the soil-plant system [14,19,20]. It was also found in a study that the biochar produced at 250 °C can promote the abiotic dissipation rate of antibiotics in soils [21]. However, the effect of SL-BC in attenuating antibiotic concentration was not studied before. CMHR is generally defined as herbal wastes or dregs extracted through decoction. Chinese herbs are mainly derived from fibrous plants, which have a high content of carbon. As compared with SL and BC, the utilization of CMHR is much less investigated [22]. It has been suggested as a bulking agent during composting [23] and as an organic fertilizer [24]. Apart from its nutrient potential, CHMR contributes to the alternation of microbial communities in soils by, for instance, strengthening the antagonistic and mycoparasitic abilities of bacteria and fungi towards the specific soilborne phytopathogens [25]. It was also proven that it can minimize the spread of antibiotic resistance genes by inhibiting horizontal gene transfer when co-composting with manure [26]. Yet, the effect of CHMR on attenuating antibiotic concentrations in the soil-plant system was still unknown.
In this study, we explored the effectiveness of the soil amendments, SL-BC and SL-CMHR-BC, in reducing the exposure risk of human to antibiotics through the consumption of crops grown in contaminated soils. One potential factor that affects the accumulation of antibiotics is plant species. In this study, tomato (Lycopersicon esculentum) was used as the target crop species, as it is a widely consumed species that can be representative of fruit vegetables. Therefore, the objectives of this study were to (1) quantify the effect of the application of SL-BC and SL-CMHR-BC the on bioaccumulation of antibiotics in tomato; and (2) assess the human exposure and their corresponding risks of antibiotics in the edible part of different crops using the experimental data.
2. Materials and Methods
2.1. Antibiotics
Six antibiotics that are from different classes, commonly used in human therapy and animal husbandry and frequently detected in different matrixes were selected for the study. They were amoxicillin (AMX) in the penicillin class, tetracycline (TC) in the tetracycline class, sulfamethazine (SMZ) in the sulfonamide class, norfloxacin (NOR) in the fluoroquinolone class, erythromycin (ERY) in the macrolide class and chloramphenicol (CAP) in the amphenicol class (Table 1). The antibiotics used in the experiment were obtained from Sigma-Aldrich (Merck Ltd., Taipei, Taiwan).

Table 1.
Molecular formula, molecular weight, acid dissociation constant (Ka), octanol/water partition coefficient (Kow) and soil adsorption coefficient (Koc) of the six selected antibiotics in this study.
2.2. Soil Amendments: Co-Application of SL-BC and SL-CMHR-BC
SL, CMHR and BC were used to prepare the soil amendments. SL was collected from a local sewage treatment facility (Stanley Sewage Treatment Works, Hong Kong, China) and stabilized through aerobic fermentation using the solar dryer. The details of the pre-treatment method can be found in [33]. CMHR was collected from a local herbal tea company (Herbaceous Teas Ltd. Co., Hong Kong, China) and dried at 180 °C after collection. BC (8–20 mesh, CAS No. 7440-44-0) was purchased from Sigma-Aldrich (Merck Ltd., Taipei, Taiwan). To grow microbes and remove impurities, it was immersed in river water and ionic water was applied. It was then oven-dried at 105 °C and heated at 600 °C for 2 h. SL-BC and SL-CMHR-BC were prepared by mixing the respective raw materials on a dry-weight basis of 1:1 and 1:1:1 respectively.
2.3. Greenhouse Pot Experiment
The plantation experiment followed the method described in [4]. The tomatoes were grown in a greenhouse with controlled temperature (25 ± 2 °C) and relative humidity (60%) in summer. They were planted from seeds in pots with antibiotic-free soils. They were then transplanted into pots with 3–4 kg of soils with six different soil treatments. The soil treatments were soils mixed with 5%, 10% or 20% SL-BC or SL-CMHR-BC. Control soils without the addition of soil amendments were included for comparison. The tomatoes were irrigated with 200 mL of antibiotic-treated wastewater every day and the planting medium was maintained at 70% water-holding capacity. The wastewater had two different levels of antibiotic concentration: 3 μg/L (IW3) and 30 μg/L (IW30). These two spiked concentrations were decided according to the range of commonly detected concentrations of the target antibiotics in surface water [34,35].
There were three replicates for each combination of treatment group (i.e., IW3 5% SL-BC, IW3 10% SL-BC, IW3 20% SL-BC, IW30 5% SL-CMHR-BC, IW30 10% SL-CMHR-BC, IW30 20% SL-CMHR-BC) and the two control groups (IW3 control and IW30 control). The soils were collected and tomatoes were harvested after reaching marketable size (160 days) for the determination of the antibiotic concentration of different crop tissues (roots, shoots/leaves and fruits).
2.4. Extraction and Analytical Methods for Determination of Antibiotic Concentration
The extraction and determination methods for antibiotics in soils and plant tissues as proposed by [36] were modified and applied in this study.
One gram of soil samples was freeze-dried and spiked with the internal standard of each target antibiotic to reach 1 mg/L. The extraction buffer used was acetonitrile and 0.2 M citric acid (v:v = 1:1, pH 4.4). Each soil sample was vortex-mixed and extracted using ultrasonication followed by centrifugation. The combined supernatant was evaporated to near dryness. Solid phase extraction (SPE) was applied. The Oasis HLB cartridges (Waters, Milford, CT, USA) were preconditioned by using methanol and Milli-Q water, and methanol was then used to elute target analytes from the cartridge. The analytes were concentrated by using a gentle nitrogen stream and then redissolved in methanol. Finally, they were filtered into an amber glass vial and stored at −18 °C.
