Abstract
Wetlands are recognized as hotspots of biodiversity and providers of several ecosystem services, including water purification, sediment stabilization, and flood, erosion, and climate regulation. This article aims to investigate the floristic diversity of the wetlands the Haho River watershed in southern Togo. Spatial data from Astrium service and Google Earth were collected, and phytosociological data were classified following the Braun–Blanquet approach. The findings indicate that 72 families in total have evolved in this environment, with Poaceae (14.95%) and Fabaceae (11.98%) dominating. The number of species was estimated to be 323; the three species that were most prevalent in the wetland’s habitats were Elaeis guineensis Jacq (2.44%), Panicum maximum Jacq (2.29%), and Lonchocarpus sericeus (Poir) H. B. K. (1.71%). The most prevalent and abundant life forms in these moist habitats were micro-phanerophytes (34.70%) and therophytes (23.50%). However, the most common and abundant chorological categories included pantropical (31.05%) and Guinean-Congolese species (21.46%). Principal Component Analysis (PCA) was used to examine how abiotic parameters, including depth/degree of immersion, influence the distribution of plant species in a wetland landscape. This research has the potential to be developed into a more robust action study for wetland classification and recognition.
1. Introduction
During the last 150 years, 50% of the worldwide wetland habitats have vanished, and the remnants now cover only 6% of the land surface area [1,2]. Wetlands around the globe have been altered, degraded, or lost through a wide range of human activities altering their biological productivity [3,4]. These particular ecosystems link aquatic and terrestrial ecosystems as they provide specific ecosystem services, including water filtration, protection from ecological disturbances such as floods and hurricanes, support for food chains to maintain the biological cycle, and carbon sequestration [5,6]. Wetlands constitute an enormously diversified landscape component that spans a wide range of geographic locations and hydrographic networks in both aquatic (inland and coastal marshes) and terrestrial (lakes, pools, ponds, and dams) systems [7,8]. Wetlands are green infrastructures that serve as territorial resources and can deliver ecosystem services for the local population [8,9]. Due to their unique characteristics and complexities, these ecosystems are traditionally landscapes that need to be managed [10,11]. When wetlands are present in a metropolitan area experiencing economic growth and rapid population growth, policymakers have been especially concerned about planning to avoid degradation.
The production of wetland ecosystem knowledge, including the creation of various legal and technical tools, has advanced significantly in recent decades in the context of conservation and restoration programs on a global and regional scale [12]. Since the Ramsar Convention [13] on the recognition of wetlands, followed by the Convention on Biological Diversity [14], and the Millennium Ecosystem Assessment [15], there has been a resurgence in interest in the reclamation of wetlands for ecological, historical, and economic reasons. Therefore, the Ramsar treaty established precise principles/criteria for classifying wetland areas and drew increased local or regional attention to their sustainable monitoring. However, all methods of wetland appropriation require rational and multidisciplinary reasoning [16,17]. The paradigm of reclassifying wetlands as structural and functional spatial units that constitute a crucial component of the territorial network was developed through multi-actor talks with stakeholders [17]. Wetland management has not always been approached by scientists, planners, and water managers in the same way [18], raising concerns about the value of biological variety and the impact of suitable management methods [19,20].
The rehabilitation and protection of wetlands throughout the world, particularly in developing countries, has been a source of social conflict; these conflicts have been accentuated by institutional weakness, followed by the lack of adequate knowledge of these critical ecosystems [21,22]. To effectively and efficiently manage wetland ecosystems, researchers agree that territorial sovereignty over wetlands must consider the local populations’ participation and socio-economic resilience [23]. However, a significant barrier to the effective adoption of sustainable management strategies is the low degree of local government decentralization in the domain of wetlands.
In Togo, infrequent studies have shown interest in floodplains’ biology to understand these ecosystems better. Studies have examined the hydrochemistry, phytosociology, and spatiotemporal dynamics of mangrove regions in the littoral belt [24]. Additionally, research has shown that Sorindeia warneckei Engl. has a positive economic impact on the people living in the Lama Depression [25].
However, little research has examined the floristic diversity, phytosociology, or production dynamics of wetlands at an eco-floristic or watershed scale; thus, the primary purpose of the present study is to fill this gap in our research area. Wetlands can contribute enormously to landscape-level biodiversity because of their species richness and spatial variation in community composition [26]. This article contributes to a greater understanding of Togo’s wetland ecosystems and attracts the attention of natural resource managers interested in their sustainable management. Notably, South Togo’s Haho River watershed’s wetland’s floristic diversity and ecological classification require in-depth analysis.
2. Materials and Methods
2.1. Study Area
The study area is a complex hydrological system, approximately 4165.62 km2 (Figure 1), made up of rivers (Haho, Yoto, Lili, and Boko) and lakes (Togo and Zowla). The northernmost two-thirds are peneplains shared by Benin and Togo, typified by low elevations (below 200 m), but are also interspersed with hills and inselbergs. At a height of less than 100 m, the southern portion is a part of the coastal plain [27].
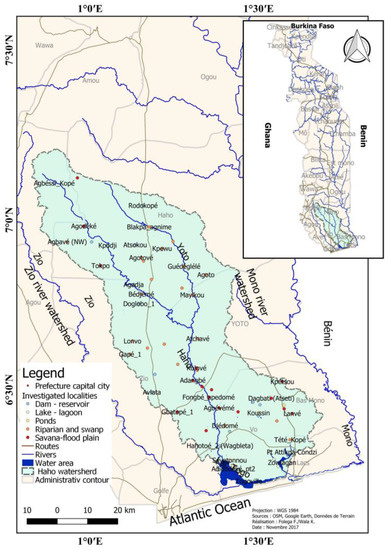
Figure 1.
Location of wetlands sampled in the Haho basin (Togo).
The area has a tropical Guinean climate, with 1000 and 1400 mm of rain yearly. Although it can occasionally reach extremes of 30 °C, the average temperature is around 27 °C. The majority of soils are shallowly leached tropical ferruginous types.
The study area is located in the coastal plain, the fifth phytogeographical domain V, and is characterized by a mosaic of severely degraded plant formations, including forest islands with Milicia excelsa (Welw.) C. C. Berg., Antiaris africana Engl., Cola gigantea A. Chev. Land cover is impacted by pedology and hydrography, which are the sources of physiognomic units, including agrarian landscapes, mining areas, swamps and water bodies, natural vegetation, plantations, and urban areas [28].
The Adja, Ewé, Watchis, Pedas, Gins, and Minas [29] make up the key socio-cultural groups in a country with a population of over one million. Socio-economic development is supported by family-based agricultural production, cattle keeping, artisanal fishing, market gardening, small trading, and processing agricultural products. The lack of innovative capacity and inadequate technical support for rural development impacts yields and production performance in this area.
2.2. Data Collection
Identification and Geolocation of Wetlands in the Watershed
By comparing the spatial data (hydrographic, geomorphological, pedological, and vegetation maps) available, the wetlands to be surveyed were identified [30]. First, the geographical extent of the Haho River watershed could be defined using tools for visual analyses and interpretations, with geoprocessing in the QGIS software (Figure 1). Next, a KLM (Keyhole Markup Language) was created by geolocating the surface entities and delineating the observable bodies of water in the basin representing the potential wetlands to be studied. Before the floristic inventory was formed, the latter was put into Google Earth Pro to verify the efficacy and typology of wetlands. Sixty-four (64) sampling points were ultimately chosen after being compared to high-resolution photos that Astrium service had made available from Google Earth. The sampling points are divided into riparian forests (38), ponds and dams (15), around lakes and lagoons (6), and ponds (5). It should be emphasized that riparian forests are complexes that include riparian forests and nearby marshland landforms. In general, there are two main types of wetlands: those that are natural (such as riparian forests, lakes, lagoons, and ponds) and those that are constructed (ponds and dams).
2.3. Floristic and Ecological Inventories of Wetlands
The phytosociological data of the wetlands in our sample were collected following the sigmatist approach of Braun–Blanquet [30]. The floristic surveys were conducted in 100 m2 plots [31,32]. This survey method is appropriate for examining formations that are more or less spatially extensive [33,34] and composed of weed communities, ruderal vegetation, and deteriorated forested formations. The size of the surveys was determined using descriptive criteria that were in line with the condition of the habitats sampled. Each site was equipped with a 100 m2 transect of floristic surveys that ran parallel to the banks of streams and other bodies of water. All the species present for each survey were noted and assigned an abundance-dominance coefficient. The floristic nomenclature used was that of Brunel and Akoegninou [35,36]. Ecological descriptors, such as the overall vegetation cover, the composition and texture of the soil, and the remnants of human activity, were added to the floristic data to supplement them.
2.4. Data Processing and Analysis
The acquired floristic data were recorded and adjusted following the national nomenclature. Based on the surveys, a general list of species was produced. Each species’ biological and chorological type was recorded, and then the raw and weighted spectrum frequencies were assessed. Afterward, the data were encoded in various matrices in preparation for statistical analysis. The overall floristic balance was established by assessing the specific richness, the spectrum of families, biological forms, and phytogeographical types [37,38].
To perform an indirect analysis of the abiotic ecological factors influencing the distribution of vegetation, the matrix (64 samples plots × 324 species) was subjected to a multivariate principal component test (PCA) using the Statistica 7 software [39]. The floristic data of the four categories of identified wetlands were then subjected to the above analyses.
3. Results
3.1. Overview of Local Floristic Diversity
Seventy-two (72) plant families were present in total, according to wetlands prospection in the Haho River watershed (Figure 2). This taxonomic procession is dominated by Poaceae (14.95%), followed by Fabaceae (11.98%), Euphorbiaceae (6.20%), Rubiaceae (4.48%), Asteraceae (4.01%), Mimosaceae (3.90%), Cyperaceae (3.85), Arecaceae (3.80%) and Onagraceae (2.76%). Additionally, it should be emphasized that there are families present that are less common but yet typical of moist biotopes, such as Nymphaeaceae (0.93%), Polygonaceae (0.88%), Convolvulaceae (0.83%), Lemnaceae (0.36%), Zingiberaceae (0.26%), Ceratophyllaceae (0.15%), Araliceae (0.10%), and Hydrophyllaceae (0.05%).
