Effects of Fungi on Soil Organic Carbon and Soil Enzyme Activity under Agricultural and Pasture Land of Eastern Türkiye
Abstract
1. Introduction
2. Material and Methods
2.1. Study Site
2.2. Soil Sampling
2.3. Fungal Cultures and Soil Treatments
2.4. Soil Analysis
2.5. Statistical Analysis
3. Results and Discussion
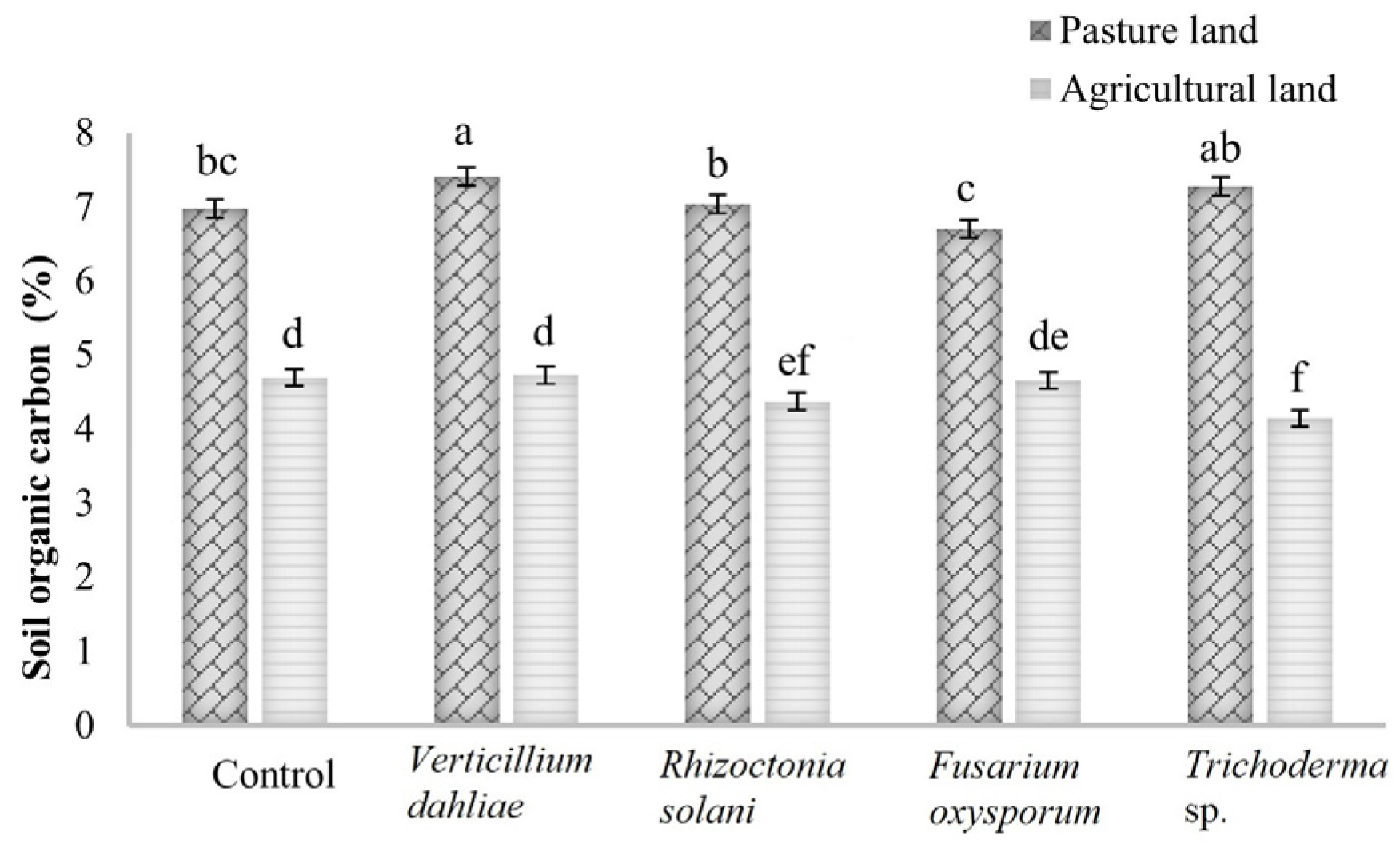
3.1. Soil Organic Carbon (SOC)
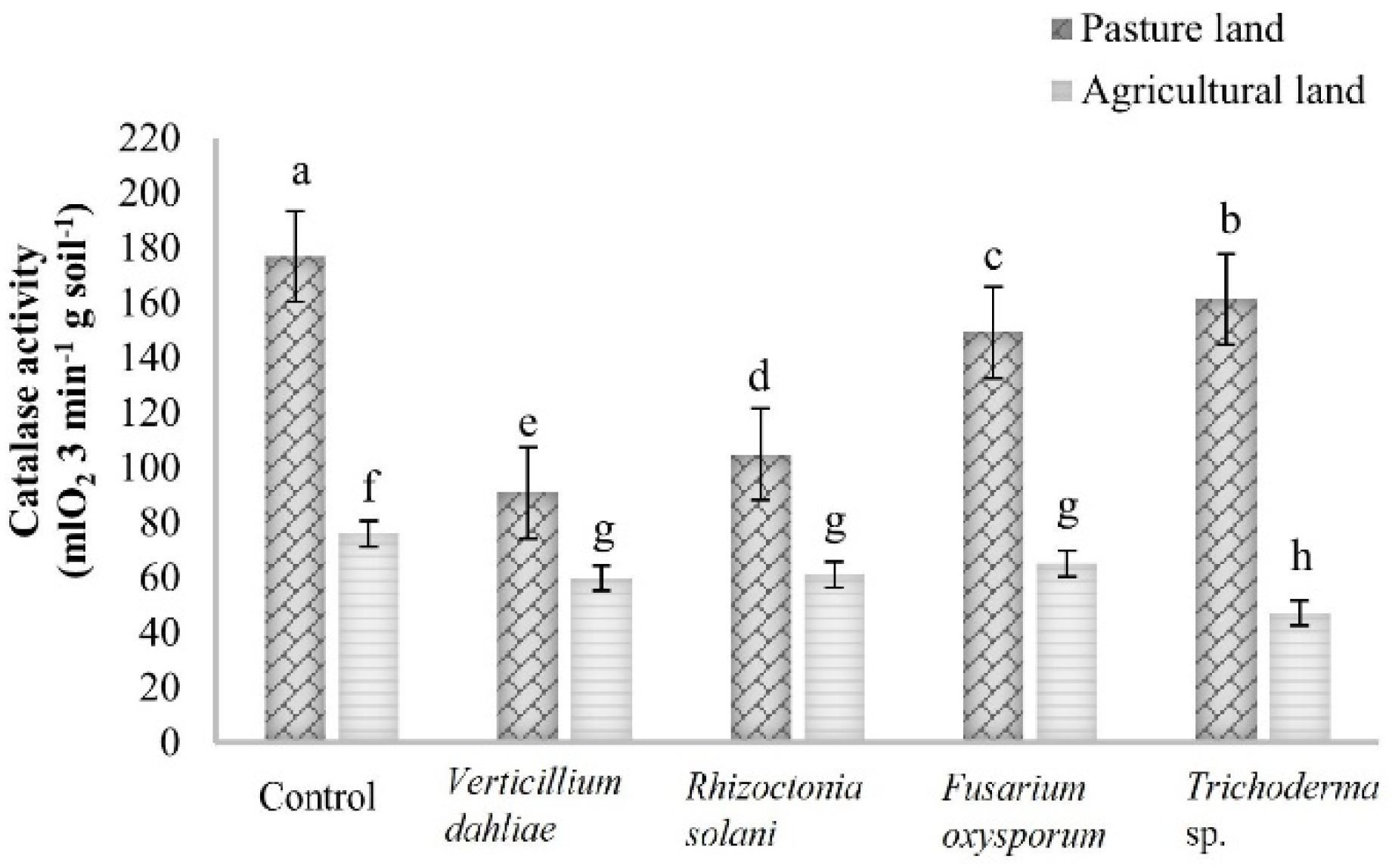
3.2. Soil Enzyme Activity
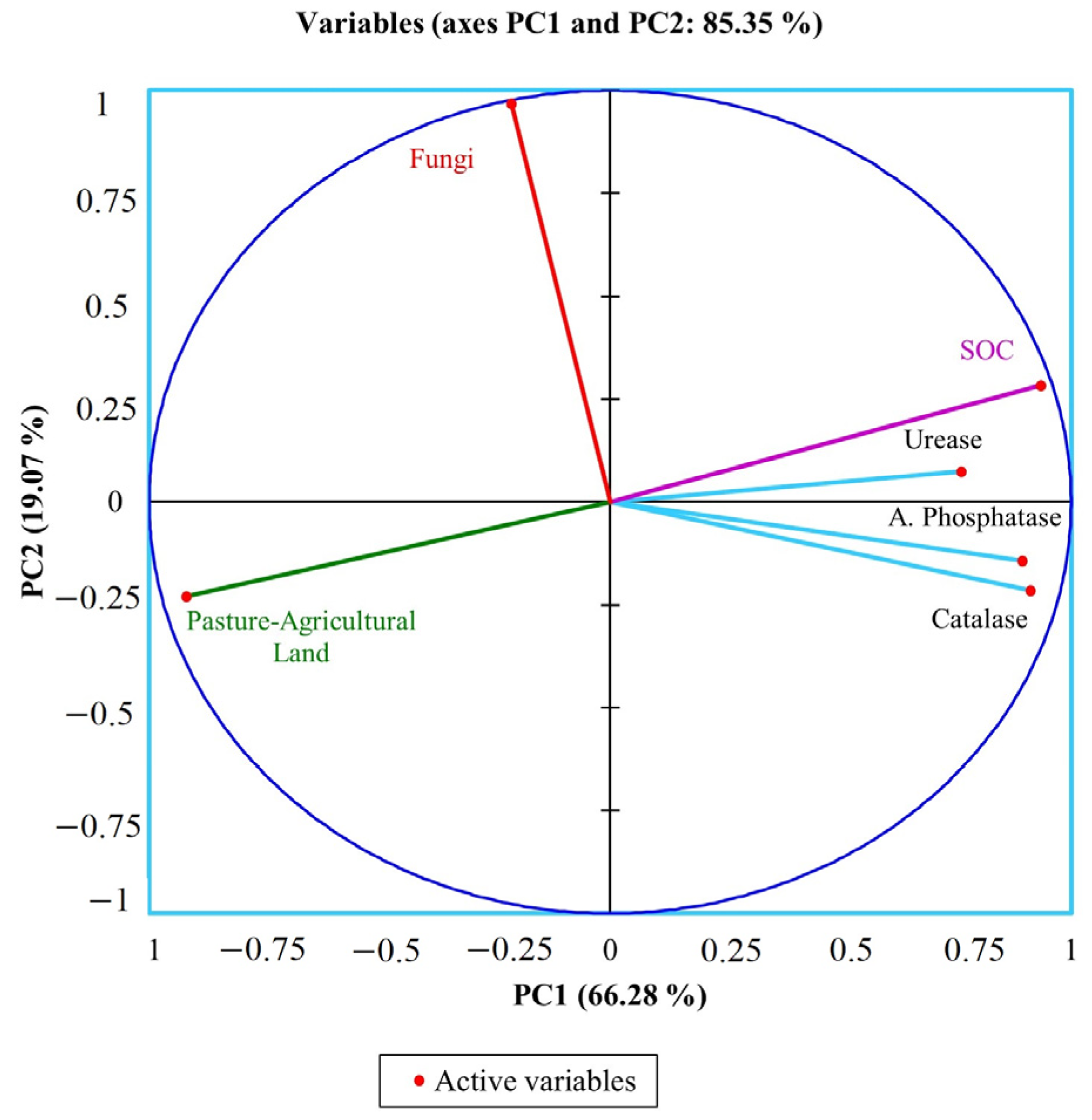
3.3. Correlation of Variables
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Courcelles, V.R.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Schweinle, J.; Rodl, A.; Borjesson, P.; Neary, D.G.; Langeveld, J.W.; Berndes, G.; Cowie, A.; Ahlgren, S.; Margni, M.; Gaudreault, C. Assessing the Environmental Performance of Biomass Supply Chains: Methods, Results, Challenges and Limitations; IEA Bioenergy Task 43. Report 2015:TR01; IEA Bioenergy: Paris, France, 2015; p. 121. [Google Scholar]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The ecology of soil carbon: Pools, vulnerabilities, and biotic and abiotic controls. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 419–445. [Google Scholar] [CrossRef]
