Elaboration and Characterization of a Biochar from Wastewater Sludge and Olive Mill Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Biochar
2.3. Physicochemical Characterization of Biochar
2.4. Surface Chemical Characterization of Biochar
2.5. Spectroscopic Characterization of Biochar
3. Results and Discussions
3.1. Physicochemical Characterization of the Biochar
3.2. Surface Characterization of Biochar
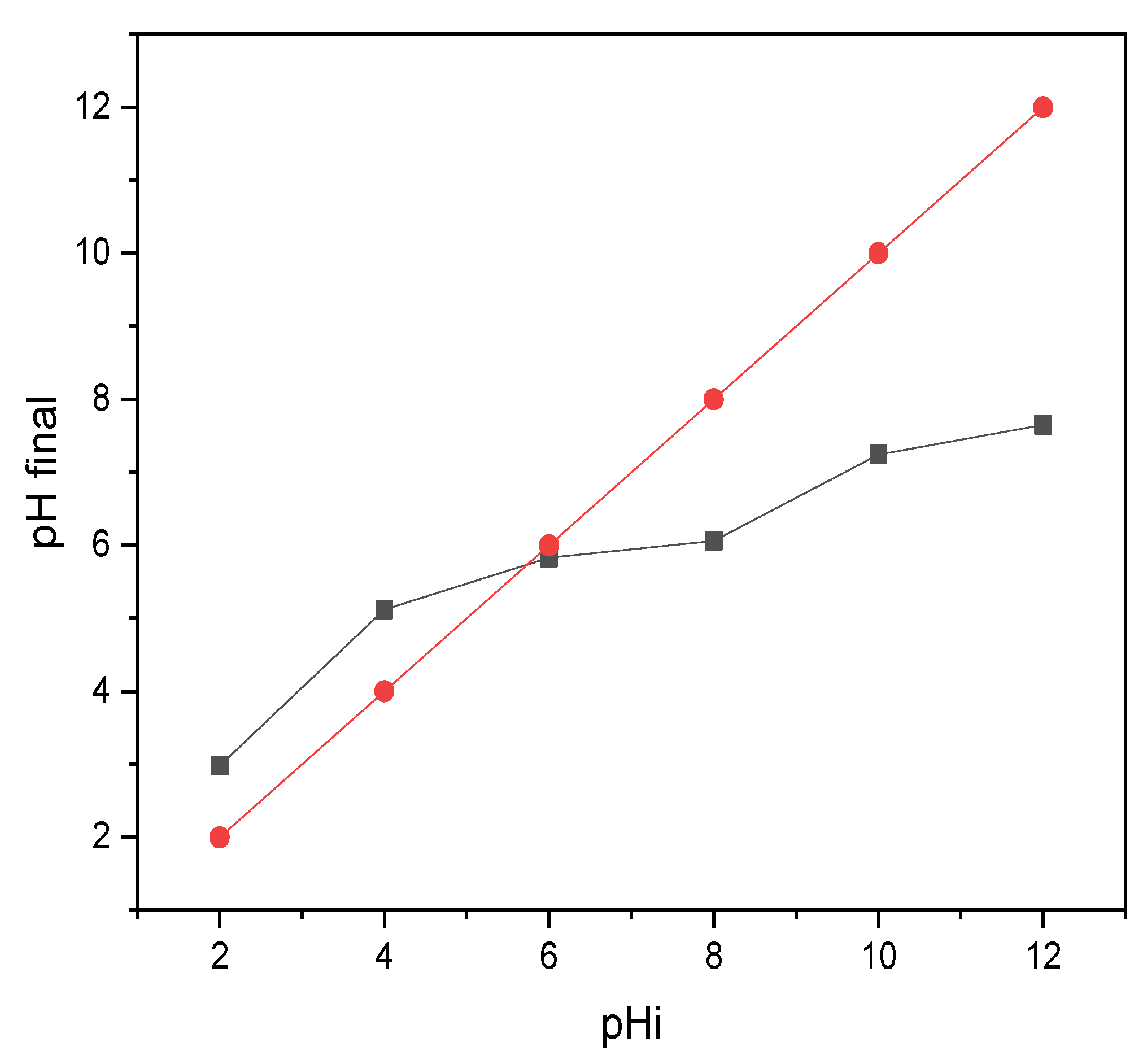
3.2.1. Zero Charge Point pHpzc of Biochar
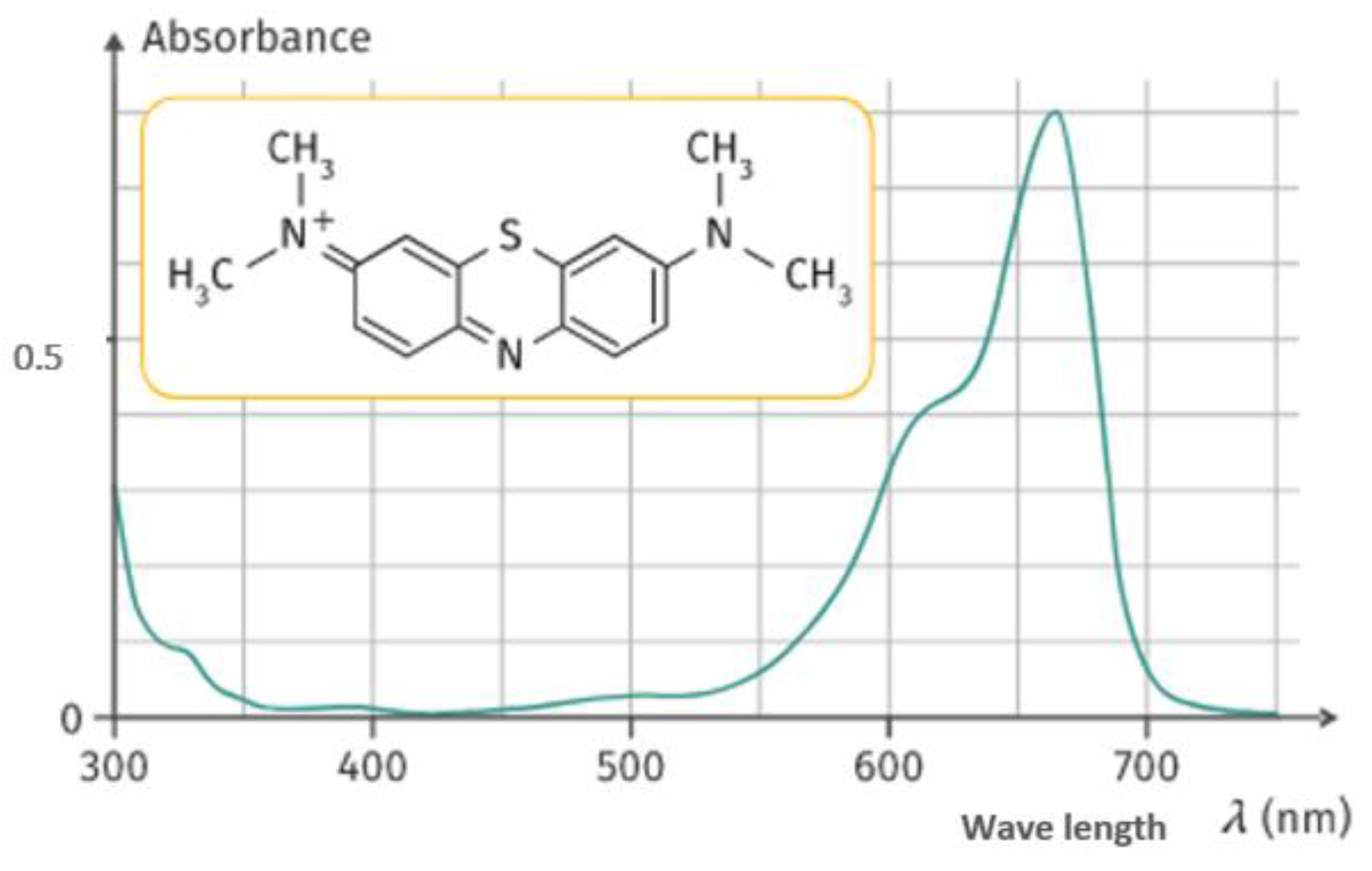
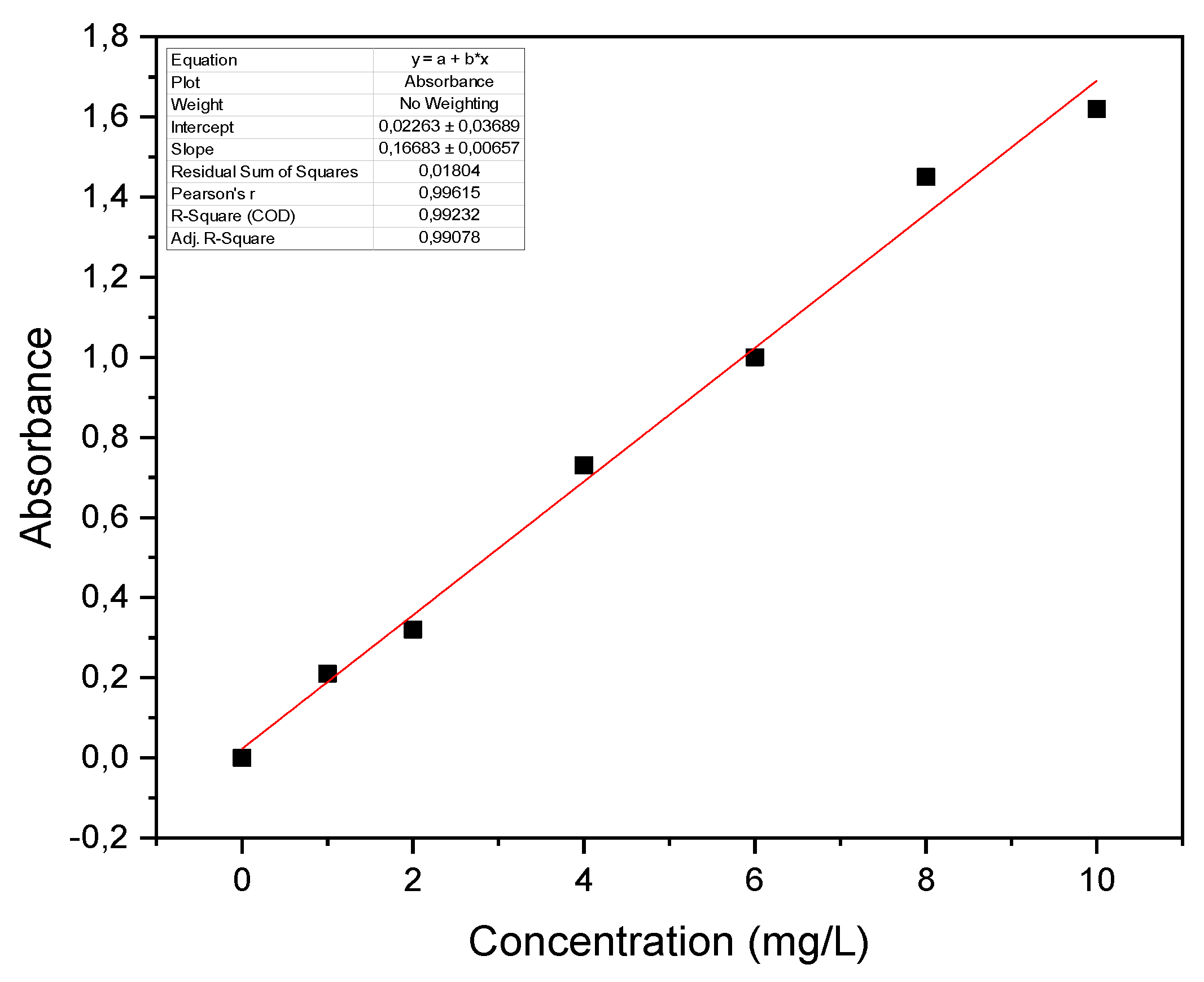
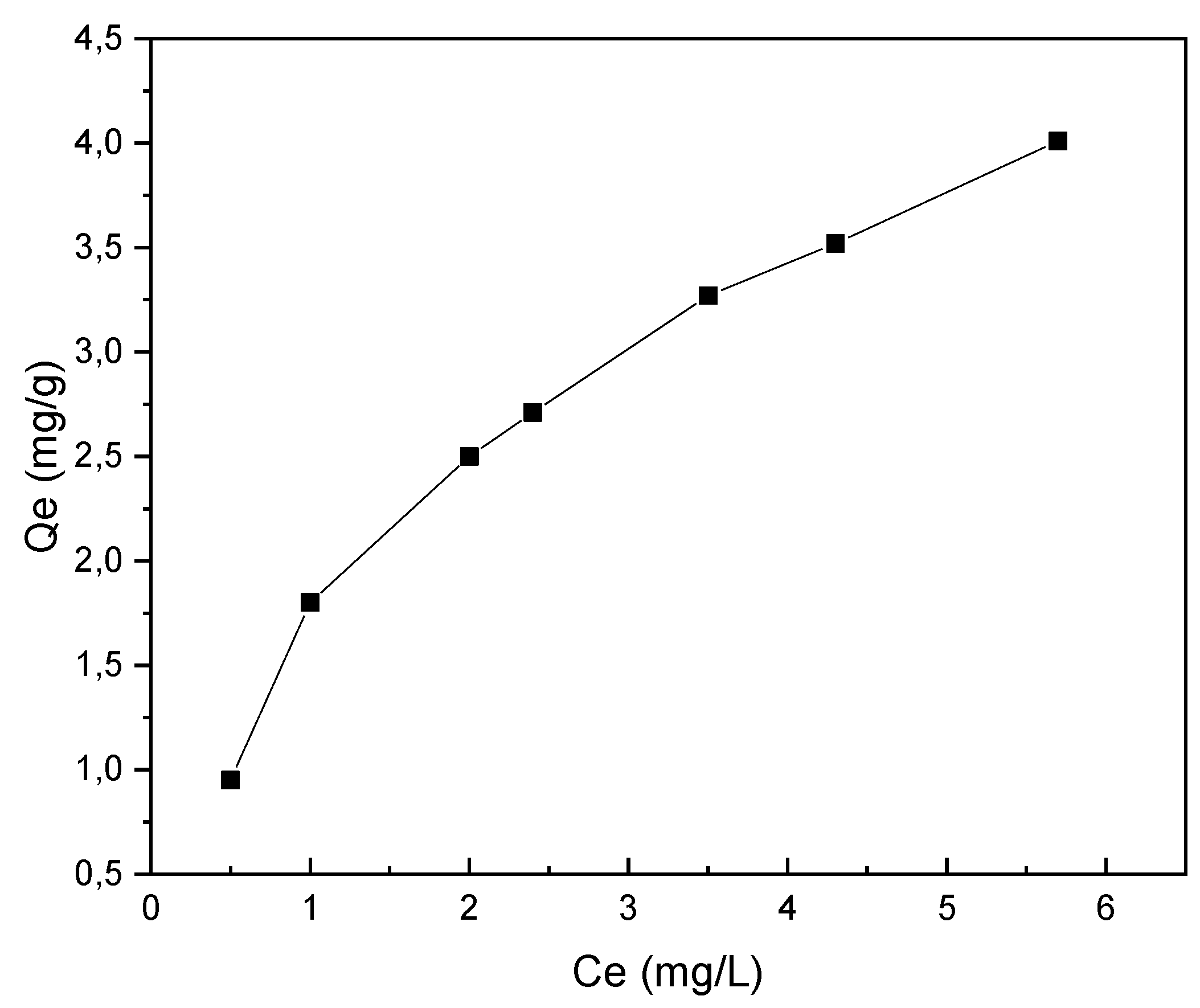
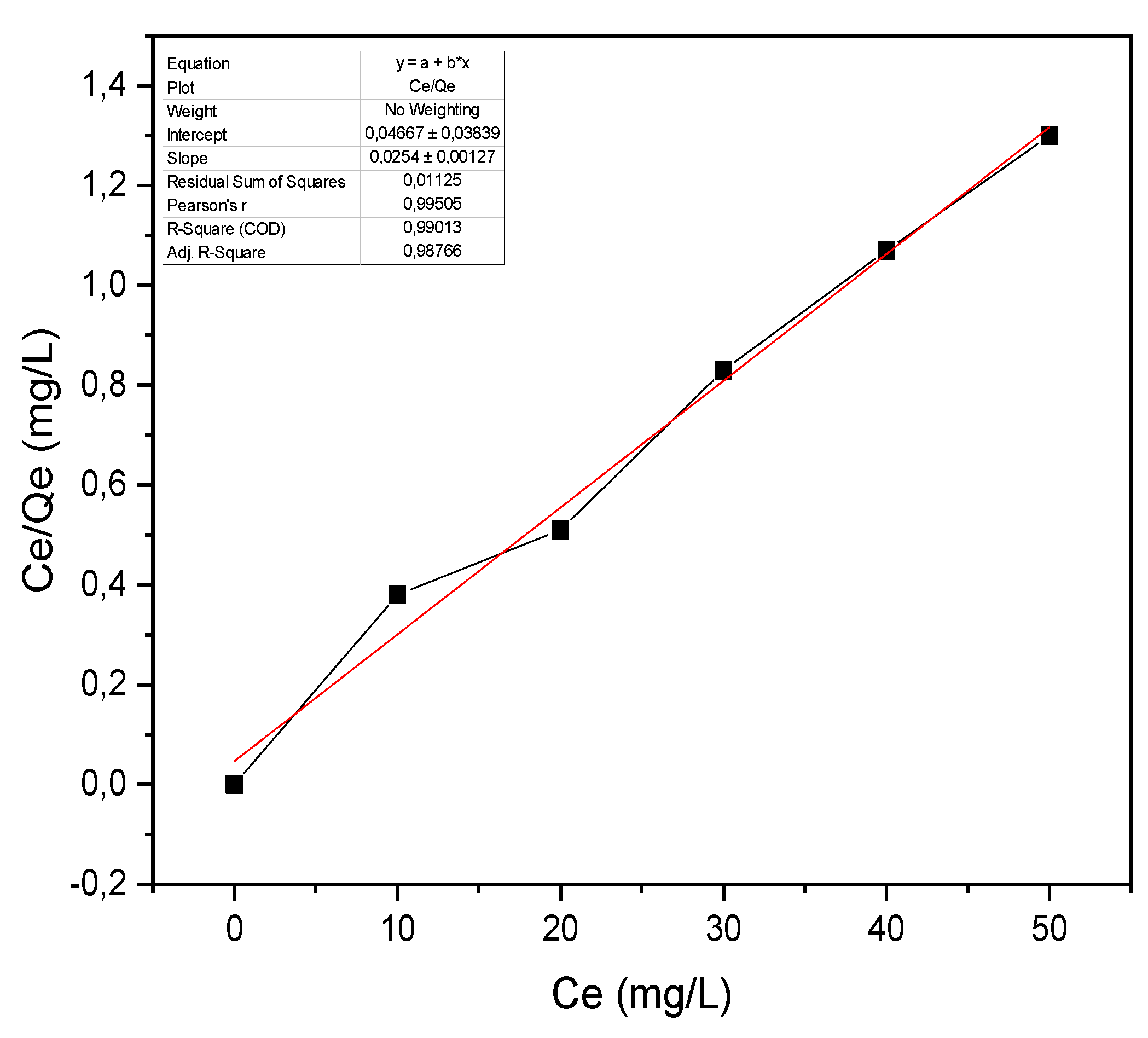
3.2.2. Specific Surface of Biochar by the Methylene Blue Method (BM)