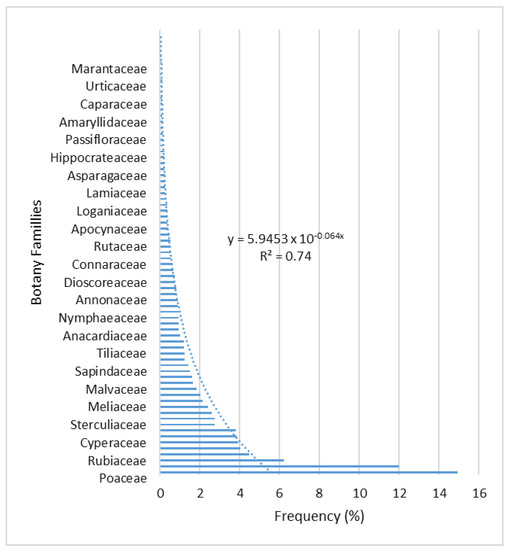
Figure 2.
Distribution of the relative frequencies of the families identified in the wetlands.
From a genus point of view, the floristic diversity of the wetlands of this basin comprises two hundred and thirty-six (236) genera, the most frequent of which are, among others, Ludwigia (3.12%), Panicum (2.60%), Cyperus (2.44%), Elaeis (2.44%), Acacia (2.34%), Paspalum (2.03%), Commelina (1.71%), and Lonchocarpus (1.71%).
The species richness is estimated at three hundred and twenty-three (323) species. The most frequent species seen in this flora are E. guineensis Jacq. (2.44%), P. maximum Jacq (2.29%), L. sericeus (Poir) H. B. K. (1.71%), Azadirachta indica A. Juss (1.61%), Flueggea virosa Roxb, ex Willd (1.45%), Millettia thonningii Schumach. And Thonn (1.45%), Acacia polyacantha Willd. (1.30%), Phyllanthus amarus Schum. and Thonn (1.19%), Typha domingensis Pers. (1.19%), and Spermacoce ruelliae DC (1.14%).
Based on occurrence, micro-phanerophytes (34.70%), followed by therophytes (23.50%), and nano-phanerophytes (10.78%), represent the most frequent biological forms, justifying the character of savannah mosaics that prevail around these wetlands (Figure 3). The weighted spectrum of life forms shows that micro-phanerophytes (38.76%), followed by hemicryptophytes (33.51%), hydrophytes (12.40%), and therophytes (9.45%), dominate and abound in these wetland ecosystems (Figure 3).
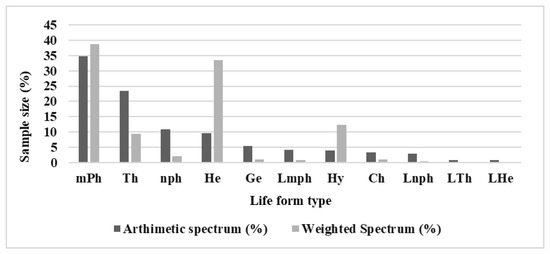
Figure 3.
Frequency of distribution of species richness by biological types. mPh: micro-phanerophytes. Th: therophytes. nph: nano-phanerophytes. He: hemicrypto-phytes. Ge: geophytes. Lmph: liana-micro-phanerophytes. Hy: hydrophytes. Ch: chamaephytes. Lnph: nano-phanerophyte creepers. LTh: therophyte creepers. LHe: hemicryptophyte.
The raw spectrum of phytochoria (Figure 4) indicates a strong presence of pantropical (31.05%), Guineo-Congolese (21.46%), Sudano-Zambezian (13.86%), and Afro-tropical (10.99%) species. In terms of cover and abundance, Guineo-Congolese (56.97%) and pantropical (20.55%) species almost dominate the flora of these wetlands (Figure 4).
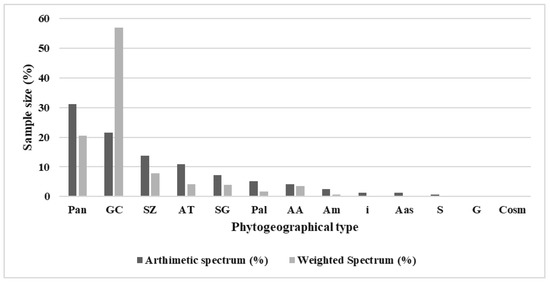
Figure 4.
Frequency of distribution of species richness by phytogeographical types. Pan: pantropical. GC: Guinean-Congolese species. SZ: Sudano-Zambezian. AT: Afro-tropical. SG: Sudano-Guinean. Pal: paleo-tropical. AA: African-American. Am: Afro-Malagasy. i: introduced. Aas: Afro-Asian. S: Sudanian. G: Guinean. Cosm: Cosmopolitan.
The matrix (324 species × 64 samples plots) was projected onto principal components (Figure 5). It illustrates the distribution of species richness in the composite plane of axes 1 and 2, for which the eigenvalues were significant. Two main gradients influence the distribution of species in the wetland landscape. The first is concerned with the level of immersion indicated by axis 1, which depicts a diminishing gradient of species immersion from right to left, with species on the far right immersed or floating and those on the far left temporarily submerged, depending on the season. A gradient of diminishing edaphic humidity is represented on axis 2. In contrast to stations with negative values, those with positive values have higher relative humidity levels. In other words, the water factor significantly affects how well a species adapts and survives.
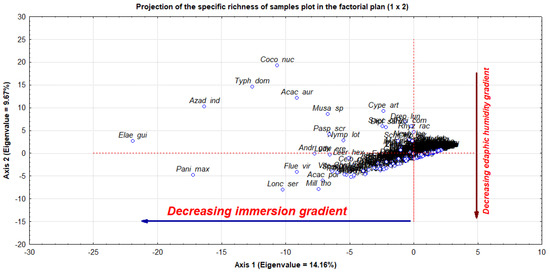
Figure 5.
Projection of the 324 species using a principal component factorial design based on immersion and relative humidity gradients.
3.2. Biological Properties of Main Wetlands Types
3.2.1. Riparian Forests
Riparian wetlands are made up of riparian vegetation on the banks of watercourses and marshy vegetation adjacent to riparian vegetation. Riparian forests were found to harbor two hundred and ninety (290) species, the most common of which are E. guineensis Jacq. (2.63%), P. maximum Jacq. (2.32%), L. sericeus (Poir.) Kunth ex DC. (1.85%), Flueggea virosa (Roxb. ex Willd.) Royle (1.70%), M. thonningii (Schumach. & Thonn.) Baker (1.70%) and Paulinia pinnata L. (1.47%). The floristic procession is divided into two hundred and nineteen (219) genera and sixty-six (66) families. The most frequent families are Poaceae (13.85%), followed by Fabaceae (11.68%), Euphorbiaceae (6.96%), Mimosaceae (4.48%), and Cyperaceae (3.86%).
The raw spectrum of the most prevalent life forms (Figure 6a) was determined to comprise micro-phanerophytes (37.46%), therophytes (21.13%), and nano-phanerophytes (10.52%). As for the most abundant species, the weighted spectrum of life forms shows the dominance of micro-phanerophytes (44.02%), nano-phanerophytes (35.65%), and therophytes (8.13%).
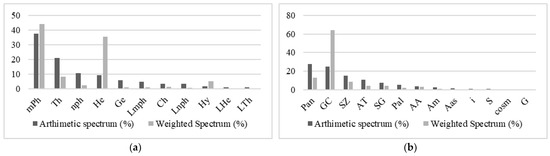
Figure 6.
Distribution of biological and chorological types in riparian forests: (a) LFT: life forms types: mPh: micro-phanerophytes. Th: therophytes. nph: nano-phanerophytes. He: hemicryptophytes. Ge: geophytes. Lmph: liana-micro-phanerophytes. Hy: hydrophytes. Ch: chamephytes. Lnph: lianes nano-phanerophytes. LTh: liana therophytes. LHe: liana hemicryptophytes. (b) TP: phytogeographical types: Pan: pantropicals. GC: Guineo-Congolese species. SZ: Soudano-Zambezian species. AT: Afro-tropicales. SG: Soudano-Guinean species. Pal: paleo-tropicals. AA: Afro-American. Am: Afro-Malgash. i: introduced. Aas: Afro-Asiatics. S: Soudanian, G: Guinean, cosm: cosmopolitan.
The biogeography in this type of wetland shows a high prevalence of raw spectra (Figure 6b) from pantropical (60.91%), Guineo-Congolese (24.84%), and Sudano-Zambezian (15.01%) species. The weighted spectrum is dominated by Guineo-Congolese species (64.21%), followed by pantropical (13.04%) and Sudano-Zambezian (8.60%).
Riparian forests often exhibit three physiognomic units that are significantly connected to the drainage gradient. The first consists of a mosaic lawn of herbaceous plants and grasses with Ludwigia sp., Echinochloa sp., Paspalum sp., and Cyperus sp., which grows on the inner banks of rivers close to the flow. The second, which is highly disturbed, is distinguished by an abundance of lianas (Cissus sp., Ampelocissus sp., Dioscorea sp.) in the ligneous formations, with Pterocarpus santalinoides L’Hér. ex DC., Cynometra megalophyla Harms, Cola laurifolia Mast., Bambusa vulgaris Schrad. ex J.C.Wendl. in the riparian forests. Finally, the third unit consists of highly disturbed habitats (fallow, savannah, parks) with E. guineensis Jacq., L. sericeus (Poir.) Kunth ex DC., P. erinaceus Poir., V. paradoxa C. F. Gaertn., and M. thonningii (Schumach. & Thonn.) Baker when the land is relatively drained or of marshy vegetation with M. inermis (Willd.) Kuntze, Polygonum sp., Ipomea sp., Merinia sp. and Typha domingensis (Pers.) Steud provides a natural receptacle for river overflow during floods. It is associated with Acacia sp, Entada sp, Terminalia sp, and Combretum sp on the periphery.
3.2.2. The Ponds
The wetland habitats formed by the ponds are also a sanctuary of diversity, made up of physiognomic facies linked to hydrological and edaphic gradients. The majority of sampled ponds have been historically exploited by residents, revealing a low floristic richness and a sizeable presence of plant species with anthropogenic preference.
The specific richness of the ponds consisted of 26 species divided into 26 genera and 18 families. The most representative families were Arecaceae (16.66), followed by Poaceae (16.66) and Cyperaceae (10.41).
The species of Cocos nucifera L. (12.50%), Cyperus articulatus L. (10.41%), T. domingensis, (Pers.) Steud, A. indica A. Juss., Drepanocarpus lunantus (L.) G. Mey. and P. scrobiculatum L. (6.25% each) were the most frequent.
Phytogeographically, the ponds were dominated by pantropical species (46.95%) and Guineo-Congolese (32.44%) (Figure 7b). The most frequent phytochoria were pantropical species (47.91%), followed by Afro-American (12.50%) and introduced species (10.41%). The biological forms frequently encountered were micro-phanerophytes (35.41%), nano-phanerophytes (25%), and hydrophytes (18.75%) (Figure 7b); however, the dominant biological types around the ponds were made up of hydrophytes (37.84%), micro-phanerophytes (35.94%), and hemicryptophytes (20.07%).
