Ecological Indicative Stressors of Native vs. Non-Native Fish in an Ultra-Oligotrophic Region of the Mediterranean Sea
Abstract
1. Introduction
2. Materials and Methods
3. Results
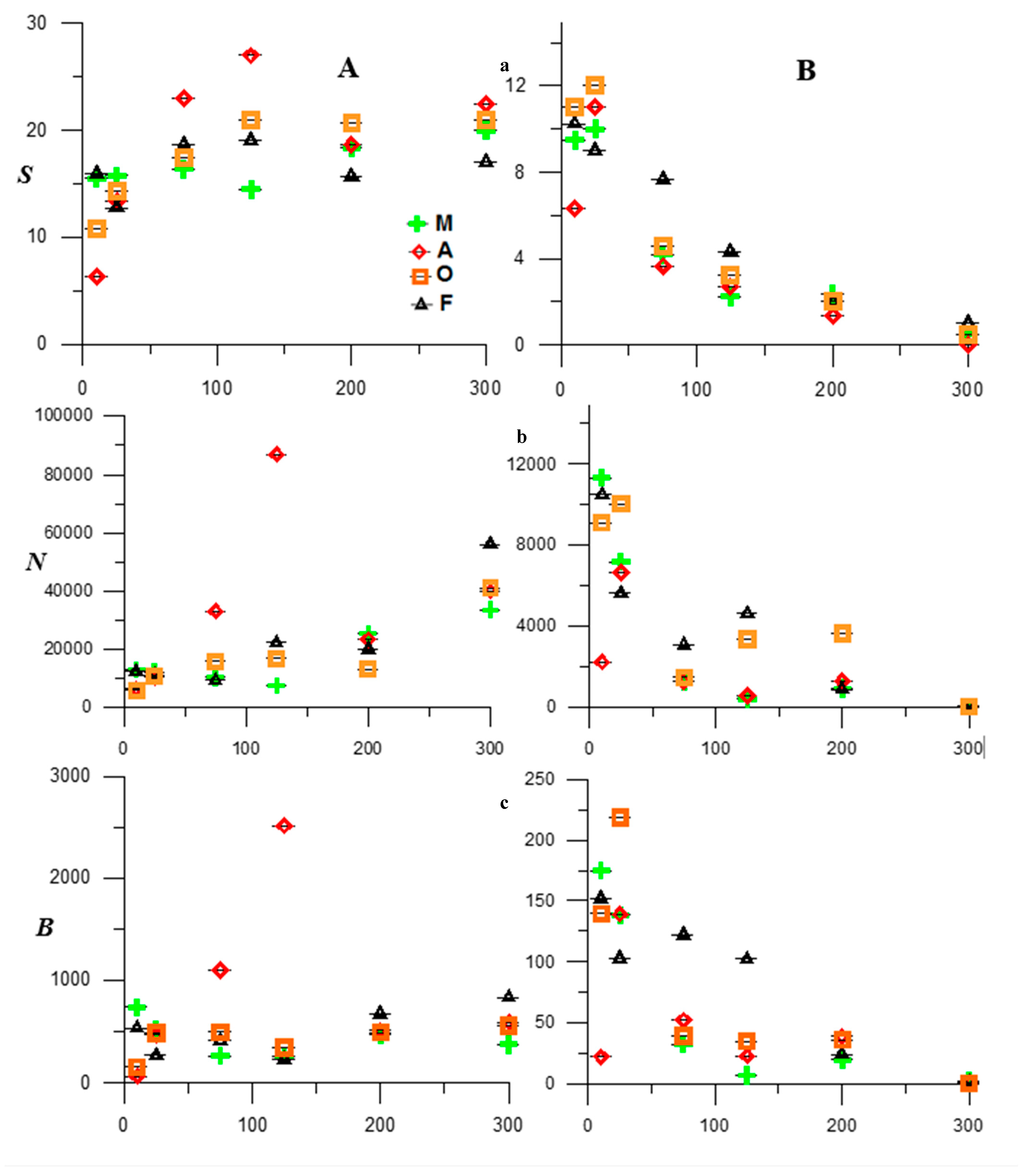
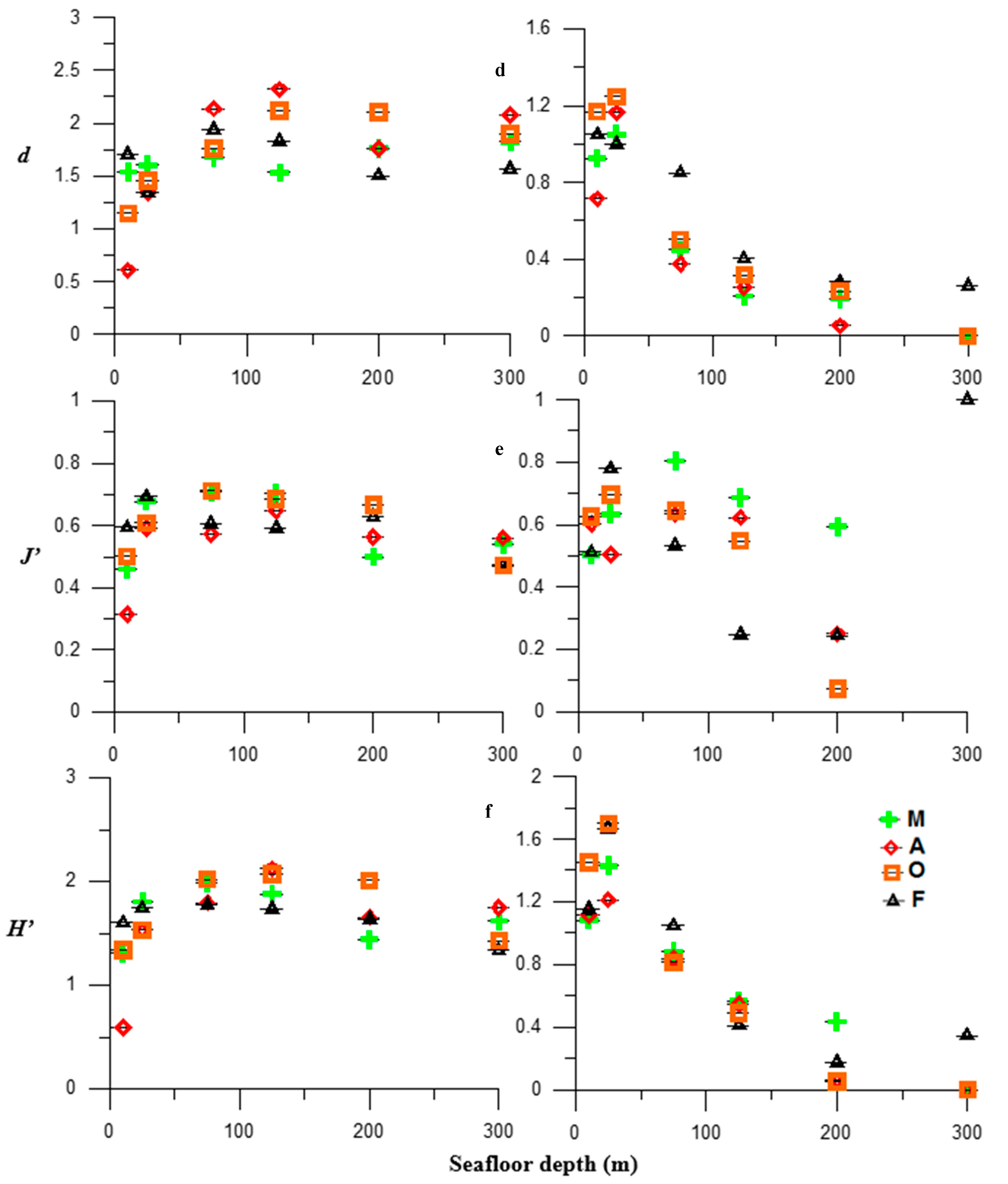
3.1. Faunistic Indicators
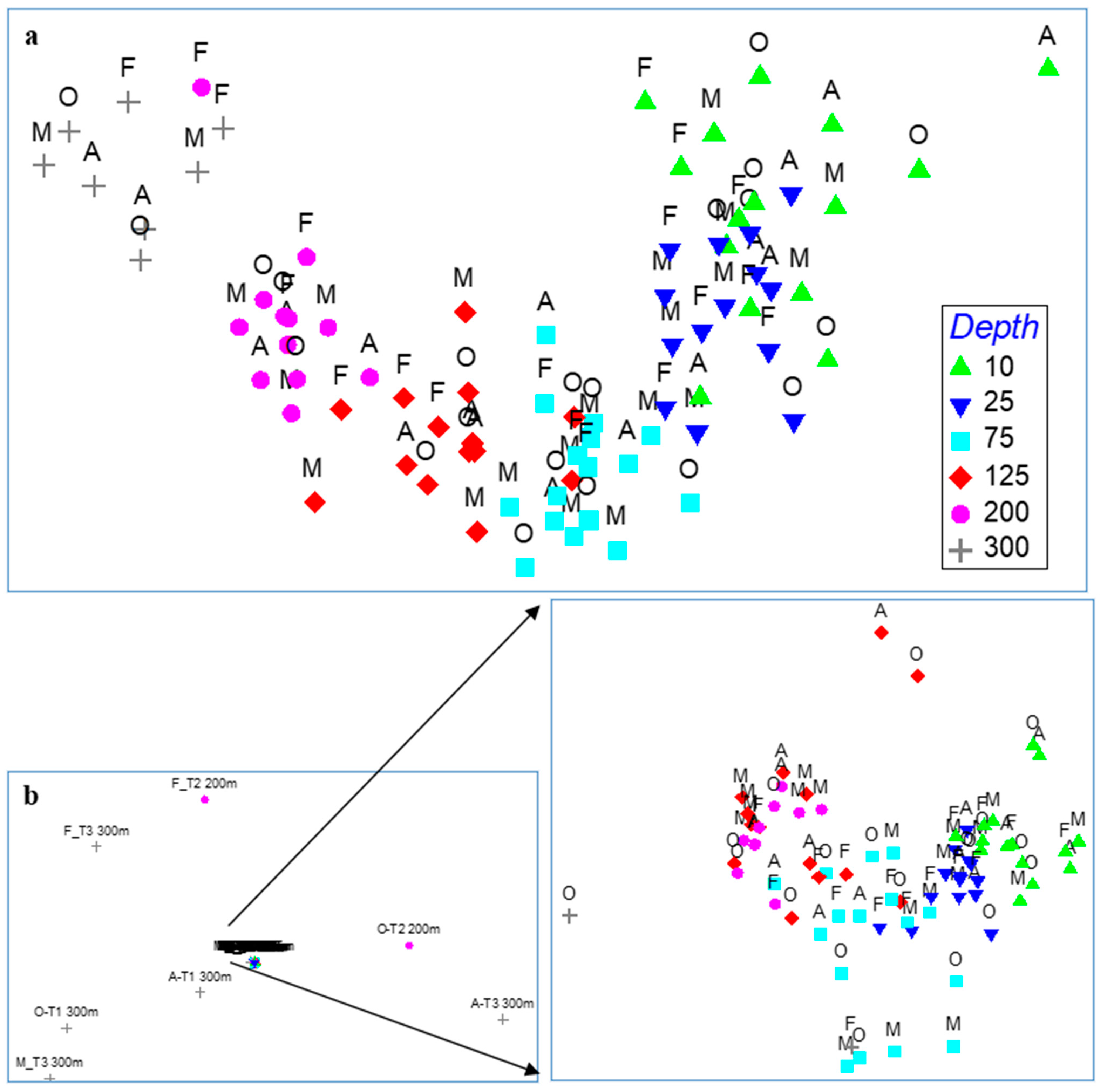
3.2. Fish Assemblages
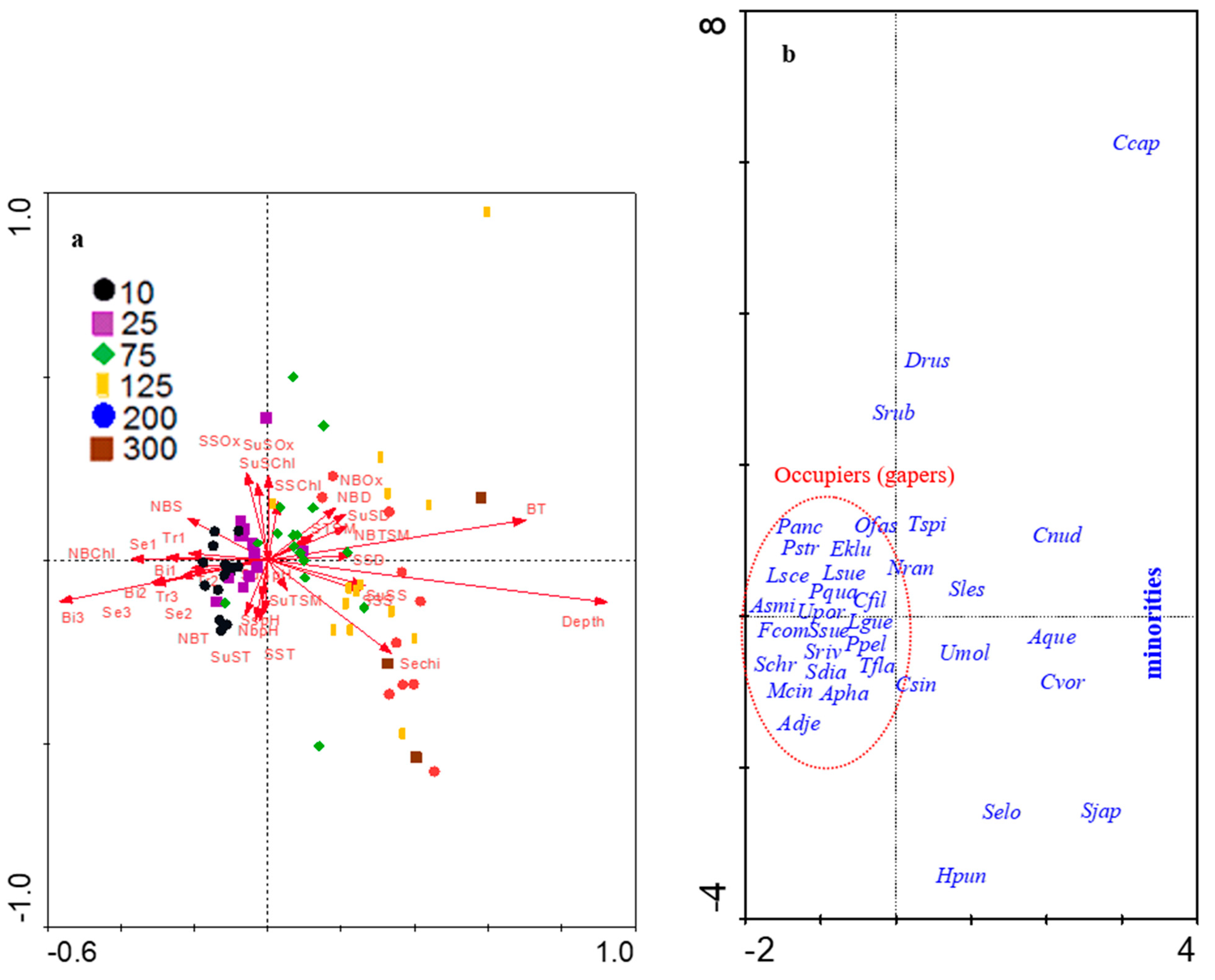
3.3. Fish Assemblage–Environment Relation
3.4. Abundance/Biomass Relation
3.5. Biometric Indicators
3.5.1. Length–Weight Relationship
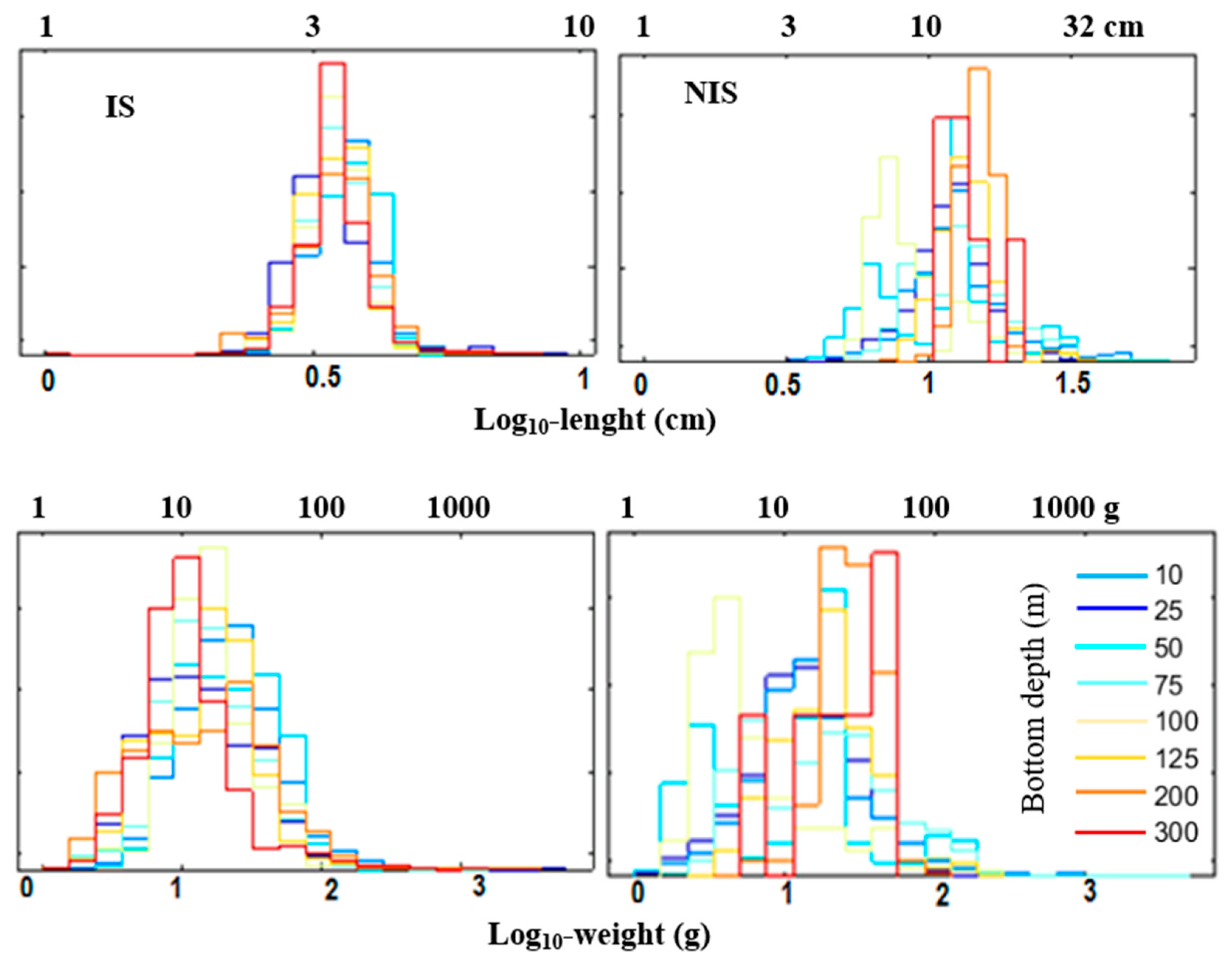
3.5.2. Length and Weight Histograms
4. Discussion
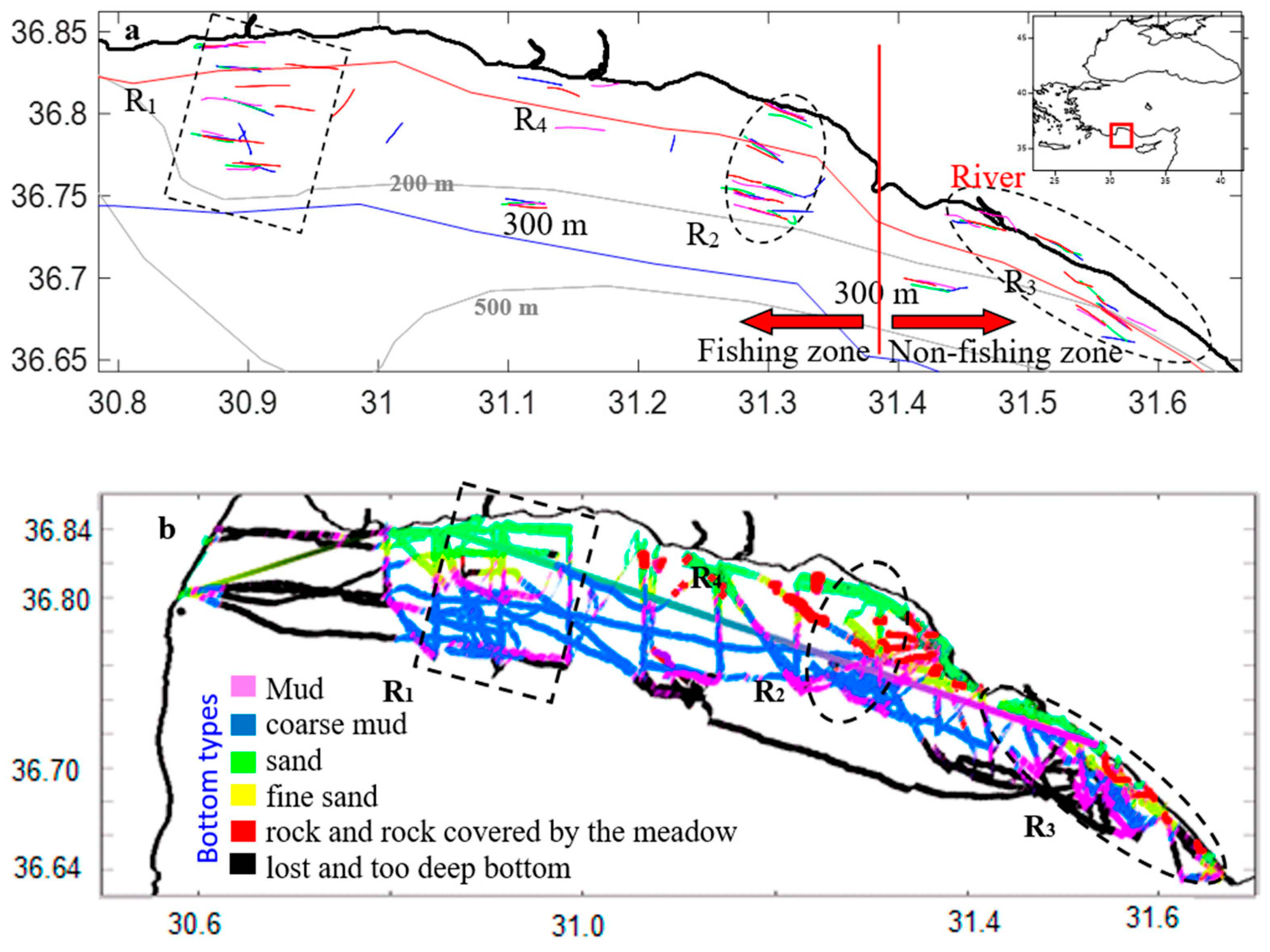
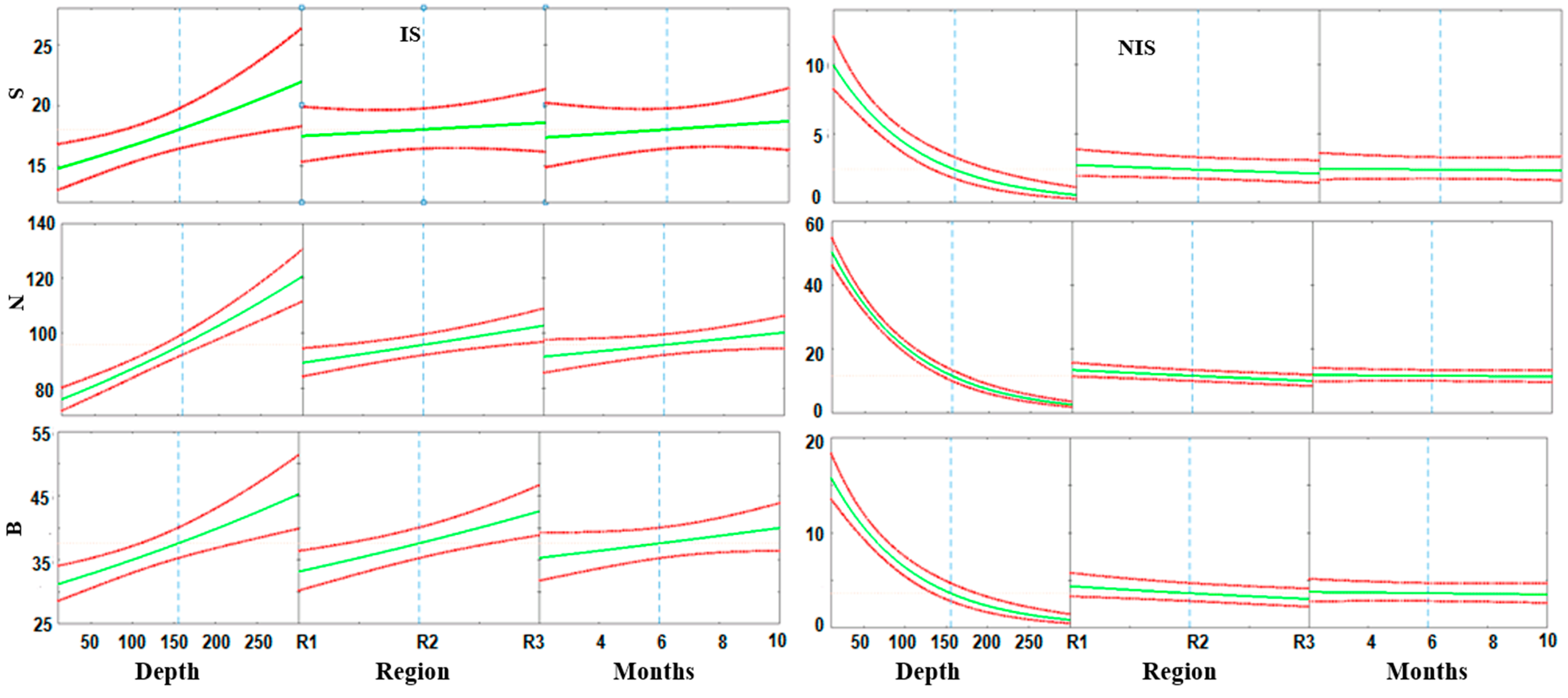
4.1. Bottom Depth
4.2. Bottom Vegetation Status
4.3. Hierarchy
4.4. Key Fish Species
4.5. Water Productivity (Food Web)
4.6. Fish Trophic Level
4.7. Fish Life Strategy
4.8. Fish Morphometrical Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Abb | Origin | Within All Species | Within IS and NIS | Abundance (ind/km2) | Biomass (kg/km2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D% | FO% | NO% | FO% | NO% | Mn | X ± SD | Mx | Depth Range, MxD | Mn | X ± SD | Mx | MxD | |||
| Aetomylaeus bovinus | Abov | A-M | 5.06 | 0.23 | 0.011 | 0.30 | 0.013 | 19 | 2.6 ± 1.6 | 114 | 8.5–13.5, 8.5 | 56.76 | 10.13 ± 6.44 | 472.20 | 8.5 |
| Alosa fallax | Afal | A-M | 1.27 | 0.06 | 0.001 | 0.07 | 0.002 | 26 | 0.3 ± 0.3 | 26 | 11–11, 11 | 0.45 | 0.01 ± 0.01 | 0.45 | |
| Anthias anthias | Aant | A-M | 6.33 | 0.28 | 0.070 | 0.37 | 0.084 | 7 | 16.7 ± 9.7 | 594 | 77.7–128.8, 128.8 | 0.07 | 0.18 ± 0.10 | 5.71 | 128.8 |
| Argentina sphyraena | Asph | A-M | 31.65 | 1.41 | 5.422 | 1.87 | 6.489 | 299 | 1288.2 ± 329.2 | 16348 | 115–299, 193.1 | 2.17 | 11.08 ± 2.66 | 111.34 | 186.3 |
| Arnoglossus imperialis | Aimp | A-M | 5.06 | 0.23 | 0.004 | 0.30 | 0.005 | 13 | 0.9 ± 0.5 | 24 | 76.5–287.3, 77.7 | 0.09 | 0.01 ± 0.00 | 0.16 | 77.7 |
| Arnoglossus laterna | Alat | A-M | 29.11 | 1.30 | 0.126 | 1.72 | 0.151 | 17 | 30.0 ± 7.3 | 317 | 11.5–193.1, 35 | 0.15 | 0.23 ± 0.07 | 3.02 | 84.2 |
| Arnoglossus rueppelii | Arue | A-M | 20.25 | 0.90 | 0.109 | 1.19 | 0.130 | 22 | 25.8 ± 7.9 | 458 | 84.6–299, 299 | 0.02 | 0.10 ± 0.03 | 1.72 | 286.4 |
| Arnoglossus thori | Atho | A-M | 18.99 | 0.85 | 0.341 | 1.12 | 0.409 | 26 | 81.1 ± 26.7 | 1222 | 35–130, 74 | 0.23 | 0.56 ± 0.19 | 9.78 | 74 |
| Balistes capriscus | Bcap | C | 11.39 | 0.51 | 0.015 | 0.67 | 0.018 | 13 | 3.5 ± 1.3 | 77 | 8.5–25.7, 12.1 | 0.27 | 2.46 ± 2.10 | 166.01 | 11.2 |
| Blennius ocellaris | Boce | A-M | 24.05 | 1.07 | 0.131 | 1.42 | 0.157 | 22 | 31.1 ± 13.7 | 922 | 67–137, 111.9 | 0.15 | 0.48 ± 0.22 | 13.02 | 111.9 |
| Boops boops | Bboo | A-M | 63.29 | 2.82 | 3.329 | 3.73 | 3.984 | 16 | 791.0 ± 165.9 | 6836 | 8.5–286.4, 111.9 | 0.21 | 19.68 ± 4.16 | 200.75 | 111.9 |
| Bothus podas | Bpod | A-M | 43.04 | 1.92 | 4.035 | 2.54 | 4.829 | 26 | 958.7 ± 230.3 | 10910 | 8.5–289.5, 8.5 | 0.34 | 11.41 ± 3.10 | 194.59 | 8.5 |
| Callionymus maculatus | Cmac | A-M | 3.80 | 0.17 | 0.005 | 0.22 | 0.006 | 20 | 1.2 ± 0.7 | 38 | 115–194, 115 | 0.06 | <0.01 ± 0.00 | 0.16 | 115 |
| Capros aper | Aape | A-M | 22.78 | 1.01 | 1.065 | 1.34 | 1.275 | 20 | 253.0 ± 137.5 | 10589 | 127–299, 265.1 | 0.01 | 1.82 ± 1.20 | 93.73 | 265.1 |
| Caranx crysos | Ccry | A-M | 2.53 | 0.11 | 0.011 | 0.15 | 0.014 | 65 | 2.7 ± 2.0 | 148 | 11–11.2, 11.2 | 3.10 | 0.11 ± 0.08 | 5.38 | 11.2 |
| Carapus acus | Cacu | M | 2.53 | 0.11 | 0.002 | 0.15 | 0.003 | 19 | 0.5 ± 0.4 | 23 | 125–133.9, 125 | 0.02 | <0.01 ± 0.00 | 0.02 | 133.9 |
| Carcharhinus plumbeus | Cplu | M | 1.27 | 0.06 | 0.001 | 0.07 | 0.002 | 24 | 0.3 ± 0.3 | 24 | 74–74, 74 | 36.67 | 0.46 ± 0.46 | 36.67 | 74 |
| Centracanthus cirrus | Ccir | A-M | 11.39 | 0.51 | 0.878 | 0.67 | 1.050 | 24 | 208.5 ± 86.7 | 4101 | 117.7–193.1, 141.1 | 0.16 | 1.57 ± 0.69 | 41.39 | 141.1 |
| Cepola macrophtalma | Cmac | A-M | 3.80 | 0.17 | 0.007 | 0.22 | 0.008 | 19 | 1.6 ± 1.0 | 65 | 84.6–133.9, 87 | 0.09 | 0.01 ± 0.00 | 0.21 | 87 |
| Chelidonichthys cuculus | Ccuc | A-M | 7.59 | 0.34 | 0.112 | 0.45 | 0.134 | 16 | 26.5 ± 22.7 | 1792 | 125–299, 299 | 0.26 | 0.55 ± 0.44 | 34.17 | 299 |
| Chelidonichthys lucerna | Cluc | A-M | 17.72 | 0.79 | 0.048 | 1.04 | 0.057 | 13 | 11.4 ± 3.9 | 211 | 8.5–193.1, 12.1 | 0.36 | 0.39 ± 0.15 | 9.96 | 12.1 |
| Chlorophthalmus agassizi | Caga | C | 17.72 | 0.79 | 9.011 | 1.04 | 10.783 | 25 | 2140.8 ± 1216.0 | 85848 | 141.1–299, 290 | 0.02 | 20.45 ± 12.04 | 870.97 | 290 |
| Citharus linguatula | Clin | A-M | 64.56 | 2.87 | 3.049 | 3.81 | 3.648 | 17 | 724.3 ± 288.6 | 21866 | 10.5–289.5, 111.9 | 0.18 | 15.58 ± 6.09 | 455.76 | 111.9 |
| Coelorinchus caelorhincus | Ccae | A-M | 6.33 | 0.28 | 2.284 | 0.37 | 2.734 | 865 | 542.7 ± 319.2 | 18189 | 258.3–299, 299 | 5.09 | 4.48 ± 2.81 | 196.69 | 299 |
| Conger conger | Ccon | A-M | 12.66 | 0.56 | 0.012 | 0.75 | 0.015 | 16 | 3.0 ± 0.9 | 46 | 23.1–298.6, 298.6 | 0.02 | 0.34 ± 0.17 | 12.00 | 298.6 |
| Dactylopterus volitans | Dvol | C | 7.59 | 0.34 | 0.009 | 0.45 | 0.010 | 16 | 2.0 ± 0.8 | 39 | 11–117.7, 11 | 0.32 | 0.07 ± 0.04 | 2.73 | 94 |
| Dasyatis centroura | Dcen | A-M | 1.27 | 0.06 | 0.003 | 0.07 | 0.003 | 49 | 0.6 ± 0.6 | 49 | 186.3–186.3, 186.3 | 200.05 | 2.53 ± 2.53 | 200.05 | 186.3 |
| Dasyatis pastinaca | Dpas | A-M | 32.91 | 1.46 | 0.115 | 1.94 | 0.137 | 13 | 27.3 ± 7.3 | 478 | 8.5–141.1, 11 | 18.11 | 40.88 ± 9.63 | 450.06 | 11 |
