Abstract
Phytoplankton and bacteria play key roles in material cycling and their consequent eco-functions in lakes, which are threatened by anthropogenic pressures, especially agricultural activities, which, in the watershed, are effective in changing the material composition and hydrodynamic conditions of the lake through material input and water withdrawal. This process theoretically changes the interaction and assembly pattern of microorganisms, which are important factors driving the structural and functional evolution of ecological communities in lakes. In this research, the community structure, interactions, and assembly of phytoplankton and bacteria were investigated during agro-irrigation seasons in a typical agricultural drainage receiving lake, Wuliangsuhai. The results showed that the seasonal variations in the community were driven by nitrogen and phosphorus. In particular, Cyanobacteria increased significantly during the seasons with the regulation of TP (λ = 0.56, p < 0.01, n = 30). The TN positively drove Chlorophyta and Bacillariophyta (λ = 0.42 and 0.65, p < 0.05, n = 30). Furthermore, MENA showed that planktonic algae and bacterial community interactions were enhanced, and interspecific competition increased at high trophic levels. The community assembly is primarily a stochastic process that is mostly related to hydrodynamic conditions. The second related factor, nitrogen and phosphorus inputs, had obvious effects on community assembly, which responded to its effects on species diversity, niche width, and interactions, and they jointly controlled community assembly. This study reveals that the assembly processes of bacteria and planktonic algae were driven by different environmental factors in specific ways, which provides a new view for understanding agriculture’s impacts on microecology and helps in developing lake protection strategies.
1. Introduction
Lakes carry important ecological processes and provide essential ecological and societal services, in which microorganisms play an important role as basic components. Revealing the dynamics of the microbial community can deepen our understanding of the ecological process in aquatic environments. However, lake microorganisms are strongly affected by anthropogenic pressures, among which agricultural production is one of the most concerning [1,2]. Previous studies have demonstrated that the worldwide application of agricultural fertilizers makes up considerable anthropogenic inputs of 22–26 Tg P and ~ 118 Tg N per year, of which 18% are lost into water bodies [3]. The excessive N and P inputs may affect primary productivity, community structure evolution, community construction, and biotic relationships in lakes [2]. Among them, the relationship between phytoplankton and bacteria is the basic ecological relationship in the aquatic environment, which may be regulated by controlling nutrient cycling and biomass production at the base of the food web [4]. Theoretically, considering that the chemical characteristics of substrates are related to microorganisms, excessive N and P inputs from agricultural production will change the composition of microorganisms [5]. It is important to note that microbes do not work independently [6]. They communicate with each other through material, energy, and information exchanges, creating complex interactions such as competition, symbiosis, collaboration, and predation [7]. This composition and the resulting interactions largely determine their material cycling capacity and ecological function [8].
The community assembly processes that shape microbial diversity have important ecological significance in aquatic environments. However, studying community assembly mechanisms is difficult due to the astonishing diversity. In addition, classic eco-process theories may help to explain it [9]. Previous studies have provided theories, niche-based theory and neutral theory, for microorganism gathering (i.e., assembly) [10]. Traditional niche-based theories hypothesize that community selection is constrained by environmental conditions, species characteristics, and interspecies interactions [11,12]. In contrast, neutral theory assumes that community structures are independent of species traits and governed by drift, dispersal, random speciation, death, and extinction [13]. Although it is widely accepted that deterministic and stochastic processes operate simultaneously in community assembly, a central debate is their relative importance in controlling community succession [13]. Recently, some scholars have developed several models, such as the neutral model and null the model, to quantify the importance of processes [14,15]. Among them, it makes sense to infer community assembly mechanisms by a phylogenetic-bin-based null model (iCAMP) to consider ecological processes at the level of a single taxon/lineage rather than an entire community [16]. In addition, previous studies have also found that dynamic environmental conditions affect the microbial community in pronounced ways [17], but the planktonic microbial community assembly processes of lakes driven by nitrogen and phosphorus from agricultural drainage remain unclear. Meanwhile, most studies on community assembly have focused on bacteria [17,18], and little is known about microeukaryotes. Thus, there is a surge of interest in understanding the distinct assembly process between bacteria and the microeukaryotic community and gaining insights into factors that contribute to variation.
Acknowledging the above, this research focuses on investigating the community composition, interactions, and assembly processes of planktonic bacteria and microeukaryotes in a receiving lake in combination with typical agricultural irrigation and drainage with seasonal variations. The famous Wuliangsuhai Lake in China [19], which is strongly influenced by agricultural activities, was taken as the research object. This is also the first study involving interactions and assembly processes among the planktonic bacterial and microeukaryotic communities in Wuliangsuhai. The objectives of this work were to (1) link seasonal agro-irrigation with the variations in planktonic bacterial and microeukaryotic community structure in an agricultural drainage receiving lake, (2) explore the key environmental factors driving the alteration of biotic community composition, and (3) reveal the contributions of N and P inputs to the interactions and assembly processes of bacterial and microeukaryotic communities. The results obtained provide a view on how agricultural drainage affects freshwater lake microecology and support the development of strategies for agro-irrigation management and water-receiving lake conservation.
2. Materials and Methods
2.1. Study Sites and Sampling
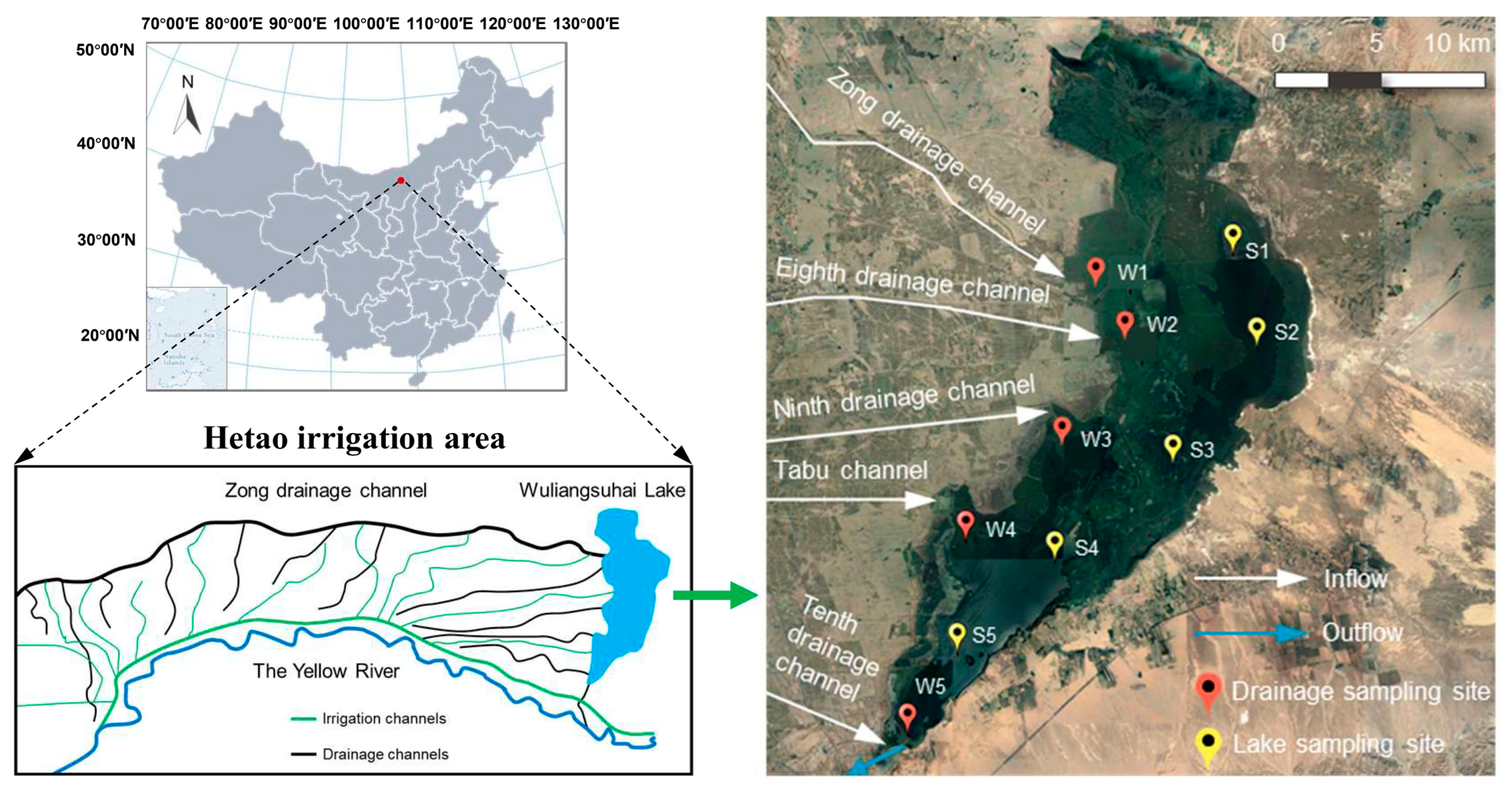
Wuliangsuhai Lake (40°46′–41°03′ N, 108°43′–108°57′ E, as shown in Table S1) is connected to the largest irrigated region (the Hetao irrigation area) and the upper reaches of the Yellow River (Figure 1), with a storage capacity of 2.5–3 × 108 m3 [20,21]. The depth of the lake is 0.5–3 m, and the average depth is 0.7 m [22,23]. It is the most important wetland in the Yellow River Basin and was officially listed as an internationally important wetland by the Ramsar Convention in 2002 [20]. It has extremely high biodiversity compared with that of other semidesert lakes [21], and receives 1.69, 1.03, and 1.00 billion cubic meters of agricultural drainage water from the Hetao irrigation area [22] during May (summer irrigation), July (autumn irrigation), and October (autumn watering), respectively (Implementation plan of 2019 summer irrigation work in Hetao Irrigation Area, Inner Mongolia. http://www.zghtgq.com/plus/view.php?aid=6217 accessed on 1 January 2021). The outflow of water drains into the Yellow River and affects the downstream water quality. The lake has been frozen for up to five months, and the ice is thick. Due to its unique hydroclimatic characteristics and important watershed functions, the freshwater ecosystem of Wuliangsuhai has attracted much attention.