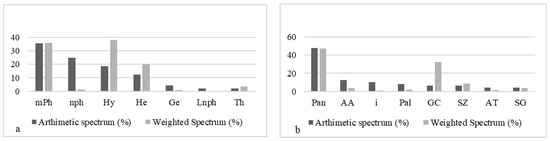
Figure 7.
Distribution of biological types and phytogeographical types in ponds: (a) LFT: life forms types: mPh: micro-phanero0phytes. Th: therophytes. nph: nano-phanerophytes. He: hemicryptophytes. Ge: geophytes. Hy: hydrophytes. Lnph: lianes nano-phanerophytes. (b) TP: phytogeographical types: Pan: pantropicals. GC: Guineo-Congolese species. SZ: Soudano-Zambezian species. AT: Afro-tropicales. SG: Soudano-Guinean species. Pal: paleo-tropicals. AA: Afro-American. i: introduced. S: Soudanian. G: Guinean.
While water lilies can be seen in some spots, the ponds are mostly covered with grass, Typha sp., in immersion. The pond borders are generally occupied by Polygonum sp., Saccharum sp. and Ludwigia sp. The banks are planted with A. auriculiformis A.Cunn. ex Benth., C. nucifera L., E. guineensis Jacq. and M. esculenta Crantz, controlling maintained crops or fallows when they are in operation.
3.2.3. Dams and Reservoirs
Reservoirs and dams are artificial wetlands created by mining phosphates and gravel and building dykes (dams) along the banks of rivers and streams. The main purpose of artificial dams and ponds is to produce agricultural goods.
The artificial reservoirs and dams have a specific richness estimated at 184 species divided between 50 families and 147 genera. The most diverse families are Poaceae (17.89%), Fabaceae (13.55%), Mimosaceae, Cyperaceae, and Asteraceae (5.46% each). However, the most frequent species are P. maximum Jacq (2.25%), A. indica A. Juss., L. sericeus (Poir.) Kunth ex DC., L. erecta (L.) H. Hara, T. domingensis (Pers.) Steud, (1.69% each) and A. polyacantha. (1.50%).
The biological forms most frequently encountered are composed of therophytes (33.33%), micro-phanerophytes (27.11%), and hemicryptophytes (10.73%) (Figure 8a); however, the dominant biological types around these artificial water points are made up of hemicryptophytes (34.46%), micro-phanerophytes (28.29%), and hydrophytes (17.78%). Phytogeographically (Figure 8b), wetlands resulting from human activities are dominated by Guineo-Congolese (48.41%) and pantropical (26.52%) species; however, the most frequent phytochoria are pantropical species (36.34%), followed by Guineo-Congolese (15.81%) and Sudano-Zambezian (12.99%).
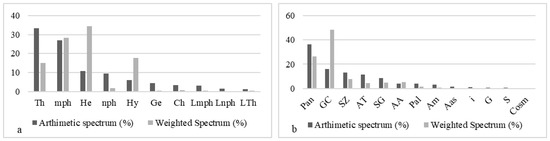
Figure 8.
Distribution of biological and chorological types of reservoirs (a) LFT: life forms types: mph: micro-phanerophytes. Th: therophytes. nph: nano-phanerophytes. He: hemicryptophytes. Ge: geophytes. Lmph: liana-micro-phanerophytes. Hy: hydrophytes. Ch: chamephytes. Lnph: lianes nano-phanerophytes. LTh: liana therophytes. (b) TP: phytogeographical types: Pan: pantropicals. GC: Guineo-Congolese species. SZ: Sudano-Zambezian species. AT: Afro-tropicales. SG: Sudano-Guinean species. Pal: paleo-tropicals. AA: Afro-American. Am: Afro-Malgash. i: introduced. Aas: Afro-Asiatics. S: Sudanese. G: Guinean. Cos: cosmopolitan.
Following the increasing drainage gradient, we found on the banks a tree stratum with Acacia sp., Entada sp. and P. thonningii (Schumach) Milne. Redh. The intense runoff gives rise to Ludwigia sp., Paspalum sp., Spermacoce sp., Cyperus sp. and Echinochloa sp. Water lilies have taken over the reservoirs. P. lanigerum R. Br. grasses penetrate undeveloped wetlands, then T. domingensis Pers. grasses take over the water’s surface.
3.2.4. Lakes and Lagoons
The lagoon and lake complex wetlands are closely associated with the ponds in the south of the basin. These types of wetlands are fed by the Boko, Haho, and Zio rivers in connection with the marine system. The floristic report reveals the presence of 21 species divided into 21 genera and 13 families. The most frequent species are C. nucifera (12.50%), T. domingensis Pers. (10.41%), E. guineensis Jacq, R. racemosa and C. articulatus L. (6.25% each), Arecaceae (18.75%), Mimosaceae, Poaceae and Typhaceae (10.41% each).
Phytogeographically lakes and lagoons are dominated by pantropical species (46.82%) and Guineo-Congolese (26.70%). However, the most frequent phytochoria are pantropical (50%) species, followed by Afro-tropical species (16.66%) and African-American species (10.41%) (Figure 9b). The biological forms frequently encountered are composed of micro-phanerophytes (43.75%), hydrophytes (22.91%), and nano-phanerophytes (18.75%) (Figure 9a); however, the dominant biological types around lakes and lagoons are made up of hydrophytes (40.82%), micro-phanerophytes (25.48%), and hemicryptophytes (10.44%).
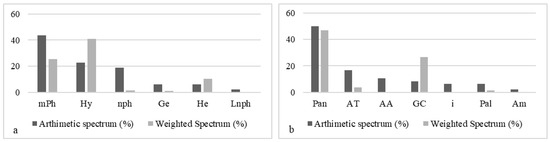
Figure 9.
Distribution of biological types and chorological types of lakes and lagoons. (a) LFT: life forms types: mPh: micro-phanerophytes. nph: nano-phanerophytes. He: hemicryptophytes. Ge: geophytes. Hy: hydrophytes. Lnph: lianes nano-phanerophytes. (b) TP: phytogeographical types: Pan: pantropicals. GC: Guineo-Congolese species. AT: Afro-tropicales. Pal: paleo-tropicals. AA: Afro-American. Am: Afro-Malgash. i: introduced.
Where the bodies of water are brackish, the banks are occupied by degraded mangroves with Rhizophora sp., and lawns of Saccharum sp., Paspalum sp., and C. articulatus L. A. aureum vegetation is also noticeable in the brackish landscape. In locations with moderate or mild salinity, meadows of T. domingensis cover the water bodies, and the banks are colonized by a lawn of Polygonum sp., Saccharum sp., Paspalum sp., and C. articulatus L. On hydromorphic soil, the tree stratum is characterized by a strong dominance of coconut groves, palm groves, and reforestation (A. auriculiformis A. Cunn. ex Benth).
4. Discussion
With 72 families, 236 genera, and 324 species, the wetlands in the Haho River basin have a high floristic potential. Its specific richness exceeds that of the mangroves and associated vegetation of the lagoon–lacustrine complex of the littoral in Togo (23 species), the swamp forests of Lokoli in Zogbodomey (241 species) in southern Benin [40], and the riparian forests of Burili ulcer (216 species) with a high rate of endemicity in Côte d’Ivoire [41]. Therefore, according to the third criterion of the Ramsar wetland classification related to wetland biodiversity, the study area can be considered a wetland of international importance. This criterion stipulates that: a wetland may be considered of international importance if it supports populations of plant and/or animal species critical to maintaining the diversity of a biogeographic region [42,43]. A further article on the classification of this wetland may be necessary.
Our findings demonstrate that Poaceae and Fabaceae are the dominant plant families in the southern wetlands, whether in Benin or Côte d’Ivoire. Furthermore, the southern wetlands of these three West African nations share Ludwigia, Panicum, and Cyperus species. The research areas’ shared latitudinal positions and similar climatic regimes are unquestionably responsible for their substantial taxonomic similarities (families and genera) based on occurrence. However, part of the discrepancy in species richness is probably explained by the typological diversity of the wetlands prospected in our study, as opposed to those undertaken in the two other sub-regions [44].
According to the results of several phytosociological studies conducted in the sub-region, biological types are one of the criteria used to evaluate the physiognomy of plant formations [45,46]. The results of this study reveal that micro-phanerophytes and therophytes are the predominant biological forms; this pattern was also seen in the riparian forests in the southern part of Côte d’Ivoire [47]. The strong representation of microphanerophytes would suggest the presence of shrubby formations or open forests close to the sampled locations [33,48]. This is particularly evident in riparian forests and ponds, where woody formations contain forest species such as P. santalinoides L’Hér. ex DC., C. megalophyla Harms, Cola laurifolia Mast., Bambusa vulgaris Schrader ex Wendl., L. sericeus (Poir.) H. B. K., and M. thonningii (Schumach. Et Thonn.) Baker and M. inermis (Willd.) Kuntze.
When it comes to therophytes, this preponderance highlights a period of severe vegetation degradation brought on by a sizeable anthropogenic impact on ecosystems [49]. This therophyzation of the floristic richness may indicate the extent pastoralism is expanding in and around these wetlands [50]. The permanence of water, and the expanses of open meadows, explain the growing number of camps of transhumant herders in the catchment area. The abundance of hemicryptophytes around several wetlands in the watershed indicates stable soils and suitable humidity levels, which justify the off-season agriculture that has been recorded there [51,52].
The pantropical and Guineo-Congolese type phytochoria determine the phytogeographical landscape of the study area. The wetlands under investigation exhibit damaged ecosystems and contain signs of human activity. Therefore, ongoing human interference with the ecological dynamism of wetlands has led to the domination of widely dispersed chorological types (pantropical) [53,54]. Additionally, this illustrates how tropical species of the widespread range have enriched the endemic flora of this phytogeographical region (pantropical, Afro-tropical, paleo-tropical). The importance of the Guinean-Congolese groups confirms that the wetlands sampled are at the transition between the Guinean and Congolese climatic domains. However, species having Sudanese characteristics would be found more toward the Sudanese climate region in terms of latitude.
