Soil Organic Matter Composition in Urban Soils: A Study of Wrocław Agglomeration, SW Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Objects of the Studies and Sampling
2.2. Basic Physical, Physicochemical and Chemical Properties
- pH in 1 mol dm–3 KCl using potentiometric method (m:v ratio as 1:2.5);
- content of total organic carbon (TOC) and total nitrogen (TN) using a Vario Macro Cube CN analyser (Elementar Analysensysteme GmbH, Germany).
2.3. Comprehensive Studies on Soil Humic Substances (HS)
- ash content after ignition at 550 °C;
- elemental composition with 2400 CHN Perkin Elmer (United Kingdom) analyser. Based on the results, the atomic ratios (H/C, N/C, O/C and O/H) and oxidation ratio (ω) were calculated according to the formula [49]:
- 13C NMR spectra on the Bruker Advance III 300 MHz spectrometer (Germany) with CP-MAS unit for the range 0–210 ppm. The shares of carbon present in defined organic bonds [9,11,30] were determined: Calkyl (0–45 ppm), CO-alkyl (45–110 ppm), Clig (14–160 ppm), Ccarbox (160–200 ppm), Caliph (0–110ppm), Carom (110–160 ppm). Based on the obtained data, the degree of aromaticity [11,47] was calculated according to the formula:
2.4. Statistical Analysis
3. Results and Discussion
3.1. Basic Physical and Chemical Properties of the Investigated Soils
3.2. Fractional Analysis of Soil Organic Matter
3.3. Elemental Composition of Humic Acids
3.4. 13C NMR Spectra of the Investigated HA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biasioli, M.; Berberis, R.; Ajmone-Marsan, F. The influence of large city on some soil properties and metal content. Sci. Total Environ. 2006, 356, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, W. Soils in urban and industrial environments. J. Plant Nutr. Soil Sci. 1994, 157, 205–214. [Google Scholar] [CrossRef]
- Kumar, K.; Hundal, L.S. Soil in the City: Sustainably Improving Urban Soils. J. Environ. Qual. 2016, 45, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Winiwarter, W.; Amon, B.; Bai, Z.; Greinert, A.; Kaltenegger, K.; Ma, L.; Myszograj, S.; Schneidergruber, M.; Suchowski-Kisielewicz, M.; Wolf, L.; et al. Urban nitrogen budgets: Flows and stock changes of potentially polluting nitrogen compounds in cities and their surroundings–a review. J. Integr. Environ. Sci. 2020, 17, 57–71. [Google Scholar] [CrossRef]
- Lehmann, A.; Stahr, K. Nature and significance of anthropogenic urban soils. J. Soils Sediments 2007, 7, 247–260. [Google Scholar] [CrossRef]
- Kabala, C.; Galka, B.; Labaz, B.; Anjos, L.; Cavassani, R.D. Towards more simple and coherent chemical criteria in a classification of anthropogenic soils: A comparison of phosphorus tests for diagnostic horizons and properties. Geoderma 2018, 320, 1–11. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Lloyd, J.E.; Johnson-Maynard, J.L. Distinguishing urban soils with physical, chemical and biological properties. Pedobiologia 2005, 49, 283–296. [Google Scholar] [CrossRef]
- Beyer, L.; Blume, H.-P.; Elsner, D.-C.; Willnow, A. Soil organic matter composition and microbal activity in urban soils. Sci. Total Environ. 1995, 168, 267–278. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Howe, K.; Parkhurst, D.F.; Pouyat, R.V. Variation in quality and decomposibility of red oak leaf litter along an urban-rural gradient. Biol. Fertil. Soils 1999, 30, 258–268. [Google Scholar] [CrossRef]
- Lorenz, K.; Preston, C.M.; Kandeler, E. Soil organic matter in urban soils: Estimation of elemental carbon by thermal oxidation and characterization of organic matter by solid-state 13C nuclear magnetic resonance (NMR) specroscopy. Geoderma 2006, 130, 312–323. [Google Scholar] [CrossRef]