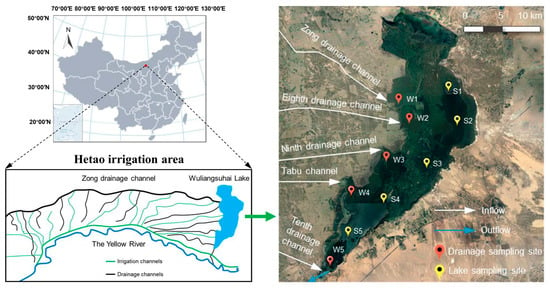
Figure 1.
Study area of Wuliangsuhai Lake and main drainage channels. Red pins represent sampling sites near the drainage channels and yellow pins represent sampling sites in the lake.
The samples were collected in the study area in May, July, and October 2021. Ten sampling sites were selected along the drainage channels and lake area of Wuliangsuhai, as shown in Figure 1. Site W (W1–W5) refers to the outlets of those drainage channels (Zong drainage channel, the Eighth drainage channel, the Ninth drainage channel, the Tabu channel, and the Tenth drainage channel, respectively). Sites labeled S (S1–S5) represent five sampling points evenly distributed from the north of the lake to the outlet. The water temperature (T, °C) and dissolved oxygen concentration (DO, mg/L) were measured on site with a portable oxygen dissolving instrument (HACH HQ30D, USA). The water samples were taken in triplicate at each site with a stainless-steel sampler, and 3 L water was taken in each. All samples were collected into glass bottles and immediately preserved in a 4 °C refrigerator.
2.2. Chemical Analysis
Prior to laboratory analysis, all samples were cold-stored. The water samples were used for the determination of water quality indicators within 24 h. Ammonia nitrogen (NH3-N), total nitrogen (TN), total phosphorus (TP), and the permanganate index (CODMn) were analyzed according to the standard method (A.P.H. A, 1998, and ISO6060).
2.3. Biological Analysis
The 1 L water samples were first filtered (0.22 μm, water phase filtration membrane, Jinteng, China) to collect planktonic microorganisms on the membrane, which was then cut into pieces with sterilized medical scissors and put into a mortar. Liquid nitrogen was added in small amounts many times until the sample was covered, and then it was ground to powder with a pestle. For DNA extraction, refer to the FastDNA ®SPIN Kit instructions. All samples were stored at −80 °C for further use after extraction, and the quality was detected by 1% agarose gel electrophoresis.
The primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were selected for the V3-V4 highly variable region of 16S rRNA, and primers 573F (5′-CGCGGTAATTCCAGCTCCA-3′) and 951R (5′-TTGGYRAATGCTTTCGC-3′) were used as primers for the V4 highly variable region of 18S rRNA to amplify the target band [24]. The PCR products from the parallel samples at each site were mixed, and electrophoresis was performed on a 1.5% agarose gel for detection.
The constructed amplicon library was sequenced on the Illumina MiSeq 2500 platform, and 400–450 bp paired-end reads were generated. The effective sequences were assigned to operational taxonomic units (OTUs) at 97% sequence identity by the QIIME 2 pipeline. Representative sequences for each OTU were chosen for taxonomic annotation of each sequence using the Greengene (version 13_5) and Silva 138_18S databases by the RDP classifier, with a confidence threshold of 0.7 [25]. All original sequences have been uploaded to the GSA database with submission numbers subCRA014002 and subCRA014005.
2.4. Statistical Analysis
The experimental results (except microbial community structure data) were expressed as three parallel means and standard errors, and all basic data calculations, chart drawing, and microbial analyses were completed using the open-source software R (version 4.1.2). Trend analysis was performed using Excel 2020 (Microsoft Corp., Hyannis, Massachusetts, USA). Statistical analysis (analysis of variance, t test) was carried out using R. Principal component analysis (PCA), cluster analysis (CA), and redundancy analysis (RDA) were also performed, and their results were plotted using R.
The molecular ecological network was constructed using packages in R (‘WGCNA’, ‘multtest’, ‘reshape 2′, and ‘tidyverse’), and the co-occurrence network was constructed using the OTUs observed in the samples (≥10). When the Spearman correlation coefficient |ρ| was greater than 0.6 and the p value was less than 0.05, we considered the correlation between OTUs significant [26]. To describe the topology of the network, a set of metrics (nodes, edges, averageness, network diameter, network density, modularity, average clustering coefficient, and average path length) were calculated, and Cytoscape software (version 3.9.1.0) was used to generate and visualize the results [27,28].
The iCAMP revealed the assembly mechanisms of different microorganisms. A series of calculations were performed using an in-house Galaxy software platform (IEG Statistical Analysis Pipeline3, http://ieg3.rccc.ou.edu:8080/ accessed on 1 January 2021) [16]. Multiple interactions between environmental variables and microorganisms were explored by RDA and variance partitioning analysis (VPA). Path analysis of direct and indirect effects between environmental variables and planktonic algae was carried out based on structural equation modeling (SEM) and linear regression, and all graphs were created by R.
3. Results
3.1. Variations in Bacterial Community Structure during the Seasons
A total of 536920 OTUs were obtained by sorting out the effective sequences. According to the species annotation, as shown in Figure S1A–C, the bacterial community was composed of 39 phyla. Proteobacteria, Actinobacteriota, Bacteroidota, and Cyanobacteria were the dominant phyla at all sites. Obviously, the abundance of Cyanobacteria was significantly higher in July and October than in May (average abundance was 5.82%, 23.80% and 16.44% in May, July, and October, respectively, p < 0.05).
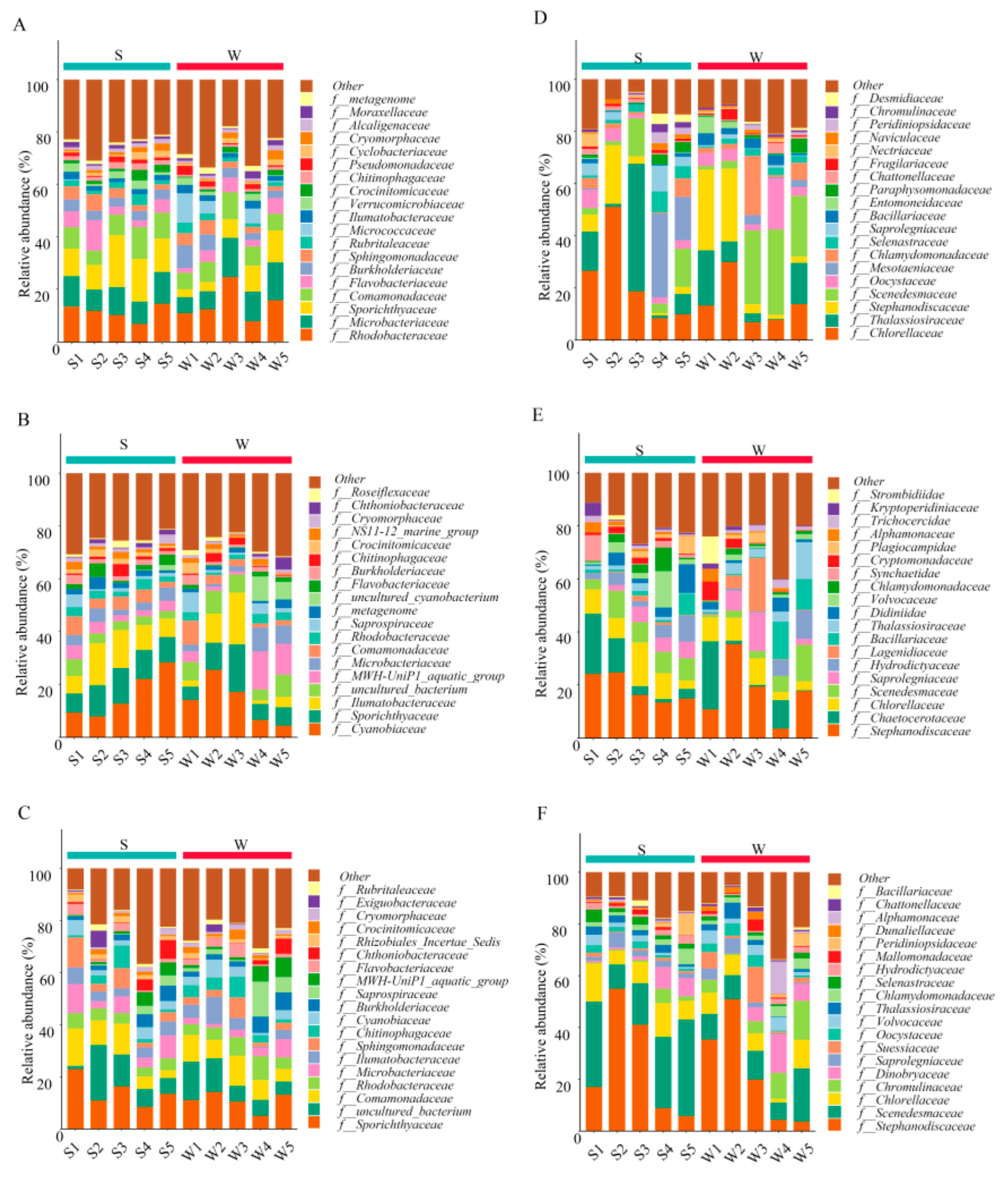
At the family level, 415 bacterial lineages were assigned 97% identity, and the 20 most abundant families are illustrated in Figure 2A–C. In May, Rhodobacteraceae and Microbacteriaceae were the dominant families at all sites (6.72–24.65% and 6.08–14.91%, respectively), and W3 accounted for the largest proportion. The relative abundance of Sporichthyaceae at the S sites was higher than that at the W sites (averages of 13.74% and 7.15%, respectively, p < 0.05). In July, the abundance of Cyanobiaceae (4.41–28.26%) was higher and significantly different from that in May (p < 0.05), followed by Sporichthyaceae, which accounted for a considerable proportion at all sites (4.85–17.98%). However, in October, Sporichthyaceae was the dominant family (5.07–22.95%), while the percentage of cyanoacetate decreased to 0.92–6.90%. In addition, the proportion of Comamonadaceae cannot be ignored and was significantly higher than that in July (1.41–8.98% and 2.93–14.32% in July and October, respectively, p < 0.05). Moreover, compared with that in May, the abundance of Rhodobacteraceae and Microbacteriaceae in October decreased to 3.52–9.12% and 1.32–11.09%, respectively (p < 0.01).