Different parts of the fresh tomato tissues (roots, leaves/shoots and fruits) were rinsed, freeze-dried and ground to powder. Internal standards were added to the tomato samples for the calculation of antibiotics concentration (1 mg/L) before extraction. The tomato samples were extracted three times by using a mixed solution of acidified acetonitrile and acetone. Following the mixing, ultrasonication and centrifugation, the extracts were analyzed by high-performance liquid chromatography mass spectrometry (HPLC-MS/MS) and Agilent Liquid Chromatography 1100 series HPLC system coupled to an Agilent 6410 triple quadrupole MS, equipped with an electrospray ionization (ESI) source (Agilent, Santa Clara, CA, USA) in multiple-reaction monitoring (MRM) mode. The chromatographic column was a Waters BEH T3 (2.1 × 100 mm, 1.7 µm) column with a poroshell 120 pre-column filter (3.0 mm, 0.2 mm). For ESI+, mobile phase A was 5 mM acetic acid and 0.1% formic acid, and mobile phase B was 5 mM acetic acid and acetonitrile. The settings of the gradient conditions were: 0 min, 20% B; 4.5 min, 35% B; 5 min, 60% B; 10 min, 70% B; 11 min, 100% B and 13.1 min, 20% B.
The recoveries of the target compounds were 73–126% for soil extracts and 79–114% for crop extracts, and the limit of quantification (LOQ) were 0.70–4.63 mg/kg and 0.70–4.63 mg/kg, respectively. The calibration curves (r2 > 0.999) were calculated by using ten concentrations (0.5–500 mg/L) of each individual antibiotic.
2.5. Estimation of Bioaccumulation and Translocation of Antibiotics
Bioaccumulation factor (BCF) of antibiotic concentration in crops was determined by the ratio of the antibiotic concentration the plant tissues to that in the soil [4], i.e., . Translocation factor (TF) was determined by the ratio of the antibiotic concentration in shoots/leaves or fruits to that in roots [4], i.e., .
2.6. Assessment of Potential Human Exposure to Antibiotics
To assess human exposure to antibiotics, the estimated daily intake (EDI) of antibiotics per kg of body weight through the consumption of edible parts of carrot, lettuce and tomato was calculated using the equation:
where C is the antibiotic concentration in edible tissue, D is the average daily consumption of edible tissue and is the wet-to-dry conversion factor. The equation was adapted from [5].
The antibiotic concentrations in the edible part of carrot, i.e., carrot root, and lettuce, i.e., lettuce shoot/leaf, were obtained from the results of our previous study (Table S1), while that of tomato, i.e., tomato fruit, was obtained from the results of this study. The antibiotic concentrations were converted to a fresh weight basis using the wet-to-dry conversion factor, as derived from the average water content of each crop (Pan and Chu, 2017b). Both the average water content of each crop (88.29% for carrot, 94.61% for lettuce, 93.95% for tomato) and the average daily consumption of the edible tissue (0.31 kg/kg bw/day for carrot, 0.42 kg/kg bw/day for lettuce, 0.81 kg/kg bw/day for tomato) was obtained from [37]. Additionally, the hazard quotient (HQ) was adopted to better assess the exposure risk to antibiotics from different crops by comparing the EDI of the different antibiotics of the three crops with the acceptable daily intake (ADI) of the different antibiotics. The equation is [38]. According to FAO [39], the ADI levels are as follows: AMX is 2 μg/kg bw, TC is 30 μg/kg bw, SMZ is 50 μg/kg bw and ERY is 0.7 μg/kg bw. For NOR, the ADI followed data provided by [5], which is 11.4 μg/kg bw. For CAP, the ADI was suggested to be impossible to derive [40], so we used the proposed Threshold of Toxicological Concern (TTC) [41] as a substitute for the ADI. The TTC of CAP was proposed as 0.15 μg/person/day in their study, which is 0.002 μg/kg bw for a person weighing 70 kg.
2.7. Statistical Analysis
The statistical tests were conducted using RStudio 2021.09.2. The data were analyzed by one-way analysis of variance (ANOVA), with the Dunnett test as a post host test to determine the effects of soil amendments on antibiotic attenuation. A p-value ≤ 0.5 indicated a significant difference.
3. Results and Discussion
3.1. Effect of SL-BC and SL-CMHR-BC on Antibiotics Concentration in Soils
All six studied antibiotics were detected in the IW3 and IW30 soils (Figure 1). NOR had the highest concentration (185.0–1176.0 ng/g) in soils, followed by ERY (37.1–530.0 ng/g) (Figure 1). On the other hand, the concentrations of SMZ (8.0–46.9 ng/g) and AMX (3.8–24.9 ng/g) were very low in soils. The differences among the final antibiotic concentrations in soils in a similar environment are mainly attributed to the differences in the physio-chemical properties of the antibiotics, which affect their adsorption rate, degradation rate and leaching rate [42]. The results matched with the previous findings that NOR has a relatively high half-life value or DT50 as compared with other antibiotics [43,44].
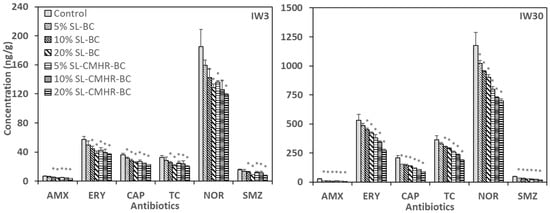
Figure 1.