- Lal, R. Managing soils and ecosystems for mitigating anthropogenic carbon emissions and advancing global food security. BioScience 2010, 60, 708–721. [Google Scholar] [CrossRef]
- Chow, A.T.; Tanji, K.K.; Gao, S.; Dahlgren, R.A. Temperature, water content and wet–dry cycle effects on DOC production and carbon mineralization in agricultural peat soils. Soil Biol. Biochem. 2006, 38, 477–488. [Google Scholar] [CrossRef]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef]
- Adair, E.C.; Parton, W.J.; Del Grosso, S.J.; Silver, W.L.; Harmon, M.E.; Hall, S.A.; Burke, I.C.; Hart, S.C. Simple three-pool model accurately describes patterns of long term litter decomposition in diverse climates. Glob. Chang. Biol. 2008, 14, 2636–2660. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef]
- Aciego Pietri, J.C.; Brookes, P.C. Substrate availability and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol. Biochem. 2008, 41, 1396–1405. [Google Scholar] [CrossRef]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Liang, C.; Kao-Kniffin, J.; Sanford, G.R.; Wickings, K.; Balser, T.C.; Jackson, R.D. Microorganisms and their residues under restored perennial grassland communities of varying diversity. Soil Biol. Biochem. 2016, 103, 192–200. