Deformation Characteristics of Combined Heavy Metals-Contaminated Soil Treated with nZVI through the Modified Slurry Consolidation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Soil
2.1.2. Contaminants and nZVI
2.1.3. Toxic Leaching Reagent
2.2. Methods
2.2.1. Preparation of Soil Samples
2.2.2. Toxicity Characteristic Leaching Procedure (TCLP)
2.2.3. Oedometer Tests
2.2.4. Microscopic Characterization
- 1.
- XRD analysis
- 2.
- Atterberg limits
- 3.
- Particle-size distribution (PSD)
3. Results and Discussion
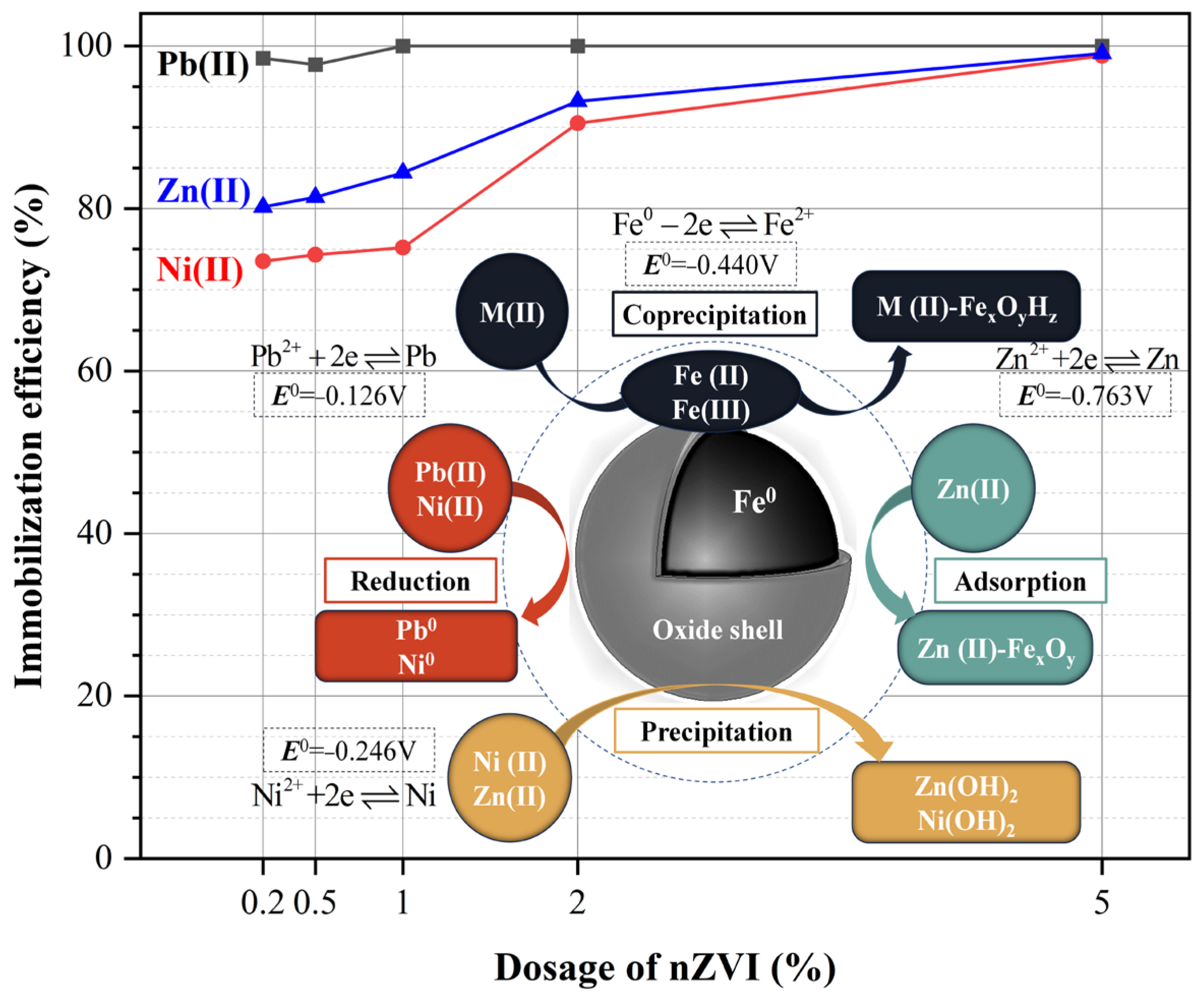