- Pouyat, R.; Groffman, P.; Yesilonis, I.; Hernandez, L. Soil carbon pools and fluxes in urban ecosystems. Environ. Pollut. 2002, 116, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Kocowicz, A.; Bekier, J.; Jamroz, E.; Tyszka, R.; Debicka, M.; Parylak, D.; Kordas, L. The effect of a sandy soil amendment with municipal solid waste (MSW) compost on nitrogen uptake efficiency by plants. Eur. J. Agron. 2014, 54, 54–60. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Sciences 2014, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Lutzow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions-a review. Eur. J. Soil. Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- De Nobili, M.; Bravo, C.; Chen, Y. The spontaneous secondary synthesis of soil organic matter components: A critical examination of the soil continuum model theory. Appl. Soil Ecol. 2020, 154, 103655. [Google Scholar] [CrossRef]
- Frąc, M.; Weber, J.; Gryta, A.; Debicka, M.; Kocowicz, A.; Jamroz, E.; Oszust, K.; Żołnierz, L. Microbial functional diversity in Podzol ectohumus horizons affected by alkaline fly ash in the vicinity of electric power plant. Geomicrobiol. J. 2017, 34, 579–586. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; De Santo, A.V.; Alfani, A.; Bartoli, G.; De Cristofaro, A. Effects of urban heavy metal pollution on organic matter decomposition in quercus ilex l. woods. Environ. Pollut. 1995, 89, 81–87. [Google Scholar] [CrossRef]
- Kögel–Knabner, I. Analytical approaches for characterizing soil organic matter. Org. Geochem. 2000, 31, 609–625. [Google Scholar] [CrossRef]
- Zhu, W.X.; Carreiro, M.M. Chemoautotrophic nitrification in acidic forest soils along an urban–to–rural transect. Soil Biol. Biochem. 1999, 31, 1091–1100. [Google Scholar] [CrossRef]
- Beyer, L.; Kahle, P.; Kretschmer HWu, Q. Soil organic matter composition of man- impacted urban sites in North Germany. J. Plant Nutr. Soil Sci. 2001, 164, 359–364. [Google Scholar] [CrossRef]
- Lorenz, K.; Kandeler, E. Biochemical characterization of urban soil profiles from Stuttgart, Germany. Soil Biol. Biochem. 2005, 37, 1373–1385. [Google Scholar] [CrossRef]
- Waksman, S.A. Humus: Origin, Chemical Composition and Importance in Nature; Williams and Wilkins: Baltimore, MD, USA, 1936; Available online: https://soilcarboncoalition.org/files/Waksman-Humus.pdf (accessed on 25 May 2022).
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Schnitzer, M.; Monreal, C.M. Chapter Three-Quo Vadis Soil Organic Matter Research? A Biological Link to the Chemistry of Humification. Adv. Agron. 2011, 113, 143–217. [Google Scholar]
- Adani, F.; Ricca, G.; Tambone, F.; Genevini, P. Isolation of the stable fraction (the core) of the humic acid. Chemosphere 2006, 65, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.-P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- De Nobili, M. Comment on ‘‘humic substances extracted by alkali are invalid proxies for the dynamics and functions of organic matter in terrestrial and aquatic ecosystems’’, by Kleber and Lehmann. J. Environ. Qual. 2019, 48, 787–789. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Swift, R.S. Vindication of humic substances as a key component of organic matter in soil and water. Adv. Agron. 2020, 163, 1–37. [Google Scholar]
- Myneni, S.C.B. Chemistry of natural organic matter— the next step: Commentary on a humic substances debate. J. Environ. Qual. 2019, 48, 233–235. [Google Scholar] [CrossRef]
- International Humic Substances Society. Available online: http://humic-substances.org (accessed on 30 June 2022).