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organicmatter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Wright, D.; Goulding, K.W.T.; Jones, D.L. pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biol. Biochem. 2006, 38, 898–911. [Google Scholar] [CrossRef]
- Fontaine, S.; Henault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Trivedi, P.; Anderson, I.C.; Singh, B.K. Microbial modulators of soil carbon storage: Integrating genomic and metabolic knowledge for global prediction. Trends Microbiol. 2013, 21, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Hu, H.; Liu, Z.; Dong, Q.; Sun, K.; Feng, Y.; Li, G.; Ning, T. Shifts in microbial community and carbon sequestration in farmland soil under long-term conservation tillage and straw returning. Appl. Soil Ecol. 2019, 136, 43–54. [Google Scholar] [CrossRef]
- Degrune, F.; Theodorakopoulos, N.; Dufrêne, M.; Colinet, G.; Bodson, B.; Hiel, M.P.; Taminiau, B.; Nezer, C.; Daube, G.; Vandenbol, M. No favorable effect of reduced tillage on microbial community diversity in a silty loam soil (Belgium). Agric. Ecosyst. Environ. 2016, 224, 12–21. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B. Priming effects of sugars, amino acids, organic acids and catechol on the mineralization of lignin and peat. J. Plant Nutr. Soil Sci. 2002, 165, 261–268. [Google Scholar] [CrossRef]