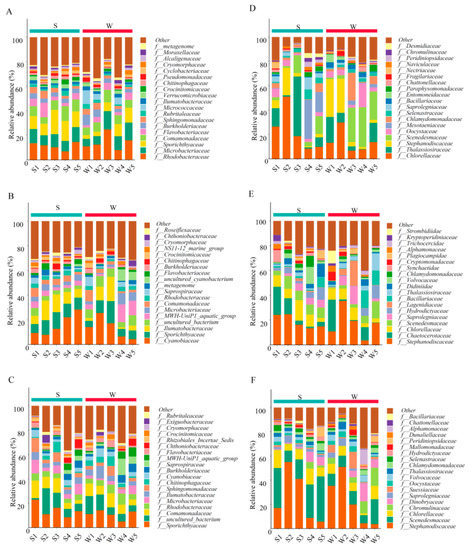
Figure 2.
The stacked bar diagram of relative species abundance shows the community structure at the family level at 10 sampling sites. (A–C) represent the community composition of planktonic bacteria in May, July and October respectively. (D–F) represent the community composition of microeukaryotes in May, July and October, respectively.
In addition, α-diversity analysis showed that the Chao 1 and richness indices of the bacterial community decreased in October (p < 0.05), while the Shannon index did not (p > 0.05, Figure S2A). The β-diversity in the bacterial community was analyzed for different groups (Figure S3A–C). The PCA score plot showed that all samples in the confidence ellipses of Figure S3A clustered together in the same seasons, demonstrating that the bacterial community structure differed significantly in different seasons (p < 0.01, Table S2A). However, in terms of spatial effect, it was found that the confidence ellipses overlapped each other at the S and W sites (Figure S3B), suggesting that the bacterial community was similar in spatial locations (p > 0.05, Table S2B). In addition, the ANOSIM analysis was also in line with the PCA results (Figure S3C, p < 0.05).
3.2. Variations in Microeukaryotic Community Structure during the Seasons
At the phylum level, Chlorophyta accounted for a large percentage of the microbes at all sites (from 23.20% to 80.97%, with an average of 52.87%, Figure S1D–F), which was consistent with studies on its widespread existence in freshwater habitats [29]. The abundance of Bacillariophyta was higher at W1, W2, S2, and S3 in May and October (22.98–54.87%) and increased significantly in July (11.19–59.58%, p < 0.05). The proportion of Streptophyta in May was higher than that in July and October (average abundance of 7.55%, 1.78%, and 2.63%, respectively, p > 0.05).
At the family level (Figure 2D–F), in May, the abundance of Chlorellaceae at the S sites was higher (9.99–51.08%), and S2 accounted for the largest proportion. Thalassiosiraceae had the highest abundance at S3 (48.84%). Stephanodiscaceae was the dominant family at W1, W2, and S2 (30.97%, 27.86%, and 22.44%, respectively), but its proportion at other sites was negligible. In July, Chaetocerotaceae accounted for the largest proportion in S1 (22.71%) and W1 (22.57%) and significantly increased compared with May and October (p < 0.05). In October, the relative abundance of Chromulinaceae significantly increased compared with that in May (0.99% in May, 4.47% in October, p < 0.05), while that of Chlorellaceae decreased (18.67% in May, 8.34% in October, p < 0.05). In general, the composition and abundance of the dominant species during the three seasons differed considerably.
The α-diversity of microeukaryotes varied significantly in the three seasons and showed an increasing trend (p < 0.05, Figure S2B), which was contrary to the variations in the bacterial community. There were similarities between the S and W sites (p > 0.05). The results of β-diversity analysis were consistent with those of bacterial communities (Figure S3D–F), and no significant variations in community structure were detected for the effects of lake locations (Table S2B,D, p > 0.05), demonstrating that the sensitivity of planktonic microorganisms to spatial changes was not notable in Wuliangsuhai Lake, which was consistent with the findings of studies in the Nanliu River Basin ecosystem [30]. The above results showed that seasonality was more important than spatial variability (Table S2A,C) for the microbial communities [31]. Previous studies also indicated that the variations in community structure were related to spatial variations and the strength of environmental gradients [31]. Consequently, the grouping under the combined effects of season and space (Figure S3C,F) was also statistically significant (p < 0.05). Results obtained revealed that season dominated the variations in microbial community structure.
3.3. Seasonal Variations in Physicochemical Factors and Their Correlation with Microorganisms
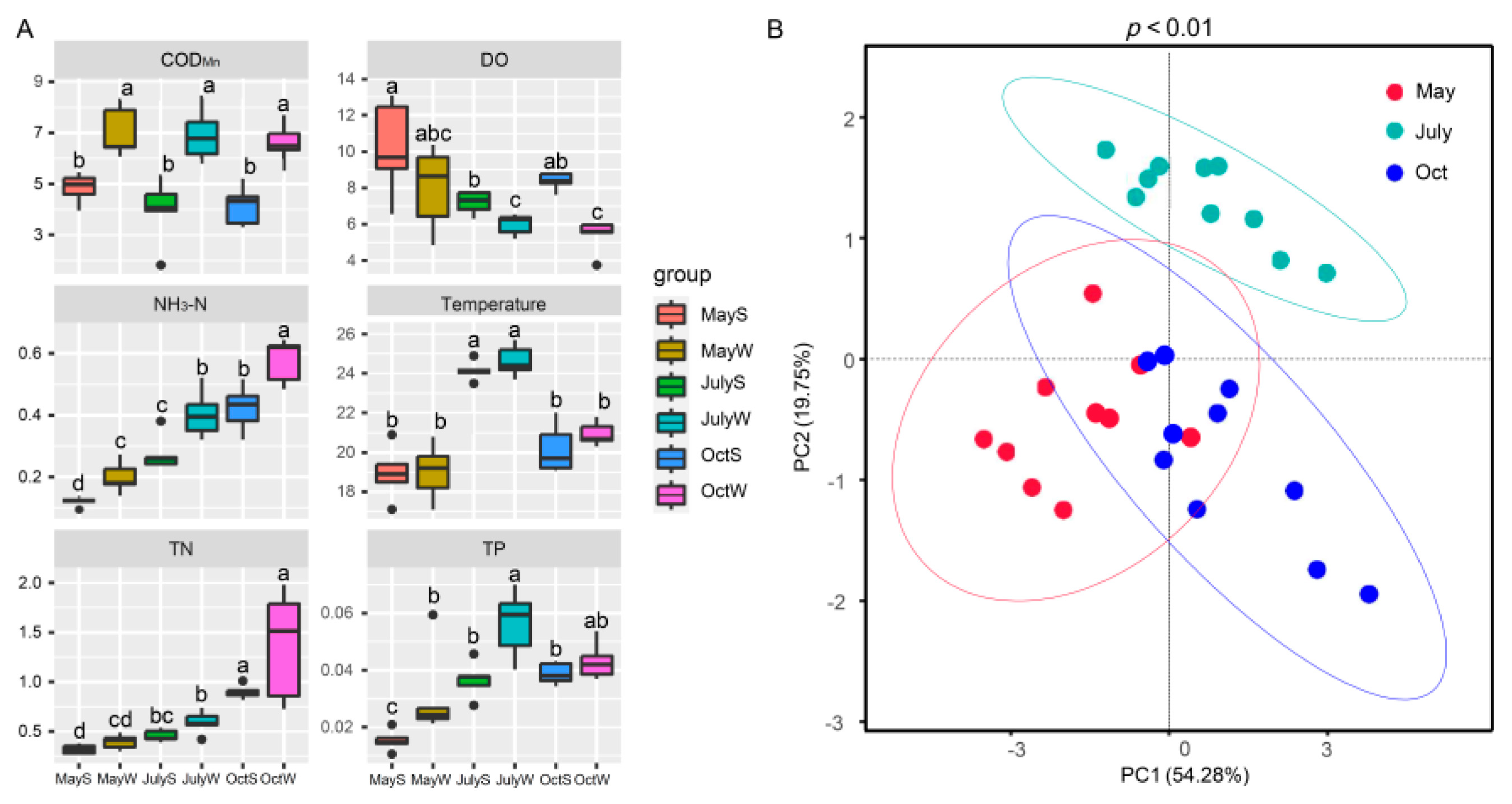
The variations in NH3-N, TN, TP, CODMn, DO, and temperature are shown in Table 1, Figure 3 and Figure S4. Overall, the concentration of NH3-N was 0.10–0.64 mg/L. TN ranged from 0.27 to 1.98 mg/L, and TP ranged from 0.01–0.07 mg/L. The CODMn and DO were 1.82–8.46 mg/L and 3.75–13.09 mg/L, respectively. The water temperature was in the range of 17.10–25.70 °C, with an average of 21.31 °C.

Table 1.
Seasonal variations of physicochemical parameters of each site.
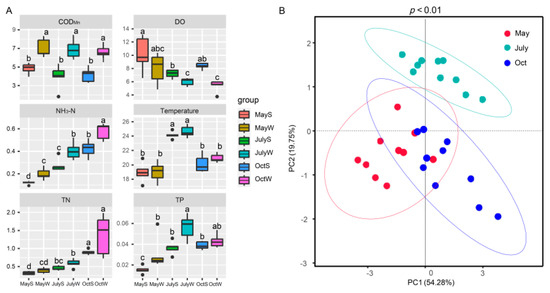
Figure 3.
Spatiotemporal differences of water quality parameters. (A) The top and bottom boundaries of each box represent the 75th and 25th quartile values, respectively; and the line within each box represents the median values. Different letters indicate significant differences at the p < 0.05 level. (B) the principal component (PCA) scores of indices at each sampling point in seasons.