- Doichinova, V.; Zhiyanski, M.; Hursthouse, A. Impact of urbanisation on soil characteristics. Environ. Chem. Lett. 2006, 3, 160–163. [Google Scholar] [CrossRef]
- Hiller, D.A. Properties of Urbic Anthrosols from an abandoned shunting yard in the Ruhr area, Germany. Catena 2000, 39, 245–266. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Futa, B.; Ukalska-Jaruga, A.; Weber, J.; Chmielewski, S.; Wesołowska, S.; Mocek-Płóciniak, A.; Patkowski, K.; Mielnik, L. Mutual relations between PAHs derived from atmospheric deposition, enzymatic activity, and humic substances in soils of differently urbanized areas. J. Soils Sediments 2018, 18, 2682–2691. [Google Scholar] [CrossRef]
- Oktaba, L.; Odrobinska, D.; Uzarowicz, L. The impact of different land uses in urban area on humus quality. J. Soils Sediments 2018, 18, 2823–2832. [Google Scholar] [CrossRef]
- Schleuß, U.; Wu, Q.; Blume, H.-P. Variability of soils in urban and periurban areas in Northern Germany. Catena 1998, 33, 255–270. [Google Scholar] [CrossRef]
- Licznar, S.E.; Licznar, M. Wrocław agglomeration impact on humus horizons of the Szczytnicki Park’s soil. Soil Sci. Ann. 2005, 56, 113–118. [Google Scholar]
- Walenczak, K.; Licznar, S.E.; Licznar, M. The role of organic matter and colloidal clay in forming of buffer properties of soils of Szczytnicki Park. Soil Sci. Ann. 2009, 60, 102–107. [Google Scholar]
- Konieczny, R.; Madej, P.; Siudak, M. Local Flood Hazard Reduction Plans in Poland—Problems and Perspectives. In Coping With Flash Floods; Gruntfest, E., Handmer, J., Eds.; NATO Science Series; Springer: Dordrecht, NL, USA, 2001. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W. Summer 1997 flood in Poland in perspective. In Extreme Hydrological Events: New Concepts for Security; Vasiliev, O., van Gelder, P., Plate, E., Bolgov, M., Eds.; NATO Science Series; Springer: Dordrecht, NL, USA, 2006. [Google Scholar] [CrossRef]
- USS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Kaszubkiewicz, J.; Papuga, K.; Kawałko, D.; Woźniczka, P. Particle size analysis by an automated dynamometer method integrated with an x-y sample changer. Measurement 2020, 157, 107680. [Google Scholar] [CrossRef]
- Papuga, K.; Kaszubkiewicz, J.; Kawałko, D. Do we have to use suspensions with low concentrations in determination of particle size distribution by sedimentation methods? Powder Technol. 2021, 389, 507–521. [Google Scholar] [CrossRef]
- Polish Society of Soil Science (PSSS). Particle size distribution and textural classes of soils and mineral materials–clasification of Polish Society of Soil Science 2008. Soil Sci. Ann. 2009, 60, 5–16. [Google Scholar]