- Sullivan, B.W.; Hart, S.C. Evaluation of mechanisms controlling the priming of soil carbon along a substrate age gradient. Soil Biol. Biochem. 2013, 58, 293–301. [Google Scholar] [CrossRef]
- Ritz, K.; Young, I.M. Interactions between soil structure and fungi. Mycologist 2004, 18, 52–59. [Google Scholar] [CrossRef]
- Amin, F.; Razdan, V.K. Potential of Trichoderma species as biocontrol agents of soil borne fungal propagules. J. Phytol. 2010, 2, 10. [Google Scholar]
- Khalid, S.A.L. Trichoderma as biological control weapon against soil borne plant pathogens. Afr. J. Biotechnol. 2017, 16, 2299–2306. [Google Scholar] [CrossRef]
- Okungbowa, F.I.; Shittu, H.O. Fusarium wilts: An overview. Environ. Res. J. 2012, 6, 83–102. [Google Scholar]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Soil Science Society of America, Inc.: Madison, WI, USA, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Li, Y.; Nie, C.; Liu, Y.; Du, W.; He, P. Soil microbial community composition closely associates with specific enzyme activities and soil carbon chemistry in a long-term nitrogen fertilized grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Turkish State Meteorological Service. Available online: https://www.mgm.gov.tr/ (accessed on 28 December 2018).