| Deltentosteus quadrimaculatus | Dqua | A-M | 18.99 | 0.85 | 0.105 | 1.12 | 0.126 | 13 | 25.0 ± 10.5 | 726 | 12–286.4, 130.6 | 0.01 | 0.04 ± 0.02 | 1.01 | 130.6 |
| Dentex dentex | Dden | A-M | 2.53 | 0.11 | 0.002 | 0.15 | 0.003 | 13 | 0.5 ± 0.4 | 26 | 35–76.5, 35 | 25.38 | 0.92 ± 0.68 | 47.55 | 35 |
| Dentex macrophthalmus | Dmac | A-M | 7.59 | 0.34 | 0.184 | 0.45 | 0.220 | 35 | 43.7 ± 25.2 | 1656 | 74–163.3, 117.7 | 1.07 | 0.64 ± 0.34 | 19.56 | 74 |
| Dentex maroccanus | Dmar | A-M | 27.85 | 1.24 | 6.232 | 1.64 | 7.457 | 20 | 1480.5 ± 756.3 | 56264 | 78.3–287.3, 111.9 | 0.58 | 45.63 ± 20.76 | 1351.00 | 111.9 |
| Dicologlossa cuneata | Dcun | A-M | 3.80 | 0.17 | 0.009 | 0.22 | 0.011 | 43 | 2.2 ± 1.3 | 87 | 11.5–29.4, 11.7 | 0.25 | 0.02 ± 0.01 | 0.95 | 11.5 |
| Diplodus annularis | Dann | A-M | 26.58 | 1.18 | 1.790 | 1.57 | 2.142 | 13 | 425.3 ± 209.9 | 15267 | 8.5–121.8, 11.2 | 0.31 | 10.28 ± 4.51 | 308.30 | 11.2 |
| Diplodus vulgaris | Dvul | A-M | 1.27 | 0.06 | 0.008 | 0.07 | 0.009 | 148 | 1.9 ± 1.9 | 148 | 13.5–13.5, 13.5 | 7.40 | 0.09 ± 0.09 | 7.40 | 13.5 |
| Dipturus oxyrinchus | Doxy | A-M | 6.33 | 0.28 | 0.020 | 0.37 | 0.024 | 23 | 4.8 ± 2.7 | 193 | 193.1–287.3, 286.4 | 2.28 | 5.17 ± 3.82 | 290.00 | 286.4 |
| Echeneis naucrates | Enau | C | 1.27 | 0.06 | 0.001 | 0.07 | 0.001 | 22 | 0.3 ± 0.3 | 22 | 11.7–11.7, 11.7 | 21.83 | 0.28 ± 0.28 | 21.83 | 11.7 |
| Engraulis encrasicolus | Eenc | A-M | 7.59 | 0.34 | 0.066 | 0.45 | 0.078 | 26 | 15.6 ± 9.2 | 631 | 35–193.1, 73.7 | 0.13 | 0.24 ± 0.15 | 9.21 | 73.7 |
| Epinephelus aeneus | Eaen | A-M | 27.85 | 1.24 | 0.217 | 1.64 | 0.260 | 15 | 51.6 ± 15.7 | 934 | 11.2–84.2, 25.7 | 3.89 | 5.85 ± 1.55 | 80.65 | 25 |
| Epinephelus haifensis | Ehai | A-M | 1.27 | 0.06 | 0.004 | 0.07 | 0.005 | 77 | 1.0 ± 1.0 | 77 | 77–77, 77 | 0.93 | 0.01 ± 0.01 | 0.93 | 77 |
| Etrumeus teres | Eter | A-M | 3.80 | 0.17 | 0.006 | 0.22 | 0.007 | 19 | 1.4 ± 0.8 | 46 | 8.5–125, 125 | 0.38 | 0.02 ± 0.01 | 0.56 | 121.8 |
| Gadiculus argenteus | Garg | A-M | 1.27 | 0.06 | 0.002 | 0.07 | 0.003 | 43 | 0.5 ± 0.5 | 43 | 258.3–258.3, 258.3 | 0.22 | <0.01 ± 0.00 | 0.22 | 258.3 |
| Glossanodon leioglossus | Glei | A-M | 22.78 | 1.01 | 6.199 | 1.34 | 7.418 | 17 | 1472.8 ± 626.5 | 33887 | 127–299, 130 | 0.04 | 10.12 ± 4.19 | 213.07 | 194 |
| Gnathophis mystax | Gmys | A-M | 8.86 | 0.39 | 0.017 | 0.52 | 0.020 | 22 | 4.0 ± 1.6 | 64 | 25.7–299, 137 | 0.27 | 0.40 ± 0.18 | 9.67 | 121.8 |
| Gobius geniporus | Ggen | M | 1.27 | 0.06 | 0.001 | 0.07 | 0.001 | 15 | 0.2 ± 0.2 | 15 | 57–57, 57 | 0.11 | <0.01 ± 0.00 | 0.11 | 57 |
| Gobius niger | Gnig | A-M | 2.53 | 0.11 | 0.003 | 0.15 | 0.004 | 19 | 0.8 ± 0.6 | 41 | 13.5–25, 25 | 0.17 | 0.01 ± 0.01 | 0.46 | 25 |
| Gymnura altavela | Galt | A-M | 13.92 | 0.62 | 0.038 | 0.82 | 0.045 | 19 | 8.9 ± 3.7 | 248 | 8.5–121.8, 8.5 | 53.67 | 35.42 ± 14.75 | 835.50 | 8.5 |
| Helicolenus dactylopterus | Hdac | A-M | 13.92 | 0.62 | 0.371 | 0.82 | 0.444 | 21 | 88.2 ± 45.5 | 3084 | 74–299, 299 | 0.12 | 0.89 ± 0.42 | 26.04 | 299 |
| Hoplostethus mediterraneus | Hmed | C | 2.53 | 0.11 | 0.007 | 0.15 | 0.008 | 22 | 1.6 ± 1.4 | 108 | 258.3–290, 290 | 0.22 | 0.01 ± 0.01 | 0.63 | 290 |
| Hymenocephalus italicus | Hita | A-M | 5.06 | 0.23 | 0.402 | 0.30 | 0.481 | 42 | 95.4 ± 69.3 | 4945 | 258.3–299, 258.3 | 0.25 | 0.28 ± 0.21 | 15.05 | 258.3 |
| Lepidopus caudatus | Lcau | A-M | 1.27 | 0.06 | 0.001 | 0.07 | 0.001 | 22 | 0.3 ± 0.3 | 22 | 258.3–258.3, 258.3 | 0.75 | 0.01 ± 0.01 | 0.75 | 258.3 |
| Lepidorhombus whiffiagonis | Lwhi | A-M | 12.66 | 0.56 | 0.089 | 0.75 | 0.106 | 21 | 21.1 ± 8.1 | 485 | 186.3–299, 298.6 | 0.25 | 3.41 ± 1.79 | 131.73 | 298.6 |
| Lepidotrigla cavillone | Lcav | A-M | 44.30 | 1.97 | 2.895 | 2.61 | 3.464 | 18 | 687.8 ± 281.8 | 16060 | 12.2–289.5, 111.9 | 0.02 | 8.45 ± 3.65 | 217.03 | 111.9 |
| Lepidotrigla dieuzeidei | Ldie | A-M | 16.46 | 0.73 | 0.727 | 0.97 | 0.870 | 41 | 172.6 ± 73.9 | 4737 | 141.1–298.6, 265.1 | 0.16 | 3.12 ± 1.57 | 108.93 | 265.1 |
| Lithognathus mormyrus | Lmor | A-M | 25.32 | 1.13 | 1.063 | 1.49 | 1.272 | 19 | 252.6 ± 86.3 | 4433 | 8.5–84.6, 11 | 1.00 | 9.68 ± 3.16 | 156.14 | 25.7 |
| Liza saliens | Lsal | A-M | 2.53 | 0.11 | 0.011 | 0.15 | 0.013 | 39 | 2.6 ± 2.2 | 167 | 11–13.5, 13.5 | 2.32 | 0.28 ± 0.25 | 19.99 | 13.5 |
| Lophius budegassa | Lbud | A-M | 13.92 | 0.62 | 0.022 | 0.82 | 0.027 | 21 | 5.3 ± 1.8 | 108 | 111.9–299, 258.3 | 0.20 | 3.89 ± 1.55 | 66.69 | 195 |
| Macroramphosus scolopax | Msco | A-M | 41.77 | 1.86 | 3.082 | 2.46 | 3.688 | 20 | 732.2 ± 183.9 | 9441 | 10.7–286.4, 111.9 | 0.07 | 3.00 ± 0.76 | 43.41 | 111.9 |
| Merluccius merluccius | Mmer | A-M | 35.44 | 1.58 | 0.308 | 2.09 | 0.368 | 13 | 73.1 ± 15.6 | 624 | 78.3–299, 258.3 | 0.73 | 6.61 ± 1.52 | 86.00 | 258.3 |
| Microchirus ocellatus | Moce | A-M | 16.46 | 0.73 | 0.077 | 0.97 | 0.092 | 21 | 18.3 ± 8.2 | 539 | 10.7–137, 121.8 | 0.46 | 0.49 ± 0.26 | 19.60 | 121.8 |
| Microchirus variegatus | Mvar | A-M | 1.27 | 0.06 | 0.001 | 0.07 | 0.002 | 25 | 0.3 ± 0.3 | 25 | 84.2–84.2, 84.2 | 0.17 | <0.01 ± 0.00 | 0.17 | 84.2 |
| Mullus barbatus | Mbar | A-M | 82.28 | 3.66 | 11.392 | 4.85 | 13.632 | 18 | 2706.4 ± 540.1 | 28673 | 8.5–197.1, 74 | 0.28 | 73.34 ± 14.29 | 598.20 | 84.6 |
| Mullus surmuletus | Msur | A-M | 17.72 | 0.79 | 0.076 | 1.04 | 0.091 | 16 | 18.2 ± 7.5 | 441 | 23.1–286.4, 121.8 | 0.45 | 1.01 ± 0.40 | 24.01 | 121.8 |
| Nettastoma melanurum | Nmel | A-M | 8.86 | 0.39 | 0.033 | 0.52 | 0.040 | 16 | 7.9 ± 5.7 | 441 | 81–299, 82.3 | 0.03 | 0.03 ± 0.02 | 1.89 | 82.3 |