Concentration of the six studied antibiotics in control and amended soils under the irrigation of wastewater with antibiotic concentration of 3 μg/L (IW3) and 30 μg/L (IW30). * indicates significance at the p < 0.05 compared to the control (n = 3).
The control soils captured the highest antibiotic concentration from the soil treatments. Meanwhile, 20% SL-CMHR-BC led to the lowest antibiotic concentration in soils, which was a decrease of 35.0–48.9% under IW3 and 40.5–75.2% under IW30 when compared to the control. A high proportion of soil amendments and SL-CMHR-BC were the favorable factors for antibiotic attenuation. The differences in effects between SL-BC and SL-CMHR-BC on antibiotic attenuation were more varied under IW30 than under IW3. For example, the reduction of NOR was 13.8–30.6% for SL-BC and 26.5–35.6% for SL-CMHR-BC under IW3. Alternatively, the reduction of NOR under IW30 was 13.0–23.3% for SL-BC, but 32.0–40.5% for SL-CMHR-BC. It suggested that the choice of soil amendments has a greater role in antibiotic attenuation to soils when there is a higher level of antibiotic contamination.
It was expectable that the inclusion of BC in soil amendments can lead to a decrease in the soil concentration of antibiotics, which was proven in previous studies [20,45,46]. The main factors were the provision of living space and nutrients for the bacteria to colonize and grow. Meanwhile, our findings further implied that CMHR enhanced the effect of antibiotic attenuation in soils. This was possibly due to the increased degradation of antibiotics caused by the nutrient enrichment effect of CMHR [24], which promoted the microbial dissipation process of antibiotics in soil by enhancing the growth of microorganisms.
3.2. Effect of SL-BC and SL-CMHR-BC on Antibiotics Concentration in Tomato Tissues
All six studied antibiotics were detected in the tomato roots, except for AMX under IW3 (Figure 2). NOR (4.0–35.9 ng/g) and CAP (3.0–31.1 ng/g) were the antibiotics with the highest concentration in the roots. The roots of 20% SL-CMHR-BC had a marked decrease in antibiotic concentration across all antibiotics (60.0–100.0% under IW3; 63.0–100.0% under IW30), as compared with the control. The antibiotic concentrations in the roots of SL-CMHR-BC were lower than that of SL-BC. For example, the NOR concentrations in the roots of SL-BC were 8.4–9.5 ng/g and those of SL-CHMR-BC were 4.0–8.0 ng/g under IW3. Similarly, the NOR concentrations in the roots of SL-BC were 22.3–28.3 ng/g and those of SL-CHMR-BC were 13.3–21.3 ng/g under IW30. Meanwhile, a higher proportion of soil amendment resulted in a lower antibiotic concentration in the roots.
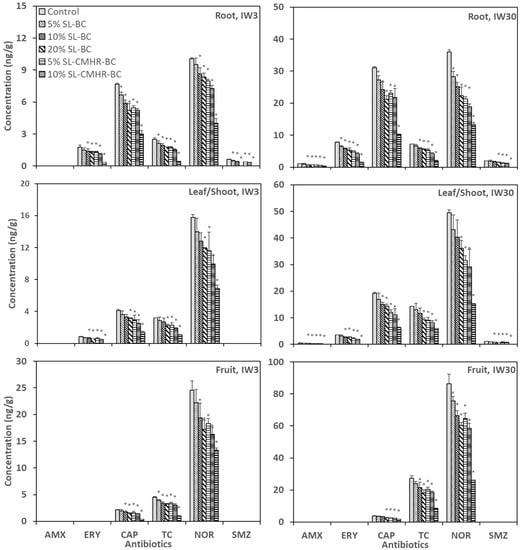
Figure 2.
Concentration of the six studied antibiotics in tomato roots, leaves/shoots and fruits grown in control and amended soils under the irrigation of wastewater with antibiotic concentrations of 3 μg/L (IW3) and 30 μg/L (IW30). * indicates significance at the p < 0.05 compared to the control (n = 3).
Four antibiotics were detected in the tomato leaves/shoots under IW3, with AMX and SMZ absent in all soil treatments. On the other hand, all six studied antibiotics were found in the leaves/shoots under IW30, but AMX (0–0.5 ug/g) and SMZ (0–1.1 ug/g) had very low concentration levels. NOR had a particularly high concentration (6.9–49.5 ng/g) in the leaves/shoots among all the antibiotics. The concentrations of CAP and TC were only around one-fourth of NOR under IW3 and one-third of NOR under IW30. Antibiotic concentrations in the leaves/shoots of 20% SL-CMHR-BC were the lowest among the different soil treatments, showing a 56.4–100.0% reduction under IW3 and 58.4–100.0% reduction under IW30, as compared with the control. ERY was undetectable in leaves/shoots under both IW3 and IW30 when 20% SL-CMHR-BC was applied. Similar to the roots, SL-CMHR-BC and a higher proportion of soil amendments contributed to a lower final concentration of antibiotics in the leaves/shoots.