3.1. Immobilization of Heavy Metal Ions
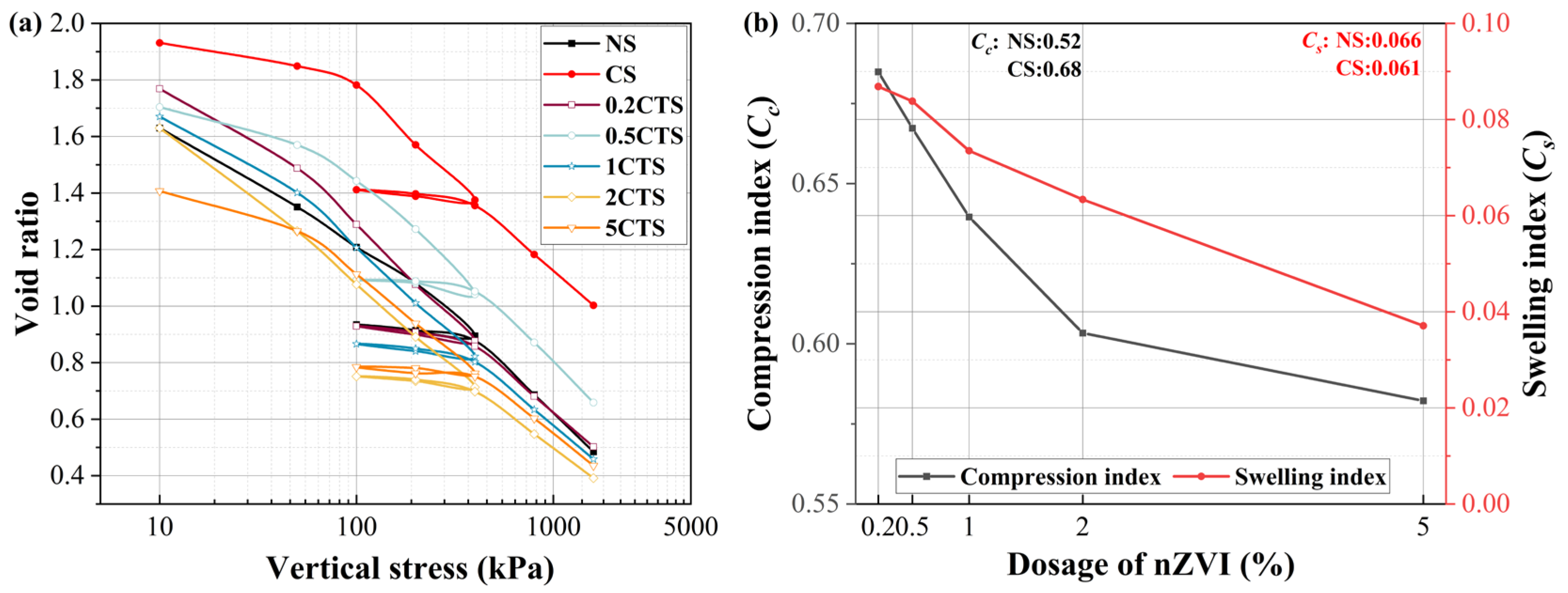
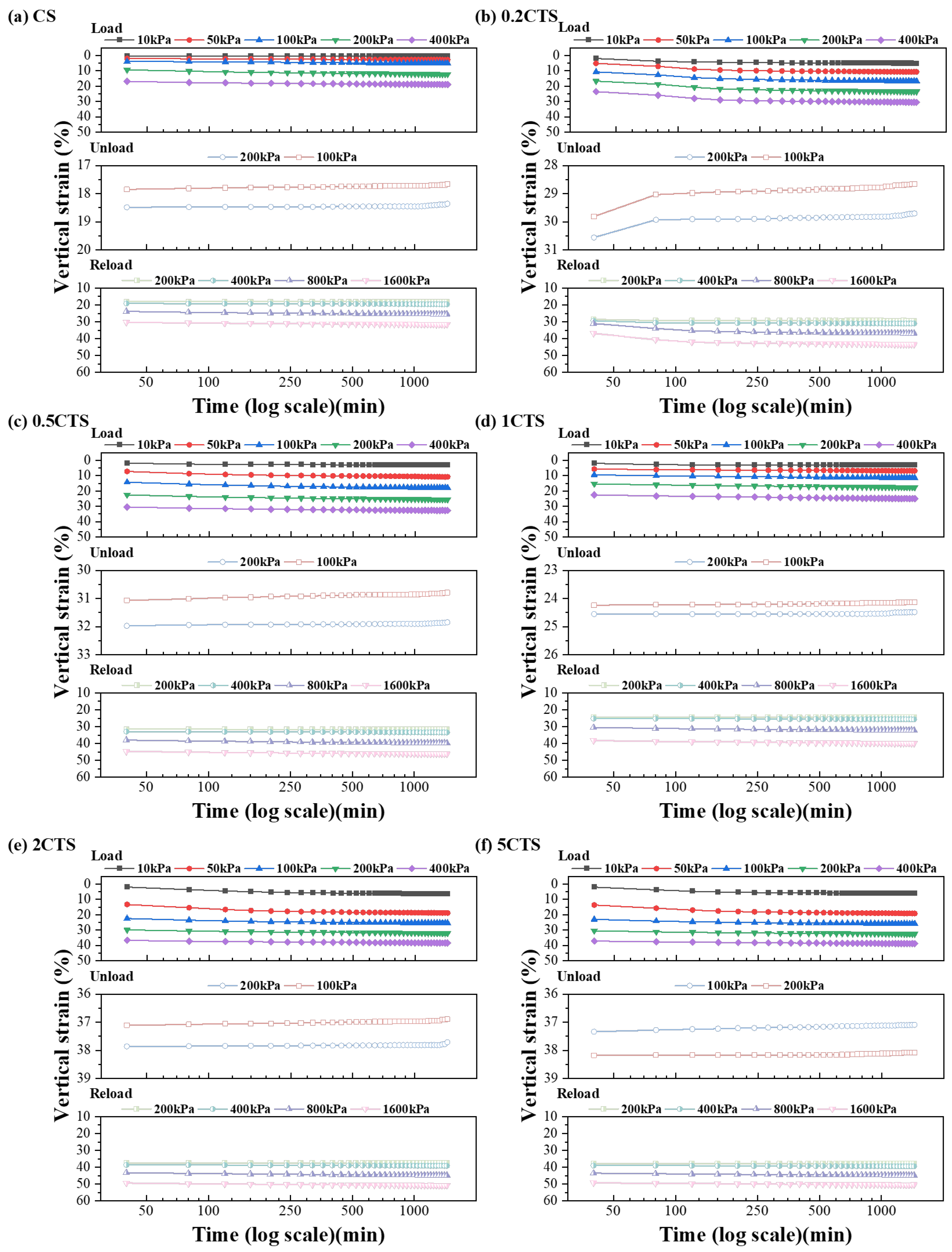
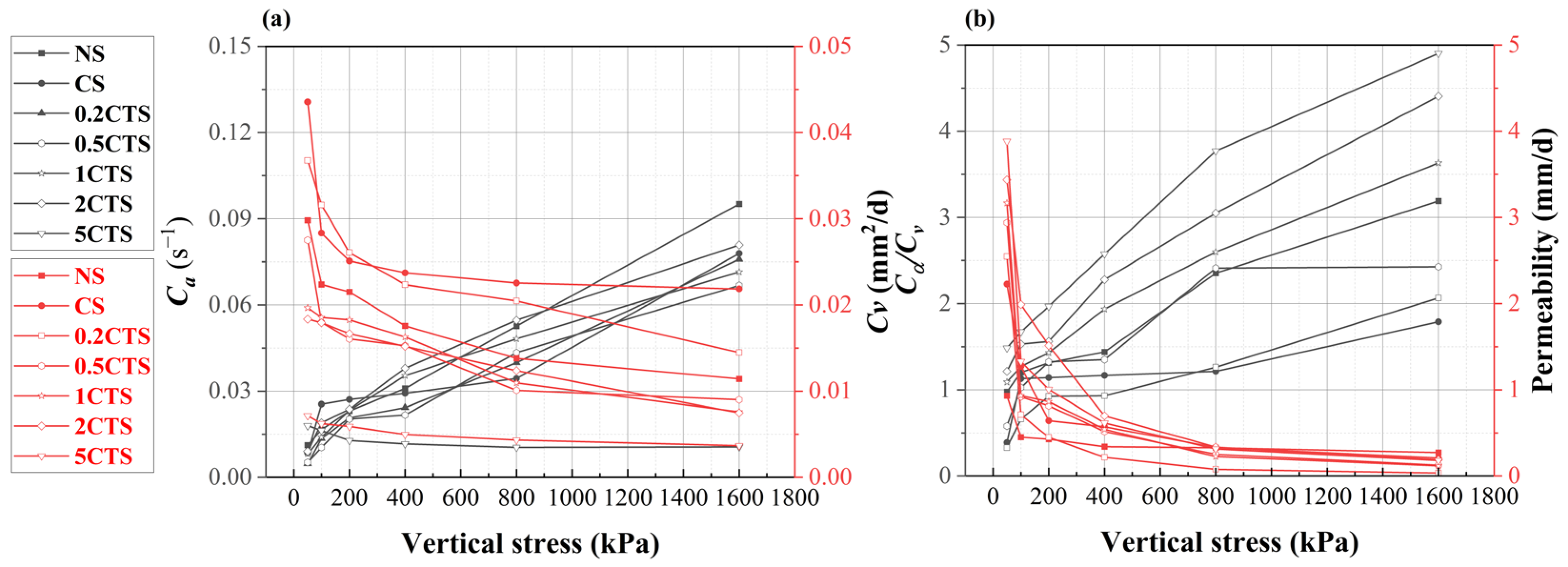