| Pagellus acarne | Paca | A-M | 43.04 | 1.92 | 3.104 | 2.54 | 3.715 | 13 | 737.5 ± 262.6 | 14627 | 8.5–289.5, 84.6 | 0.24 | 19.44 ± 8.29 | 535.43 | 84.2 |
| Pagellus erythrinus | Pery | A-M | 67.09 | 2.99 | 3.781 | 3.96 | 4.525 | 13 | 898.3 ± 216.6 | 12533 | 8.5–298.6, 111.9 | 0.60 | 40.37 ± 11.05 | 737.89 | 111.9 |
| Pagrus auriga | Paur | A-M | 2.53 | 0.11 | 0.002 | 0.15 | 0.002 | 19 | 0.5 ± 0.3 | 19 | 8.5–13.5, 8.5 | 0.21 | 0.01 ± 0.00 | 0.32 | 8.5 |
| Pagrus bogaraveo | Pbog | A-M | 1.27 | 0.06 | 0.009 | 0.07 | 0.011 | 168 | 2.1 ± 2.1 | 168 | 77.7–77.7, 77.7 | 1.42 | 0.02 ± 0.02 | 1.42 | 77.7 |
| Pagrus caeruleostictus | Pcae | A-M | 22.78 | 1.01 | 0.227 | 1.34 | 0.272 | 18 | 54.0 ± 20.7 | 1407 | 8.5–31.6, 13.5 | 0.16 | 3.19 ± 1.05 | 52.52 | 25.7 |
| Pagrus pagrus | Ppag | A-M | 18.99 | 0.85 | 0.349 | 1.12 | 0.418 | 18 | 82.9 ± 58.2 | 4579 | 11–289.5, 128.8 | 0.04 | 6.10 ± 4.78 | 376.47 | 128.8 |
| Peristedion cataphractum | Pcat | A-M | 8.86 | 0.39 | 0.331 | 0.52 | 0.396 | 22 | 78.6 ± 58.0 | 4529 | 258.3–299, 287.3 | 0.22 | 0.72 ± 0.54 | 42.07 | 287.3 |
| Phycis blennoides | Pble | A-M | 2.53 | 0.11 | 0.003 | 0.15 | 0.004 | 22 | 0.8 ± 0.6 | 42 | 258.3–299, 299 | 1.29 | 0.07 ± 0.06 | 4.17 | 299 |
| Pomadasys incisus | Pinc | A-M | 3.80 | 0.17 | 0.007 | 0.22 | 0.009 | 30 | 1.7 ± 1.0 | 56 | 11.2–21.9, 13.5 | 2.48 | 0.30 ± 0.23 | 17.79 | 11.2 |
| Raja asterias | Rast | A-M | 1.27 | 0.06 | 0.003 | 0.07 | 0.004 | 57 | 0.7 ± 0.7 | 57 | 115–115, 115 | 64.68 | 0.82 ± 0.82 | 64.68 | 115 |
| Raja clavata | Rcla | A-M | 25.32 | 1.13 | 0.191 | 1.49 | 0.228 | 19 | 45.4 ± 22.6 | 1736 | 11–299, 111.9 | 0.07 | 14.53 ± 5.19 | 366.78 | 111.9 |
| Raja miraletus | Rmir | A-M | 25.32 | 1.13 | 0.098 | 1.49 | 0.117 | 16 | 23.3 ± 6.4 | 254 | 73.7–298.6, 128.8 | 1.01 | 11.00 ± 3.77 | 260.60 | 195 |
| Rhinobatos rhinobatos | Rrhi | A-M | 1.27 | 0.06 | 0.001 | 0.07 | 0.001 | 16 | 0.2 ± 0.2 | 16 | 23.1–23.1, 23.1 | 132.43 | 1.68 ± 1.68 | 132.43 | 23.1 |
| Sardina pilchardus | Spil | A-M | 10.13 | 0.45 | 0.150 | 0.60 | 0.179 | 21 | 35.6 ± 21.6 | 1641 | 25.7–163.3, 73.7 | 0.55 | 0.39 ± 0.19 | 13.54 | 73.7 |
| Sardinella aurita | Saur | A-M | 6.33 | 0.28 | 0.011 | 0.37 | 0.014 | 21 | 2.7 ± 1.3 | 78 | 11–25.7, 25.7 | 0.15 | 0.11 ± 0.08 | 5.84 | 25.7 |
| Sardinella maderensis | Smad | A-M | 1.27 | 0.06 | 0.001 | 0.07 | 0.002 | 26 | 0.3 ± 0.3 | 26 | 11–11, 11 | 0.26 | <0.01 ± 0.00 | 0.26 | |
| Scorpaena elongata | Selo | A-M | 11.39 | 0.51 | 0.070 | 0.67 | 0.084 | 22 | 16.6 ± 7.9 | 550 | 35–298.6, 35 | 0.42 | 1.48 ± 0.96 | 73.96 | 35 |
| Scorpaena porcus | Spor | A-M | 5.06 | 0.23 | 0.019 | 0.30 | 0.023 | 22 | 4.5 ± 2.8 | 160 | 82.3–141.1, 82.3 | 0.36 | 0.04 ± 0.02 | 1.39 | 82.3 |
| Scorpaena scrofa | Sscr | A-M | 8.86 | 0.39 | 0.028 | 0.52 | 0.033 | 13 | 6.6 ± 3.2 | 163 | 35–299, 111.9 | 0.06 | 0.41 ± 0.20 | 10.00 | 111.9 |
| Scorpana notata | Snot | A-M | 6.33 | 0.28 | 0.050 | 0.37 | 0.060 | 74 | 11.9 ± 5.5 | 252 | 35–195, 82.3 | 1.46 | 0.31 ± 0.23 | 18.03 | 111.9 |
| Scyliorhinus canicula | Scan | A-M | 11.39 | 0.51 | 0.084 | 0.67 | 0.101 | 20 | 20.0 ± 9.0 | 485 | 165.8–299, 298.6 | 0.38 | 1.69 ± 0.85 | 48.46 | 298.6 |
| Serranus cabrilla | Scab | A-M | 31.65 | 1.41 | 0.661 | 1.87 | 0.791 | 19 | 157.1 ± 50.2 | 2932 | 10.7–133.9, 35 | 0.19 | 4.69 ± 1.53 | 74.87 | 77 |
| Serranus hepatus | Shep | A-M | 46.84 | 2.08 | 0.968 | 2.76 | 1.159 | 13 | 230.0 ± 44.2 | 1576 | 22.7–194, 25 | 0.09 | 1.93 ± 0.37 | 16.38 | 25 |
| Serranus scriba | Sscr | A-M | 1.27 | 0.06 | 0.001 | 0.07 | 0.001 | 19 | 0.2 ± 0.2 | 19 | 13.5–13.5, 13.5 | 1.14 | 0.01 ± 0.01 | 1.14 | 13.5 |
| Solea senegalensis | Ssen | A | 1.27 | 0.06 | 0.002 | 0.07 | 0.002 | 39 | 0.5 ± 0.5 | 39 | 10.5–10.5, 10.5 | 0.19 | <0.01 ± 0.00 | 0.19 | 10.5 |
| Solea vulgaris | Svul | A-M | 18.99 | 0.85 | 0.041 | 1.12 | 0.049 | 13 | 9.7 ± 2.8 | 125 | 11–130.6, 23.1 | 0.26 | 1.71 ± 0.58 | 25.48 | 25.7 |
| Sparisoma cretense | Scre | A-M | 1.27 | 0.06 | 0.003 | 0.07 | 0.004 | 57 | 0.7 ± 0.7 | 57 | 8.5–8.5, 8.5 | 1.33 | 0.02 ± 0.02 | 1.33 | 8.5 |
| Sparus aurata | Saura | A-M | 2.53 | 0.11 | 0.003 | 0.15 | 0.003 | 13 | 0.6 ± 0.5 | 38 | 8.5–11, 8.5 | 1.09 | 0.07 ± 0.05 | 4.19 | 8.5 |
| Sphoeroides pachygaster | Spac | C | 2.53 | 0.11 | 0.007 | 0.15 | 0.009 | 20 | 1.8 ± 1.5 | 120 | 165.8–189, 189 | 15.94 | 0.57 ± 0.41 | 28.69 | 189 |
| Sphyraena sphyraena | Ssph | A-M | 3.80 | 0.17 | 0.005 | 0.22 | 0.006 | 19 | 1.2 ± 0.8 | 59 | 11.2–25, 11.2 | 1.24 | 0.09 ± 0.05 | 3.56 | 11.2 |
| Sphyraena viridensis | Svir | A-M | 5.06 | 0.23 | 0.005 | 0.30 | 0.006 | 13 | 1.2 ± 0.6 | 41 | 25.7–78.3, 25.7 | 1.19 | 0.10 ± 0.05 | 3.39 | 25.7 |
| Spicara maena | Smae | A-M | 39.24 | 1.75 | 0.638 | 2.31 | 0.763 | 15 | 151.5 ± 38.2 | 1948 | 11.2–298.6, 133.9 | 0.36 | 6.10 ± 2.37 | 171.56 | 111.9 |
| Spicara smaris | Ssma | A-M | 60.76 | 2.70 | 5.506 | 3.58 | 6.588 | 21 | 1308.0 ± 287.2 | 12000 | 10.7–298.6, 111.9 | 0.09 | 24.39 ± 6.98 | 479.52 | 111.9 |
| Squatina oculata | Socu | A-M | 3.80 | 0.17 | 0.009 | 0.22 | 0.011 | 25 | 2.1 ± 1.4 | 102 | 12.1–189.4, 189.4 | 12.26 | 2.64 ± 1.76 | 107.23 | 189.4 |
| Squatina squatina | Ssqu | A-M | 3.80 | 0.17 | 0.009 | 0.22 | 0.011 | 22 | 2.2 ± 1.4 | 92 | 82.3–141.1, 141.1 | 15.64 | 5.20 ± 3.65 | 252.17 | 82.3 |
| Synchiropus phaeton | Spha | A-M | 12.66 | 0.56 | 0.331 | 0.75 | 0.396 | 20 | 78.6 ± 36.3 | 2125 | 141.1–299, 299 | 0.02 | 0.45 ± 0.23 | 14.58 | 299 |