In tomato fruits, only three antibiotics were detected under both IW3 and IW30. They were, in ascending order of concentrations, CAP (0.4–3.7 ug/g), TC (1.0–27.4 ug/g) and NOR (13.3–86.3 ug/g). Again, the antibiotic concentrations in fruits were lower when SL-CMHR-BC and a higher proportion of soil amendments were applied. Accordingly, 20% SL-CMHR-BC produced the most prominent effect in the reduction of antibiotics in fruits, among all the soil treatments. Except for 20% SL-CMHR-BC, the effects of soil amendments on antibiotic reduction in fruits were similar between IW3 and IW30 in terms of percentage decrease. The concentrations of CAP, TC and NOR in fruits of 20% SL-CMHR-BC were 18.0%, 22.7% and 54.2% of that of the control under IW3 and 49.2%, 30.6% and 30.4% of that of the control under IW30.
The addition of BC was found to reduce the concentration of antibiotics in plant tissues [14,19]. In this study, the final concentrations of antibiotics in tomato tissues were primarily affected by the antibiotic types and soil treatments, which affected the root absorption rate and bioavailability of antibiotics in the soils. It was proven that there are dose-response effects for the plant uptake of antibiotics from soils [47]. This should be one primary factor of the observed lower antibiotic concentrations in different plant tissues when 20% SL-CHMR-BC was applied, leading to the lowest antibiotic concentrations in the soils among the various soil treatments.
Additionally, it was observed in the previous study that the uptake potential of soil contaminants was highest for leafy vegetables but lowest in fruit vegetables [48]. In another study, it was found that pepper fruits had a lower accumulation of antibiotics than carrot roots and lettuce leaves [7]. Similarly, Hussain et al. [49] observed that the antibiotic concentration of different plant tissues was lowest in fruits. However, the accumulation of NOR and TC in the tomato fruit in our experiment was comparable to or even higher than the amount observed in the carrot root and lettuce leaves/shoots (Table S1) in our previous experiment with similar settings. Indeed, the higher accumulation of antibiotics in tomato fruits than in the lettuce leaf and carrot roots of both NOR and TC was also observed in [4].
3.3. Effect of SL-BC and SL-CMHR-BC on Bioaccumulation and Translocation
The BCFroot of all detected antibiotics was generally lower under IW30, except for SMZ (Figure 3). For example, BCFroot of NOR was 0.034–0.065 under IW3, but 0.025–0.031 under IW30. CAP had the highest BCFroot (0.13–0.21 under IW3 and 0.11–0.20 under IW30). 20% SL-CMHR-BC led to a significant reduction in BCFroot for most of the antibiotics, except for AMX. On the other hand, some soil treatments were associated with a higher BCFroot, especially under IW30. For example, 5% and 10% SL-CMHR-BC were both linked with increased BCFroot of CAP.
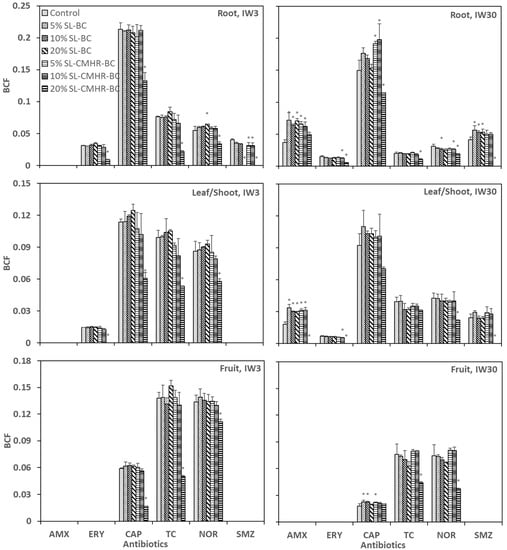
Figure 3.
Bioconcentration factor (BCF) of the six studied antibiotics in tomato roots, leaves/shoots and fruits grown in control and amended soils under the irrigation of wastewater with antibiotic concentration of 3 μg/L (IW3) and 30 μg/L (IW30). * indicates significance at the p < 0.05 compared to the controls (n = 3).
The BCFleaf/shoot of CAP (0.060–0.12), TC (0.053–0.10) and NOR (0.058–0.093) were similarly high under IW3, with CAP having a slightly higher value. The BCFleaf/shoot under IW30 was lower than that under IW3, especially for TC and NOR, which decreased to 0.031–0.040 and 0.022–0.042, respectively. The BCFleaf/shoot of the antibiotics were markedly lower when 20% SL-CMHR-BC was applied. Under IW30, the BCFleaf/shoot of AMX was lowest for the control among the different soil treatments, excluding 20% SL-CMHR-BC.
TC and NOR were similar with BCFfruit, except for 20% SL-CMHR-BC. CAP had the lowest BCFfruit among the three detected antibiotics (CAP, TC and NOR) in fruits. Again, BCFfruit of antibiotics were higher under IW3 than under IW30. A concentration of 20% SL-CMHR-BC was linked with a lower BCFfruit, with CAP under IW30 as an exception. Conversely, the soil amendment tended to cause a higher BCFfruit of CAP under IW30.
The BCF of antibiotics in tomatoes was observed to be higher than that of the lettuce and carrot observed in our previous study (Figure 3). The relatively high BCF of tomatoes was in accord with a previous study that focused on the bioaccumulation of residual fluoroquinolones in different crop species [11]. The study suggested that this was due to the stronger uptake ability of roots and the transport ability of tomatoes.
The higher BCF for IW3 than IW30 accorded with the findings of Azanu et al. [50], who suggested that the sorption capacity of crops for the organic contaminants would become saturated at a high concentration. The variations of BCF among different antibiotics are mainly attributed to the different physiochemical properties of the antibiotics [42]. Overall, CAP, TC and NOR were the three antibiotics that were most abundant in plant tissues in this study. It was found in a field monitoring study that NOR had a strong accumulation ability in plants [51].