3.2.3. Methylene Blue Index (MBI)
3.2.4. Iodine Index (Ii)
3.2.5. Surface Chemical Characteristics of Biochar
3.3. Spectroscopic Characterization
3.3.1. Fourier Transform Infrared
3.3.2. X-ray Diffraction
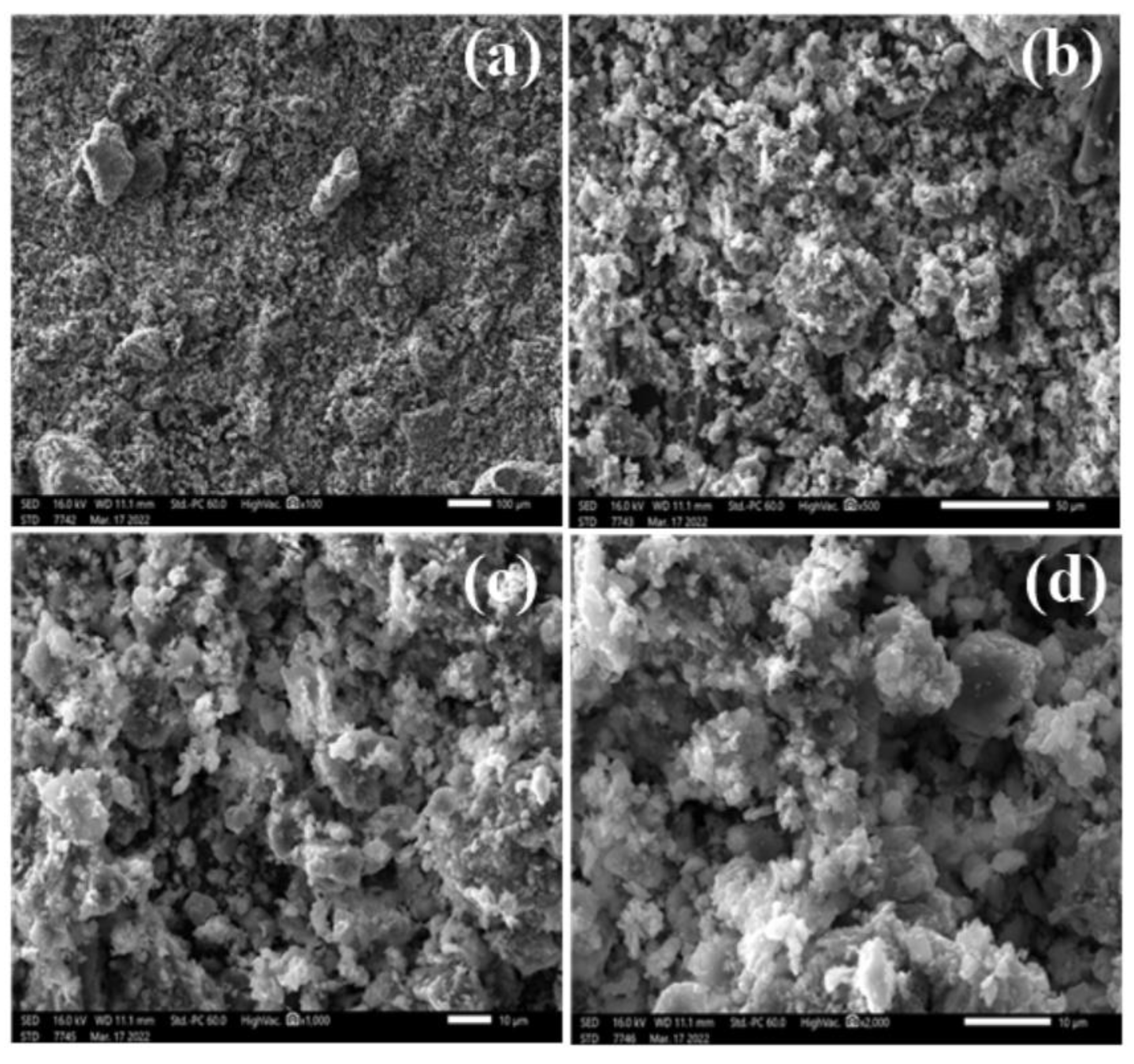
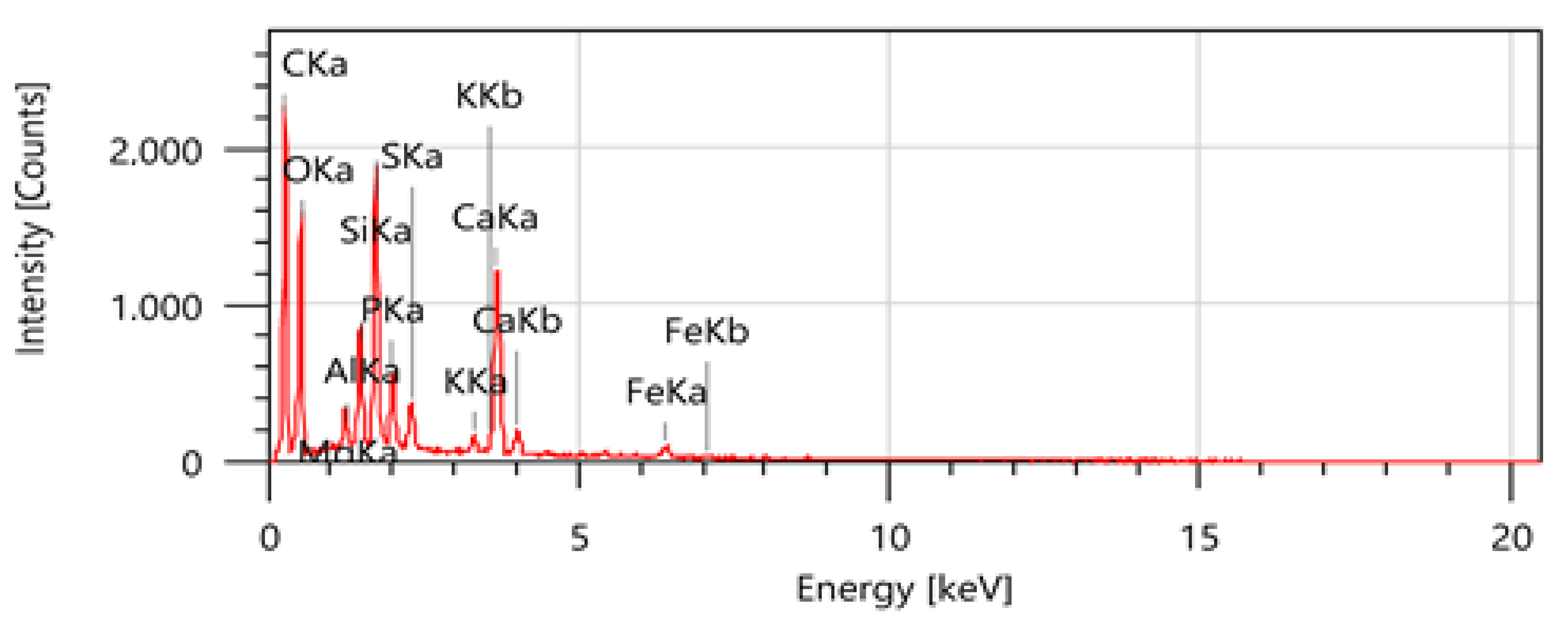
3.3.3. Scanning Electron Microscopy Coupled to an EDX Probe
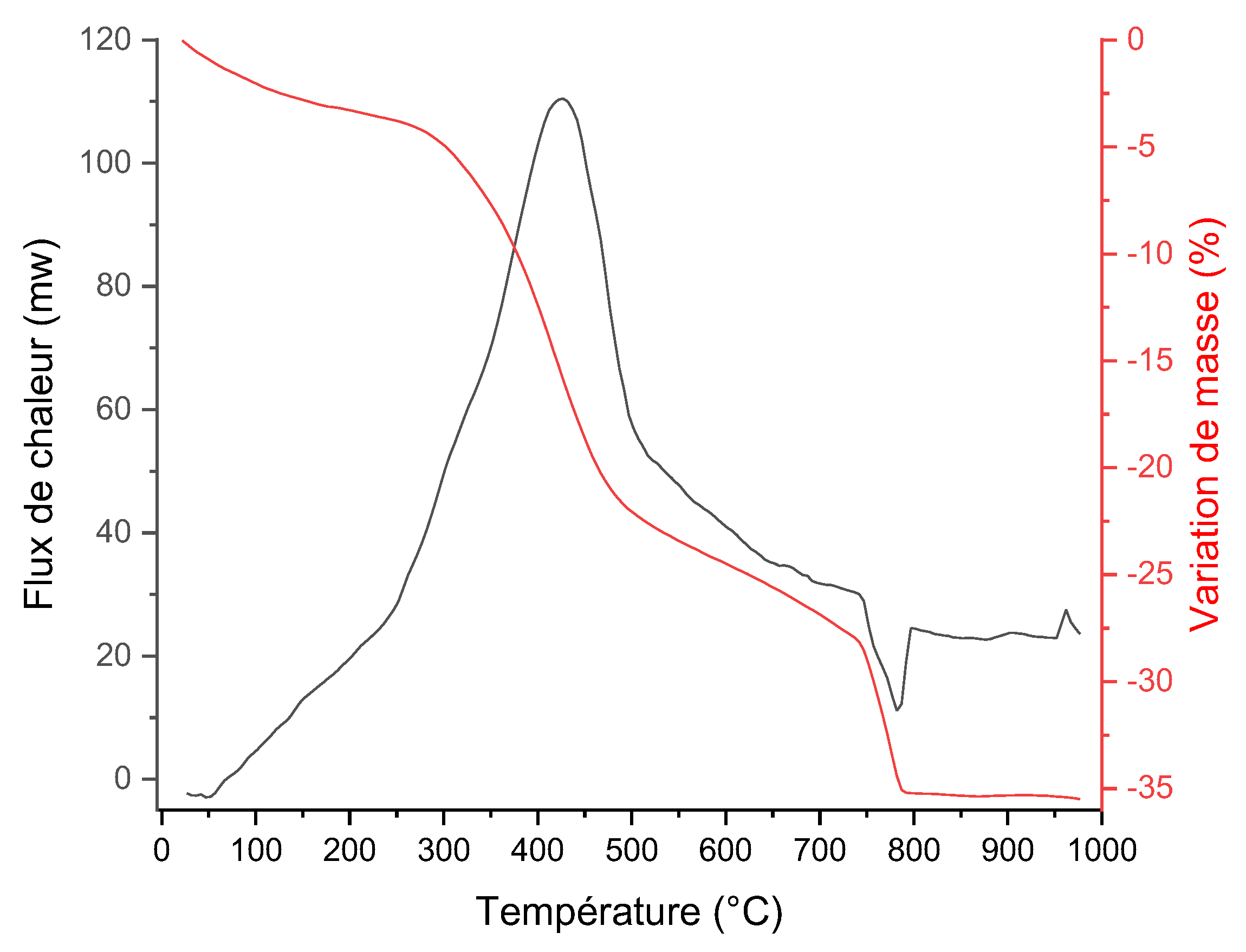
3.3.4. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bairoch, P. Révolution Industrielle et Sous-Développement; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2017; Volume 9. [Google Scholar]
- Belhadj, M.Z. Qualité des Eaux de Surface et Leur Impact sur L’environnement Dans la Wilaya de Skikda. Ph.D. Thesis, Université Mohamed Khider-Biskra, Biskra, Algeria, 2017. [Google Scholar]