| Synodus saurus | Ssau | A-M | 21.52 | 0.96 | 0.091 | 1.27 | 0.108 | 13 | 21.5 ± 7.5 | 391 | 11.5–130, 74 | 0.32 | 1.23 ± 0.43 | 18.23 | 128.8 |
| Torpedo marmorata | Tmar | A-M | 1.27 | 0.06 | 0.001 | 0.07 | 0.001 | 19 | 0.2 ± 0.2 | 19 | 25.7–25.7, 25.7 | 0.58 | 0.01 ± 0.01 | 0.58 | 25.7 |
| Trachinus draco | Tdra | A-M | 3.80 | 0.17 | 0.004 | 0.22 | 0.005 | 24 | 1.0 ± 0.6 | 32 | 121.8–137, 137 | 0.85 | 0.03 ± 0.02 | 0.91 | 130 |
| Trachurus mediterraneus | Tmed | A-M | 34.18 | 1.52 | 0.260 | 2.01 | 0.311 | 13 | 61.8 ± 27.4 | 1953 | 8.5–287.3, 111.9 | 0.09 | 1.82 ± 0.68 | 43.41 | 111.9 |
| Trachurus trachurus | Ttra | A-M | 20.25 | 0.90 | 0.151 | 1.19 | 0.181 | 16 | 35.8 ± 13.6 | 922 | 81–286.4, 133.9 | 0.08 | 0.65 ± 0.26 | 16.61 | 189 |
| Trichiurus lepturus | Tlep | C | 8.86 | 0.39 | 0.058 | 0.52 | 0.070 | 19 | 13.9 ± 8.0 | 574 | 11–163.3, 13.5 | 0.09 | 0.58 ± 0.39 | 29.61 | 13.5 |
| Trigla lyra | Tlyr | A-M | 5.06 | 0.23 | 0.146 | 0.30 | 0.175 | 19 | 34.7 ± 26.8 | 2064 | 67–128.8, 77 | 0.88 | 0.44 ± 0.33 | 25.80 | 77 |
| Trigloporus lastoviza | Tlas | A-M | 30.38 | 1.35 | 0.386 | 1.79 | 0.462 | 16 | 91.7 ± 28.2 | 1393 | 11.5–141.1, 74 | 0.14 | 2.91 ± 0.93 | 43.41 | 111.9 |
| Umbrina cirrosa | Ucir | A-M | 1.27 | 0.06 | 0.001 | 0.07 | 0.001 | 13 | 0.2 ± 0.2 | 13 | 11–11, 11 | 0.75 | 0.01 ± 0.01 | 0.75 | |
| Uranoscopus scaber | Usca | A-M | 15.19 | 0.68 | 0.026 | 0.90 | 0.031 | 13 | 6.2 ± 2.6 | 177 | 11–197.1, 11.5 | 0.14 | 0.50 ± 0.19 | 8.14 | 141.1 |
| Xyrichthys novacula | Xnov | A-M | 7.59 | 0.34 | 0.014 | 0.45 | 0.017 | 19 | 3.4 ± 1.5 | 74 | 10.5–111.9, 12 | 0.17 | 0.04 ± 0.02 | 1.36 | 11 |
| Zeus faber | Zfab | C | 21.52 | 0.96 | 0.049 | 1.27 | 0.059 | 16 | 11.7 ± 3.1 | 113 | 23–287.3, 67 | 0.08 | 0.76 ± 0.42 | 32.53 | 163.3 |
| Alepes djedaba | Adje | I-P | 3.80 | 0.17 | 0.004 | 0.69 | 0.025 | 19 | 1.0 ± 0.6 | 38 | 8.5–31.6, 8.5 | 0.57 | 0.04 ± 0.02 | 1.57 | 31.6 |
| Apogonichthyoides pharaonis | Apha | I-P | 6.33 | 0.28 | 0.005 | 1.15 | 0.033 | 18 | 1.3 ± 0.6 | 26 | 10.7–23, 11 | 0.04 | 0.01 ± 0.00 | 0.27 | 23 |
| Callionymus filamentosus | Cfil | I-P | 41.77 | 1.86 | 0.271 | 7.59 | 1.646 | 18 | 64.3 ± 16.3 | 824 | 8.5–186.3, 12.1 | 0.07 | 0.63 ± 0.16 | 7.47 | 12.1 |
| Champsodon capensis | Ccap | I-P | 1.27 | 0.06 | 0.007 | 0.23 | 0.043 | 133 | 1.7 ± 1.7 | 133 | 115–115, 115 | 0.95 | 0.01 ± 0.01 | 0.95 | 115 |
| Champsodon nudivittis | Cnud | I-P | 16.46 | 0.73 | 0.068 | 2.99 | 0.411 | 21 | 16.0 ± 5.2 | 274 | 57–195, 73.7 | 0.06 | 0.14 ± 0.04 | 2.07 | 73.7 |
| Champsodon vorax | Cvor | I-P | 13.92 | 0.62 | 0.028 | 2.53 | 0.170 | 13 | 6.6 ± 2.3 | 113 | 73.7–265.1, 127 | 0.15 | 0.06 ± 0.02 | 1.02 | 127 |
| Cynoglossus sinusarabici | Csin | I-P | 21.52 | 0.96 | 0.038 | 3.91 | 0.230 | 17 | 9.0 ± 2.4 | 109 | 10.5–197.1, 111.9 | 0.05 | 0.06 ± 0.02 | 0.62 | 23.1 |
| Decapterus russelli | Drus | I-P | 1.27 | 0.06 | 0.002 | 0.23 | 0.011 | 33 | 0.4 ± 0.4 | 33 | 117.7–117.7, 117.7 | 3.83 | 0.05 ± 0.05 | 3.83 | 117.7 |
| Equulites klunzingeri | Eklu | I-P | 34.18 | 1.52 | 5.165 | 6.21 | 31.427 | 22 | 1227.0 ± 364.2 | 15356 | 8.5–192, 11.2 | 0.07 | 8.80 ± 2.84 | 115.61 | 11.2 |
| Fistularia commersonii | Fcom | I-P | 29.11 | 1.30 | 0.296 | 5.29 | 1.803 | 18 | 70.4 ± 25.1 | 1381 | 8.5–77.7, 25.7 | 0.08 | 1.29 ± 0.59 | 38.90 | 25.7 |
| Herklotsichthys punctatus | Hpun | I-P | 1.27 | 0.06 | 0.001 | 0.23 | 0.008 | 24 | 0.3 ± 0.3 | 24 | 74–74, 74 | 0.16 | <0.01 ± 0.00 | 0.16 | 74 |
| Jaydia queketti | Aque | I-P | 2.53 | 0.11 | 0.002 | 0.46 | 0.011 | 13 | 0.4 ± 0.3 | 20 | 78.3–189, 189 | 0.08 | <0.01 ± 0.00 | 0.09 | 189 |
| Jaydia smithii | Asmi | I-P | 3.80 | 0.17 | 0.006 | 0.69 | 0.035 | 20 | 1.4 ± 0.8 | 50 | 21.9–25.7, 21.9 | 0.21 | 0.03 ± 0.02 | 1.24 | 21.9 |
| Lagocephalus guentheri | Lgue | I-P | 13.92 | 0.62 | 0.104 | 2.53 | 0.633 | 13 | 24.7 ± 11.3 | 685 | 10.5–78.3, 11 | 0.30 | 1.27 ± 0.49 | 24.17 | 11 |
| Lagocephalus sceleratus | Lsce | I-P | 17.72 | 0.79 | 0.045 | 3.22 | 0.275 | 13 | 10.8 ± 3.4 | 146 | 11–78.3, 78.3 | 0.11 | 2.52 ± 1.76 | 135.27 | 78.3 |
| Lagocephalus suezensis | Lsue | I-P | 41.77 | 1.86 | 1.167 | 7.59 | 7.100 | 13 | 277.2 ± 98.2 | 5512 | 8.5–128, 21.9 | 0.10 | 5.86 ± 2.10 | 148.98 | 21.9 |
| Muraenesox cinereus | Mcin | I-P | 1.27 | 0.06 | 0.001 | 0.23 | 0.006 | 19 | 0.2 ± 0.2 | 19 | 8.5–8.5, 8.5 | 2.42 | 0.03 ± 0.03 | 2.42 | 8.5 |
| Nemipterus randalli | Nran | I-P | 31.65 | 1.41 | 0.284 | 5.75 | 1.726 | 23 | 67.4 ± 15.5 | 596 | 21.5–298.6, 78.3 | 0.31 | 2.89 ± 0.70 | 30.86 | 87 |
| Ostorhinchus fasciatus | Ofas | I-P | 17.72 | 0.79 | 0.086 | 3.22 | 0.526 | 16 | 20.5 ± 7.1 | 370 | 11.5–94, 25.7 | 0.13 | 0.14 ± 0.05 | 3.14 | 25.7 |
| Pelates quadrilineatus | Pqua | I-P | 6.33 | 0.28 | 0.040 | 1.15 | 0.244 | 19 | 9.5 ± 6.9 | 529 | 8.5–77.2, 29.4 | 0.24 | 0.24 ± 0.18 | 13.23 | 29.4 |
| Petroscirtes ancylodon | Panc | I-P | 1.27 | 0.06 | 0.001 | 0.23 | 0.007 | 22 | 0.3 ± 0.3 | 22 | 11.7–11.7, 11.7 | 0.22 | <0.01 ± 0.00 | 0.22 | 11.7 |
| Pomadasys stridens | Pstr | I-P | 6.33 | 0.28 | 0.023 | 1.15 | 0.139 | 13 | 5.4 ± 4.2 | 329 | 11–26, 25.7 | 0.06 | 0.14 ± 0.11 | 8.22 | 25.7 |
| Pteragogus trispilus | Ppel, Ptri | I-P | 5.06 | 0.23 | 0.016 | 0.92 | 0.099 | 20 | 3.9 ± 2.8 | 220 | 11.5–29.4, 29.4 | 0.08 | 0.02 ± 0.01 | 1.13 | 29.4 |
| Sargocentron rubrum | Srub | I-P | 1.27 | 0.06 | 0.003 | 0.23 | 0.016 | 49 | 0.6 ± 0.6 | 49 | 22.7–22.7, 22.7 | 5.40 | 0.07 ± 0.07 | 5.40 | 22.7 |