The generally lower BCF of different parts of the tomato tissues of 20% SL-CMHR-BC suggested that the soil treatment reduced the relative rate of plant absorption of antibiotics from soils. The lack of a similar trend for 20% SL-BC reflected that CMHR was the crucial factor for the decreased BCF, although it was also reported in some previous studies that BC can reduce BCF [45,52]. It was proposed that the antibiotics can be strongly adsorbed to soils via the organic matter and, hence, a lower availability of the antibiotics to plants would result [53]. Therefore, the higher organic content of 20% SL-CMHR-BC may cause the antibiotics to be more strongly bound to soils. Meanwhile, the metabolism of the antibiotics in plant cells may also affect the accumulation in plant tissues, as the enzymes for the metabolism of organic contaminants are produced in response to antibiotic exposure [54]. The lower exposure of tomatoes to antibiotics in the case of 20% SL-CMHR-BC may lead to a lower rate of metabolism, comparatively [55]. Nevertheless, our results suggested that 20% SL-CMHR-BC can promote antibiotic reduction in tomato tissues not only by attenuating the soil concentration of antibiotics, but also through the other pathways.
The translocation factor (TF) represented the tendency of antibiotics to be accumulated in different parts of the tomato tissue. TC and NOR were the two antibiotics that had a TF > 1 for both TFleaf/shoot and TFfruit (Figure 4). For these two antibiotics, the accumulation levels were fruit > shoots/leaves > roots. Additionally, the TF of NOR was comparable between IW3 (1.4–1.7 for TFleaf/shoot and 2.1–3.3 for TFfruit) and IW30 (1.2–1.6 for TFleaf/shoot and 2.0–3.1 for TFfruit). Oppositely, the TF of TC (1.2–2.4 for TFleaf/shoot and 1.7–2.3 for TFfruit) under IW3 was obviously lower than that of the IW30 treatment (1.9–3.0 for TFleaf/shoot and 3.3–4.4 for TFfruit), particularly for TFfruit. On the other hand, the remaining four antibiotics (AMX, ERY, CAP and SMZ) had a TF < 1. Their accumulation patterns were roots > shoots/leaves > fruit.
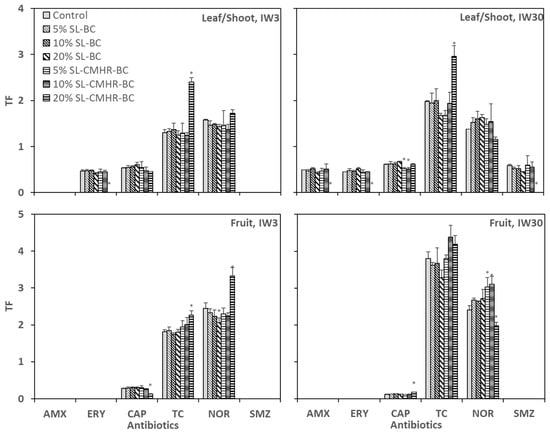
Figure 4.
Translocation factor (TF) of the six studied antibiotics of leaf/shoot to root, and fruit to the root of tomatoes grown in control and amended soils under the irrigation of wastewater with antibiotic concentration of 3 μg/L (IW3) and 30 μg/L (IW30). * indicates significance at the p < 0.05 compared to the controls (n = 3).
One critical factor in determining the degree of translocation is the physicochemical properties of the antibiotics [42]. The negative log Kow of TC and NOR reflected their higher solubility in water and, hence, they were more readily transported away from roots by the transpiration stream. In our previous study, it was observed that in addition to TC and NOR, CAP also had a TF > 1 in tomatoes [4]. The discrepancy may be due to the differences in soil texture between the two studies. On the other hand, Ahmed et al. [8] observed a very low concentration of TC in the fruit of tomatoes as compared with the other plant tissues. However, there were only 45 days of cultivation in their study, which was fewer than this study (160 days).
Regarding the effects of soil treatments on TF, a significantly higher TFleaf/shoot and TFfruit of TC, and TFfruit of NOR were observed for 20% SL-CMHR-BC under IW3, as compared with the control. Alternatively, a significantly higher TFleaf/shoot was observed for TC only, while TFfruit of NOR for 20% SL-CMHR-BC was significantly lower under IW30, as compared with the control.
The lowering of TFfruit of antibiotics in tomatoes is preferable, as it indicates that the antibiotics do not accumulate in the edible parts of tomatoes. The varied effects of 20% SL-CMHR-BC on TF of different plant tissues (i.e., fruit and leaf/shoot) and different antibiotics under different exposure levels to antibiotics suggested the complexity in predicting the influences of soil amendments. Keerthanan et al. [52] observed that the addition of BC led to the decrease of TF of sulfamethoxazole in water spinach at a low-contaminated environment, but no effect in a high one, which was not consistently observed in this study. On the other hand, the lack of effects of most of the other soil treatments on TF reflected that the soil environment did not affect TF to a great extent, in general. In other words, influences of soil amendments on TF played a rather minor role in minimizing the antibiotic level in crop tissues.