- Gee, G.W.; Bauder, J.W. Particle-Size Analysis. In Methods of Soil Analysis. Part 1. Physical and Minerological Methods, 2nd ed.; Agronomy Monographs; American Society of Agronomy: Madison, WI, USA, 1986; Volume 9, pp. 383–441. [Google Scholar]
- Walkley, A.; Black, L.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Mclean, E.O. Soil pH and Lime Requirement. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Agronomy Monographs; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9, pp. 199–224. [Google Scholar]
- Rhoades, J.D. Soluble Salts. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Agronomy Monographs; American Society of Agronomy: Madison, WI, USA, 1983; Volume 9, pp. 167–179. [Google Scholar]
- Beck, T.H. Die messung der katalaseaktivitaet von Böden. Z. Pflanz. Und Bodenkd. 1971, 130, 68–81. (In German) [Google Scholar] [CrossRef]
- Hoffmann, G.G.; Teicher, K. Ein kolorimetrisches verfahren zur bestimmung der urease aktivitat in böden. Z. Pflanz. Düngung Bodenkd. 1961, 95, 55–63. (In German) [Google Scholar] [CrossRef]
- Hofmann, E.D.; Hoffmann, G.G. Die bestimmug der biologischen tatigheit in böden mit enzymethoden. Adv. Enzymol. Relat. Areas Mol. Biol. 1966, 28, 365–390. (In German) [Google Scholar]
- Adugna, A.; Abegaz, A. Effects of land-use changes on the dynamics of selected soil properties in northeast Wellega, Ethiopia. Soil 2016, 2, 63–70. [Google Scholar] [CrossRef]
- Yang, S.; Sheng, D.; Adamowski, J.; Gong, Y.; Zhang, J.; Cao, J. Effect of land-use change on soil carbon storage over the last 40 years in the Shi Yang River Basin China. Land 2018, 7, 11. [Google Scholar] [CrossRef]
- Alvarez, R.; Alvarez, C.R.; Daniel, P.E.; Richter, V.; Blotta, L. Nitrogen distribution in soil density fractions and its relation to nitrogen mineralisation under different tillage systems. Soil Res. 1998, 36, 247–256. [Google Scholar] [CrossRef]
- Singh, P.; Heikkinen, J.; Ketoja, E.; Nuutinen, V.; Palojärvi, A.; Sheehy, J.; Esala, M.; Alakukku, L.; Regina, K.; Mitra, S. Tillage and crop residue management methods had minor effects on the stock and stabilization of topsoil carbon in a 30-year field experiment. Sci. Total Environ. 2015, 518, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Erdel, E. Soil Enzyme Activities Affecting by Different Tillage Systems and Cover Crops Following Corn Cultivation. J. Inst. Sci. Technol. 2022, 12, 1134–1142. [Google Scholar] [CrossRef]
- Martinez, J.M.; Galantini, J.A.; Duval, M.E.; Lopez, F.M. Tillage effects on labile pools of soil organic nitrogen in a semi-humid climate of Argentina: A long-term field study. Soil Tillage Res. 2017, 169, 71–80. [Google Scholar] [CrossRef]
- Calegari, A.; Hargrove, W.L.; Rheinheimer, D.D.S.; Ralisch, R.; Tessier, D.; de Tourdonnet, S.; de Fatima Guimarães, M. Impact of long-term no-tillage and cropping system management on soil organic carbon in an Oxisol: A model for sustainability. J. Agron. 2008, 100, 1013–1019. [Google Scholar] [CrossRef]
- Van Eerd, L.L.; Congreves, K.A.; Hayes, A.; Verhallen, A.; Hooker, D.C. Long-term tillage and crop rotation effects on soil quality, organic carbon, and total nitrogen. Can. J. Soil Sci. 2014, 94, 303–315. [Google Scholar] [CrossRef]