Changes in the characteristics of the floristic composition of wetland ecosystems result from several mutations connected to the forms of use and production [55]. It is acknowledged that wetlands possess a functional dynamic on which the viability of local economies depend (Appendix A). It has also been established that this resilience has spatial and temporal boundaries, where seasonal climate fluctuations also affect the ecological functions of the microorganisms in these particular biotopes by changing the dynamics of wetland formation. In order to stop the disturbance of the food chain in response to the trend of diminishing quality in the services and goods given by wetlands, many studies encourage in-depth research on the ecotoxicology and biological contamination of these biotopes [56].
5. Conclusions
This study on the floristic diversity of the wetlands in the Haho River basin found 324 species distributed among 236 genera and 72 families, indicating that this region has high floristic potential. This region could be included in the list of wetland areas of international importance upon confirmation of additional studies in accordance with the Ramsar criteria. The species found belong to the widely dispersed tropical flora (pantropical, Afro-tropical, and paleo-tropical) found to have been substantially increased by the endogenous species. Several factors, including water availability during the season and anthropogenic influences through agriculture, influence the distribution of these species. It should be noted that the potential of wetlands is undervalued, mainly because the actors are poorly organized and have limited technical and financial resources when implementing the integrated development projects from which these floodable ecosystems can benefit. If wetlands are to be managed sustainably, short- and medium-term actions are required to inform the public authorities of the need for regulation and usage regulation. An integrated management strategy requires action research to categorize wetlands by function (water resource, biodiversity, hunting, or tourism development).
Author Contributions
Conceptualization, F.F. and K.W.; methodology, F.F., M.K. and K.F.; resources, M.K.; data curation, K.B. and K.W.; writing—original draft preparation, F.F.; writing—review and editing, E.B. and K.F.; visualization, F.F.; supervision, K.W., K.B. and K.A.; project administration, K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Acknowledgments
We are very grateful to the Botany and Plant Ecology Laboratory members who contributed to the data collection. TWAS (The World Academy of Sciences), Matsumae International Foundation (MIF), and The Ecosystems Dynamic and Productions (Kyoto University) deserve our gratitude for their hospitality and financial support. Finally, we are indebted to the reviewers of our text, who remain anonymous, for their advice.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Plant Diversity Follow Life Form, Phytogeographical Type and It Endogenous Uses
LFT: life forms types
mPh: micro-phanerophytes. Th: therophytes. nph: nano-phanerophytes. He: hemicryptophytes. Ge: geophytes. Lmph: liana-micro-phanerophytes. Hy: hydrophytes. Ch: chamaephytes. Lnph: nano-phanerophyte lianas. LTh: lianas therophytes. LHe: hemicryptophyte lianas.
TP: phytogeographical types
Pan: pantropical. GC: Guinean-Congolese species. SZ: Sudano-Zambezian. AT: Afro-tropical. SG: Sudano-Guinean. Pal: paleo-tropical. AA: African-American. Am: Afro-Malagasy. i: introduced. Aas: Afro-Asian. S: Sudanian. G: Guinean. Cos: cosmopolitan.
| No | Species | LFT | TP | EB | L-L | M | R |
| 1 | Abutilon mauritianum (Jacq) Medik. *** | Th | AT | 1 | 1 | ||
| 2 | Acacia auriculiformis A. Cunn. ex Benth. **** ***** | mPh | AT | 1 | 1 | 1 | 1 |
| 3 | Acacia dudgeoni Craib ex Hall. ** *** **** ***** | mPh | SZ | 1 | |||
| 4 | Acacia polyacantha Willd. campylacantha (Hochst. ex A. Rich.) Brenan ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 5 | Acacia sieberiana DC.var.villosa ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 6 | Acroceras amplectens Stapf *** | nph | SG | 1 | |||
| 7 | Acroceras zizanioides (Kunth) Dandy *** | He | Pan | 1 | |||
| 8 | Adansonia digitata L. * ** *** ***** ****** | mPh | SZ | 1 | 1 | ||
| 9 | Adenia rumicifolia Engl. &Harms ** *** | Lnph | GC | 1 | |||
| 10 | Aframomum sceptrum (Olïv. & D.Ranb.) K.Schum. ** | Ge | GC | 1 | |||
| 11 | Ageratum conyzoides L. ** | Th | Pan | 1 | 1 | ||
| 12 | Albizia lebbeck (L.) Benth. ** *** **** ***** | mPh | Pan | 1 | |||
| 13 | Albizia zygia (DC.) J.F. Macbr. ** *** **** ***** | mPh | GC | 1 | 1 | ||
| 14 | Alchornea cordifolia (Schum. & Thonn.) Mull. Arg. ** | mPh | GC | 1 | 1 | ||
| 15 | Allophylus africanus P. Beauv. ** *** | mPh | GC | 1 | |||
| 16 | Alternanthera nodiflora R.Br. ** | Ch | Pan | 1 | 1 | ||
| 17 | Alternanthera sessilis (L.) R. Br.ex Roth ** | Th | Pan | 1 | 1 | ||
| 18 | Ampelocissus grantii (Baker) Planch. * ** | Lmph | SG | 1 | 1 | ||
| 19 | Ampelocissus leonensis (Hook.f.) Planch. * ** | Lmph | GC | 1 | |||
| 20 | Ampelocissus pentaphylla (GuilI. & Perr.) Gilg & Brandt * ** | Lmph | SZ | 1 | |||
| 21 | Anchomanes difformis (BIume) Engl. ** | Ge | GC | 1 | 1 | ||
| 22 | Andropogon gayanus Kunth ** *** | He | SG | 1 | 1 | 1 | |
| 23 | Annona senegalensis Pers. * ** *** **** ***** | nph | SZ | 1 | 1 | ||
| 24 | Anogeisus leiocarpa (DC) Guill. Et Perr * ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 25 | Anthocleista djalonensis A. Chev. ** *** | mPh | GC | 1 | |||
| 26 | Antiaris toxicaria Lesch. ssp. welwitschii (Engl.) C.C.Berg ** *** **** ***** | mPh | GC | 1 | 1 | ||
| 27 | Antidesma venosum E. Mey. Ex Tul. ** *** **** ***** | mPh | AT | 1 | |||
| 28 | Artocarpu saltilis (Parkinson) Fosberg * ** *** **** ***** | mPh | i | 1 | |||
| 29 | Asparagus africanus Lam. ** | nph | SZ | 1 | |||
| 30 | Aspilia rudus Oliv. & Hiern ** | Th | SG | 1 | |||
| 31 | Asystasia gangetica (L.) T. Anders. ** *** **** | nph | Pan | 1 | |||
| 32 | Axonopus compressus (Sw.) P.Beauv. ** | Th | Pan | 1 | 1 | ||
| 33 | Azadirachta indica A. Juss. ** *** **** ***** | mPh | Pal | 1 | 1 | 1 | 1 |
| 34 | Bambusa vulgaris Schrader ex Wendl. | Ge | SG | 1 | |||
| 35 | Berlinia grandiflora (Vah) Hutch. Et Dalziel ** *** **** ***** | mPh | AT | 1 | |||
| 36 | Blighia sapida Konig * ** *** **** ***** | mPh | Pan | 1 | |||
| 37 | Boerhavia erecta L. ** *** | Th | cosm | 1 | |||
| 38 | Borassus aethiopum Mart. * ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 39 | Brachiaria deflexa (Schumach) C. E. Hubbard ex Robyns | Th | Am | 1 | |||
| 40 | Bridelia ferruginea Benth ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 41 | Caesalpinia benthamiana (Baill.) Herend. Et Zarucchi | nph | AT | 1 | |||
| 42 | Calopogonium mucoïdes Desv. ** | LTh | Am | 1 | 1 | ||
| 43 | Calotropis procera (Aiton) W. T. Aiton ** *** **** ***** ****** | nph | Pal | 1 | 1 | ||
| 44 | Capsicum frutescens L. * ** | nph | Pan | 1 | |||
| 45 | Carica papaya L. ** *** | mPh | AA | 1 | 1 | ||
| 46 | Carissa edulis Bahl. * ** | nph | Pal | 1 | |||