- Schnitzer, M.; Khan, S.U. Humic Substances in the Environment; Marcel Dekker Inc.: New York, NY, USA, 1972. [Google Scholar]
- Spark, D.L. Methods of soil analysis. Part 3: Chemical methods. Soil Sci. Soc. Am. 1996, 5, 961–1010. [Google Scholar]
- Weber, J.; Jamroz, E.; Kocowicz, A.; Debicka, M.; Bekier, J.; Ćwieląg-Piasecka, I.; Ukalska-Jaruga, A.; Mielnik, L.; Bejger, R.; Jerzykiewicz, M. Optimized isolation method of humin fraction from mineral soil material. Environ Geochem Health 2022, 44, 1289–1298. [Google Scholar] [CrossRef]
- Zdanov, J.A. Mean oxidation degree of carbon and amino acids. Biochimija 1965, 30, 1257–1259. [Google Scholar]
- Simpson, A.J.; McNally, D.J.; Simpson, M.J. NMR spectroscopy in environmental research: From molecular interactions to global processes. Prog. Nucl. Magn. Reason. Spectrosc. 2011, 58, 97–175. [Google Scholar] [CrossRef] [PubMed]
- Jim, C.Y. Soil Characteristics and Management in an Urban Park in Hong Kong. Environ. Manag. 1998, 22, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Pantano, G.; Tadini, A.M.; Bisinoti MCMoreira, A.B. Seasonal variability of a conditional stability constant and the characterization of sedimentary humic substances from typical agricultural and urban areas. J. Soils Sediments 2014, 14, 385–393. [Google Scholar] [CrossRef]
- Greinert, A. The heterogeneity of urban soils in the light of their properties. J. Soils Sediments 2015, 15, 1725–1737. [Google Scholar] [CrossRef]
- Blume, H.P. Böden städtisch–industrieller Verdichtungsräume. In Handbuch der Bodenkunde; Blume, H.-P., Stahr, K., Fischer, W., Guggenberger, G., Horn, R., Frede, H.-G., Felix-Henningsen, P., Eds.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Beyer, L.; Cordsen, E.; Blume, H.-P.; Schleuss, U.; Vogt, B.; Wu, Q. Soil organic matter composition in urbic anthrosols in the city of Kiel, NW-Germany, as revealed by wet chemistry and CPMAS 13C-NMR spectroscopy of whole soil samples. Soil Technol. 1996, 9, 121–132. [Google Scholar] [CrossRef]
- Oktaba, L.; Paziewski, K.; Kwasowski, W.; Kondras, M. The effect of urbanization on soil properties and soil organic carbon accumulation in topsoil of Pruszków-a medium-sized city in the Warsaw Metropolitan Area, Poland. Soil Sci. Ann. 2014, 65, 10–17. [Google Scholar] [CrossRef]
- Tomaszewicz, T.; Chudecka, J. Evaluation of soils from the Old Town in Szczecin as a potential forest habitat using Forest Soil Trophism Index. Sylwan 2018, 162, 343–350. [Google Scholar]
- Ghosh, K.; Schnitzer, M. Macromolecular structures of humic substances. Soil Sci. 1980, 129, 266–276. [Google Scholar] [CrossRef]