- Morin-Crini, N.; Crini, G.; Roy, L. Eaux industrielles contaminées. Besançon PUFC 2017, 513, 37–47. [Google Scholar]
- Kandalaft, J.P. L’accès à l’eau douce dans le monde: Ses principaux enjeux. Proche-Orient Études Manag. 2017, 29, 159–185. [Google Scholar]
- Sauquet, E.; Arama, Y.; Blanc-Coutagne, E.; Bouscasse, H.; Branger, F.; Braud, I.; Vidal, J.P. Le partage de la ressource en eau sur la Durance en 2050: Vers une évolution du mode de gestion des grands ouvrages duranciens ? Houille Blanche. 2016, 5, 25–31. [Google Scholar] [CrossRef]
- Bouleau, G.; Deuffic, P.; Sergent, A.; Paillet, Y.; Gosselin, F. Entre logique de production et de préservation: L’évo- lution de l’information environnementale dans les domaines de l’eau et de la forêt. VertigO Rev. Électronique En Sci- 403 Ences L’environnement 2016, 16. [Google Scholar] [CrossRef]
- ONEE National Office of Electricity. 2018. Available online: https://www.fedenerg.ma/2018/12/27/lonee-ambitionne-de-franchir-le-cap-de-160-stations-depuration-des-eaux-usees-a-lhorizon-2023/ (accessed on 7 November 2022).
- Arisily, T.; Hajji, A. Technologie de séchage solaire des boues des stations d’épuration des eaux usées et son impact sur la gestion des boues au Maroc. Rev. Maroc. Sci. Agron. Vet. 2020, 8. Available online: https://agrimaroc.org/index.php/Actes_IAVH2/article/view/785/1008#info (accessed on 7 November 2022).