3.4. Human Exposure Implications
Table 2 revealed the varied EDI and HQ of different antibiotics through the consumption of carrot, lettuce and tomato. The carrot was the only species that contained AMX and SMZ in its edible parts. Consumption of carrots also led to higher exposure to ERY and CAP than the other two crops grown under IW3 and IW30, except for tomatoes grown under IW30. Higher exposure to TC and NOR resulted when the tomatoes were consumed. When comparing EDI with ADI, the risk of human exposure was almost negligible for all antibiotics except CAP, even for the control soils. HQ () is a better estimation of health risk, as there are different pathways of human exposure to antibiotics, and a HQ of 0.1 is regarded as the threshold at which a human is considered to be exposed to the potential hazard [5]. HQs of CAP from the consumption of carrots grown under IW30 were >0.1, which reached the level of concern.

Table 2.
Estimated daily intake (EDI) and human exposure risk as expressed in terms of hazard quotient (HQ) to the six studied antibiotics through consumption of carrot, lettuce and tomato.
Although the exposure risk as derived from the experimental data was very low, it is always preferable to minimize the risk as far as possible. Specifically, there is usually more than one type of antibiotic in the plant tissues. For example, it is suggested that the daily intake of NOR has the potential to pose a health risk [10]. The risk of expoure to a mixture of different types of antibiotics is unknown [5]. Therefore, the use of 20% SL-CMHR-BC in growing crops is promising in reducing the exposure risk of human to antibiotics, particularly for antibiotics such as NOR and CAP.
4. Conclusions
This study was the first study to evaluate the effectiveness of the co-application of SL-CMHR-BC as a new type of soil amendment in attenuating the antibiotic concentration of the soil-plant system of a fruit crop species. The result indicated that both SL-BC and SL-CMHR-BC attenuated the antibiotic concentration in a contaminated soil-plant system of tomatoes, with 20% SL-CMHR-BC producing the best results. The concentration of 20% SL-CMHR-BC led to a lower BCFfruit of different antibiotics in general, while its effects on TFfruit were more varied. The promotion of biotic degradation of antibiotics and the lowering of bioavailability of antibiotics for plant uptake were likely the main two mechanisms that allowed 20% SL-CMHR-BC to minimize antibiotic concentration in tomato fruits. The human risk of exposure to antibiotics through consumption of the three crop species, carrots, lettuce and tomatoes, was in general negligible at the set level of antibiotic contamination in the experiments. Meanwhile, CAP is the antibiotic that may potentially raise more health concern, particularly for the exposure to CAP from the consumption of carrot. Nevertheless, our findings have shown that that high antibiotic attenuation ability of 20% SL-CMHR-BC can help minimize the human exposure risk to edible crops grown from soils that are contaminated by antibiotics. It has proven that SL-CMHR-BC is a promising soil amendment for the purpose of antibiotic attenuation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15042980/s1, Table S1: Concentration of the six studied antibiotics in the carrot root and lettuce leaf/shoot grown in control and amended soils under the irrigation of wastewater with antibiotic concentration of 3 μg/L (IW3) and 30 μg/L (IW30).
Author Contributions
Conceptualization, M.P.; methodology, M.P. and P.-C.Y.; validation, M.P. and P.-C.Y.; formal analysis, M.P. and P.-C.Y.; investigation, M.P.; resources, M.P. and L.-W.L.; data curation, M.P.; writing—original draft preparation, M.P. and L.-W.L.; writing—review and editing, M.P. and H.Z.; visualization, H.Z.; supervision, M.P.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Grants Council of Hong Kong; grant numbers UGC/FDS25(16)/M02/19 and UGC/FDS25(16)/M01/20.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data sets used during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, K.; Gupta, S.C.; Chander, Y.; Singh, A.K. Antibiotic use in agriculture and its impact on the terrestrial environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Hanjra, M.A.; Blackwell, J.; Carr, G.; Zhang, F.; Jackson, T.M. Wastewater irrigation and environmental health: Implications for water governance and public policy. Int. J. Hyg. Environ. Health 2012, 215, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Transfer of antibiotics from wastewater or animal manure to soil and edible crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Prosser, R.S.; Sibley, P.K. Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation. Environ. Int. 2015, 75, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.T.; Sheng, G.; Manes, M. A partition-limited model for the plant uptake of organic contaminants from soil and water. Environ. Sci. Technol. 2001, 35, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Tasho, R.P.; Ryu, S.H.; Cho, J.Y. Effect of sulfadimethoxine, oxytetracycline, and streptomycin antibiotics in three types of crop plants—Root, leafy, and fruit. Appl. Sci. 2020, 10, 1111. [Google Scholar] [CrossRef]
- Ahmed, M.B.M.; Rajapaksha, A.U.; Lim, J.E.; Vu, N.T.; Kim, I.S.; Kang, H.M.; Lee, S.S.; Ok, Y.S. Distribution and accumulative pattern of tetracyclines and sulfonamides in edible vegetables of cucumber, tomato, and lettuce. J. Agric. Food Chem. 2015, 63, 398–405. [Google Scholar] [CrossRef]
- Christou, A.; Kyriacou, M.C.; Georgiadou, E.C.; Papamarkou, R.; Hapeshi, E.; Karaolia, P.; Michael, C.; Fotopoulos, V.; Fatta-Kassinos, D. Uptake and bioaccumulation of three widely prescribed pharmaceutically active compounds in tomato fruits and mediated effects on fruit quality attributes. Sci. Total Environ. 2019, 647, 1169–1178. [Google Scholar] [CrossRef]
- Ben, Y.; Hu, M.; Zhong, F.; Du, E.; Li, Y.; Zhang, H.; Andrews, C.B.; Zheng, C. Human daily dietary intakes of antibiotic residues: Dominant sources and health risks. Environ. Res. 2022, 212, 113387. [Google Scholar] [CrossRef]