- Barančíková, G.; Klučáková, M.; Madaras, M.; Makovníková, J.; Pekař, M. Chemical structure of humic acids isolated from various soil types and lignite. Humic Subst. Environ. 2003, 3, 3–8. [Google Scholar]
- van Krevelen, D.W. Graphical-statistical method for investigation of the structure of coal. Fuel 1950, 26, 269–284. [Google Scholar]
- Adani, F.; Spagnol, M. Humic acid formation in artificial soils amended with compost at different stages of organic matter evolution. J. Environ. Qual. 2008, 37, 1608–1616. [Google Scholar] [CrossRef]
- Drosos, M.; Jerzykiewicz, M.; Deligiannakis, Y. H-binding groups in lignite νs.soil humic acids: NICA-Donnan and spectroscopic parameters. J. Colloid Interface Sci. 2009, 332, 78–84. [Google Scholar] [CrossRef]
- Keeler, C.; Maciel, G.E. Quantitation in the solid-state 13C NMR analysis of soil and organic soil fractions. Anal. Chem. 2003, 75, 2421–2432. [Google Scholar] [CrossRef] [PubMed]
- Keeler, C.; Kelly, E.F.; Maciel, G.E. Chemical-structual information from solid-state 13C NMR studies of a suite of humic materials from a lower montane forest soil, Colorado, USA. Geoderma 2006, 130, 124–140. [Google Scholar] [CrossRef]
- Bekier, J.; Drozd, J.; Jamroz, E.; Jarosz, B.; Kocowicz, A.; Walenczak, K.; Weber, J. Changes in selected hydrophobic components during composting of municipal solid wastes. J. Soils Sediments 2014, 14, 305–311. [Google Scholar] [CrossRef]
- Weber, J.; Chen, Y.; Jamroz, E.; Miano, T. Preface: Humic substances in the environment. J. Soils Sediments 2018, 18, 2665–2667. [Google Scholar] [CrossRef]
- Kaluza-Haladyn, A.; Jamroz, E.; Bekier, J. Humic substances of differently matured composts produced from municipal solid wastes and biomass of energetic plants. Soil Sci. Annu. 2019, 70, 292–297. [Google Scholar] [CrossRef]

| Profile No | GPS Coordinates | WRB Soil Unit | Depth of Debris and Artefacts Layer | Land Use Type |
|---|---|---|---|---|
| cm | ||||
| Control | N 51. 115.11; E 17. 042556 | Fluvic Cambisol | walking area. park | |
| 1 | N 51. 113659; E 17.047577 | Mollic Urbic Technosol | 40–70 | lawn |
| 2 | N 51.107843; E 17.043274 | Urbic Technosol | 25–50 | walking area. lawn |
| 3 | N 51.114444; E 17.050090 | Urbic Technosol | 20–65 | walking area. lawn |
| Profile No. | Depth of A Horizon [cm] | pH (KCl) | Particles > 2.0 mm | Particles < 0.002 mm | USDA Textural Class | TOC | TN | TOC/TN |
|---|---|---|---|---|---|---|---|---|
| % | g kg−1 | |||||||
| Control | 0–20 | 6.48 | 5.5 | 11.0 | loamy sand | 22.39 a | 2.09 a | 10.71 a |
| 1 | 0–30 | 6.50 | 3.3 | 12.0 | sandy loam | 66.08 b | 4.63 b | 14.27 b |
| 2 | 0–15 | 6.52 | 6.1 | 14.0 | sandy loam | 53.45 c | 2.81 c | 19.02 c |