- Cerra, I.; Desagnat, M.; Dubart, R.; Juven, L.; Zhou, N.; Ziani, H. Traitement des Boues des Stations D’épuration des Petites Collectivités; Montpellier, France. 2014. Available online: https://reseau-eau.educagri.fr/files/fichierRessource2_Rapport_bibliographique_traitement_boues.pdf (accessed on 7 November 2022).
- Belloulid, M.O. National program of sanitation and wastewater treatment in morocco: Objectives, achievements and challenges. Environ. Water Sci. Public Health Territ. Intell. J. 2018, 2, 67–76. [Google Scholar]
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Carnevale Miino, M.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legis- lation for the reuse of biosolids on agricultural land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef]
- Cherfouh, R.; Lucas, Y.; Derridj, A.; Merdy, P. Long-Term, low technicality sewage sludge amendment and irrigation with treated wastewater under Mediterranean climate: Impact on agronomical soil quality. Environ. Sci. Pollut. Res. 2018, 25, 35571–35581. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M. Examen National de L’Export vert du Maroc: Produits Oléicoles; Romarin et ym; United Nations: Geneva, Switzerland, 2017. [Google Scholar]
- Idrissi, M. Trituration des Olives: Comment Arrêter la Pollution par les Margines. 2016. Available online: https://leseco.ma/maroc/trituration-des-olives-comment-arreter-la-pollution-par-les-margines.html (accessed on 7 November 2022).