- Dalal, R.C.; Thornton, C.M.; Allen, D.E.; Owens, J.; Kopittke, P.M. Long-term land use change in Australia from native forest decreases all fractions of soil organic carbon, including resistant organic carbon, for cropping but not sown pasture. Agric. Ecosyst. Environ. 2021, 311, 107326. [Google Scholar] [CrossRef]
- Gebresamuel, G.; Molla, B.; Teka, K.; Negash, E.; Haile, M.; Okolo, C.C. Changes in soil organic carbon stock and nutrient status after conversion of pasture land to cultivated land in semi-arid areas of northern Ethiopia. Arch. Agron. Soil Sci. 2022, 68, 44–60. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Glaser, B.; Turrión, M.B.; Alef, K. Amino sugars and muramic acid—Biomarkers for soil microbial community structure analysis. Soil Biol. Biochem. 2004, 36, 399–407. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter, a key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Ludwig, M.; Achtenhagen, J.; Miltner, A.; Eckhardt, K.U.; Leinweber, P.; Emmerling, C.; Thiele-Bruhn, S. Microbial contribution to SOM quantity and quality in density fractions of temperate arable soils. Soil Biol. Biochem. 2015, 81, 311–322. [Google Scholar] [CrossRef]
- Lan, L. The status quo and development of China’s edible mushroom industry. Chin. Agr. Sci. Bull. 2009, 25, 205–208. [Google Scholar]
- Khan, K.S.; Mack, R.; Castillo, X.; Kaiser, M.; Joergensen, R.G. Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 2016, 271, 115–123. [Google Scholar] [CrossRef]
- Shi, S.; Wang, X.; Ye, Z.; Chen, W.; Li, T.; Chen, J.; Li, J. Effect of the combined application of fungal residue and chemical fertilizers on the mineralization of soil organic carbon in paddy fields. Environ. Sci. Pollut. Res. Int. 2019, 26, 23292–23304. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, J.; Zhang, D.; Cheng, H.; Hao, B.; Cao, A.; Yan, D.; Wang, Q.; Li, Y. Beneficial effect on the soil microenvironment of Trichoderma applied after fumigation for cucumber production. PLoS ONE 2022, 17, e0266347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Chen, L.J.; Chen, X.H.; Tan, M.L.; Duan, Z.H.; Wu, Z.J.; Li, X.J.; Fan, X.H. Response of soil enzyme activity to long-term restoration of desertified land. Catena 2015, 133, 64–70. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Gruba, P. Enzymatic activity and stabilization of organic matter in soil with different detritus inputs. Soil Sci. Plant Nutr. 2017, 63, 242–247. [Google Scholar]
- Tang, F.; Yao, Y.; Song, J.; Wang, C.; Liu, Y. Interactive Influence of Soil Erosion and Cropland Revegetation on Soil Enzyme Activities and Microbial Nutrient Limitations in the Loess Hilly-Gully Region of China. Agronomy 2022, 12, 2796. [Google Scholar] [CrossRef]
- Li, Q.; Liang, J.H.; He, Y.Y.; Hu, Q.J.; Yu, S. Effect of land use on soil enzyme activities at karst area in Nanchuan, Chongqing, Southwest China. Plant Soil Environ. 2014, 60, 15–20. [Google Scholar] [CrossRef]
- Blonska, E.; Lasota, J.; da Silva, G.R.V.; Vanguelova, E.; Ashwood, F.; Tibbett, M.; Watts, K.; Lukac, M. Soil organic matter stabilization and carbon-cycling enzyme activity are affected by land management. Ann. For. Res. 2020, 63, 71–86. [Google Scholar]
- Yang, Y.; Yang, Y.; Geng, Y.; Huang, G.; Cui, X.; Hou, M. Effects of different land types on soil enzyme activity in the Qinghai Lake region. Wetlands 2018, 38, 711–721. [Google Scholar] [CrossRef]