| 47 | Cassia hirsuta L. ** | Pan | 1 | ||||
| 48 | Ceiba pentandra (L.) Gaertn. * ** *** **** ***** ****** | mPh | Pan | 1 | 1 | 1 | |
| 49 | Celosia argentea L.var.argentea | Th | Pal | 1 | |||
| 50 | Celosia trigyna L. ** | Th | Pal | 1 | |||
| 51 | Ceratophyllum demersum L. ** | Hy | Pan | 1 | |||
| 52 | Chamaecrista mimosoides (L.) Greene ** | Th | Pal | 1 | 1 | ||
| 53 | Chassalia kolly (Schumach.) Hepper ** | nph | GC | 1 | |||
| 54 | Chloris barbata Sw. ** *** | Th | Pan | 1 | 1 | ||
| 55 | Chloris pilosa Schum. *** | Th | Pan | 1 | |||
| 56 | Chromolaena odorata DC. ** *** | nph | Pan | 1 | 1 | 1 | |
| 57 | Cisampelos mucronata A. Rich * ** | Lmph | SZ | 1 | |||
| 58 | Cissus petiolata Hook.f. * ** | Lnph | GC | 1 | |||
| 59 | Cissus populnea Guill. Et Perr * ** | LHe | SZ | 1 | |||
| 60 | Clausena anisata (Willd) Hook. F. ex Benth ** *** **** ***** | mPh | AT | 1 | |||
| 61 | Cleome rutidesperma DC. ** | Th | SG | 1 | |||
| 62 | Clitoria falcata Lam ** | Th | AA | 1 | 1 | ||
| 63 | Cnestis ferruginea DC. ** *** **** ***** | mPh | GC | 1 | |||
| 64 | Cocos nucifera L. * ** *** **** ***** | mPh | Pan | 1 | 1 | 1 | 1 |
| 65 | Cola gigantea A, Chev. Var. gigantea ** *** **** ***** ****** | mPh | GC | 1 | 1 | ||
| 66 | Cola laurifolia Mast. ** *** **** ***** | mPh | GC | 1 | |||
| 67 | Cola millenii K. Schum. ** *** **** ***** | mPh | GC | 1 | |||
| 68 | Colocasia esculenta (L.) Schoot * ** | Ge | AT | 1 | |||
| 69 | Combretum adenogonium Steud. Ex A. Rich. ** *** **** ***** | mPh | SG | 1 | |||
| 70 | Combretum collinum Fresen ** *** **** ***** | mPh | SG | 1 | |||
| 71 | Combretum glutinosum Perr.ex De. ** *** **** ***** | mPh | SZ | 1 | |||
| 72 | Combretum hispidum Law. ** *** **** ***** | mPh | GC | 1 | |||
| 73 | Combretum molle R. Br. ex G. Don ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 74 | Commelina bracteosa Hassk. *** | He | Pan | 1 | |||
| 75 | Commelina diffusa Burm.f. *** | He | Pan | 1 | 1 | ||
| 76 | Commelina erecta L. *** | Ch | AT | 1 | 1 | ||
| 77 | Crinum jagus (J. Thomps.) Dandy. ** | Ge | GC | 1 | 1 | ||
| 78 | Crossopteryx febrifuga (Afzel. ex G. Don) Benth ** *** **** ***** | mPh | SZ | 1 | |||
| 79 | Crotalaria cephalotes Steud. ex A.Rich. *** | Th | Pan | 1 | 1 | ||
| 80 | Crotalaria retusa Linn *** | Ch | Pan | 1 | 1 | ||
| 81 | Croton lobatus L. *** | Th | Pan | 1 | 1 | ||
| 82 | Culcasia scandens P. Beauv. *** | Lmph | GC | 1 | |||
| 83 | Cussonia kirkii Seeman var. kirkii ** *** **** ***** | mPh | SZ | 1 | |||
| 84 | Cyanotis lanata Benth. ** | He | SG | 1 | |||
| 85 | Cynodon dactylon (L.) Pers ** | Th | Pan | 1 | |||
| 86 | Cynometra megalophylla Harms ** *** **** ***** | mPh | GC | 1 | |||
| 87 | Cyperus alternifolius L. *** | Ge | i | 1 | 1 | ||
| 88 | Cyperus articulatus L. *** | Hy | i | 1 | 1 | 1 | 1 |
| 89 | Cyperus distans L.f. s.l. *** | Ge | Pan | 1 | 1 | ||
| 90 | Cyperus haspan Linn *** | Ge | Pan | 1 | 1 | ||
| 91 | Cyperus latifolius Poir. *** | Ge | Pan | 1 | |||
| 92 | Cyperus rhotondus Linn. *** | Ge | Pan | 1 | 1 | ||
| 93 | Cyperus tenuiculmis Boeck.s.l. *** | He | SG | 1 | 1 | ||
| 94 | Cyphostemma adenocole (Steud.) Desc. ** | Lmph | AT | 1 | |||
| 95 | Cyphostemma sokodense (Gilg & Brandt) Desc. ** | Lmph | G | 1 | |||
| 96 | Dactyloctenium aegyptium (L.) Willd. *** | Th | Pan | 1 | 1 | ||
| 97 | Daniellia oliveri (Rolfe) Hutch & Dalz ** *** **** ***** ****** | mPh | SG | 1 | |||
| 98 | Delonix regia (Boj. Ex Hook.) Raf. **** ***** | mPh | Am | 1 | |||
| 99 | Desmodium adscendens (Sw.) DC var. adscendens ** | Th | AA | 1 | |||
| 100 | Desmodium barbatum (L.) Benth. var. dimorphum ** | Th | AT | 1 | |||
| 101 | Desmodium salicifolium (Poir.) DC. ** | nph | GC | 1 | |||
| 102 | Desmodium tortuosum (Sw.) DC. ** | nph | Pan | 1 | 1 | ||
| 103 | Desmodium triflorum (L.) DC ** | Lnph | Pan | 1 | 1 | ||
| 104 | Desmodium velutinum (Willd.) DC. ** | Th | Pal | 1 | |||
| 105 | Dialium guineense Willd. * ** *** **** ***** ****** | mPh | GC | 1 | |||
| 106 | Dicrostachys cynera (L.) Wight & Arn *** | nph | AT | 1 | 1 | 1 | |
| 107 | Digitaria ciliaris (Retz.) Koeler *** | Th | Pan | 1 | |||
| 108 | Digitaria horizontalis Willd. *** | Th | AA | 1 | 1 | ||
| 109 | Dioscorea bulbilfera L. ** | Ge | Pan | 1 | 1 | ||
| 110 | Dioscorea togoensis Knuth ** | Ge | GC | 1 | |||
| 111 | Diospyros mespiliformis Hochst.ex A.DC. * ** *** **** ***** ****** | mPh | SZ | 1 | |||
| 112 | Diospyros monbuttensis Gürke * ** *** **** ***** ****** | mPh | GC | 1 | |||
| 113 | Diplazium sammatii (Kuhn) C. Chr. ** | Hy | AT | 1 | 1 | 1 | |
| 114 | Dracaena arborea (Willd.) Link. ** ****** | mPh | GC | 1 | |||
| 115 | Drepanocarpus lunantus ** | nph | AA | 1 | 1 | ||
| 116 | Drypetes floribunda (Muell.–Arg.) Hutch. *** *** **** ***** | mPh | GC | 1 | |||
| 117 | Echinochloa colona (L.) Link ** *** | Th | Pan | 1 | 1 | ||
| 118 | Echinochloa obtusiflora Stapf ** *** | Th | Pan | 1 | |||
| 119 | Echinochloa pyramidalis (Lam.) Hitchc. & Chase ** *** | Th | Pan | 1 | 1 | ||
| 120 | Eclipta prostata L. ** *** | Th | GC | 1 | 1 | ||
| 121 | Ehretia cymosa Thonn. Ex Sehum. var. cymosa Brenan ** *** | mPh | GC | 1 | |||
| 122 | Eichhornia crassipes (Mart.) Solms-Laub.** *** | Hy | Pan | 1 | 1 | ||
| 123 | Elaeis guineensis Jacq. * ** *** **** ***** | mPh | GC | 1 | 1 | 1 | 1 |
| 124 | Eleusine indica Gaertn *** | Th | Pan | 1 | 1 | ||
| 125 | Emilia coccinea (Sims.) G. Don *** | Th | SG | 1 | 1 | ||
| 126 | Entada africana Guill et Perr ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 127 | Entidesma venosum E. Mey. Ex Tul. *** | nph | AT | 1 | |||
| 128 | Eragrostis ciliaris (Linn.) R. Br.*** | Th | Pan | 1 | |||
| 129 | Eragrostis tremula (Lam.) Steud. *** | Th | Pan | 1 | |||
| 130 | Eriosema griseum Baker var. griseum | Th | AA | 1 | 1 | ||
| 131 | Euphorbia heterophylla L. *** | Th | Pan | 1 | 1 | ||
| 132 | Euphorbia hirta Linn. ** *** | Th | Pan | 1 | 1 | ||
| 133 | Euphorbia hyssopifolia Linn ** *** | Th | Pan | 1 | |||
| 134 | Ficus asperlifolia Miq. ** *** **** ***** | nph | GC | 1 | |||
| 135 | Ficus dicranostyla Mildbr. * ** *** **** ***** ****** | mPh | GC | 1 | |||
| 136 | Ficus elastica Roxb. ** *** **** ***** | mPh | AT | 1 | |||
| 137 | Ficus exasperata Vahl. ** *** **** ***** | mPh | AT | 1 | |||
| 138 | Ficus lutea Vahl ** *** **** ***** | mPh | i | 1 | |||
| 139 | Ficus mucuso Ficalho ** *** **** ***** | mPh | GC | 1 | 1 | ||
| 140 | Ficus sur Forssk. * ** *** **** ***** ****** | mPh | SG | 1 | 1 | ||
| 141 | Ficus sycomorus L. * ** *** **** ***** ****** | mPh | SZ | 1 | |||
| 142 | Fimbristylis littoralis Gaudet *** | He | Pan | 1 | 1 | ||
| 143 | Flacourtia indica (Burm.f.) Merr ** *** **** | nph | Aas | 1 | |||
| 144 | Fleurya aestuans (Linn.) ex Miq ** *** | Th | Pan | 1 | |||
| 145 | Flueggea virosa (Roxb, ex Willd) Voigt ** | nph | Pan | 1 | 1 | 1 | |
| 146 | Fuirena umbellata Rottb.** | Ge | Pan | 1 | 1 | ||
| 147 | Gmelina arborea Roxb ** *** **** ***** | mPh | i | 1 | |||
| 148 | Gomphrena celosioides Mart. ** | Ch | cosm | 1 | 1 | ||
| 149 | Grewia venusta fresen * ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 150 | Griffonia simplicifolia (Val. Ex DC) Baill. | Lmph | AT | 1 | |||