Table 1 shows that the NH3-N, TN, and TP of the S sites were 0.10–0.52 mg/L, 0.27–1.01 mg/L, and 0.01–0.05 mg/L, respectively, which were lower than those of the W sites (0.14–0.64 mg/L, 0.31–1.98 mg/L, and 0.02–0.07 mg/L for NH3-N, TN and TP, respectively, p < 0.05). Statistical analysis revealed that all the physicochemical parameters, excluding temperature, were significantly different between the S and W sites (p < 0.05). This result is expected since the W sites are close to the drainage channels and are greatly affected by nutrient inputs [32].
In terms of seasons, the concentrations of NH3-N and TN were highest in October (0.32–0.64 mg/L and 0.73–1.98 mg/L, respectively), TP and temperature were highest in July (0.03–0.07 mg/L and 23.50–25.70 °C), while DO was higher in May (4.85–13.09 mg/L), and the variation in CODMn was not obvious. Water quality parameters (except CODMn, DO, and TP) were significantly different in the three seasons (p < 0.05, Figure S5). The PCA score plot explained 74.03% of the total variance, which also demonstrated that the differences between the groups were significant (p < 0.01, Figure 3B).
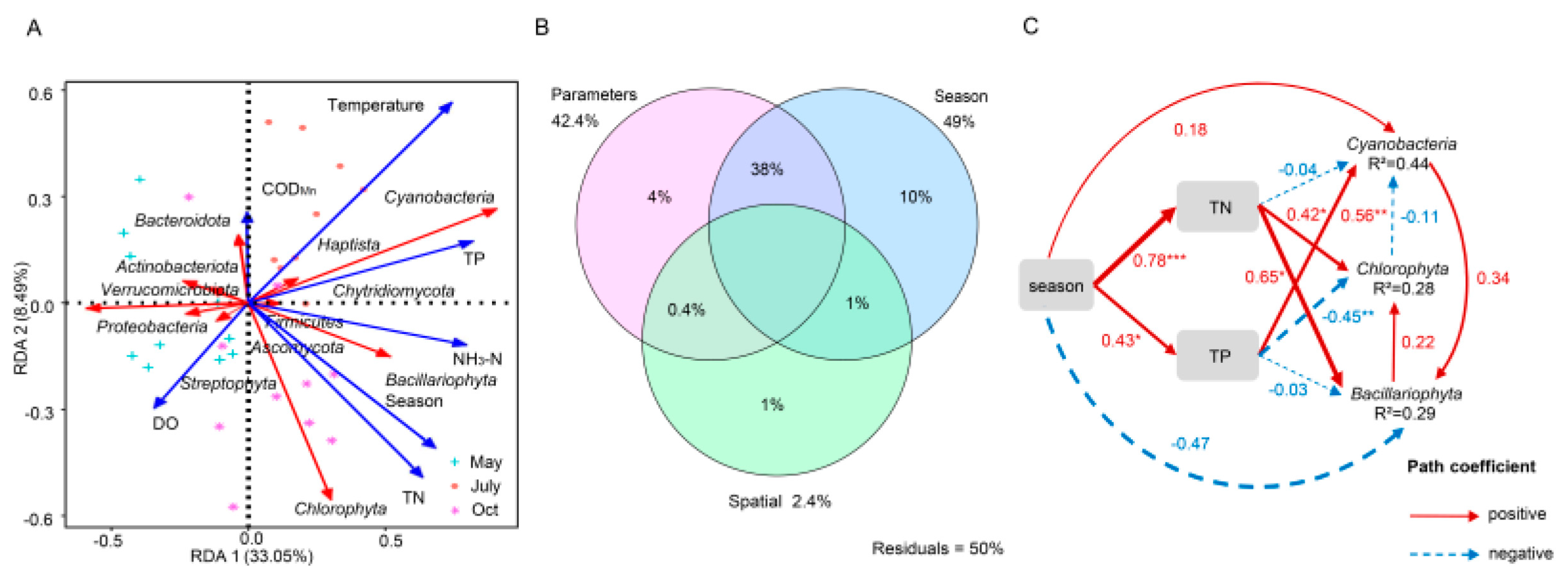
The relationship between microorganisms and physicochemical factors based on the Bray–Curtis distance was analyzed by the Mantel test (Figure S6 and Table S3). The results indicated that the abundances of species were more strongly correlated with NH3-N, TP, and temperature (p < 0.01), followed by TN and DO (p < 0.05). Meanwhile, RDA was used to reveal the interactions between the dominant microorganisms and physicochemical parameters. The results showed that the eigenvalues of the x- and y-axes were 0.33 and 0.08, respectively, which explained 41.54% of the total variance (Figure 4A). NH3-N, TN, TP, temperature, and season were the environmental factors that had a great influence on microorganisms. Among them, the correlations between autotrophic microorganisms (Cyanobacteria, Chlorophyta, and Bacillariophyta) and nutrients (NH3-N, TN, and TP) in the water body showed a positive correlation, which is consistent with the findings of studies on their widespread existence in freshwater habitats [2,33]. It is worth mentioning that the abundance of Cyanobacteria was strongly correlated with NH3-N, TN, and TP (p < 0.05, Figure S7). This was followed by the season, which was also strongly correlated with the algae. However, these factors were negatively correlated with heterotrophic bacteria (Proteobacteria, Actinobacteriota, Bacteroidota, and Verrucomicrobiota).
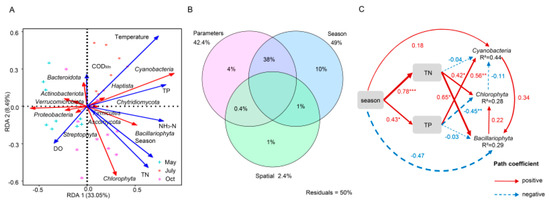
Figure 4.
Environmental variables affect the planktonic microbial community in freshwater lakes. (A) Redundancy analysis (RDA) between physicochemical indices and phylum level key species at each sampling site in May, July and October. Red arrows indicate microorganisms and blue arrows indicate physicochemical indices. (B) Variance Partitioning analysis (VPA) to distinguish the effects of water quality parameters, seasonal and spatial factors on microorganisms. (C) Path analysis based on structural equation model (SEM) shows direct and indirect effects between environmental variables and the key planktonic microorganisms. The continuous (red) and dotted lines (blue) indicate positive and negative relationships respectively. The significance levels were * p < 0.05, ** p < 0.01 and *** p < 0.001, respectively. The number next to the path line is the path coefficient (normalized regression weight), and the width of the line is proportional to the strength of the path coefficient. The model parameters are assumed as follows: Fisher’s C = 2.85, P = 0.58, AIC = 48.85, BIC = 81.08.
The variables affecting species were further divided into physicochemical parameters and seasonal and spatial factors, which were used for VPA, as shown in Figure 4B. The seasonal explanation rate of community variations was higher than that of physicochemical parameters (10% and 4%, respectively), and the interactive explanation of seasonal and physicochemical parameters was higher than that of them alone (38%, 10%, and 4%, respectively), while the spatial effect had the lowest explanation rate (1%). SEM also showed that TN and TP had great effects on microorganisms (Figure 4C). The season directly and significantly affected TN and TP, and TN and TP further significantly affected microorganisms. Simultaneously, the season also had direct effects on Cyanobacteria and Bacillariophyta but not on Chlorophyta. These results indicated that season, N, and P inputs jointly explained the variations in community structure.
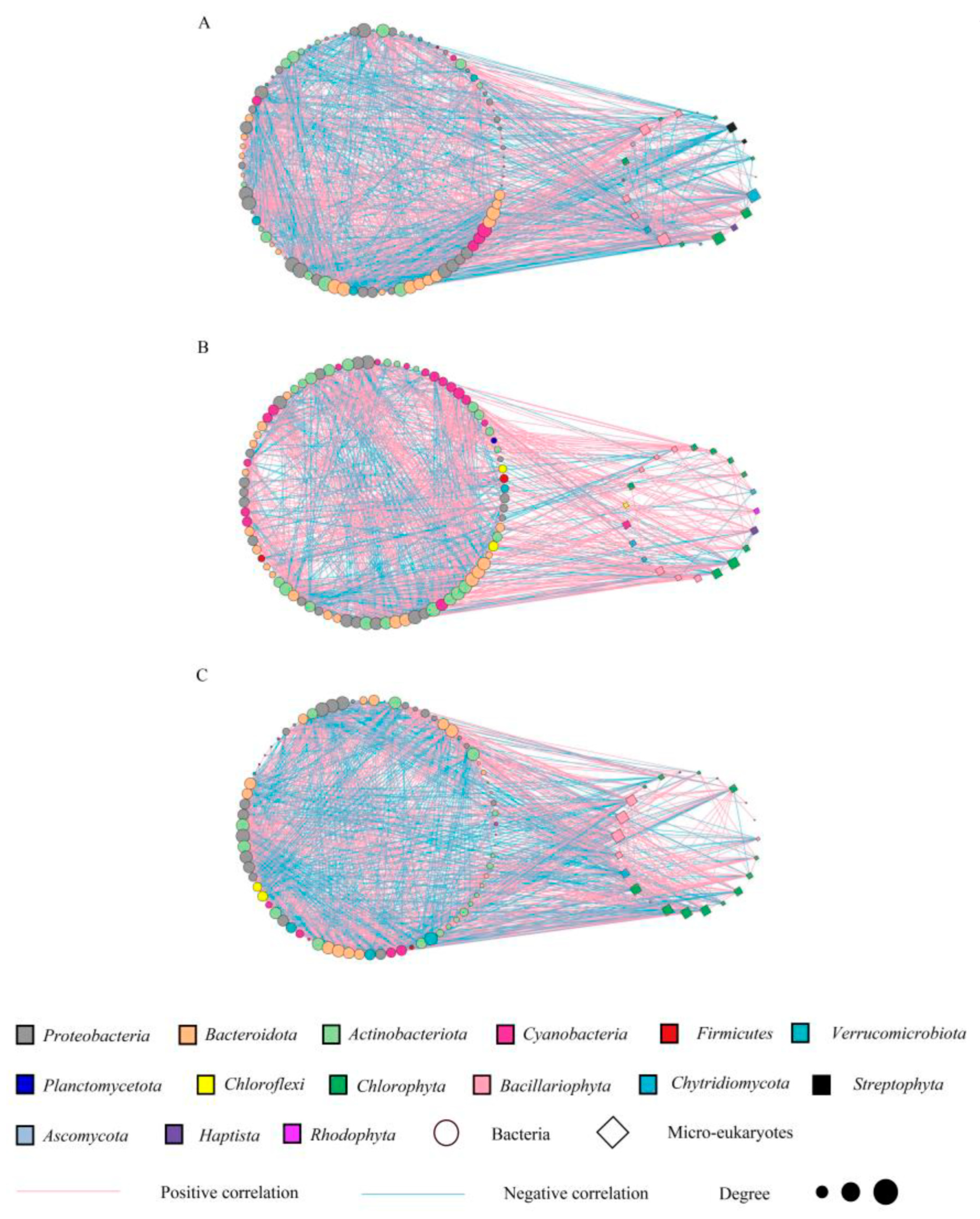
3.4. Interactions of the Bacterial and Microeukaryotic Communities
The determination of the interactions between different species is critical to a greater understanding of the diversity and function of communities. A molecular ecological network (MEN) was constructed to explore the co-occurrence patterns of planktonic microorganisms in different seasons (Figure 5A–C). To compare the network characteristics of communities, some topological properties commonly used in MENA (Table 2) were calculated. In terms of season, we obtained 108, 104, and 99 nodes in the network of May, July, and October, respectively. The number of edges representing correlation is between 1162 and 1499. Moreover, the modularity of the cooccurrence networks was 1.39–2.93 (4, 7, and 5 modules in May, July, and October, respectively) Table 2, and the average clustering coefficient (avgCC) was 0.65–0.69. These network topology properties indicated that the interactions between species were strong and that the communities were stable [34,35]. In addition, the average degree, density, modularity, and average clustering coefficient of the networks were the highest in October, followed by July, and the lowest in May. The above results indicated that the microorganisms had a stronger co-occurrence pattern in October. Meanwhile, the interactions of the bacterial and microeukaryote networks were significantly different (p < 0.05, Table S4), and all of them were based on a cooperative relationship (accounting for more than 50%, Table S4), especially among microeukaryotes, which was the most extensive in October (91.57%, Table S4). It is also worth mentioning that the negative correlation between bacteria and microeukaryotes was still the largest in October (46.76%, Table S4), indicating that this period drove the different survival patterns of the community as interspecific competition became more intense.