| Saurida lessepsianus | Sles | I-P | 53.16 | 2.37 | 0.551 | 9.66 | 3.353 | 13 | 130.9 ± 25.1 | 1381 | 10.5–298.6, 25.7 | 0.10 | 13.37 ± 3.18 | 186.72 | 25.7 |
| Scomber japonicus | Sjap | I-P | 2.53 | 0.11 | 0.004 | 0.46 | 0.023 | 13 | 0.9 ± 0.7 | 58 | 130.6–133.9, 133.9 | 0.53 | 0.04 ± 0.03 | 2.31 | 133.9 |
| Siganus rivulatus | Sriv | I-P | 6.33 | 0.28 | 0.032 | 1.15 | 0.198 | 22 | 7.7 ± 4.7 | 298 | 11–26, 21.9 | 0.35 | 0.16 ± 0.10 | 6.95 | 21.9 |
| Sillago suezensis | Ssue | I-P | 11.39 | 0.51 | 0.721 | 2.07 | 4.389 | 40 | 171.3 ± 101.0 | 7677 | 8.5–27.7, 11 | 1.04 | 4.26 ± 1.92 | 125.36 | 11 |
| Solea elongata | Selo | I-P | 3.80 | 0.17 | 0.006 | 0.69 | 0.035 | 20 | 1.4 ± 0.8 | 46 | 11.5–299, 141.1 | 0.04 | 0.08 ± 0.07 | 5.21 | 299 |
| Sphyraena chrysotaenia | Schr | I-P | 3.80 | 0.17 | 0.009 | 0.69 | 0.056 | 19 | 2.2 ± 1.7 | 129 | 8.5–21.9, 11 | 1.45 | 0.12 ± 0.09 | 6.59 | 11 |
| Stephanolepis diaspros | Sdia | I-P | 22.78 | 1.01 | 0.072 | 4.14 | 0.438 | 13 | 17.1 ± 5.0 | 276 | 8.5–84.2, 11.5 | 0.03 | 0.24 ± 0.08 | 4.42 | 11.5 |
| Torquigener flavimaculosus | Tfla | I-P | 21.52 | 0.96 | 0.046 | 3.91 | 0.280 | 18 | 11.0 ± 3.2 | 155 | 8.5–84.2, 77 | 0.02 | 0.11 ± 0.04 | 1.81 | 77 |
| Tylerius spinosissimus | Tspi | I-P | 2.53 | 0.11 | 0.002 | 0.46 | 0.014 | 13 | 0.6 ± 0.4 | 31 | 57–78.3, 57 | 0.03 | <0.01 ± 0.00 | 0.05 | 57 |
| Upeneus moluccensis | Umol | I-P | 67.09 | 2.99 | 3.878 | 12.18 | 23.600 | 18 | 921.4 ± 179.3 | 7268 | 8.5–197.1, 127 | 0.18 | 16.40 ± 2.77 | 124.26 | 127 |
| Upeneus pori | Upor | I-P | 34.18 | 1.52 | 3.450 | 6.21 | 20.990 | 42 | 819.5 ± 197.7 | 10347 | 8.5–87, 21.5 | 0.56 | 13.65 ± 3.75 | 213.30 | 21.5 |
References
- De Meo, I.; Miglietta, C.; Mutlu, E.; Cengiz Deval, M.; Balaban, C.; Olguner, T.M. Ecological distribution of demersal fish species in space and time on the shelf of Antalya Gulf, Turkey. Mar. Biodivers. 2018, 48, 2105–2118. [Google Scholar] [CrossRef]
- Lasram, F.B.R.; Mouillot, D. Increasing southern invasion enhances congruence between endemic and exotic Mediterranean fish fauna. Biol. Invasions 2009, 11, 697–711. [Google Scholar] [CrossRef]
- Quignard, J.P.; Tomasini, J.A. Mediterranean fish biodiversity. Biol. Mar. Mediterr. 2000, 7, 1–66. [Google Scholar]
- Golani, D. Colonization of the Mediterranean by Red Sea fishes via the Suez Canal: Lessepsian migration. In Fish Invasions of the Mediterranean Sea: Change and Renewal; Golani, D., Appelbaum-Golani, B., Eds.; Pensoft Publishers: Sofia, Bulgaria, 2010; pp. 145–188. [Google Scholar]
- Kalogirou, S.; Azzurro, E.; Bariche, M.; Lameed, A.G. The ongoing shift of Mediterranean coastal fish assemblages and the spread of non-indigenous species. In Biodiversity Enrichment in a Diverse World; InTech: Rijeka, Croatia, 2012; pp. 263–280. [Google Scholar]
- Biton, E. Possible implications of sea level changes for species migration through the Suez Canal. Sci. Rep. 2020, 10, 21195. [Google Scholar] [CrossRef] [PubMed]
- Zenetos, A.; Ovalis, P.; Giakoumi, S.; Kontadakis, C.; Lefkaditou, E.; Mpazios, G.; Simboura, N.; Tsiamis, K. Saronikos Gulf: A hotspot area for alien species in the Mediterranean Sea. BioInvasions Rec. 2020, 9, 873–889. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Cinar, M.; Garcia Raso, J.; Bianchi, C.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Mediterr. Mar. Sci. 2010, 11, 381. [Google Scholar] [CrossRef]
- Ergüden, D.; Gürlek, M.; Öztürk, B.; Turan, C. Alien species of the Turkish part ofthe Mediterranean. In The Turkish Part of the Mediterranean Sea; Marine Biodiversity, Fisheries, Conservation and Governance; Turan, C., Salihoğlu, B., Özgür Özbek, E., Öztürk, B., Eds.; Publication No.: 43; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2016; pp. 462–479. [Google Scholar]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Sisma-Ventura, G.; Bialik, O.M.; Yam, R.; Herut, B.; Silverman, J. pCO2 variability in the surface waters of the ultra-oligotrophic Levantine Sea: Exploring the air-sea CO2 fluxes in a fast warming region. Mar. Chem. 2017, 196, 13–23. [Google Scholar] [CrossRef]
- Corrales, X.; Coll, M.; Ofir, E.; Piroddi, C.; Goren, M.; Edelist, D.; Heymans, J.J.; Steenbeek, J.; Christensen, V.; Gal, G. Hindcasting the dynamics of an Eastern Mediterranean marine ecosystem under the impacts of multiple stressors. Mar. Ecol. Prog. Ser. 2017, 580, 17–36. [Google Scholar]
- Occhipinti-Ambrogi, A. Global change and marine communities: Alien species and climate change. Mar. Pollut. Bull. 2007, 55, 342–352. [Google Scholar] [CrossRef]
- Lasram, F.B.R.; Tomasini, J.A.; Guilhaumon, F.; Romdhane, M.S.; Do Chi, T.; Mouillot, D. Ecological correlates of dispersal success of Lessepsian fishes. Mar. Ecol. Prog. Ser. 2008, 363, 273–286. [Google Scholar] [CrossRef]
- Belmaker, J.; Parravicini, V.; Kulbicki, M. Ecological traits and environmental affinity explain Red Sea fish introduction into the Mediterranean. Glob. Change Biol. 2014, 20, 680. [Google Scholar] [CrossRef]
- Rijn, V.I.; Kiflawi, M.; Belmaker, J. Alien species stabilize local fisheries catch in a highly invaded ecosystem. Can. J. Fish. Aquat. Sci. 2020, 77, 752–761. [Google Scholar] [CrossRef]
- Edelist, D.; Rilov, G.; Golani, D.; Carlton, J.T.; Spanier, E. Restructuring the Sea: Profound shifts in the world’s most invaded marine ecosystem. Divers. Distrib. 2013, 19, 69–77. [Google Scholar] [CrossRef]