- Lebrun, J.D.; Trinsoutrot-Gattin, I.; Vinceslas-Akpa, M.; Bailleul, C.; Brault, A.; Mougin, C.; Laval, K. Assessing impacts of copper on soil enzyme activities in regard to their natural spatiotemporal variation under long-term different land uses. Soil Biol. Biochem. 2012, 49, 150–156. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, S.; Zhang, K.; Zhao, J.; Jiang, J.; Chen, F.; Fang, W. Evaluation of soil-applied chemical fungicide and biofungicide for control of the Fusarium wilt of chrysanthemum and their effects on rhizosphere soil microbiota. Agriculture 2018, 8, 184. [Google Scholar] [CrossRef]
- Christopher, D.J.; Raj, T.S.; Rani, S.U.; Udhayakumar, R. Role of defense enzymes activity in tomato as induced by Trichoderma virens against Fusarium wilt caused by Fusarium oxysporum f sp. lycopersici. J. Biopestic. 2010, 3, 158. [Google Scholar]
- Aghababaei, F.; Raiesi, F.; Hosseinpur, A. The combined effects of earthworms and arbuscular mycorrhizal fungi on microbial biomass and enzyme activities in a calcareous soil spiked with cadmium. Appl. Soil Ecol. 2014, 75, 33–42. [Google Scholar] [CrossRef]
- Vasconcellos, R.L.; Bonfim, J.A.; Baretta, D.; Cardoso, E.J. Arbuscular mycorrhizal fungi and glomalin-related soil protein as potential indicators of soil quality in a recuperation gradient of the Atlantic forest in Brazil. Land Degrad. Dev. 2016, 27, 325–334. [Google Scholar] [CrossRef]
- Huang, C.; Deng, L.; Gao, X.; Zhang, S.; Luo, T.; Ren, Q. Effects of fungal residues return on soil enzymatic activities and fertility dynamics in a paddy soil under a rice-wheat rotation in Chengdu Plain. Soil Tillage Res. 2010, 108, 16–23. [Google Scholar] [CrossRef]
- Naseby, D.C.; Pascual, J.A.; Lynch, J.M. Effect of biocontrol strains of Trichoderma on plant growth, Pythium ultimum populations, soil microbial communities and soil enzyme activities. J. Appl. Microbiol. 2000, 88, 161–169. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, Q.; Pan, J.; Jiang, S.; Liu, Y.; Bahadur, A.; Peng, Z.; Yang, Y.; Feng, H. Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur. J. Soil Sci. 2020, 71, 84–92. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Cao, H.; Fan, Y.; Du, K.; Bu, X.; Ao, D. Effects of Trichoderma harzianum biofertilizer on growth, yield, and quality of Bupleurum chinense. Plant Direct 2022, 6, e461. [Google Scholar] [CrossRef] [PubMed]
- Asghar, W.; Kataoka, R. Effect of co-application of Trichoderma spp. with organic composts on plant growth enhancement, soil enzymes and fungal community in soil. Arch. Microbiol. 2021, 203, 4281–4291. [Google Scholar] [CrossRef]
- Baazeem, A.; Almanea, A.; Manikandan, P.; Alorabi, M.; Vijayaraghavan, P.; Abdel-Hadi, A. In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J. Fungi 2021, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Liu, Z.; Liu, B.; Wang, Y.; Wang, J. The effect of Trichoderma biofertilizer on the quality of flowering Chinese cabbage and the soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Xiao, L.; Bi, Y.; Du, S.; Wang, Y.; Guo, C. Effects of re-vegetation type and arbuscular mycorrhizal fungal inoculation on soil enzyme activities and microbial biomass in coal mining subsidence areas of Northern China. Catena 2019, 177, 202–209. [Google Scholar] [CrossRef]