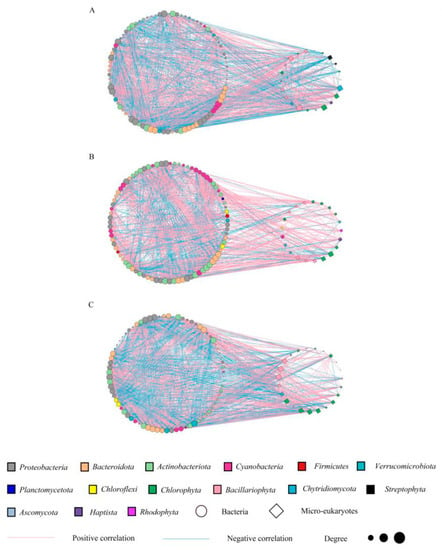
Figure 5.
The network was established by calculating the correlation between microbial communities under seasonal variations. Network nodes are colored according to species phyla. (A–C) represent the molecular ecological networks at different seasons (May, July, and October, respectively).

Table 2.
Topological properties in molecular ecological networks.
In addition, as a primary source of productivity, autotrophic algae are essential to aquatic ecosystems. In this study, under the different seasons (July vs. May and October vs. May), the associations between the autotrophic algae (Cyanobacteria, Chlorophyta) and other species increased (5.56% and 7.41% in May, 16.35% and 7.69% in July, and 9.09% and 15.15% in October, respectively, Table S5), while those of Bacillariophyta decreased (6.48% in May, 5.77% in July, and 6.06% in October). This suggested that the interactions of dominant algae (Cyanobacteria and Chlorophyta) were stronger in July and October. Moreover, the interactions between some heterotrophic bacteria (Proteobacteria, Actinobacteriota, and Verrucomicrobiota; 33.33%, 16.67%, and 5.56% in May; 19.23%, 21.15%, and 0.96% in July; and 23.23%, 16.16%, and 4.04% in October, respectively) and other species were weakened. Cyanobacteria and Chlorophyta with higher degrees in the network also showed a negative correlation (the average was 58.62%) with these bacteria (Proteobacteria, Actinobacteriota, Bacteroidota, and Verrucomicrobiota).
3.5. Community Assembly of Bacteria and Microeukaryotes
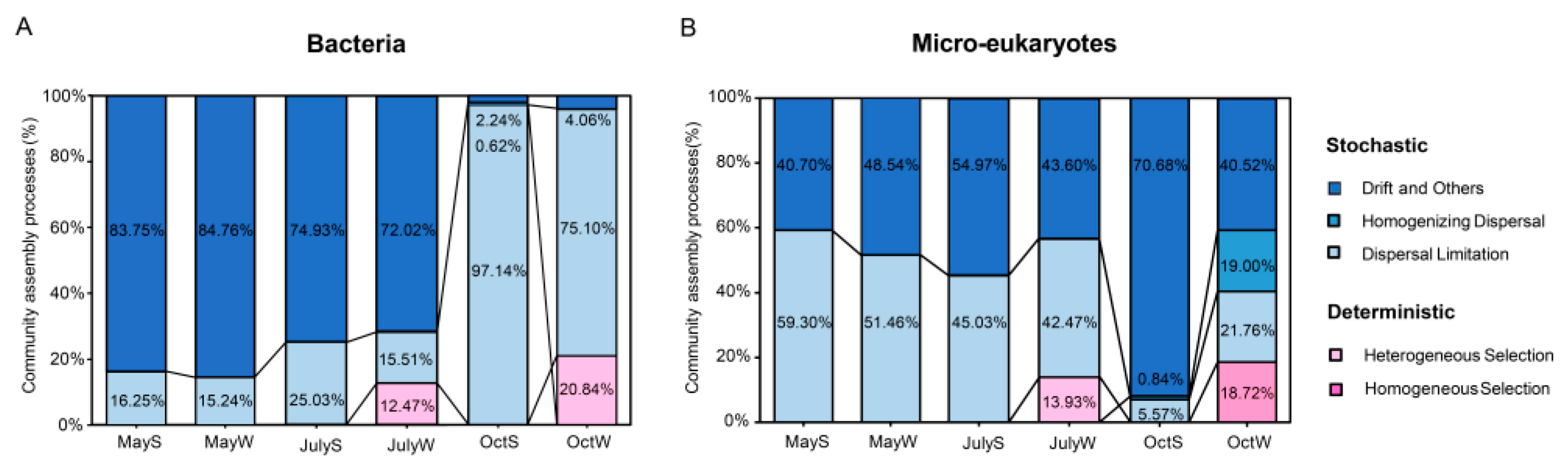
The microbial community of freshwater ecosystems often shows obvious seasonal succession [15], but the possible variations in their assembly processes and the differences between the underlying mechanisms have largely been unassessed. Thus, the iCAMP tool, which was newly developed on the Galaxy platform, was used to reveal the assembly processes of different microbiomes (called “bins”). The tool distributed the observed OTUs to 30 phylogenetic chambers, and the results are shown in Figure 6. For the bacterial community, in general, drift accounted for the largest proportion (from 2.24% to 84.76%, Table S6 and Figure 6A) and dominated in May and July (averages of 84.26% and 73.38%, respectively), while dispersal limitation dominated in October (average of 86.12%). However, the microeukaryotic community showed the opposite variation trend. Dispersal limitation dominated in May (average of 55.38%), and drift dominated in July and October (averages of 49.29% and 55.60%, respectively; Table S6 and Figure 6B). These results indicate that the community assembly processes (bacteria and microeukaryotes) also varied with the change in seasons, whereas spatial locations had a limited effect on them because they were similar at the S and W sites (p > 0.05). There was also a deterministic process in which a large proportion of heterogeneous selection (12.47%, 13.93%, and 20.84%, Figure 6A,B) and homogeneous selection (18.72%, Figure 6B) occurred at the W sites in July and October, while the effect at other sites was negligible. At the same time, the iCAMP results also showed that the bins causing this deterministic process were identified as some autotrophic algae (Cyanobacteria, Chlorophyta, and Bacillariophyta). Among them, Cyanobacteria accounted for 16.67–33.33% of the deterministic process and only 3.33–6.67% of the stochastic process. There was little difference in the amount of Chlorophyta and Bacillariophyta in assembly processes (averages of 40.00% and 26.67% in stochastic processes and 43.33% and 33.33% in deterministic processes, respectively). In summary, these results showed that stochastic processes (dispersal limitation and drift) dominated community assembly.
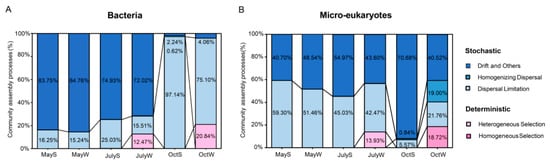
Figure 6.
The relative contributions of deterministic and stochastic processes of planktonic bacteria (A) and microeukaryotes (B) under seasonal changes. Deterministic process includes heterogeneous selection and homogeneous selection, and stochastic process includes drift, homogeneous dispersal and dispersal limitation.
4. Discussion
Agriculture is one of the largest activities affecting lake ecology [36]. In this paper, the focus was on planktonic bacteria and algae, which play an important role in the lake’s ecological environment, and the variations in community structure were studied in combination with the seasonal changes in farmland water retreat. The results showed that the N and P concentrations in the lake increased significantly under the influence of seasonal agricultural irrigation, which greatly affected the microbial community, especially for some autotrophic algae (Cyanobacteria, Chlorophyta, and Bacillariophyta) and heterotrophic bacteria (Proteobacteria, Actinobacteriota, Bacteroidota, and Verrucomicrobiota).