- Gücü, A.C.; Bingel, F. Trawlable species assemblages on the continental shelf of the northeastern Levant Sea (Mediterranean) with an emphasis on Lesseptian migration. Acta Adriat. 1994, 35, 83–100. [Google Scholar]
- Arndt, E.; Schembri, P.J. Common traits associated with establishment and spread of Lessepsian fishes in the Mediterranean Sea. Mar. Biol. 2015, 162, 2141–2153. [Google Scholar] [CrossRef]
- Corsini-Foka, M.; Mastis, S.; Kondylatos, G.; Batjakas, I.E. Alien and native fish in gill nets at Rhodes, eastern Mediterranean (2014–2015). J. Mar. Biol. Assoc. UK 2017, 97, 635–642. [Google Scholar] [CrossRef]
- Ribas, X.C. Ecosystem Modelling in the Eastern Mediterranean Sea: The Cumulative Impact of Alien Species, Fishing and Climate Change on the Israeli Marine Ecosystem. Ph.D. Thesis, Universidad Politécnica de Cataluña (UPC) dentro del Programa de Doctorado de Ciencias del Mar, Barcelona, Spain, 2019. [Google Scholar]
- Michailidis, N.; Corrales, X.; Karachle, P.K.; Chartosia, N.; Katsanevakis, S.; Sfenthourakis, S. Modelling the role of alien species and fisheries in an Eastern Mediterranean insular shelf ecosystem. Ocean Coast. Manag. 2019, 175, 152–171. [Google Scholar] [CrossRef]
- Azzurro, E.; Soto, S.; Garofalo, G.; Maynou, F. Fistularia commersonii in the Mediterranean Sea: Invasion history and distribution modeling based on presence-only records. Biol. Invasions 2013, 15, 977–990. [Google Scholar] [CrossRef]
- Wilson, R.J.; Thomas, C.D.; Fox, R.; Roy, D.B.; Kunin, W.E. Spatial patterns in species distributions reveal biodiversity change. Nature 2004, 432, 393–396. [Google Scholar] [CrossRef]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar] [CrossRef]
- Givan, O.; Edelist, D.; Sonin, O.; Belmaker, J. Thermal affinity as the dominant factor changing Mediterranean fish abundances. Glob. Change Biol. 2018, 24, e80–e89. [Google Scholar] [CrossRef] [PubMed]
- Arndt, E.; Givan, O.; Edelist, D.; Sonin, O.; Belmaker, J. Shifts in eastern Mediterranean fish communities: Abundance changes, trait overlap, and possible competition between native and non-native species. Fishes 2018, 3, 19. [Google Scholar] [CrossRef]
- Sonin, O.; Spanier, E.; Levi, D.; Patti, B.; Rizzo, P.; Andreoli, M.G. Nanism (dwarfism) in fish: A comparison between red mullet Mullus barbatus from the southeastern and the central Mediterranean. Mar. Ecol. Prog. Ser. 2007, 343, 221–228. [Google Scholar] [CrossRef]
- Edelist, D.; Golani, D.; Spanier, E. First implementation of the Large Fish Index (LFI) in the eastern Mediterranean. Sci. Mar. 2014, 78, 185–192. [Google Scholar] [CrossRef]
- Dawson, M.N.; Waples, R.S.; Bernardi, G. Phylogeography. In The Ecology of Marine Fishes: California and Adjacent Waters; Allen, L.G., Pondella, D.J., Horn, M.H., Eds.; University of California Press: Berkeley, CA, USA, 2006; pp. 26–54. [Google Scholar]
- Otero, M.; Cebrian, E.; Francour, P.; Galil, B.; Savini, D. Monitoring Marine Invasive Species in Mediterranean Marine Protected Areas (MPAs): A Strategy and Practical Guide for Managers; IUCN: Malaga, Spain, 2013; 136p. [Google Scholar]
- Azzurro, E.; Fanelli, E.; Mostarda, E.; Catra, M.; Andaloro, F. Resource partitioning among early colonizing Siganus luridus and native herbivorous fish in the Mediterranean: An integrated study based on gut-content analysis and stable isotope signatures. J. Mar. Biol. Assoc. UK 2007, 87, 991–998. [Google Scholar] [CrossRef]
- Vergés, A.; Tomas, F.; Cebrian, E.; Ballesteros, E.; Kizilkaya, Z.; Dendrinos, P.; Karamanlidis, A.A.; Spiegel, D.; Sala, E. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Whitlatch, R.B. Interactions among aliens: Apparent replacement of one exotic species by another. Ecology 2002, 83, 719–732. [Google Scholar] [CrossRef]
- Jennings, S.; Kaiser, M.J. The effects of fishing on marine ecosystems. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 1998; Volume 34, pp. 201–352. [Google Scholar]
- Jennings, S.; Kaiser, M.J.; Reynolds, J.D. Marine Fisheries Ecology; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- Tittensor, D.P.; Worm, B.; Myers, R.A. Macroecological changes in exploited marine systems. In Marine Macroecology; Witman, J.D., Roy, K., Eds.; University of Chicago Press: Chicago, IL, USA, 2009; pp. 310–337. [Google Scholar]
- Edelist, D.; Sonin, O.; Golani, D.; Rilov, G.; Spanier, E. Spatio-temporal patterns of catch and discards of the Israeli Mediterranean trawl fishery in the early 1990s: Ecological and conservation perspectives. Sci. Mar. 2011, 75, 641–652. [Google Scholar] [CrossRef]
- D’Amen, M.; Azzurro, E. Lessepsian fish invasion in Mediterranean marine protected areas: A risk assessment under climate change scenarios. ICES J. Mar. Sci. 2020, 77, 388–397. [Google Scholar] [CrossRef]
- Civitarese, G.; Gacic, M.; Lipizer, M.; Eusebi Borzelli, G.L. On the impact of the Bimodal Oscillating System (BiOS) on the biogeochemistry and biology of the Adriatic and Ionian Seas (Eastern Mediterranean). Biogeosciences 2010, 7, 3987–3997. [Google Scholar] [CrossRef]
- Poulain, P.M.; Bussani, A.; Gerin, R.; Jungwirth, R.; Mauri, E.; Menna, M.; Notarstefano, G. Mediterranean surface currents measured with drifters: From basin to sub inertial scales. Oceanography 2013, 26, 38–47. [Google Scholar] [CrossRef]
- Giangrande, A.; Pierri, C.; Del Pasqua, M.; Gravili, C.; Gambi, M.C.; Gravina, M.F. The Mediterranean in check: Biological invasions in a changing sea. Mar. Ecol. 2020, 41, e12583. [Google Scholar] [CrossRef]