| 3 | 0–20 | 6.46 | 5.8 | 13.0 | sandy loam | 58.24 d | 3.40 d | 17.13 d |
| Parameter | Clay | pH KCl | TOC | TN | CHA | CFA | CR |
|---|---|---|---|---|---|---|---|
| Clay | - | ||||||
| pH KCl | 0.841 * | - | |||||
| TOC | 0.576 * | 0.306 | - | ||||
| TN | 0.128 | −0.132 | 0.876 * | - | |||
| CHA | −0.167 | −0.395 | 0.622 * | 0.884 * | - | ||
| CFA | 0.650 * | 0.335 | 0.788 * | 0.637 * | 0.585 * | - | |
| CR | 0.534 | 0.467 | 0.654 * | 0.409 | −0.043 | 0.150 | - |
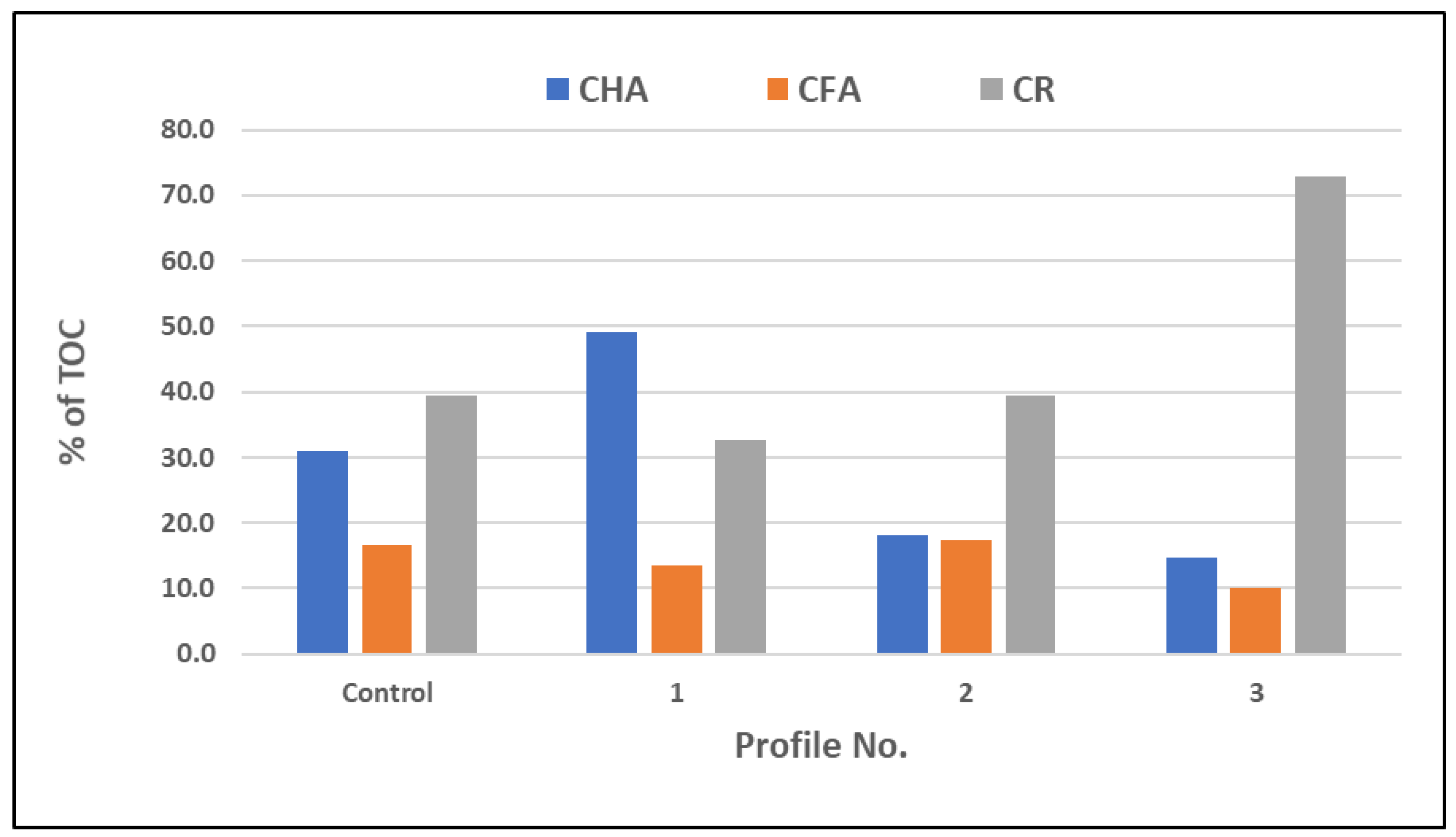
| Profile No. | CHA | CFA | CR | CHA/CFA |
|---|---|---|---|---|
| g kg−1 | ||||
| Control | 6.94 a | 3.72 a | 8.82 a | 1.90 a |
| 1 | 32.51 b | 8.99 b | 21.61 b | 3.60 b |
| 2 | 9.63 c | 9.25 b | 21.11 b | 1.04 c |
| 3 | 8.62 d | 5.82 c | 42.40 c | 1.50 d |
| Profile No | C | H | O | N | H/C | N/C | O/C | O/H | ω |
|---|---|---|---|---|---|---|---|---|---|
| Atomic % | |||||||||
| Control | 32.28 a | 42.95 a | 20.05 a | 2.81 a | 1.33 a | 0.09 a | 0.62 a | 0.47 a | 0.17 a |
| 1 | 36.85 b | 42.10 b | 19.40 b | 1.98 b | 1.16 b | 0.05 b | 0.53 b | 0.46 a,d | 0.07 b |
| 2 | 36.15 c | 42.58 c | 19.45 b | 2.96 a | 1.20 c | 0.08 a | 0.53 b | 0.44 b,c | 0.12 c |
| 3 | 36.25 c | 43.04 a | 19.31 b | 2.91 a | 1.24 d | 0.08 a | 0.56 c | 0.45 c,d | 0.13 a |
| Profile No | Calkyl | CO-Alkyl | Caliph | Carom | Clig | Ccarbox | α |
|---|---|---|---|---|---|---|---|
| [%] | |||||||
| Control | 27.62 | 27.99 | 55.61 | 22.89 | 9.13 | 21.10 | 29.16 |
| 1 | 28.51 | 29.84 | 58.35 | 21.04 | 9.72 | 20.66 | 26.50 |
| 2 | 24.24 | 30.03 | 54.27 | 22.52 | 7.76 | 23.21 | 29.33 |
| 3 | 26.57 | 27.84 | 54.41 | 22.48 | 8.24 | 23.11 | 29.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekier, J.; Jamroz, E.; Walenczak-Bekier, K.; Uściła, M. Soil Organic Matter Composition in Urban Soils: A Study of Wrocław Agglomeration, SW Poland. Sustainability 2023, 15, 2277. https://doi.org/10.3390/su15032277
Bekier J, Jamroz E, Walenczak-Bekier K, Uściła M. Soil Organic Matter Composition in Urban Soils: A Study of Wrocław Agglomeration, SW Poland. Sustainability. 2023; 15(3):2277. https://doi.org/10.3390/su15032277
Chicago/Turabian StyleBekier, Jakub, Elżbieta Jamroz, Karolina Walenczak-Bekier, and Martyna Uściła. 2023. "Soil Organic Matter Composition in Urban Soils: A Study of Wrocław Agglomeration, SW Poland" Sustainability 15, no. 3: 2277. https://doi.org/10.3390/su15032277
APA StyleBekier, J., Jamroz, E., Walenczak-Bekier, K., & Uściła, M. (2023). Soil Organic Matter Composition in Urban Soils: A Study of Wrocław Agglomeration, SW Poland. Sustainability, 15(3), 2277. https://doi.org/10.3390/su15032277