In order to understand these algae and bacterial traits, the interactions and assembly processes of communities were further analyzed. These results indicated that with the increase in seasonal N and P inputs, the competition between communities became more intense, a response to their competition for nutrient resources with each other. Community assembly was dominated by stochastic processes, which were mostly related to hydrodynamic conditions. In July and October, N and P inputs to the lake increased significantly due to the effects of autumn irrigation and watering, and the effects of these environmental conditions on community assembly were more obvious.
4.1. N and P Inputs Significantly Affected the Seasonal Variations in Community Structure
The nutrients concentrations, especially those nitrogen and phosphorus inputs, tended to increase in lakes receiving diffuse loading from agriculture [37]. In this study, it was also observed that nitrogen and phosphorus concentrations increased significantly with seasonal agro-irrigation. Simultaneously, the dynamics of the planktonic microbial community under nutrient load have received extensive attention [38]. Previous studies have shown that seasonality is an important source of stress on microbial communities, causing high fluctuations in physicochemical properties and thus strongly affecting microorganisms [39], which was further confirmed in this study.
The seasonal variations in bacterial and microeukaryotic community structure had novel findings. In particular, with the increase in N and P inputs during the agricultural irrigation seasons, at the phylum level, the abundance of Cyanobacteria increased significantly. As might be expected, because Cyanobacteria have a high demand for N and P, many of its species have an advantage in utilizing N and P [40]. However, excessive N and P inputs and seasonal variations can exacerbate eutrophication and harmful algal blooms, which is worrisome [41,42]. At the family level, the abundance of autotrophic algae capable of oxygen-producing photosynthesis (Cyanobiaceae, Stephanodiscaceae, Chaetocerotaceae, Scenedesmaceae, Chromulinaceae, and Dinobryaceae) and bacteria involved in heterotrophic denitrification (Comamonadaceae) increased, while the abundance of some heterotrophic bacteria decreased (Rhodobacteraceae, Microbacteriaceae, Flavobacteriaceae, and Burkholderiaceae). This means that agricultural irrigation may lead to algal blooms and damage the inherent characteristics and functions of the lake, which does not benefit the ecosystem.
It was also of interest that the MENA results indicate that dominant algae had a stronger interaction pattern in October. Therefore, to further explore the key environmental factors driving community variations, path analysis was conducted through SEM to clarify the relationships between algal blooms and environmental variables (Figure 4C). The SEM results reported that season had significant positive effects on TN (λ = 0.78, p < 0.001, n = 30) and TP (λ = 1.43, p < 0.05, n = 30). The season also had a direct positive effect on Cyanobacteria (λ = 0.18, p > 0.05, n = 30) but a negative effect on Bacillariophyta (λ = −0.47, p > 0.05, n = 30). Simultaneously, TN positively drove Chlorophyta and Bacillariophyta (λ = 0.42 and 0.65, p < 0.05, n = 30). TP positively regulated Cyanobacteria to the largest extent (λ = 0.56, p < 0.01, n = 30) but had a negative effect on Chlorophyta (λ = −0.45, p < 0.01, n = 30). TP also showed a weak linear correlation with Bacillariophyta (λ = −0.03, p > 0.05, n = 30). The total effect showed that the season was the most important factor because its direct and indirect effects on microorganisms were consistently the strongest (Figure 6C). The responses of seasonality to microorganisms indicated that they have different survival patterns due to the stress of environmental changes, as the seasonal effects on Cyanobacteria and Bacillariophyta were completely opposite. Of the physicochemical factors, TN and TP had a greater impact on microorganisms in this study, and these factors also affect microbial communities in other ecosystems [43,44,45]. Therefore, N and P inputs were the main factors driving the variations in the community on a seasonal scale [46]. Seasonal agro-irrigation could have an impact on the trophic state of water bodies (especially an increase in N and P inputs) and result in the differentiation of the niches of species, which ultimately causes variations in community structure [47,48,49].
4.2. Specialized Interaction Patterns between Phytoplankton and Bacteria with N and P Inputs
The biological association between phytoplankton and bacteria is the most important ecological relationship in aquatic environments [4]. Anthropogenic disturbances such as nutrient inputs can alter the interactions between phytoplankton and bacteria [50], and the results of this study also indicated that there were new changes in their interactions under the influence of agricultural activities. In different agro-irrigation seasons, interaction patterns among communities were driven by the increase in N and P inputs. In the case of autotrophic microorganisms, the cooccurrence pattern of Cyanobacteria and Chlorophyta was strengthened, while that of Bacillariophyta was weakened. This indicated not only a cooperative relationship between the dominant algae in a receiving lake but also a response to their competition for nutrient resources with each other. Thus, once a suitable environment developed (such as the increased N and P inputs and temperature in July and October), some of the more competitive algae would be prone to bloom and strengthen their association with other species. At the same time, SEM also revealed the specialized interaction relationships between species under changing environmental conditions. There was a positive correlation between Cyanobacteria and Bacillariophyta (λ = 0.34, p > 0.05, n = 30). Bacillariophyta also had a positive effect on Chlorophyta (λ = 0.22, p > 0.05, n = 30), while Chlorophyta had a direct negative one-way effect on Cyanobacteria (λ = −0.11, p > 0.05, n = 30). Therefore, specialized interaction patterns are necessary for the communities in a receiving lake to respond to the multiple environmental stresses imposed by seasonal variations. The complex interactions between different species maintain the stability of community structure. In addition, with the increase in N and P inputs, Bacillariophyta may be replaced by the more competitive Cyanobacteria and Chlorophyta, as the latter exhibited a stronger survival pattern.
On the other hand, the interaction patterns of some heterotrophic bacteria (Proteobacteria, Actinobacteriota, and Verrucomicrobiota) were also weakened, as Cyanobacteria and Chlorophyta were more competitive. Obviously, an increase in Cyanobacteria and Chlorophyta can inhibit the growth of these bacteria with adequate nutrients [2]. This result mainly reflected that the appropriate N and P inputs improved the competitiveness of photosynthetic microorganisms, while the content of organic matter on which the bacteria depended decreased. [33,51]. Second, bacteria began to proliferate in May, while the microeukaryotes had just returned to life, and the predation on bacteria was not strong [52]. In July and October, although the concentrations of N, P, and temperature increased, the predation of microeukaryotes was strengthened [52], which was also confirmed by the richness and diversity of the two species (Figure S2). This means more competition among the different species with the increase in N and P inputs [4,53]. Nevertheless, planktonic algae have an extensive mutualistic relationship with bacteria, and it can be argued that mutualism among these organisms is more common than competition [4]. Overall, the interactions between planktonic algae and bacteria are highly complex. The mineralization of bacteria can provide a large amount of nutrients for the growth of planktonic algae. However, it can also compete for inorganic nutrients [4]. In addition, some algicidal bacteria can control blooms by dissolving planktonic algal cells, and others can buffer the effects of phycotoxins [54]. However, some bacteria can promote the growth of harmful algal blooms and even increase the production of toxins [4]. In conclusion, the interactions between phytoplankton and bacteria were diverse, and their relationship depended on the supply of nutrient resources, which can be cooperative or competitive.
4.3. N and P Inputs Contributed Greatly to Community Assembly
It is important to study the community assembly to verify whether community succession has a universal law. Generally, the variations in microbial community structure are driven by both deterministic and stochastic processes, but there has been debate about the relative importance of the two processes [13]. One of the studies showed that stochastic processes dominated the microbial community variations in aquatic environments in a lotic river [55]. In contrast, another study demonstrated that deterministic processes played a key role in shaping the microbial community [56]. Similarly, this study not only revealed the dominant role of stochastic processes but also showed the importance of deterministic processes during autumn irrigation and autumn watering.
Deterministic and stochastic processes work together in microbial assembly, and their equilibrium is dependent upon the richness of the initial community [15]. Communities with small habitats, high species diversity, and strong species interactions tend to be more susceptible to stochastic processes [57], while deterministic processes dominate when species diversity is lower [58]. In this study, the richness and diversity of bacteria were the lowest in October (Figure S2A), which also supported the results of the above studies. However, the variations in α-diversity in microeukaryotes were opposite to those in bacteria. Most studies on community assembly have focused on bacteria [17,59], so this study verified that the assembly process of microeukaryotes may be different from that of bacteria [60,61]. This may be related to their differences in interaction patterns (cooperation or competition), which can strongly influence their dispersal capability and niche widths [62,63]. Meanwhile, the richness and diversity of microeukaryotes were much lower than those of bacteria (Figure S2B). The random survival, death, and extinction of species may have more intense effects on communities with smaller population sizes, thus leading to an increase in the relative importance of drift (Figure 6A,B) [57,58].
In addition, the effects of agricultural irrigation on community assembly came from two aspects: nutrient supply and hydrodynamic drive. First, the concentrations of NH3-N, TN, and TP were highest in October, followed by July. The W sites are located in the main drainage channels and are greatly affected by human activities and environmental changes [64], which may explain the large proportion of the deterministic process in community assembly at the W sites in July and October [59,65]. Meanwhile, it is noteworthy that the highly variable community structure is inseparable from heterogeneous selection when environmental conditions vary greatly over time or space [13]. The deterministic process was dominated by heterogeneous selection. Obviously, the increase in N and P inputs affected the microbial community assembly processes.
Moreover, when microorganisms disperse passively to new habitats under hydrodynamic conditions, they may colonize more easily and cause more similar community structures through dispersal and drift [66]. The community maintains the assembly processes of random growth, reproduction, and death to adapt to environmental changes [67,68]. Thus, stochasticity is more important than determinism [59]. It is believed that, in many cases, the large number of variations observed in the microbial community may be due to stochastic processes of community assembly (ecological drift, dispersal limitation, or historical chance, e.g., priority effects). When strong interactions and variable migration are combined, it is possible to form an apparently random community structure, demonstrating the importance of ecological randomness for community succession. Previous studies on microbial ecology have mostly concentrated on deterministic processes while ignoring the essentiality of stochastic processes [16]. At present, microbial communities are increasingly sensitive to stochastic processes, and their ecological predictability is increasing, indicating that stochastic processes play a crucial role in regulating the structure and function of microbial communities [16].