| 151 | Hewittia scandens (Milne) Mabberley | Lnph | Pan | 1 | 1 | ||
| 152 | Hexalobus monopetalus (A. Rich.) Engl. & Diels. * ** *** **** ***** | mPh | SZ | 1 | |||
| 153 | Hibiscus asper Hook. F. * ** | nph | Pan | 1 | 1 | 1 | |
| 154 | Hibiscus Ionchosepalus Hochr. * ** | nph | Pan | 1 | |||
| 155 | Holarrhena floribunda (G. Don) Durand et Sching ** *** **** ***** | mPh | AT | 1 | |||
| 156 | Hoslundia opposita Bahl. ** | nph | Am | 1 | |||
| 157 | Hydrolea macrosepala A.W. Benn. ** | Th | AT | 1 | |||
| 158 | Hyparrhenia involucrata Stapf. *** | Th | SG | 1 | 1 | ||
| 159 | Hyppocratea indica Planch.** | Lnph | Aas | 1 | |||
| 160 | Hyptis suaveolens (L.) Poit. ** | Th | Pan | 1 | |||
| 161 | Imperata cylindrica var africana (Aderss.) ** *** | He | Pan | 1 | 1 | ||
| 162 | Indigofera astragalirea ** | Th | Pan | 1 | |||
| 163 | Indigofera dendroides Jacq. *** | nph | SZ | 1 | |||
| 164 | Indigofera hirsuta L. var. hirsuta ** | Ch | Pan | 1 | 1 | ||
| 165 | Indigofera suffruticosa Mill. ** | nph | Pan | 1 | |||
| 166 | Indigofera tinctoria L. var. tinctoria ** | nph | Pan | 1 | 1 | ||
| 167 | Ipomea aquatica Forssk ** *** | Lnph | GC | 1 | |||
| 168 | Ipomea involucrata (P.) Beouv.** *** | Th | AT | 1 | |||
| 169 | Ipomea vagans Bak. ** *** | Th | AT | 1 | |||
| 170 | Khaya senegalensis (Desr.) A. Juss. ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 171 | Kigelia africana (Lam.) Benth. ** *** **** ***** ****** | mPh | GC | 1 | 1 | ||
| 172 | kyllinga bulbosa Beauv. ** | Ge | Pan | 1 | 1 | ||
| 173 | Kyllinga pumila Michx ** | Ge | Pan | 1 | 1 | ||
| 174 | Kyllinga squamulata Thonn. ex Vahl ** | Ge | Pan | 1 | |||
| 175 | Lannea acida A. Rich. * ** *** **** ***** | mPh | GC | 1 | |||
| 176 | Lannea barteri (Oliv.) Enhl. * ** *** **** ***** | mPh | GC | 1 | 1 | ||
| 177 | Launaea taraxacifolia (Willd.) Amin ex C. Jeffrey * ** *** | Th | GC | 1 | 1 | ||
| 178 | Lecaniodiscus cupanioides Planch. ex Benth. ** *** **** ***** | mPh | GC | 1 | |||
| 179 | Leersia hexandra Sw. ** | He | Pan | 1 | 1 | ||
| 180 | Lemna aequinoctialis Welw | Hy | Pan | 1 | 1 | ||
| 181 | Leptadenia hastata (Pers.) Decine ** *** | Lmph | SZ | 1 | 1 | ||
| 182 | Leptochloa caerulenscens Steud. ** *** | Th | SG | 1 | 1 | ||
| 183 | Leucaena leucocephala (Lam.) de Wit ** *** **** ***** | mPh | Pan | 1 | 1 | 1 | |
| 184 | Lippia multiflora Moldenke ** *** **** ***** | nph | SG | 1 | |||
| 185 | Lippia rugosa A.Chev. ** *** **** ***** | nph | SZ | 1 | |||
| 186 | Lonchocarpus sericeus (Poir.) H. B. K. ** *** **** ***** | mPh | GC | 1 | 1 | ||
| 187 | Lophira lanceolata Van Tiegh. Ex Keay ** *** **** ***** | mPh | GC | 1 | |||
| 188 | Ludwigia abyssinica A. Rich ** *** | Th | GC | 1 | 1 | ||
| 189 | Ludwigia decurrens Walt. ** *** | Th | AA | 1 | 1 | ||
| 190 | Ludwigia erecta (L.) Hara ** *** | Th | AA | 1 | 1 | 1 | |
| 191 | Ludwigia hyssopifolia (G. Don.) Exell ** *** | Th | AT | 1 | |||
| 192 | Ludwigia octavalvis (Jacq) P. Raven ** *** | Th | AT | 1 | 1 | ||
| 193 | Ludwigia stolonifera (Guill. & Perr.) Raven ** *** | Hy | Aas | 1 | 1 | ||
| 194 | Luffa cylindrica (L.) M.J. Roem. ** *** | Th | Pan | 1 | 1 | ||
| 195 | Mallotus oppositifolius (Geisel.)Müll.Arg. ** *** | mPh | Am | 1 | 1 | ||
| 196 | Malvastrum coromandelianum (L.) Garcke. ** *** | Th | Pan | 1 | |||
| 197 | Mangifera indica Sp. Pl. * ** *** **** ***** | mPh | Aas | 1 | 1 | ||
| 198 | Manihot esculenta Crantz * ** *** | nph | Pan | 1 | |||
| 199 | Margaritaria discoïdea (Baill.) Webster ** *** | mPh | AT | 1 | 1 | ||
| 200 | Mariscus alternifolius Vahl. ** *** | He | Pan | 1 | |||
| 201 | Mayetenus senegalensis (Lam.) Exell. ** *** **** ***** | mPh | SZ | 1 | |||
| 202 | Melanthera scandens (Schum. & Thonn.) Roberty | Lmph | AT | 1 | 1 | ||
| 203 | Melochia corchorifolia L. ** *** | Ch | Pan | 1 | 1 | ||
| 204 | Melochia melissifolia Benth. ** *** | nph | Pan | 1 | |||
| 205 | Merinia umbellata (L.) Hallier. F. ** *** | Th | Pan | 1 | |||
| 206 | Mezoneuron benthamianum Baill ** *** | Lmph | GC | 1 | 1 | ||
| 207 | Milicia excelsa (Welw.) C. C. Berg ** *** **** ***** ****** | mPh | GC | 1 | 1 | ||
| 208 | Millettia thonningii (Schumach. Et Thonn.) Baker ** *** **** ***** | mPh | GC | 1 | 1 | ||
| 209 | Mimosa pudica L. ** *** | nph | Pan | 1 | |||
| 210 | Mirremia aegyptiaca (L.) Urban ** *** | Th | Pan | 1 | |||
| 211 | Mitragyna inermis (Willd.) Kuntze ** *** **** ***** ****** | mPh | SZ | 1 | 1 | 1 | |
| 212 | Mnesithea granularis (L.) Koning & Sosef ** *** | Th | Pan | 1 | |||
| 213 | Momordia charantia L. ** *** | Lnph | Pan | 1 | |||
| 214 | Monechma ciliatum (Jacq.) Milne-Redh. ** *** | nph | AT | 1 | |||
| 215 | Morelia senegalensis A. Rich. Ex. DC. ** *** | nph | SG | 1 | |||
| 216 | Morinda lucida Sp. Pl. ** *** **** ***** | mPh | Pan | 1 | 1 | ||
| 217 | Musa sp L. * ** *** | Ge | Pan | 1 | 1 | 1 | 1 |
| 218 | Newbouldia laevis (P. Beauv.) Seem ** *** **** ***** ****** | mPh | GC | 1 | 1 | ||
| 219 | Nymphae lotus Linn. ** | Hy | AT | 1 | 1 | 1 | 1 |
| 220 | Nymphea maculata Schum. & Thonn. ** | Hy | AT | 1 | 1 | ||
| 221 | Oncoba gilgiana Sprague Bull. Herb. Boiss. ** *** | nph | GC | 1 | |||
| 222 | Oplimenus burmannii (Retz) P. Beauv. | Th | SZ | 1 | 1 | ||
| 223 | Oriza barthii A. Chev. * ** *** | He | SZ | 1 | 1 | 1 | |
| 224 | Oslandia opposita ** *** | nph | Am | 1 | |||
| 225 | Panicum laxum SW. ** *** | Th | AA | 1 | |||
| 226 | Panicum maximum Jacq. ** *** | He | GC | 1 | 1 | 1 | 1 |
| 227 | Panicum tenellum Lam. ** *** | Th | SZ | 1 | |||
| 228 | Parkia biglobosa (Jacq) R. Br. Ex Benth * ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 229 | Paspalum polystachion (L.) Schult.subsp.polystachion L. ** *** | Th | GC | 1 | 1 | ||
| 230 | Paspalum scrobiculatum L. ** *** | He | Pan | 1 | 1 | 1 | 1 |
| 231 | Paspalum vaginatum Sw. ** *** | Th | Pan | 1 | 1 | ||
| 232 | Passiflora foetida L. ** *** | Lnph | Pan | 1 | |||
| 233 | Paulinia pinnata L. * ** *** | Lmph | AT | 1 | 1 | ||
| 234 | Pavetta corymbosa (DC.) F. N. Williams ** *** | mPh | SG | 1 | |||
| 235 | Pennisetum hordoides (Lam.) Steud ** *** | Th | GC | 1 | 1 | ||
| 236 | Pennisetum purpureum Schumach. ** *** | Th | Pan | 1 | |||
| 237 | Pentodon pentandrus (Schum. & Thonn.) Vatke ** *** | Th | AT | 1 | 1 | ||
| 238 | Pergularia daemia (FORSSK.) Chiov. ** *** | Lmph | AT | 1 | 1 | ||
| 239 | Persicaria senegalensis (Meisn.) Sojak | nph | AT | 1 | |||
| 240 | Phoenix reclinata Jacq. *** **** | mPh | AT | 1 | 1 | ||
| 241 | Phyllanthus amarus Schum. Et Thonn ** | Th | Pan | 1 | 1 | ||
| 242 | Physali angulata L. ** *** | Th | Pan | 1 | 1 | ||
| 243 | Physalis minima L. ** *** | Th | Pan | 1 | |||
| 244 | Piliostigma thonningii (Schumach) Milne. Redh. ** *** **** ***** | mPh | S | 1 | 1 | ||
| 245 | Polygonum lanigerum R. Br. ** *** | nph | Aas | 1 | 1 | ||
| 246 | Polygonum salicifolium Brouss. Ex Willd. ** *** | Th | AT | 1 | 1 | ||
| 247 | Polygonum senegalense Meisn. ** *** | He | AT | 1 | 1 | ||
| 248 | Pouteria alnifolia (Baker) Pierre ** *** **** ***** | mPh | GC | 1 | 1 | ||