- Yapici, S.; Filiz, H. Biological aspects of two coexisting native and non-native fish species in the Aegean Sea: Pagellus erythrinus vs. Nemipterus randalli. Mediterr. Mar. Sci. 2019, 20, 594–602. [Google Scholar] [CrossRef]
- Mutlu, E.; de Meo, I.; Miglietta, C. Spatio-temporal distribution of pufferfish (Tetraodontidae) along the Turkish coast of the Mediterranean Sea. Mediterr. Mar. Sci. 2021, 22, 1–19. [Google Scholar] [CrossRef]
- Garuti, A.; Mutlu, E. Spatiotemporal and ecological distribution of megabenthic non-crustacean invertebrates in an ultra-oligotrophic gulf, the eastern Mediterranean Sea. J. Mar. Syst. 2021, 224, 103644. [Google Scholar] [CrossRef]
- Mutlu, E.; Miglietta, C.; de Meo, I.; Deval, M.C. Length-weight relationships of 107 osseous and 9 cartilaginous fish species on a shelf/break zone of the eastern Mediterranean Sea. Cah. Biol. Mar. 2022, 63, 19–27. [Google Scholar]
- Mutlu, E.; Miglietta, C.; de Meo, I.; Deval, M.C. Spatio-temporal and bioecological distribution of four commercial Mullid species in an ultraoligotrophic Mediterranean gulf. Turk. J. Zool. 2022, 46, 484–499. [Google Scholar] [CrossRef]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Soyer, J. Bionomie benthique du plateau continental de la côte catalane française Volume III—Les peuplements de Copepodes harpacticoides (Crustacea). Vie Milieu 1970, 21, 337–551. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer-e: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER V6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research); Primer-e: Plymouth, UK, 2006. [Google Scholar]
- Teer Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Micro-computer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Marsaglia, G.; Tsang, W.; Wang, J. Evaluating Kolmogorov’s Distribution. J. Stat. Softw. 2003, 8, 1–4. [Google Scholar] [CrossRef]
- Spanier, E.; Galil, B.S. Lessepsian migration? A continuous biogeographical process. Endeavour 1991, 15, 102–106. [Google Scholar] [CrossRef]
- Mavruk, S.; Avsar, D. Non-native fishes in the Mediterranean from the Red Sea, by way of the Suez Canal. Rev. Fish Biol. Fish. 2008, 18, 251–262. [Google Scholar] [CrossRef]
- Gücü, A.C. A box model for the basic elements of the northeastern Mediterranean Sea trawl fisheries. Isr. J. Zool. 2013, 41, 551–567. [Google Scholar]
- Dulčić, J.; Scordella, G.; Guidetti, P. On the record of the Lessepsian migrant Fistularia commersonii (Rüppell, 1835) from the Adriatic Sea. J. Appl. Ichthyol. 2008, 24, 101–102. [Google Scholar] [CrossRef]
- Golani, D. Impact of Red Sea fish migrants through the Suez Canal on the aquatic environment of the Eastern Mediterranean. Bull. Ser. Yale Sch. For. Environ. Stud. 1998, 103, 375–387. [Google Scholar]
- Patania, A.; Mutlu, E. Spatiotemporal and ecological distribution of megabenthic crustaceans on the shelf-shelf break of Antalya Gulf, the eastern Mediterranean Sea. Mediterr. Mar. Sci. 2021, 22, 446–465. [Google Scholar] [CrossRef]
- Gücü, A.C. Impact of depth and season on the demersal trawl discard. Turk. J. Fish. Aquat. Sci. 2012, 12, 817–830. [Google Scholar]
- Kalogirou, S.; Wennhage, H.; Pihl, L. Non-indigenous species in Mediterranean fish assemblages: Contrasting feeding guilds of Posidonia oceanica meadows and sandy habitats. Estuar. Coast. Shelf Sci. 2012, 96, 209–218. [Google Scholar] [CrossRef]
- Kalogirou, S.; Corsini-Foka, M.; Sioulas, A.; Wennhage, H.; Pihl, L. Diversity, structure and function of fish assemblages associated with Posidonia oceanica beds in an area of the eastern Mediterranean Sea and the role of non-indigenous species. J. Fish Biol. 2010, 77, 2338–2357. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.L.; Bitar, G.; Harmelin, J.G.; Monestiez, P. The littoral fish community of the Lebanese rocky coast (eastern Mediterranean Sea) with emphasis on Red Sea immigrants. Biol. Invasions 2005, 7, 625–637. [Google Scholar] [CrossRef]
- Wolfe, L.M. Why alien invaders succeed: Support for the escape-from-enemy hypothesis. Am. Nat. 2002, 160, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Castriota, L.; Falautano, M.; Battaglia, P.; Oddo, A.; Andaloro, F. New biological data on Fistularia commersonii in the central Mediterranean Sea. Cybium 2014, 38, 15–21. [Google Scholar]
- Azzurro, E.; Tuset, V.M.; Lombarte, A.; Maynou, F.; Simberloff, D.; Rodríguez-Pérez, A.; Solé, R.V. External morphology explains the success of biological invasions. Ecol. Lett. 2014, 17, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Givan, O.; Parravicini, V.; Kulbicki, M.; Belmaker, J. Trait structure reveals the processes underlying fish establishment in the Mediterranean. Glob. Ecol. Biogeogr. 2017, 26, 142–153. [Google Scholar] [CrossRef]
- Golani, D.; Edelist, D.; Lerner, A.; Sonin, O.; Motro, U. A long term (1949–2010) study of catch and effort in Israeli trawl fishery, Eastern Mediterranean Sea. Acta Adriat. Int. J. Mar. Sci. 2017, 58, 157–162. [Google Scholar] [CrossRef]
- Ben-Yami, M.; Glaser, T. The invasion of Saurida undosquamis (Richardson) into the Levant Basin-an example of biological effect of interoceanic canals. Fish. Bull. 1974, 72, 359. [Google Scholar]
- Fishelson, L. Marine animal assemblages along the littoral of the Israeli Mediterranean seashore: The Red-Mediterranean Seas communities of species. Ital. J. Zool. 2000, 67, 393–415. [Google Scholar] [CrossRef]