Niche width is also a key characteristic that affects the alignment of deterministic and stochastic processes in community assembly [63,66]. To reveal the relationship between them, the niche widths of 10 dominant species (covering approximately 90% of the bacterial and microeukaryotic communities) were calculated in agro-irrigation seasons [59], and it was found that differences in niche widths could be observed among them (Figure S9) [62]. The niche of bacteria was widest in May, followed by July and October (the average of 7.18, 7.07, and 6.61, respectively), while that of microeukaryotes had the opposite (the average of 3.78, 4.52, and 5.36, respectively). Previous studies have shown that rich nutrient resources promote a wider niche of species [69]. Meanwhile, most bacteria are heterotrophic and rely on more organic matter [70,71], while microeukaryotes are autotrophic and have higher requirements for N and P [72]. Combined with the change in C, N, and P in water quality parameters, which could explain the differences in niche widths in each group, this also may correspond with the variabilities in their dominant stochastic process in different agro-irrigation seasons [62]. Previous studies have also shown that species with a wider niche were less affected by environmental changes [66]. In this study, seasonal variations in bacterial niche width corresponded to a greater transition between stochastic and deterministic processes, and community assembly was more stochastic in May.
Simultaneously, the species interactions also influenced community assembly. As the N and P inputs increased, the deterministic processes were caused by some dominant algae with a stronger interaction. Therefore, N and P inputs from agricultural irrigation influenced species diversity, niche width, and interactions, which together controlled community construction.
5. Conclusions
This study reported the succession of planktonic bacterial and microeukaryotic community structure during three agro-irrigation seasons in a typical agricultural drainage receiving lake, namely Wuliangsuhai Lake. In addition, the obtained conclusions are as follows: Under the influence of agricultural activities, nutrient concentrations in the lake increased significantly, especially nitrogen and phosphorus inputs. In addition to significantly changing the microbial community structure, nitrogen and phosphorus were also essential for community interactions and assembly processes, which made the bacterial and microeukaryotic community composition and diversity vary greatly with agro-irrigation seasons. These results suggested that the planktonic bacterial and microeukaryotic communities respond to environmental changes in opposite ways through different interaction patterns and assembly processes. This is due to the fact that they depend on different nutrients to different degrees. Unfortunately, only one year’s worth of data was obtained for this study, which may lead to some limitations in the results. In addition, this study also showed that autumn watering is the peak of algal blooms, so the monitoring of lake nutrient status and pollution control should be strengthened. Further, improving the fertilizer utilization efficiency in the Hetao irrigation area is an effective measure to reduce the inputs of nitrogen and phosphorus to the lake. However, how to reduce fertilizer application on the premise of ensuring adequate food will be the hot spot and challenge of future researches.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15032584/s1, Figures S1–S9; Tables S1–S6.
Author Contributions
Conceptualization, L.Z.; Methodology, E.X.; Validation, J.C.; Investigation, D.H. and H.Z.; Resources, E.X.; Data curation, D.H. and J.C.; Writing–original draft, D.H. and G.W.; Writing–review & editing, E.X.; Visualization, D.H. and H.Z.; Supervision, L.Z. and E.X.; Funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D Program of China (No. 2021YFE0192500) and the National Natural Science Foundation of China (No. 51861125103).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, X.; Wei, J.; Bai, N.; Cha, H.; Cao, C.; Zheng, K.; Liu, Y. The phosphorus fractions and adsorption-desorption characteristics in the Wuliangsuhai Lake, China. Environ. Sci. Pollut. Res. Int. 2018, 25, 20648–20661. [Google Scholar] [CrossRef]
- Ji, B.; Qin, H.; Guo, S.; Chen, W.; Zhang, X.; Liang, J. Bacterial communities of four adjacent fresh lakes at different trophic status. Ecotoxicol. Environ. Saf. 2018, 157, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.R.; Amin, S.A.; Raina, J.-B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton–bacteria relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef] [PubMed]
- Tanentzap, A.J.; Fitch, A.; Orland, C.; Emilson, E.J.S.; Yakimovich, K.M.; Osterholz, H.; Dittmar, T. Chemical and microbial diversity covary in fresh water to influence ecosystem functioning. Proc. Natl. Acad. Sci. USA 2019, 116, 24689–24695. [Google Scholar] [CrossRef]
- Foo, J.L.; Ling, H.; Lee, Y.S.; Chang, M.W. Microbiome engineering: Current applications and its future. Biotechnol. J. 2017, 12, 1600099. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Khorshidi Nazloo, E.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Khan, A.; Xiong, J.; Tian, F.; Li, X. A critical review on the interaction of substrate nutrient balance and microbial community structure and function in anaerobic co-digestion. Bioresour. Technol. 2018, 247, 1119–1127. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, X.; Ren, M.; Yuan, D.; Tan, Q.; Xing, Y.; Xia, X.; Xie, E.; Ding, A. Comparing with oxygen, nitrate simplifies microbial community assembly and improves function as an electron acceptor in wastewater treatment. Environ. Pollut. 2022, 314, 120243. [Google Scholar] [CrossRef]
- Niu, K.; Yi-ning, L.; Shen, Z.; Fang-liang, H.; Jing-yun, F. Community assembly: The relative importance of neutral theory and niche theory. Biodivers. Sci. 2009, 17, 579. [Google Scholar] [CrossRef]
- Chave, J. Neutral theory and community ecology. Ecol. Lett. 2004, 7, 241–253. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, X.; Ding, A.; Yuan, D.; Tan, Q.; Xing, Y.; Xie, E. Ecological Insights Into Community Interactions, Assembly Processes and Function in the Denitrifying Phosphorus Removal Activated Sludge Driven by Phosphorus Sources. Front. Microbiol. 2021, 12, 779369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ning, D. Stochastic Community Assembly: Does It Matter in Microbial Ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ning, D.; Yang, Y.; Van Nostrand, J.D.; Zhou, J.; Wen, X. Biodegradability of wastewater determines microbial assembly mechanisms in full-scale wastewater treatment plants. Water Res. 2020, 169, 115276. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, B.; Ning, D.; Zhang, Y.; Dai, T.; Wu, L.; Li, T.; Liu, W.; Zhou, J.; Wen, X. Seasonal dynamics of the microbial community in two full-scale wastewater treatment plants: Diversity, composition, phylogenetic group based assembly and co-occurrence pattern. Water Res. 2021, 200, 117295. [Google Scholar] [CrossRef]
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, S.; Yan, R.; Wang, R.; Gao, Y.; Kong, M.; Yi, Q.; Zhang, Y. Similar geographic patterns but distinct assembly processes of abundant and rare bacterioplankton communities in river networks of the Taihu Basin. Water Res. 2022, 211, 118057. [Google Scholar] [CrossRef]
- Xu, R.; Yu, Z.; Zhang, S.; Meng, F. Bacterial assembly in the bio-cake of membrane bioreactors: Stochastic vs. deterministic processes. Water Res. 2019, 157, 535–545. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Anderson, B.; Zhang, S.; Shi, X.; Zhao, S. A modified QWASI model for fate and transport modeling of mercury between the water-ice-sediment in Lake Ulansuhai. Chemosphere 2017, 176, 117–124. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, L.; Zhao, X.; Lian, J.; Zhang, Z. Accumulation and potential sources of heavy metals in soils of the Hetao area, Inner Mongolia, China. Pedosphere 2020, 30, 244–252. [Google Scholar] [CrossRef]
- Ma, L.; Wu, J.; Abuduwaili, J. Geochemical evidence of the anthropogenic alteration of element composition in lacustrine sediments from Wuliangsu Lake, north China. Quat. Int. 2013, 306, 107–113. [Google Scholar] [CrossRef]
- Yue, W.; Meng, K.; Hou, K.; Zuo, R.; Zhang, B.-T.; Wang, G. Evaluating climate and irrigation effects on spatiotemporal variabilities of regional groundwater in an arid area using EOFs. Sci. Total Environ. 2020, 709, 136147. [Google Scholar] [CrossRef] [PubMed]
- Köbbing, J.F.; Patuzzi, F.; Baratieri, M.; Beckmann, V.; Thevs, N.; Zerbe, S. Economic evaluation of common reed potential for energy production: A case study in Wuliangsuhai Lake (Inner Mongolia, China). Biomass Bioenergy 2014, 70, 315–329. [Google Scholar] [CrossRef]
- Rojas-Jimenez, K.; Grossart, H.P.; Cordes, E.; Cortes, J. Fungal Communities in Sediments Along a Depth Gradient in the Eastern Tropical Pacific. Front. Microbiol. 2020, 11, 575207. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Barabasi, A.L.; Oltvai, Z.N. Network biology: Understanding the cell’s functional organization. Nat. Rev. Genet. 2004, 5, 101–113. [Google Scholar] [CrossRef]
- Newman, M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Liu, J.; Cheng, Y.; Zou, J.; Zhai, F.; Sun, Z.; Han, L. Soil microbial community succession and interactions during combined plant/white-rot fungus remediation of polycyclic aromatic hydrocarbons. Sci. Total Environ. 2021, 752, 142224. [Google Scholar] [CrossRef]
- Ma, J.; Qin, B.; Paerl, H.W.; Brookes, J.D.; Wu, P.; Zhou, J.; Deng, J.; Guo, J.; Li, Z. Green algal over cyanobacterial dominance promoted with nitrogen and phosphorus additions in a mesocosm study at Lake Taihu, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 5041–5049. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, L.; Zhao, Y.; Feng, Q.; Li, C.; Wei, Y. Shift of soil fungal communities under afforestation in Nanliu River Basin, southwest China. J. Environ. Manag. 2022, 302, 114130. [Google Scholar] [CrossRef]