| 249 | Premna quadrifolia Schumach. Et Thonn ** *** | nph | GC | 1 | 1 | ||
| 250 | Prosopis africana (Guill. & Perr.) Taub. ** *** **** ***** | mPh | SZ | 1 | |||
| 251 | Pseudocedrela kotschyi (Schweinf.) Harms ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 252 | Pterocarpus erinaceus Poir. ** *** **** ***** | mPh | GC | 1 | 1 | ||
| 253 | Pterocarpus santalinoides L’Hér.ex DC. ** *** **** ***** | mPh | GC | 1 | 1 | ||
| 254 | Rauvolfia vomitoria Afzel. ** *** **** ***** | mPh | GC | 1 | |||
| 255 | Reissantia indica (Willd.) N. Hallé var. loeseneriana (Hutch.&M.B.Moss) N.Hallé ** **** | Lnph | Aas | 1 | |||
| 256 | Rhyzophora racemosa G. Mey. ** *** **** ***** | mPh | AA | 1 | |||
| 257 | Ricinus communis L. ** *** | nph | Pan | 1 | 1 | ||
| 258 | Ritchiea reflexa (Thonn.) Gilg & Benedict ** *** | nph | Pan | 1 | |||
| 259 | Rottboellia cochinchinensis (Lour.) W.D.Clayton ** *** | Th | Pal | 1 | 1 | ||
| 260 | Rourea coccinea (Thom, ex Schumach) ** *** | Lnph | AT | 1 | 1 | ||
| 261 | Saba comorensis (Boj.) Pichon ** *** | Lmph | Am | 1 | |||
| 262 | Saccharum officinarum L. * ** *** **** ***** | nph | Pan | 1 | 1 | 1 | |
| 263 | Sacciolepis africana C. E. Hubb. Et Snowden | He | SZ | 1 | |||
| 264 | Samanea saman (Jacq.) Merr. *** *** **** ***** | mPh | AA | 1 | |||
| 265 | Sarcocephalus latifolus (sm) E. A. Bruce * ** *** **** ***** | mPh | AT | 1 | 1 | ||
| 266 | Schrankia leptocarpa DC. ** *** | Lnph | Pan | 1 | 1 | 1 | 1 |
| 267 | Scoparia dulcis (L.) ** *** | Th | SZ | 1 | |||
| 268 | Secamone afzelii (Schult.) K. Schum. ** *** | Lmph | GC | 1 | 1 | ||
| 269 | Securidaca longepedunculata Fresen. ** *** **** ***** | mPh | SZ | 1 | |||
| 270 | Sena siamea (Lam.) H. S. Irwin et Barneby ** *** **** ***** | mPh | Pan | 1 | 1 | ||
| 271 | Sesbania dalzielii Phillip. & Hutch ** *** | Th | Pan | 1 | |||
| 272 | Sesbania sesban (L) Merr. Var. punctata (DC) J.B. Gillett ** *** | Th | GC | 1 | |||
| 273 | Setaria barbata (Lam) Kunth ** *** | Th | Pal | 1 | 1 | ||
| 274 | Sida acuta Burm. F. ** *** | nph | Pan | 1 | 1 | ||
| 275 | Sida corymbosa R. E. Fries ** *** | Th | GC | 1 | |||
| 276 | Sida linifolia Juss ex Cav. ** *** | Th | AA | 1 | |||
| 277 | Sida rhombifolia L. ** *** | nph | GC | 1 | |||
| 278 | Solenostemon monostachyus (P.Beauv.) Briq. Ssp monostachyus ** *** | Th | GC | 1 | |||
| 279 | Sorghum arundinaceum (Willd.) Stapf * ** *** | He | SG | 1 | 1 | ||
| 280 | Spathodea campanulata P.Beauv. ** *** **** ***** | mPh | GC | 1 | |||
| 281 | Spermacoce ruelliae DC. ** *** | Th | SZ | 1 | 1 | ||
| 282 | Sphenoclea zeylanica Gaertn. ** *** | Hy | Pan | 1 | |||
| 283 | Spigelia anthelmia L. ** *** | Th | AA | 1 | 1 | ||
| 284 | Spondias cytherea Sonner. ** *** **** ***** | mPh | Pan | 1 | |||
| 285 | Spondias mombin L. * ** *** **** ***** | mPh | Pan | 1 | |||
| 286 | Sporobolus pyramidalis P. beauv ** *** | He | SZ | 1 | 1 | ||
| 287 | Stachytarpheta indica (Linn) Vahl. ** *** | Th | Am | 1 | 1 | ||
| 288 | Sterculia setigera Delile. * ** *** **** ***** ****** | mPh | SZ | 1 | |||
| 289 | Sterculia tragacantha Lindl. ** *** **** ***** | mPh | GC | 1 | |||
| 290 | Sterospermum kunthianum Cham. ** *** **** ***** | mPh | SG | 1 | 1 | ||
| 291 | Stylochaeton hypogaeum Lepr. ** *** | Ge | SZ | 1 | 1 | ||
| 292 | Synedrella nodiflora (L.) Gaertn. ** *** | Th | Pan | 1 | 1 | ||
| 293 | Tacca leontopetaloides (L.) O. Ktze. ** *** | Ge | Pal | 1 | |||
| 294 | Talinum triangulare (Jacq.) Willd. * ** *** | Ch | Pal | 1 | |||
| 295 | Tectona grandis L. F. ** *** **** ***** | mPh | Pal | 1 | |||
| 296 | Tephrosia bracteolata Guill. & Perr. | nph | SG | 1 | 1 | ||
| 297 | Tephrosia elegans Schumach. ** *** | nph | SG | 1 | |||
| 298 | Tephrosia platycarpa Guill.&Perr. ** *** | nph | SG | 1 | |||
| 299 | Tephrosia villosa (L.) Pers. ** *** | nph | Pan | 1 | |||
| 300 | Terminalia glaucescens Planch.ex Benth. ** *** **** ***** | mPh | SG | 1 | 1 | ||
| 301 | Terminalia mollis Laws. ** *** **** ***** | mPh | SZ | 1 | |||
| 302 | Tiliacora funifera (Miers) OIiv. ** *** | Lmph | GC | 1 | |||
| 303 | Trachyphrynium braunianum (K.Schum.) Bak. | Lmph | GC | 1 | |||
| 304 | Trema orientalis (L.) Blume. ** *** **** ***** | mPh | GC | 1 | |||
| 305 | Trianthema portulacastrum L. ** *** | Th | Pan | 1 | |||
| 306 | Triclisia subcordata Oliv. ** *** | Lnph | GC | 1 | |||
| 307 | Tridax procumbens L. ** *** | Th | Pan | 1 | 1 | ||
| 308 | Triumfetta rhomboidea Jacq. ** *** | nph | Pan | 1 | |||
| 309 | Typha domingensis Pers. ** *** | Hy | Pan | 1 | 1 | 1 | 1 |
| 310 | Uraria picta (Jacq.) DC. ** *** | nph | Pal | 1 | 1 | ||
| 311 | Urena lobata L. ** *** | nph | G | 1 | 1 | ||
| 312 | Uvaria chamae P. Beauv. ** *** | Lmph | GC | 1 | |||
| 313 | Vernonia amidalina Del. * ** *** | nph | GC | 1 | |||
| 314 | Vernonia cinerea (L.) Less. * ** *** | Th | Pan | 1 | 1 | ||
| 315 | Vernonia colorata (Willd) Drake. * ** *** | nph | SZ | 1 | 1 | 1 | |
| 316 | Vigna radiata (L.)R. Wilczek var.radiata | Th | Pan | 1 | 1 | ||
| 317 | Vigna trichocarpa (C.Wright) A.Delgado ** *** | Th | Pan | 1 | |||
| 318 | Vitellaria paradoxa C.F.Gaertner subsp. Paradoxa * ** *** **** ***** | mPh | SG | 1 | |||
| 319 | Vitex doniana Sweet. * ** *** **** ***** | mPh | SZ | 1 | 1 | ||
| 320 | Waltheria indica L. ** *** | nph | Pan | 1 | 1 | ||
| 321 | Xanthosoma mafaffa Sehott * ** *** | Ge | Pan | 1 | |||
| 322 | Zanha golungensis Hiern. ** *** **** ***** | mPh | SG | 1 | |||
| 323 | Zanthoxylum Zanthoxyloides (Lam.) Zepernick et Timler * ** *** **** ***** | mPh | SG | 1 | 1 | 1 | |
| EB: ponds and dams. L-L: lakes and lagoons. M: ponds. R: riparian forests. Endogenous uses: * (food), ** (medecine), *** (forrage), **** (fuel wood), ***** (agroforestry use), ****** (magio-religious use). | |||||||
References
- O´Connel, M.J. Detecting, measuring and reversing changes to wetlands. Wetl. Ecol. Manag. 2003, 11, 197–401. [Google Scholar]
- Brinson, M.M. A Hydrogeomorphic Clasification for Wetlands; U.S. Environmental Laboratory: Vicksburg, MS, USA; Engineer Research and Development Center: Vicksburg, MS, USA, 1993; Available online: https://hdl.handle.net/11681/6483 (accessed on 10 September 2021).
- Gardner, R.C.; Finlayson, C. Global Wetland Outlook: State of the World’s Wetlands and Their Services to People; Social Science Research Network: Rochester, NY, USA, 2018; Available online: https://ssrn.com/abstract=3261606 (accessed on 10 September 2021).
- Li, H.; Petric, R.; Alazzawi, Z.; Kauzlarich, J.; Mahmoud, R.H.; McFadden, R.; Perslow, N.; Rodriguez Flores, A.; Soufi, H.; Morales, K.; et al. Four Years Continuous Monitoring Reveals Different Effects of Urban Constructed Wetlands on Bats. Land 2021, 10, 1087. [Google Scholar] [CrossRef]
- Verhoeven, J.T.A.; Setter, T.L. Agricultural use of wetlands: Opportunities and limitations. Ann. Bot. 2010, 105, 155–163. [Google Scholar] [CrossRef]
- Folega, F.; Wala, K.; Kanda, M.; Batawila, K.; Akpagana, K.; Aperçu sur les Potentialités du Paysage Laguno-Lacustre du Togo. Zones Humides Infos—n° 97–98—Été. 2019. Available online: www.snpn.com/zoneshumidesinfos (accessed on 1 November 2019).