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Vannini, A.; Fedeli, R.; Guarnieri, M.; Loppi, S. Foliar Application of Wood Distillate Alleviates Ozone-Induced Dam- 458 age in Lettuce (Lactuca sativa L.). Toxics 2022, 10, 178. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of different types of biochar on the anaerobic digestion of chicken manure. Bioresour. Technol. 2019, 275, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Awasthi, S.K.; Liu, T.; Duan, Y.; Ren, X.; Zhang, Z.; Awasthi, M.K. Effects of microbial culture and chicken manure biochar on compost maturity and greenhouse gas emissions during chicken manure composting. J. Haz-Ardous Mater. 2020, 389, 121908. [Google Scholar] [CrossRef] [PubMed]
- Côrtes, L.N.; Druzian, S.P.; Streit, A.F.M.; Godinho, M.; Perondi, D.; Collazzo, G.C.; Dotto, G.L. Biochars from animal wastes as alternative materials to treat colored effluents containing basic red 9. J. Environ. Chem. Eng. 2019, 7, 103446. [Google Scholar] [CrossRef]
- Bonga, C.P.; Lima, L.Y.; Leea, C.T.; Ongb, P.Y.; Jaromír, J.; Klemešc, C.L.; Gaod, Y. Lignocellulosic biomass and food waste for biochar production and application: A review. Chem. Eng. 2020, 81, 427–432. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Waiho, K.; Azwar, E.; Fazhan, H.; Peng, W.; Ishak, S.D.; Lam, S.S. A state-of-the-art review on producing engineered biochar from shellfish waste and its application in aquaculture wastewater treatment. Chemo-Sphere 2022, 288, 132559. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Ke, X.X.; Shao, Z.D.; Zhong, L.B.; Zhao, Q.B.; Zheng, Y.M. Fish scale-based biochar with defined pore size and ultrahigh specific surface area for highly efficient adsorption of ciprofloxacin. Chemosphere 2022, 287, 131962. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Food waste to biochars through pyrolysis: A review. Resour. Conserv. Recycl. 2019, 144, 310–320. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Rene, E.R.; Nizami, A.S.; Dupont, C.; Vaccari, M.; van Hullebusch, E.D. Beneficial role of biochar addition on the anaerobic digestion of food waste: A systematic and critical review of the operational parameters and mechanisms. J. Environ. Manag. 2021, 290, 112537. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Niazi, N.K.; Hassan, N.E.; Bibi, I.; Wang, H.; Tsang, D.C.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2019, 64, 216–247. [Google Scholar] [CrossRef]
- Assouguem, A.; Kara, M.; Mechchate, H.; Al-Mekhlafi, F.A.; Nasr, F.; Farah, A.; Lazraq, A. Evaluation of the Impact of Different Management Methods on Tetranychus urticae (Acari: Tetranychidae) and Their Predators in Citrus Orchards. Plants 2022, 11, 623. [Google Scholar] [CrossRef]
- Kwapinski, W.; Byrne, C.M.; Kryachko, E.; Wolfram, P.; Adley, C.; Leahy, J.J.; Hayes, M.H. Biochar from biomass and waste. Waste Biomass Valorization 2010, 1, 177–189. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Z.; Huang, J.; Hussain, S.; Zhao, F.; Zhu, C.; Jin, Q. Biochar alleviated the salt stress of induced saline paddy soil and improved the biochemical characteristics of rice seedlings differing in salt tolerance. Soil Tillage Res. 2019, 195, 104372. [Google Scholar] [CrossRef]
- Fedeli, R.; Alexandrov, D.; Celletti, S.; Nafikova, E.; Loppi, S. Biochar improves the performance of Avena sativa L. grown in gasoline-polluted soils. Environ. Sci. Pollut. Res. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Caractérisation des Boues—Mode Opératoire de Conditionnement Chimique en Laboratoire; AFNOR NF EN 14742; 2015; 14p. Available online: https://norminfo.afnor.org/norme/nf-en-14742/caracterisation-des-boues-mode-operatoire-de-conditionnement-chimique-en-laboratoire/86637 (accessed on 7 November 2022).
- Rodier, J.; Legube, B.; Merlet, N. L’analyse de L’eau, 9th ed.; Dunod Technique Et Ingénierie, Sciences & Techniques: Paris, France, 2009; 17p. [Google Scholar]
- Kalay, N.; Torbic, Z.; Golic, M.; Matijevic, E. Determination of the isoelectic points of several metals by adhesion method. J. Phys. Chem. 1991, 95, 7028–7032. [Google Scholar] [CrossRef]
- Pelekani, C.; et Snoeyink, V.L. Competitive adsorption between atrazine and methylene blue on activated carbon: The importance of pore size distribution. Carbon. 2000, 38, 1423–1436. [Google Scholar] [CrossRef]
- Hameed, G.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef]
- ASTM D4607– 94; Standard Test Method for Determination of Iodine Number of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2006.
- Robinson, J.T.; Hansen, R.E. AWWA Standard for Powdered Activated Carbon; American National Institute, Inc.: Denver, CO, USA, 1978. [Google Scholar]
- Boehm, H. Chemical Identification of Surface Groups; Academic Press: London, UK, 1978; pp. 179–274. [Google Scholar]
- Baudu, M.; Guibaud, G.; Raveau, D.; et Lafrance, P. Prévision de l’adsorption de molécules organiques en solution aqueuse en fonctions de quelques caractéristiques physico-chimiques de charbons actifs. Water Qual. Res. J. 2001, 36, 631–657. [Google Scholar] [CrossRef]
- Natasha, N.; Shahid, M.; Khalid, S.; Bibi, I.; Naeem, M.A.; Niazi, N.K.; Rinklebe, J. Influence of biochar on trace element uptake, toxicity and detoxification in plants and associated health risks: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2803–2843. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Y.; Chen, Z.; Wang, J.; Cai, M.; Gao, J. Shape-Stabilized phase change material with highly thermal conductive matrix developed by one-step pyrolysis method. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Nanni, B.; Lombardi, N.; Rita, A.; Scala, F. Biochar as plant growth 483 promoter: Better off alone or mixed with organic amendments? Front. Plant Sci. 2017, 8, 1570. [Google Scholar] [CrossRef] [PubMed]
- Kammann, C.; Glaser, B.; Schmidt, H.P. Combining biochar and organic amendments. Biochar Eur. Soils Agric. Routledge 2016, 158–186. [Google Scholar]
- Guo, X.X.; Liu, H.T.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- IUPAC. International Union of Pure and Applied Chemistry. 2022. Available online: https://iupac.org/etoc-chemistry-international-jan-mar-2022/ (accessed on 3 January 2023).