| Soil | Soil Characteristics | |||
|---|---|---|---|---|
| Texture | SOC, % | EC, µmhos/cm | pH | |
| Pastureland | Silty clay | 6.971 | 156.33 | 6.5 |
| Agricultural land | Silty clay | 4.694 | 46.33 | 6.9 |
| Soil | Application | ||||
|---|---|---|---|---|---|
| Soilborne Pathogen | Bioagent | ||||
| Control | Fusarium oxysporum | Verticillium dahliae | Rhizoctonia solani | Trichoderma sp. | |
| Pastureland | 6.97 ± 0.2 cdA | 6.7 ± 0.13 dA | 7.46 ± 0.12 aA | 7.03 ± 0.07 bcA | 7.27 ± 0.16 abA |
| Agricultural land | 4.69 ± 0.24 aB | 4.65 ± 0.18 aB | 4.72 ± 0.12 aB | 4.37 ± 0.26 abB | 4.14 ± 0.12 bB |
| Soil | Enzyme | Application | ||||
|---|---|---|---|---|---|---|
| Control | Soilborne Pathogen | Bioagent | ||||
| Fusarium oxysporum | Verticillium dahliae | Rhizoctonia solani | Trichoderma sp. | |||
| Pastureland | Alkaline phosphatase | 117.44 ± 1.21 aA | 106.09 ± 1.03 cA | 111.18 ± 2.01 bA | 110.91 ± 1.47 bA | 116.74 ± 1.10 aA |
| Catalase | 177.03 ± 3.96 aA | 149.28 ± 8.41 cA | 90.91 ± 4.80 eA | 104.94 ± 4.89 dA | 161.29 ± 4.55 bA | |
| Urease | 12.87 ± 1.51 b | 7.67 ± 0.50 d | 12.52 ± 0.46 bcA | 10.86 ± 0.74 cA | 17.08 ± 1.30 aA | |
| Agricultural land | Alkaline phosphatase | 109.07 ± 3.17 aB | 99.69 ± 0.72 bB | 87.92 ± 3.08 cB | 103.13 ± 2.92 bB | 83.89 ± 2.59 cB |
| Catalase | 76.05 ± 5.25 aB | 65.17 ± 4.96 bB | 59.84 ± 7.19 bB | 61.21 ± 4.35 bB | 47.22 ± 2.68 cB | |
| Urease | 11.52 ± 0.69 a | 8.51 ± 1.04 b | 9.00 ± 0.80 bB | 8.13 ± 0.75 bB | 7.70 ± 0.91 bB | |
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
|---|---|---|---|---|---|---|
| Eigenvalue | 3.977 | 1.144 | 0.568 | 0.220 | 0.078 | 0.012 |
| Variability (%) | 66.283 | 19.070 | 9.467 | 3.672 | 1.303 | 0.206 |
| Cumulative (%) | 66.283 | 85.352 | 94.819 | 98.491 | 99.794 | 100.000 |
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
|---|---|---|---|---|---|---|
| Catalase | 20.894 | 4.088 | 7.665 | 18.654 | 48.087 | 0.613 |
| Alkaline phosphatase | 20.096 | 1.792 | 5.455 | 65.215 | 7.164 | 0.279 |
| Urease | 14.611 | 0.479 | 66.434 | 15.622 | 2.024 | 0.830 |
| SOC | 21.986 | 7.008 | 4.807 | 0.048 | 14.944 | 51.207 |
| Pasture-Agricultural Land | 21.260 | 4.621 | 15.189 | 0.149 | 11.799 | 46.983 |
| Fungi | 1.154 | 82.013 | 0.449 | 0.312 | 15.983 | 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdel, E.; Şimşek, U.; Kesimci, T.G. Effects of Fungi on Soil Organic Carbon and Soil Enzyme Activity under Agricultural and Pasture Land of Eastern Türkiye. Sustainability 2023, 15, 1765. https://doi.org/10.3390/su15031765
Erdel E, Şimşek U, Kesimci TG. Effects of Fungi on Soil Organic Carbon and Soil Enzyme Activity under Agricultural and Pasture Land of Eastern Türkiye. Sustainability. 2023; 15(3):1765. https://doi.org/10.3390/su15031765
Chicago/Turabian StyleErdel, Erhan, Uğur Şimşek, and Tuba Genç Kesimci. 2023. "Effects of Fungi on Soil Organic Carbon and Soil Enzyme Activity under Agricultural and Pasture Land of Eastern Türkiye" Sustainability 15, no. 3: 1765. https://doi.org/10.3390/su15031765
APA StyleErdel, E., Şimşek, U., & Kesimci, T. G. (2023). Effects of Fungi on Soil Organic Carbon and Soil Enzyme Activity under Agricultural and Pasture Land of Eastern Türkiye. Sustainability, 15(3), 1765. https://doi.org/10.3390/su15031765