- Li, X.W.; Xie, Y.F.; Li, C.L.; Zhao, H.N.; Zhao, H.; Wang, N.; Wang, J.F. Investigation of residual fluoroquinolones in a soil–vegetable system in an intensive vegetable cultivation area in Northern China. Sci. Total Environ. 2014, 468–469, 258–264. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Zaharioiu, A.; Oancea, S.; Bucura, F.; Raboaca, M.S.; Filote, C.; Ionete, R.E.; Niculescu, V.C.; Constantinescu, M. Sewage sludge derived materials for CO2 adsorption. Appl. Sci. 2021, 11, 7139. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A.K.; Kumar, A. A review on sewage sludge (biosolids) a resource for sustainable agriculture. Arch. Agric. Environ. Sci. 2017, 2, 340–347. [Google Scholar] [CrossRef]
- Bair, D.A.; Anderson, C.G.; Chung, Y.; Scow, K.M.; Franco, R.B.; Parikh, S.J. Impact of biochar on plant growth and uptake of ciprofloxacin, triclocarban and triclosan from biosolids. J. Environ. Sci. Health Part B 2020, 55, 990–1001. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Różyło, K. Co-application of sewage sludge with biochar increases disappearance of polycyclic aromatic hydrocarbons from fertilized soil in long term field experiment. Sci. Total Environ. 2017, 599, 854–862. [Google Scholar] [CrossRef]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Adsorption and desorption of heavy metals by the sewage sludge and biochar-amended soil. Environ. Geochem. Health 2019, 41, 1663–1674. [Google Scholar] [CrossRef]
- Pan, M. Biochar adsorption of antibiotics and its implications to remediation of contaminated soil. Water Air Soil Pollut. 2020, 231, 221. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Lim, J.E.; Ahmed, M.B.M.; Zhang, M.; Lee, S.S.; Ok, Y.S. Invasive plant-derived biochar inhibits sulfamethazine uptake by lettuce in soil. Chemosphere 2014, 111, 500–504. [Google Scholar] [CrossRef]
- Ye, M.; Sun, M.; Feng, Y.; Wan, J.; Xie, S.; Tian, D.; Zhao, Y.; Wu, J.; Hu, F.; Li, H.; et al. Effect of biochar amendment on the control of soil sulfonamides, antibiotic-resistant bacteria, and gene enrichment in lettuce tissues. J. Hazard. Mater. 2016, 309, 219–227. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Zhu, Y. Changes in abiotic dissipation rates and bound fractions of antibiotics in biochar-amended soil. J. Clean. Prod. 2020, 256, 120314. [Google Scholar] [CrossRef]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Chinese medicinal herbal residues as a bulking agent for food waste composting. Bioresour. Technol. 2018, 249, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Evaluation of humic substances during co-composting of food waste, sawdust and Chinese medicinal herbal residues. Bioresour. Technol. 2014, 168, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, Y.; Wang, K.; Huang, Y.; Wang, H. Re-utilization of Chinese medicinal herbal residues improved soil fertility and maintained maize yield under chemical fertilizer reduction. Chemosphere 2021, 283, 131262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Effect of Chinese medicinal herbal residues on microbial community succession and anti-pathogenic properties during co-composting with food waste. Bioresour. Technol. 2016, 217, 190–199. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, J.; Wang, X.; Sun, W.; Yin, Y.; Sun, Y.; Guo, A.; Tuo, X. Behavior of antibiotic resistance genes during co-composting of swine manure with Chinese medicinal herbal residues. Bioresour. Technol. 2017, 244, 252–260. [Google Scholar] [CrossRef]
- Mutiyar, P.K.; Mittal, A.K. Occurrences and fate of an antibiotic amoxicillin in extended aeration-based sewage treatment plant in Delhi, India: A case study of emerging pollutant. Desalin. Water Treat. 2013, 51, 6158–6164. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Dong, Y.H. Effect of low-molecular-weight organic acids on the adsorption of norfloxacin in typical variable charge soils of China. J. Hazard. Mater. 2008, 151, 833–839. [Google Scholar] [CrossRef]
- Yang, X.; Flowers, R.C.; Weinberg, H.S.; Singer, P.C. Occurrence and removal of pharmaceuticals and personal care products (PPCPs) in an advanced wastewater reclamation plant. Water Res. 2011, 45, 5218–5228. [Google Scholar] [CrossRef]
- Peng, X.; Tan, J.; Tang, C.; Yu, Y.; Wang, Z. Multiresidue determination of fluoroquinolone, sulfonamide, trimethoprim, and chloramphenicol antibiotics in urban waters in China. Environ. Toxicol. Chem. Int. J. 2008, 27, 73–79. [Google Scholar] [CrossRef]
- Xue, Q.; Qi, Y.; Liu, F. Ultra-high performance liquid chromatography-electrospray tandem mass spectrometry for the analysis of antibiotic residues in environmental waters. Environ. Sci. Pollut. Res. 2015, 22, 16857–16867. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Yau, P.C.; Lee, K.C.; Zhang, H.; Lee, V.; Lai, C.Y.; Fan, H.J. Nutrient accumulation and environmental risks of biosolids and different fertilizers on horticultural plants. Water Air Soil Pollut. 2021, 232, 480. [Google Scholar] [CrossRef]
- Hamscher, G.; Pawelzick, H.T.; Sczesny, S.; Nau, H.; Hartung, J. Antibiotics in dust originating from a pig-fattening farm: A new source of health hazard for farmers? Environ. Health Perspect. 2003, 111, 1590–1594. [Google Scholar] [CrossRef]
- Yao, L.; Wang, Y.; Tong, L.; Li, Y.; Deng, Y.; Guo, W.; Gan, Y. Seasonal variation of antibiotics concentration in the aquatic environment: A case study at Jianghan Plain, central China. Sci. Total Environ. 2015, 527–528, 56–64. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Occurrence of antibiotics and antibiotic resistance genes in soils from wastewater irrigation areas in the Pearl River Delta region, southern China. Sci. Total Environ. 2018, 624, 145–152. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (USEPA). Exposure Factors Handbook—Chapter 9. Intake of Fruits and Vegetables. Available online: https://www.epa.gov/expobox/exposure-factors-handbook-chapter-9 (accessed on 17 December 2022).