- El Fels, L.; Zamama, M.; Hafidi, M. Advantages and limitations of using FTIR spectroscopy for assessing the maturity of sewage sludge and olive oil waste co-composts. In Biodegradation and Bioremediation of Polluted Systems–New Advances and Technologies; InTech: Rang-Du-Fliers, France, 2015; pp. 127–144. [Google Scholar]
- Gueboudji, Z.; Kadi, K.; Nagaz, K. Extraction et quantification des polyphénols des eaux usées des moulins à huile issus de l’extraction à froid de l’huile d’olive dans la région de Khenchela-Algérie. Rev. Génétique Biodiversité 2021, 5, 116–122. [Google Scholar]
- Assouguem, A.; Kara, M.; Ramzi, A.; Annemer, S.; Kowalczyk, A.; Ali, E.A.; Moharram, B.A.; Lazraq, A.; Farah, A. Evaluation of the Effect of Four Bioactive Compounds in Combination with Chemical Product against Two Spider Mites Tetranychus urticae and Eutetranychus orientalis (Acari: Tetranychidae). Based Complement. Altern. Med. 2022, 2022, 2004623. [Google Scholar] [CrossRef]
- Badou, A.; Pont, S.; Auzoux-Bordenave, S.; Lebreton, M.; Bardeau, J.F. New insight on spatial localization and micro- structures of calcite-aragonite interfaces in adult shell of Haliotis tuberculata: Investigations of wild and farmed abalones by FTIR and Raman mapping. J. Struct. Biol. 2022, 214, 107854. [Google Scholar] [CrossRef]
- Bolton, L.; Joseph, S.; Greenway, M.; Donne, S.; Munroe, P.; Marjo, C.E. Phosphorus adsorption onto an enriched biochar substrate in constructed wetlands treating wastewater. Ecol. Eng. 2019, 142, 100005. [Google Scholar] [CrossRef]
- Assouguem, A.; Kara, M.; Mechchate, H.; Alzain, M.N.; Noman, O.M.; Imtara, H.; Hano, C.; Ibrahim, M.N.; Benmessaoud, S.; Farah, A.; et al. Evaluation of the Effect of Different Concentrations of Spirotetramat on the Diaspine Scale Parlatoria ziziphi in Citrus Orchards. Agronomy 2021, 11, 1562. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and characterization of a novel 506 magnetic biochar for arsenic removal. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Li, T. Phosphate adsorption on lanthanum loaded biochar. Chemosphere 2016, 150, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ouafi, R.; Atemni, I.; Mehdaoui, I.; Asri, M.; Taleb, M.; Rais, Z. Spectroscopic Analysis of Chemical Compounds De- 509 rived from the Calcination of Snail Shells Waste at Different Temperatures. Chem. Afr. 2021, 4, 923–933. [Google Scholar] [CrossRef]
| Parameters | pH | EC (μS/cm) | H (%) | DM (%) | MM (%) | OM (%) | Polyphénols (%) | FM (%) | TOC (%) | NTK (%) | C/N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 6.65 | 1300 | 1.58 | 98.42 | 78.31 | 21.69 | 1.09 | 2.16 | 12.58 | 1.05 | 11.98 |
| Elements | Al | Ca | Cu | Fe | K | Mg | P | Mn | Na | Zn |
| Concentration (mg/g) | 4.9396 | 64.009 | 0.2535 | 7.4737 | 3.6947 | 18.646 | 22.588 | 0.3118 | 25.336 | 0.7603 |
| Elements | B | Ag | Cd | Co | Cr | Ni | Pb | Se | Ti | As |
| Concentration (mg/g) | <0.01 | 0.0458 | <0.01 | <0.01 | 1.0165 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Groups | Carboxylic | Phenolics | Lactones | Carbonyls | Total Oxygenates | Total Bases | |
| Value (meq/g) | 0.007 | 1.814 | 0.005 | 2.302 | 0.018 | 0.416 | |
| Parameters | pHPZC | SBM (m2/g) | Oxygenated Functional Groups (%) | Iodine Index (mg/g) | BM Index (mg/g) | ||
| Value | 5.56 | 694.71 | 9.6 | 462.35 | 9.22 | ||
| Elements | Mass Percentage | Atomic Percentage |
|---|---|---|
| C | 43.59 ± 0.15 | 57.90 ± 0.21 |
| O | 29.24 ± 0.25 | 29.15 ± 0.25 |
| Mg | 0.97 ± 0.03 | 0.64 ± 0.02 |
| Al | 2.78 ± 0.05 | 1.64 ± 0.03 |
| Si | 6.60 ± 0.07 | 3.75 ± 0.04 |
| P | 2.53 ± 0.04 | 1.30 ± 0.02 |
| S | 1.47 ± 0.03 | 0.73 ± 0.02 |
| K | 0.63 ± 0.03 | 0.26 ± 0.01 |
| Ca | 10.26 ± 0.10 | 4.08 ± 0.04 |
| Fe | 1.94 ± 0.07 | 0.55 ± 0.02 |
| Total | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaga, Y.; Mehdaoui, I.; Kara, M.; Assouguem, A.; Al-Hashimi, A.; Ragab AbdelGawwad, M.; Elshikh, M.S.; Saoudi Hassani, E.M.; Alwahibi, M.S.; Bahhou, J.; et al. Elaboration and Characterization of a Biochar from Wastewater Sludge and Olive Mill Wastewater. Sustainability 2023, 15, 2409. https://doi.org/10.3390/su15032409
Gaga Y, Mehdaoui I, Kara M, Assouguem A, Al-Hashimi A, Ragab AbdelGawwad M, Elshikh MS, Saoudi Hassani EM, Alwahibi MS, Bahhou J, et al. Elaboration and Characterization of a Biochar from Wastewater Sludge and Olive Mill Wastewater. Sustainability. 2023; 15(3):2409. https://doi.org/10.3390/su15032409
Chicago/Turabian StyleGaga, Younes, Imane Mehdaoui, Mohammed Kara, Amine Assouguem, Abdulrahman Al-Hashimi, Mohamed Ragab AbdelGawwad, Mohamed S. Elshikh, El Mokhtar Saoudi Hassani, Mona S. Alwahibi, Jamila Bahhou, and et al. 2023. "Elaboration and Characterization of a Biochar from Wastewater Sludge and Olive Mill Wastewater" Sustainability 15, no. 3: 2409. https://doi.org/10.3390/su15032409
APA StyleGaga, Y., Mehdaoui, I., Kara, M., Assouguem, A., Al-Hashimi, A., Ragab AbdelGawwad, M., Elshikh, M. S., Saoudi Hassani, E. M., Alwahibi, M. S., Bahhou, J., Taleb, M., & Rais, Z. (2023). Elaboration and Characterization of a Biochar from Wastewater Sludge and Olive Mill Wastewater. Sustainability, 15(3), 2409. https://doi.org/10.3390/su15032409