- Quero, G.M.; Perini, L.; Pesole, G.; Manzari, C.; Lionetti, C.; Bastianini, M.; Marini, M.; Luna, G.M. Seasonal rather than spatial variability drives planktonic and benthic bacterial diversity in a microtidal lagoon and the adjacent open sea. Mol. Ecol. 2017, 26, 5961–5973. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Hu, Y.; Zhang, S.; Wu, R.; Guo, X. Microplastics in the surface water of Wuliangsuhai Lake, northern China. Sci. Total Environ. 2020, 723, 137820. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, Y.; Ma, T.; Yang, H.; He, J. Arsenic associations in sediments from shallow aquifers of northwestern Hetao Basin, Inner Mongolia. Environ. Earth Sci. 2011, 64, 2001–2011. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, J.; Zhang, K.; Wang, Y.; Xiao, R.; Campos, M.; Acuña, J.; Jorquera, M.A. Occurrence, bioaccumulation and ecological risks of antibiotics in the water-plant-sediment systems in different functional areas of the largest shallow lake in North China: Impacts of river input and historical agricultural activities. Sci. Total Environ. 2023, 857, 159260. [Google Scholar] [CrossRef] [PubMed]
- Räike, A.; Pietiläinen, O.P.; Rekolainen, S.; Kauppila, P.; Pitkänen, H.; Niemi, J.; Raateland, A.; Vuorenmaa, J. Trends of phosphorus, nitrogen and chlorophyll a concentrations in Finnish rivers and lakes in 1975–2000. Sci. Total Environ. 2003, 310, 47–59. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P.; et al. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef]
- Shang, Y.; Wu, X.; Wang, X.; Wei, Q.; Ma, S.; Sun, G.; Zhang, H.; Wang, L.; Dou, H.; Zhang, H. Factors affecting seasonal variation of microbial community structure in Hulun Lake, China. Sci. Total Environ. 2022, 805, 150294. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Gardner, W.S.; Newell, S.E.; McCarthy, M.J.; Hoffman, D.K.; Lu, K.; Lavrentyev, P.J.; Hellweger, F.L.; Wilhelm, S.W.; Liu, Z.; Bruesewitz, D.A.; et al. Community Biological Ammonium Demand: A Conceptual Model for Cyanobacteria Blooms in Eutrophic Lakes. Environ. Sci. Technol. 2017, 51, 7785–7793. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.H.; Hamilton, D.P.; Etemad-Shahidi, A.; Helfer, F. Individual-based modelling of cyanobacteria blooms: Physical and physiological processes. Sci. Total Environ. 2021, 792, 148418. [Google Scholar] [CrossRef] [PubMed]
- Xie, E.; Zhao, X.; Li, K.; Zhang, P.; Zhou, X.; Zhao, X. Microbial community structure in the river sediments from upstream of Guanting Reservoir: Potential impacts of reclaimed water recharge. Sci. Total Environ. 2021, 766, 142609. [Google Scholar] [CrossRef]
- Yu, M.; Liu, S.; Li, G.; Zhang, H.; Xi, B.; Tian, Z.; Zhang, Y.; He, X. Municipal wastewater effluent influences dissolved organic matter quality and microbial community composition in an urbanized stream. Sci. Total Environ. 2020, 705, 135952. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zheng, L.; Tan, Q.; Wang, X.; Xing, Y.; Wang, H.; Wang, S.; Zhu, G. Comammox activity dominates nitrification process in the sediments of plateau wetland. Water Res. 2021, 206, 117774. [Google Scholar] [CrossRef]
- Tian, L.; Yan, Z.; Wang, C.; Xu, S.; Jiang, H. Habitat heterogeneity induces regional differences in sediment nitrogen fixation in eutrophic freshwater lake. Sci. Total Environ. 2021, 772, 145594. [Google Scholar] [CrossRef]
- Shen, C.; He, J.Z.; Ge, Y. Seasonal dynamics of soil microbial diversity and functions along elevations across the treeline. Sci. Total Environ. 2021, 794, 148644. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, J.; Nawaz, M.Z.; Mahboob, S.; Al-Ghanim, K.A.; Khan, I.A.; Lu, Z.; Chen, T. Seasonal succession and spatial distribution of bacterial community structure in a eutrophic freshwater Lake, Lake Taihu. Sci. Total Environ. 2019, 669, 29–40. [Google Scholar] [CrossRef]
- Hongxia, M.; Jingfeng, F.; Jiwen, L.; Su, j.; Zhiyi, W.; Yantao, W.; Dongwei, L.; Mengfei, L.; Tingting, S.; Yuan, J.; et al. Full-length 16S rRNA gene sequencing reveals spatiotemporal dynamics of bacterial community in a heavily polluted estuary, China. Environ. Pollut. 2021, 275, 116567. [Google Scholar] [CrossRef]
- Pringault, O.; Bouvy, M.; Carre, C.; Mejri, K.; Bancon-Montigny, C.; Gonzalez, C.; Leboulanger, C.; Hlaili, A.S.; Goni-Urriza, M. Chemical contamination alters the interactions between bacteria and phytoplankton. Chemosphere 2021, 278, 130457. [Google Scholar] [CrossRef]
- Jia, R.; Qu, Z.; You, P.; Qu, D. Effect of biochar on photosynthetic microorganism growth and iron cycling in paddy soil under different phosphate levels. Sci. Total Environ. 2018, 612, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Fenchel, T.M.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.-A.; Thingstad, F.J.M.E.P.S. The Ecological Role of Water-Column Microbes in the Sea*. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Santi, I.; Tsiola, A.; Dimitriou, P.D.; Fodelianakis, S.; Kasapidis, P.; Papageorgiou, N.; Daffonchio, D.; Pitta, P.; Karakassis, I. Prokaryotic and eukaryotic microbial community responses to N and P nutrient addition in oligotrophic Mediterranean coastal waters: Novel insights from DNA metabarcoding and network analysis. Mar. Environ. Res. 2019, 150, 104752. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Bigalke, A.; Kaulfuß, A.; Pohnert, G. Strategies and ecological roles of algicidal bacteria. FEMS Microbiol. Rev. 2017, 41, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ren, K.; Isabwe, A.; Chen, H.; Liu, M.; Yang, J. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 2019, 7, 138. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, W.; Yang, J.; Lin, Y.; Yu, Z.; Lin, S. Biogeographic patterns of abundant and rare bacterioplankton in three subtropical bays resulting from selective and neutral processes. ISME J. 2018, 12, 2198–2210. [Google Scholar] [CrossRef]
- Lin, Q.; De Vrieze, J.; Li, C.; Li, J.; Li, J.; Yao, M.; Hedenec, P.; Li, H.; Li, T.; Rui, J.; et al. Temperature regulates deterministic processes and the succession of microbial interactions in anaerobic digestion process. Water Res. 2017, 123, 134–143. [Google Scholar] [CrossRef]
- Xun, W.; Li, W.; Xiong, W.; Ren, Y.; Liu, Y.; Miao, Y.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Diversity-triggered deterministic bacterial assembly constrains community functions. Nat. Commun. 2019, 10, 3833. [Google Scholar] [CrossRef]
- Wang, K.; Yan, H.; Peng, X.; Hu, H.; Zhang, H.; Hou, D.; Chen, W.; Qian, P.; Liu, J.; Cai, J.; et al. Community assembly of bacteria and archaea in coastal waters governed by contrasting mechanisms: A seasonal perspective. Mol. Ecol. 2020, 29, 3762–3776. [Google Scholar] [CrossRef]
- Li, H.; Song, C.; Yang, L.; Qin, H.; Cao, X.; Zhou, Y. Nutrients regeneration pathway, release potential, transformation pattern and algal utilization strategies jointly drove cyanobacterial growth and their succession. J. Environ. Sci. 2021, 103, 255–267. [Google Scholar] [CrossRef]
- Logares, R.; Tesson, S.V.M.; Canback, B.; Pontarp, M.; Hedlund, K.; Rengefors, K. Contrasting prevalence of selection and drift in the community structuring of bacteria and microbial eukaryotes. Environ. Microbiol. 2018, 20, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lu, H.P.; Sastri, A.; Yeh, Y.C.; Gong, G.C.; Chou, W.C.; Hsieh, C.H. Contrasting the relative importance of species sorting and dispersal limitation in shaping marine bacterial versus protist communities. ISME J. 2018, 12, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Sexton, J.P.; Montiel, J.; Shay, J.E.; Stephens, M.R.; Slatyer, R.A. Evolution of Ecological Niche Breadth. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 183–206. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Chen, J. Anthropogenic activities change the relationship between microbial community taxonomic composition and functional attributes. Environ. Microbiol. 2021, 23, 6663–6675. [Google Scholar] [CrossRef]
- Legendre, P.; Mi, X.; Ren, H.; Ma, K.; Yu, M.; Sun, I.-F.; He, F. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 2009, 90, 663–674. [Google Scholar] [CrossRef]
- Pandit, S.N.; Kolasa, J.; Cottenie, K. Contrasts between habitat generalists and specialists: An empirical extension to the basic metacommunity framework. Ecology 2009, 90, 2253–2262. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Stegen, J.C.; van Elsas, J.D.; Salles, J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. USA 2015, 112, E1326–E1332. [Google Scholar] [CrossRef]
- Ofiteru, I.D.; Lunn, M.; Curtis, T.P.; Wells, G.F.; Criddle, C.S.; Francis, C.A.; Sloan, W.T. Combined niche and neutral effects in a microbial wastewater treatment community. Proc. Natl. Acad. Sci. USA 2010, 107, 15345–15350. [Google Scholar] [CrossRef]
- Feng, K.; Wang, S.; Wei, Z.; Wang, Z.; Zhang, Z.; Wu, Y.; Zhang, Y.; Deng, Y. Niche width of above- and below-ground organisms varied in predicting biodiversity profiling along a latitudinal gradient. Mol. Ecol. 2020, 29, 1890–1902. [Google Scholar] [CrossRef]
- Sánchez-Clemente, R.; Guijo, M.I.; Nogales, J.; Blasco, R. Carbon Source Influence on Extracellular pH Changes along Bacterial Cell-Growth. Genes 2020, 11, 1292. [Google Scholar] [CrossRef]
- Barnett Samuel, E.; Youngblut Nicholas, D.; Koechli Chantal, N.; Buckley Daniel, H. Multisubstrate DNA stable isotope probing reveals guild structure of bacteria that mediate soil carbon cycling. Proc. Natl. Acad. Sci. USA 2021, 118, e2115292118. [Google Scholar] [CrossRef] [PubMed]
- Nehls, U.; Plassard, C. Nitrogen and phosphate metabolism in ectomycorrhizas. New Phytol. 2018, 220, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).