- Beltrame, C.; Perennou, C.; Guelmami, A. Évolution de l’occupation Du Sol Dans Les Zones Humides Littorales Du Bassin Méditerranéen de 1975 à 2005. Méditerranée 2015, 97–111. [Google Scholar] [CrossRef]
- Mermet, L. Les Infrastructures Naturelles: Statut, Principe, Concept, Ou Slogan? Zones Humides Infos. 1995, 7, 7–9. [Google Scholar]
- Angéliaume-Descamps, A.; Blot, F.; Leroy, D. Dynamique Récente Des Relations Aux Zones Humides Des Páramos Andins Vénézuéliens: Entre Fonctionnalisme et Mystique. Géocarrefour 2013, 88, 285–298. [Google Scholar] [CrossRef]
- Emeterio, J.L.S.; Alexandre, F.; Andrieu, J.; Génin, A.; Mering, C. Changements Socio-Environnementaux et Dynamiques Des Paysages Ruraux Le Long Du Gradient Bioclimatique Nord-Sud Dans Le Sud-Ouest Du Niger (Régions de Tillabery et de Dosso). VertigO 2013. [Google Scholar] [CrossRef]
- Maughan, N. Nicolas Dynamiques Spatio-Temporelles et Évolution Des Modes de Gestion Des Milieux Humides de l’est de l’étang de Berre (Sud-Est de La France, Xviiie-. Méditerranée 2015, 113–132. [Google Scholar] [CrossRef]
- Secrétariat Convention de Ramsar Le Manuel de La Convention de Ramsar. Guide de La Convention Sur Les Zones Humides (Ramsar, Iran, 1971), 6th ed.; Secrétariat Convention de Ramsar Le Manuel de La Convention de Ramsa: Gland, Switzerland, 2013. [Google Scholar]
- Secretariat CBD. Handbook of the Convention on Biological Diversity Including Its Cartagena Protocol on Biosafety, 3rd ed.; Secretariat CBD: Montreal, QC, Canada, 2005. [Google Scholar]
- MEA Cosystems and Human Wellbeing. A Framework for Assessment; Island Press: Washington DC, USA, 2003. [Google Scholar]
- Gramond, D. Delphine Requalifier Les Zones Humides Continentales: Logiques et Paradoxes. Géocarrefour 2013, 88, 247–256. [Google Scholar] [CrossRef]
- Franchomme, M.; Dubois, J.-J. Documenter Les Zones Humides: Vers Une Meilleure Compréhension Des Paysages d’eau Du XIXe Au XXe s. Géocarrefour 2010, 7–16. [Google Scholar] [CrossRef]
- Abalo, M.; Badabaté, D.; Kperkouma, W.; Fousseni, F.; Koffi, A. Caractérisation phytosociologique des zones humides de la plaine de l’Ogou. Rev. Écosyst. Paysages 2021, 1, 43–57. [Google Scholar]
- Scarwell, H.; Franchomme, M. Autour Des Zones Humides: Espaces Productifs d’hier et Conflits d’aujourd’hui. VertigO 2005. [Google Scholar] [CrossRef]
- Diedhiou, A.; Sambou, A. Serigne Modou SARR Perception des populations sur les services écosystémiques des aires protégées: Cas de l’Aire Marine Protégée d’Abéné, Sénégal. Rev. Écosyst. Paysages 2021, 1, 73–84. [Google Scholar]
- Durand, M. Les Zones Humides Urbaines à Bogota, Conflits d’usage et Patrimonialisation. Géogr. Cult. 2008, 43–59. [Google Scholar] [CrossRef]
- Franchomme, M.; Kergomard, C. Diversité Régionale de La Prise En Compte Des Zones Humides et de Leurs Dynamiques. Dév. Durable Territ. 2006. [Google Scholar] [CrossRef]
- Beaudouin, A. Siècles Cahiers Du Centre d’Histoire Espaces et Cultures; Centre d’Histoire Espaces et Cultures: Paris, France, 2016. [Google Scholar]
- Fousseni, F.; Andrianamenoso, R.M.; Kperkouma, W.; Agbelessessi, W.Y.; Madjouma, K.; Hodabalo, P.; Aniko, P.-A.; Komlan, B.; Koffi, A. Écologie et Dynamique Spatio-Temporelle Des Mangroves Au Togo. VertigO 2017, 6. [Google Scholar] [CrossRef]
- Akodewou, A.; Akpavi, S.; Dourma, M.; Batawila, K.; Amegnaglo, K.; Atakpama, W.; Akpagama, K. Sorindeia Warneckei Engl. (Anacardiaceae), Une Espèce Multi-Usagère de La Dépression de La Lama Au Togo. Afr. Sci. 2014, 10, 450–461. [Google Scholar]
- Nicolet, P.J.; Biggs, G.; Fox, M.J.; Hodson, C.; Reynolds, M.W.; Williams, P. The wetland plant and macroinvertebrate assemblages of temporary ponds in England and Wales. Biol. Conserv. 2004, 120, 261–278. [Google Scholar]
- Gù-Konu, Y.E. Atlas Du Togo; 1981; ISBN 9782852582248. [Google Scholar]
- Moussa, A. Climate Classification Based on Vegetation, Rainfall and Temperature (Togo); University of Lomé: Lomé, Togo, 2008. [Google Scholar]
- Eeou, E.; Takou, P.W.; Boukpessi, T. Analyse des changements par télédétection de la couverture végétale du bassin de Zio (sud-ouest Togo). Rev. Écosyst. Paysages 2022, 2, 126–139. [Google Scholar]
- ISEED-TOGO Recensement Général de La Population et de L’habitat (Du 06 Au 21 Novembre 2010); Résultats Définitifs République Togolaise: Lome, Togo, 2011.
- Gorenflot, R.; De Foucault, B. Biologie Végétale—Les Cormophytes, 7th ed.; Dunod: Malakoff, France, 2005. [Google Scholar]
- Westhoff, V.; van der Maarel, E. The Braun-Blanquet Approach; Whittaker, R.H., Ed.; Ordination and Classification of Communities, Dr. W. Junk: Dordrecht, The Netherlands, 1973; pp. 617–626. [Google Scholar]
- Megharbi, A.; Abdoun, F.; Belgherbi, B. Diversité Floristique En Relation Avec Les Gradients Abiotiques Dans La Zone Humide de La Macta (Ouest d’Algérie). Rev. D’écol. (Terre Vie) 2016, 71, 142–155. [Google Scholar] [CrossRef]
- Delpech, R. Phytosociologie. Available online: https://www.tela-botanica.org/thematiques/phytosociologie/#methodes-phytosociologiques (accessed on 27 August 2018).
- Akoègninou, A.; van der Burg, W.J.; Maesen, L.J.G. Flore Analytique Du Bénin; Université d’Abomey-Calavi & Wageningen: Godomey, Benin; Wageningen Agricultural University: Wageningen, The Netherlands, 2006. [Google Scholar]
- Brunel, J.F.; Hiepko, P.; Scholz, H. Flore Analytique Du Togo: Phanerogames. Englera 1984, 3–751. [Google Scholar] [CrossRef]
- Raunkiær, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: Oxford, UK, 1934. [Google Scholar]
- White, F. La Vegetation de !’Afrique. Recherches Sur Les Ressources Naturelles; ORSTOM-UNESCO: Paris, France, 1986; Volume 20. [Google Scholar]
- Jolliffe, I.T.; Trendafilov, N.T.; Uddin, M. A Modified Principal Component Technique Based on the Lasso. J. Comput. Graph. Stat. 2003, 12, 531–547. [Google Scholar] [CrossRef]
- Dan, B.S.C.; Ahoudji, M.C.; Houinato, M.R.B.; Mensah, G.A.; Sinsin, B.A.; Lejoly, J. Flore de La Forêt Marécageuse de Lokoli à Zogbodomey Au Sud–Bénin. Bull. Rech. Agron. Bénin 2016, 80, 58–77. [Google Scholar]
- Boni, C.C.; Ehouman, E.; Soro, D.; Kone, M.W.; Bakayoko, A.; Dembele, F.; Bauthire, K.; Dosso, M. Etude Comparative de La Flore Aux Abords Des Cours d’eau Dans Les Zones Hypo et Hyper Endémiques d’ulcère de Buruli En Côte d’Ivoire. Int. J. Biol. Chem. Sci. 2017, 11, 1254. [Google Scholar] [CrossRef]
- Ramsar. Wetlands of International Importance. Gland. Ramsar Convention on Wetlands. Available online: https://www.ramsar.org/sites-countries/wetlands-of-international-importance (accessed on 7 December 2020).
- Minkilabe, D. Flore et communautés végétales des mares des carrières de phosphates abandonnées du sud-est du Togo. Rev. Écosyst. Paysages 2022, 2, 212–222. [Google Scholar]
- Nacoulma, B.M.I.; Schumann, K.; Traoré, S.; Bernhardt-Römermann, M.; Hahn, K.; Wittig, R.; Thiombiano, A. Impacts of Land-Use on West African Savanna Vegetation: A Comparison between Protected and Communal Area in Burkina Faso. Biodivers. Conserv. 2011, 20, 3341–3362. [Google Scholar] [CrossRef]
- Natta, A.K.; Sinsin, B.; van der Maesen, L.J.G. Riparian Forests, a Unique but Endangered Ecosystem in Benin. Bot. Jahrbücher 2002, 124, 55–69. [Google Scholar]
- Ali, A.; Idrissa, S.; Saley, K.; Issa, C.; Ali, M.; Mahamane, S. Flore et Végétation Des Parcours Naturels de La Région de Maradi, Niger. J. Anim. Plant Sci. 2017, 34, 5354–5375. [Google Scholar]
- Fousseni, F.; Marra, D.; Wala, K.; Batawila, K.; Zhao, X.; Zhang, C.; Akpagana, K. Basic Overview of Riparian Forest in Sudanian Savanna Ecosystem: Case Study of Togo. Rev. D’ecol. (Terre Vie) 2014, 69, 24–38. [Google Scholar] [CrossRef]
- Bammi, J.; Mouhiddine, M.; Fassi, D.; Douira, A. Contribution à La Connaissance de La Végétation Des Doukkala-Abda (Maroc Atlantique): Approche Éco-Géomorphologique. J. Anim. Plant Sci. 2014, 20, 3202–3211. [Google Scholar]
- Achard, F.; Hiernaux, P.; Banoin, M. Les Jachères Fourragères Naturelles et Améliorées En Afrique de l’Ouest. Lajachère Afr. Trop. 2001, 6, 201–239. [Google Scholar]
- Fournier, A.; Yoni, M.; Zombre, P. Les Jachères à Andropogon Gayanus En Savane Soudanienne Dans l’ouest Du Burkina Faso: Flore, Structure, Déterminants et Fonction Dans l’écosystème. Etudes flor. Vég. Burkina Faso 2000, 5, 3–32. [Google Scholar]
- Fousseni, F.; Yu, Z.C.; Hai, Z.X. Floristic Diversity in Most Dry and Environmentally Disturbed Areas of Northern Togo. Energy 2011, 1, 241–243. [Google Scholar]
- Folega, F.; Zhang, C.Y.; Woegan, Y.A.; Wala, K.; Dourma, M.; Batawila, K.; Seburanga, J.L.; Zhao, X.L.; Akpagana, K. Structure and Ecology of Forest Plant Community in Togo. J. Trop. For. Sci. 2014, 26, 225–239. [Google Scholar]
- Fousseni, F.; Xiuhai, Z.; Chunyu, Z.; Kperkouma, W.; Koffi, A. Ecological and Numerical Analyses of Plant Communities of the Most Conserved Protected Area in North-Togo. Int. J. Biodivers. Conserv. 2010, 2, 359–369. [Google Scholar]
- Ndiaye, S.; Ndiaye, M.; Sambou, A.; Koffi, E.E. Perceptions des populations sur les services écosystémiques de l’aire marine protégée de Kayar à l’ouest du Sénégal. Rev. Écosyst. Paysages 2022, 2, 53–68. [Google Scholar]
- Garchitorena, A.; Guégan, J.-F.; Léger, L.; Eyangoh, S.; Marsollier, L.; Roche, B. Mycobacterium Ulcerans Dynamics in Aquatic Ecosystems Are Driven by a Complex Interplay of Abiotic and Biotic Factors. eLife 2015, 4, e07616. [Google Scholar] [CrossRef]
- Millet, B. Hydrologie et Hydrochimie d’un Milieu Lagunaire Tropical: Le Lac Togo; Universite de Paris SudCentre d’Orsay: Paris, France, 1983. [Google Scholar]
- Marsollier, L.; Brodin, P.; Jackson, M.; Korduláková, J.; Tafelmeyer, P.; Carbonnelle, E.; Aubry, J.; Milon, G.; Legras, P.; André, J.-P.; et al. Impact of Mycobacterium Ulcerans Biofilm on Transmissibility to Ecological Niches and Buruli Ulcer Pathogenesis. PLoS Pathog. 2007, 3, e62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).