- Zhou, L.J.; Wang, W.X.; Lv, Y.J.; Mao, Z.G.; Chen, C.; Wu, Q.L. Tissue concentrations, trophic transfer and human risks of antibiotics in freshwater food web in Lake Taihu, China. Ecotoxicol. Environ. Saf. 2020, 197, 110626. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization of the United Nations (FAO). Residues of Some Veterinary Drugs in Foods and Animals. Available online: https://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-vetdrugs/en/ (accessed on 17 December 2022).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on chloramphenicol in food and feed. EFSA J. 2014, 12, 3907. [Google Scholar] [CrossRef]
- Hanekamp, J.C.; Bast, A. Antibiotics exposure and health risks: Chloramphenicol. Environ. Toxicol. Pharmacol. 2015, 39, 213–220. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Wang, J.; Lin, H.; Sun, W.; Xia, Y.; Ma, J.; Fu, J.; Zhang, Z.; Wu, H.; Qian, M. Variations in the fate and biological effects of sulfamethoxazole, norfloxacin and doxycycline in different vegetable–soil systems following manure application. J. Hazard. Mater. 2016, 304, 49–57. [Google Scholar] [CrossRef]
- Duan, M.; Li, H.; Gu, J.; Tuo, X.; Sun, W.; Qian, X.; Wang, X. Effects of biochar on reducing the abundance of oxytetracycline, antibiotic resistance genes, and human pathogenic bacteria in soil and lettuce. Environ. Pollut. 2017, 224, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Du, R.; Ye, M.; Sun, M.; Feng, Y.; Wan, J.; Zhao, Y.; Zhang, Z.; Huang, D.; Du, D.; et al. Agricultural waste to treasure–Biochar and eggshell to impede soil antibiotics/antibiotic resistant bacteria (genes) from accumulating in Solanum tuberosum L. Environ. Pollut. 2018, 242, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, L.; Chen, L.; Li, S.; Sun, L. Bioaccumulation of antibiotics in crops under long-term manure application: Occurrence, biomass response and human exposure. Chemosphere 2019, 219, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Papadavid, G.; Dalias, P.; Fotopoulos, V.; Michael, C.; Bayona, J.M.; Piña, B.; Fatta-Kassinos, D. Ranking of crop plants according to their potential to uptake and accumulate contaminants of emerging concern. Environ. Res. 2019, 170, 422–432. [Google Scholar] [CrossRef]
- Hussain, S.; Naeem, M.; Chaudhry, M.N.; Iqbal, M.A. Accumulation of residual antibiotics in the vegetables irrigated by pharmaceutical wastewater. Expos. Health 2016, 8, 107–115. [Google Scholar] [CrossRef]
- Azanu, D.; Mortey, C.; Darko, G.; Weisser, J.J.; Styrishave, B.; Abaidoo, R.C. Uptake of antibiotics from irrigation water by plants. Chemosphere 2016, 157, 107–114. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.; Chen, J.; Li, Y.; Liu, X.; Feng, Y.; Sun, Y. Source, occurrence and risks of twenty antibiotics in vegetables and soils from facility agriculture through fixed-point monitoring and numerical simulation. J. Environ. Manag. 2022, 319, 115652. [Google Scholar] [CrossRef]
- Keerthanan, S.; Jayasinghe, C.; Bolan, N.; Rinklebe, J.; Vithanage, M. Retention of sulfamethoxazole by cinnamon wood biochar and its efficacy of reducing bioavailability and plant uptake in soil. Chemosphere 2022, 297, 134073. [Google Scholar] [CrossRef]
- Camacho-Arévalo, R.; García-Delgado, C.; Mayans, B.; Antón-Herrero, R.; Cuevas, J.; Segura, M.L.; Eymar, E. Sulfonamides in tomato from commercial greenhouses irrigated with reclaimed wastewater: Uptake, translocation and food safety. Agronomy 2021, 11, 1016. [Google Scholar] [CrossRef]
- Mathews, S.; Reinhold, D. Biosolid-borne tetracyclines and sulfonamides in plants. Environ. Sci. Pollut. Res. 2013, 20, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, J.; Cheng, D.; Feng, Y.; Liu, Y.; Aly, H.M.; Li, Z. Uptake, translocation and distribution of three veterinary antibiotics in Zea mays L. Environ. Pollut. 2019, 250, 47–57. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).