- Bariche, M.; Letourneur, Y.; Harmelin-Vivien, M. Temporal fluctuations and settlement patterns of native and Lessepsian herbivorous fishes on the Lebanese coast (eastern Mediterranean). Environ. Biol. Fishes 2004, 70, 81–90. [Google Scholar] [CrossRef]
- Giakoumi, S. Distribution patterns of the invasive herbivore Siganus luridus (Rüppell, 1829) and its relation to native benthic communities in the central Aegean Sea, Northeastern M editerranean. Mar. Ecol. 2014, 35, 96–105. [Google Scholar] [CrossRef]
- Edelist, D.; Golani, D.; Spanier, E. Ecological Indicators for Overfishing in Israel’s Trawl Fishery; Technical Report; Israel Ministry Environmental Protection: Jerusalem, Israel, 2012; 50p. (In Hebrew)
- Massuti, E.; Valls, M.; Ordines, F. Changes in the western Mediterranean ichthyofauna: Signs of tropicalization and meridianization. In Fish Invasions of the Mediterranean Sea: Change and Renewal; Golani, D., Appelbaum-Golani, B., Eds.; Pensoft Publishers: Sofia, Bulgaria; Moscow, Russia, 2010; pp. 293–312. [Google Scholar]
- Guidetti, P.; Giardina, F.; Azzurro, E. A new record of Cephalopholis taeniops in the Mediterranean Sea, with considerations on the Sicily channel as a biogeographical crossroad of exotic fish. Mar. Biodivers. Rec. 2010, 3, E13. [Google Scholar] [CrossRef]
- Turan, C.; Gürlek, M.; Başusta, N.; Ali, U.Y.A.N.; Doğdu, S.A.; Karan, S. A checklist of the non-indigenous fishes in Turkish marine waters. Nat. Eng. Sci. 2018, 3, 333–358. [Google Scholar] [CrossRef]
- Bilge, G.; Filiz, H.; Yapıcı, S.; Tarkan, A.S.; Vilizzi, L. A risk screening study on the potential invasiveness of Lessepsian fishes in the south-western coasts of Anatolia. Acta Ichthyol. Piscat. 2019, 49, 23–31. [Google Scholar] [CrossRef]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- MacArthur, R. Fluctuations of animal populations and a measure of community stability. Ecology 1955, 36, 533–536. [Google Scholar] [CrossRef]
- Levine, J.M.; D’Antonio, C.M. Elton revisited: A review of evidence linking diversity and invasibility. Oikos 1999, 87, 15–26. [Google Scholar] [CrossRef]
- Wonham, M.J.; Carlton, J.T.; Ruiz, G.M.; Smith, L.D. Fish and ships: Relating dispersal frequency to success in biological invasions. Mar. Biol. 2000, 136, 1111–1121. [Google Scholar] [CrossRef]
- Safriel, U.N.; Ritte, U. Criteria for the identification of potential colonizers. Biol. J. Linn. Soc. 1980, 13, 287–297. [Google Scholar] [CrossRef]
- Moyle, P.B.; Marchetti, M.P. Predicting invasion success: Freshwater fishes in California as a model. BioScience 2006, 56, 515–524. [Google Scholar] [CrossRef]
- Bianchi, G.; Gislason, H.; Graham, K.; Hill, L.; Jin, X.; Koranteng, K.; Manickchand-Heileman, S.; Payá, I.; Sainsbury, K.; Sanchez, F.; et al. Impact of fishing on size composition and diversity of demersal fish communities. ICES J. Mar. Sci. 2000, 57, 558–571. [Google Scholar] [CrossRef]
- Reynolds, J.D.; Dulvy, N.K.; Goodwin, N.B.; Hutchings, J.A. Biology of extinction risk in marine fishes. Proc. Biol. Sci. 2005, 272, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Strona, G.; Galli, P.; Montano, S.; Seveso, D.; Fattorini, S. Global-scale relationships between colonization ability and range size in marine and freshwater fish. PLoS ONE 2012, 7, e49465. [Google Scholar] [CrossRef] [PubMed]
- Buba, Y.; van Rijn, I.; Blowes, S.A.; Sonin, O.; Edelist, D.; DeLong, J.P.; Belmaker, J. Remarkable size-spectra stability in a marine system undergoing massive invasion. Biol. Lett. 2017, 13, 20170159. [Google Scholar] [CrossRef] [PubMed]
- Edelist, D.; Golani, D.; Rilov, G.; Spanier, E. The invasive venomous striped eel catfish Plotosus lineatus in the Levant: Possible mechanisms facilitating its rapid invasional success. Mar. Biol. 2012, 159, 283–290. [Google Scholar] [CrossRef]
- Ketchum, B.H. Estuaries and Enclosed Seas (Ecosystems of the World); Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1983; p. 500. [Google Scholar]


| TL | TW | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | a | b | GT | N | ||
| IS | Mean | 9.9 | 21.3 | 14.9 | 28.6 | 187.1 | 63.7 | 0.015 | 2.988 | I | 28,987 |
| SD | 6.8 | 12.1 | 7.9 | 195.2 | 578.4 | 218.9 | 0.015 | 0.442 | |||
| NIS | Mean | 8.6 | 19.0 | 13.2 | 10.6 | 170.2 | 41.3 | 0.015 | 2.951 | I | 7642 |
| SD | 6.1 | 13.0 | 7.1 | 21.4 | 562.5 | 123.5 | 0.012 | 0.360 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutlu, E.; Meo, I.d.; Miglietta, C.; Deval, M.C. Ecological Indicative Stressors of Native vs. Non-Native Fish in an Ultra-Oligotrophic Region of the Mediterranean Sea. Sustainability 2023, 15, 2726. https://doi.org/10.3390/su15032726
Mutlu E, Meo Id, Miglietta C, Deval MC. Ecological Indicative Stressors of Native vs. Non-Native Fish in an Ultra-Oligotrophic Region of the Mediterranean Sea. Sustainability. 2023; 15(3):2726. https://doi.org/10.3390/su15032726
Chicago/Turabian StyleMutlu, Erhan, Ilaria de Meo, Claudia Miglietta, and Mehmet Cengiz Deval. 2023. "Ecological Indicative Stressors of Native vs. Non-Native Fish in an Ultra-Oligotrophic Region of the Mediterranean Sea" Sustainability 15, no. 3: 2726. https://doi.org/10.3390/su15032726
APA StyleMutlu, E., Meo, I. d., Miglietta, C., & Deval, M. C. (2023). Ecological Indicative Stressors of Native vs. Non-Native Fish in an Ultra-Oligotrophic Region of the Mediterranean Sea. Sustainability, 15(3), 2726. https://doi.org/10.3390/su15032726