3.2. Deformation Analysis
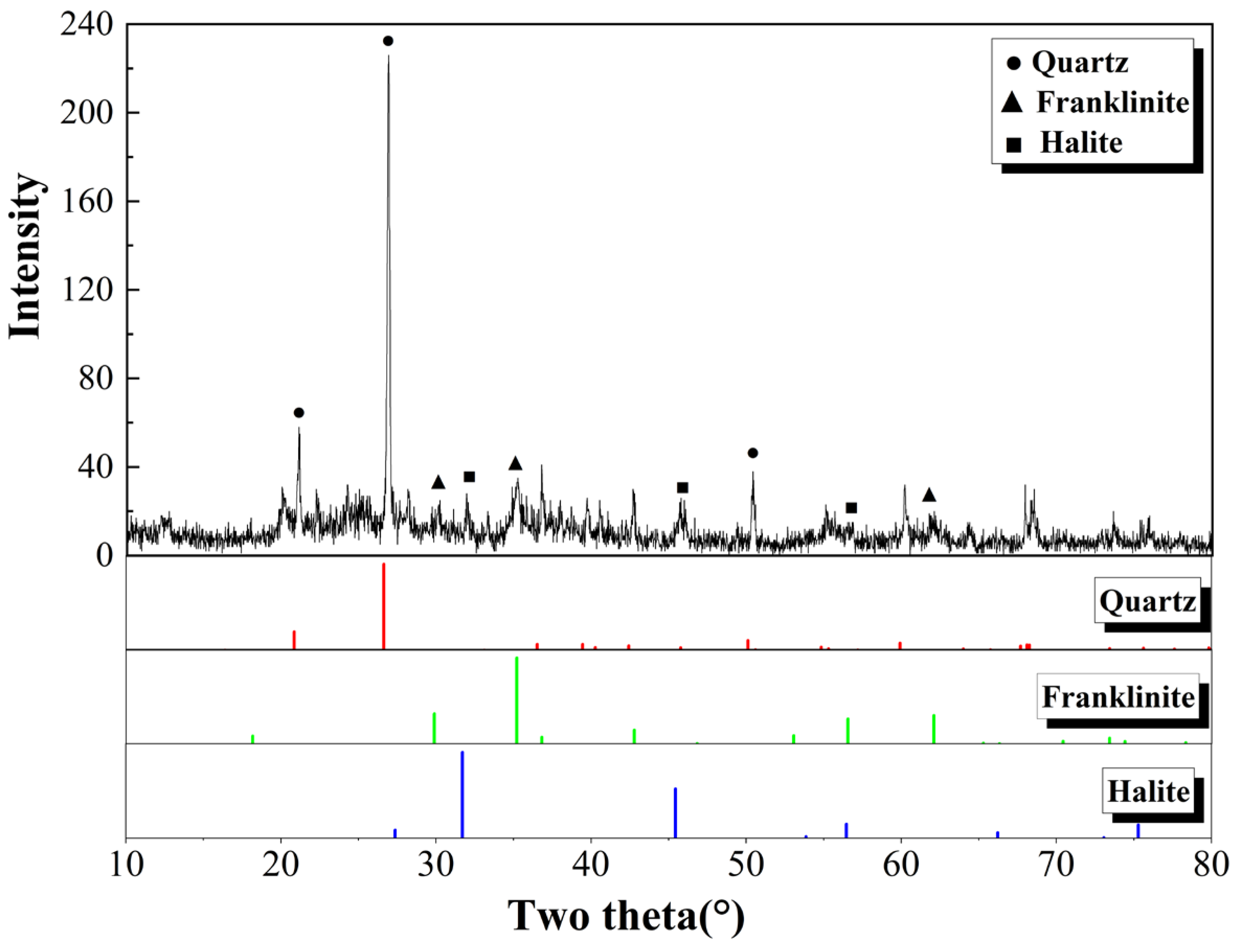
3.3. Microscopic Characteristics of Soil
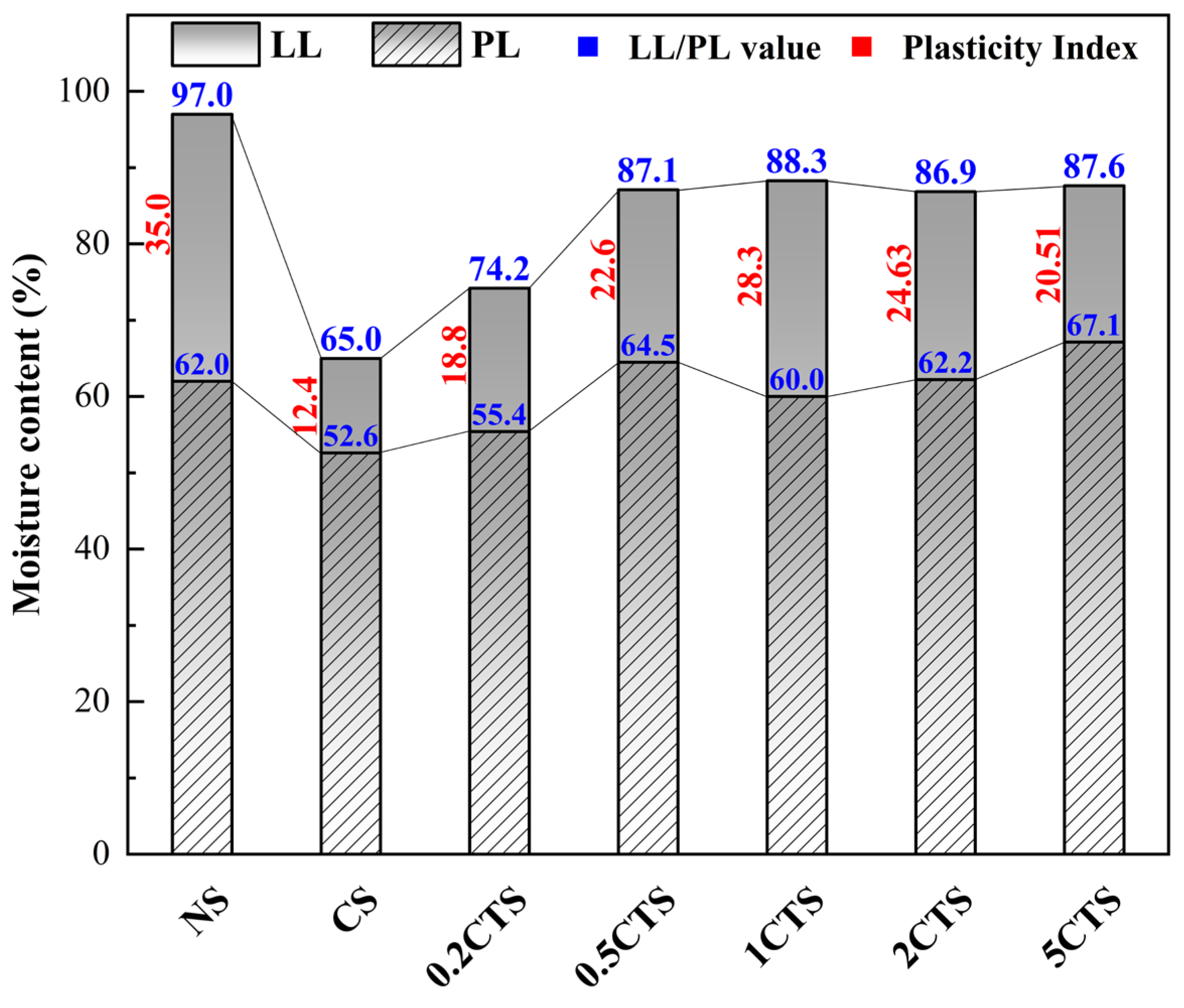
3.3.1. Plasticity of Soil Samples
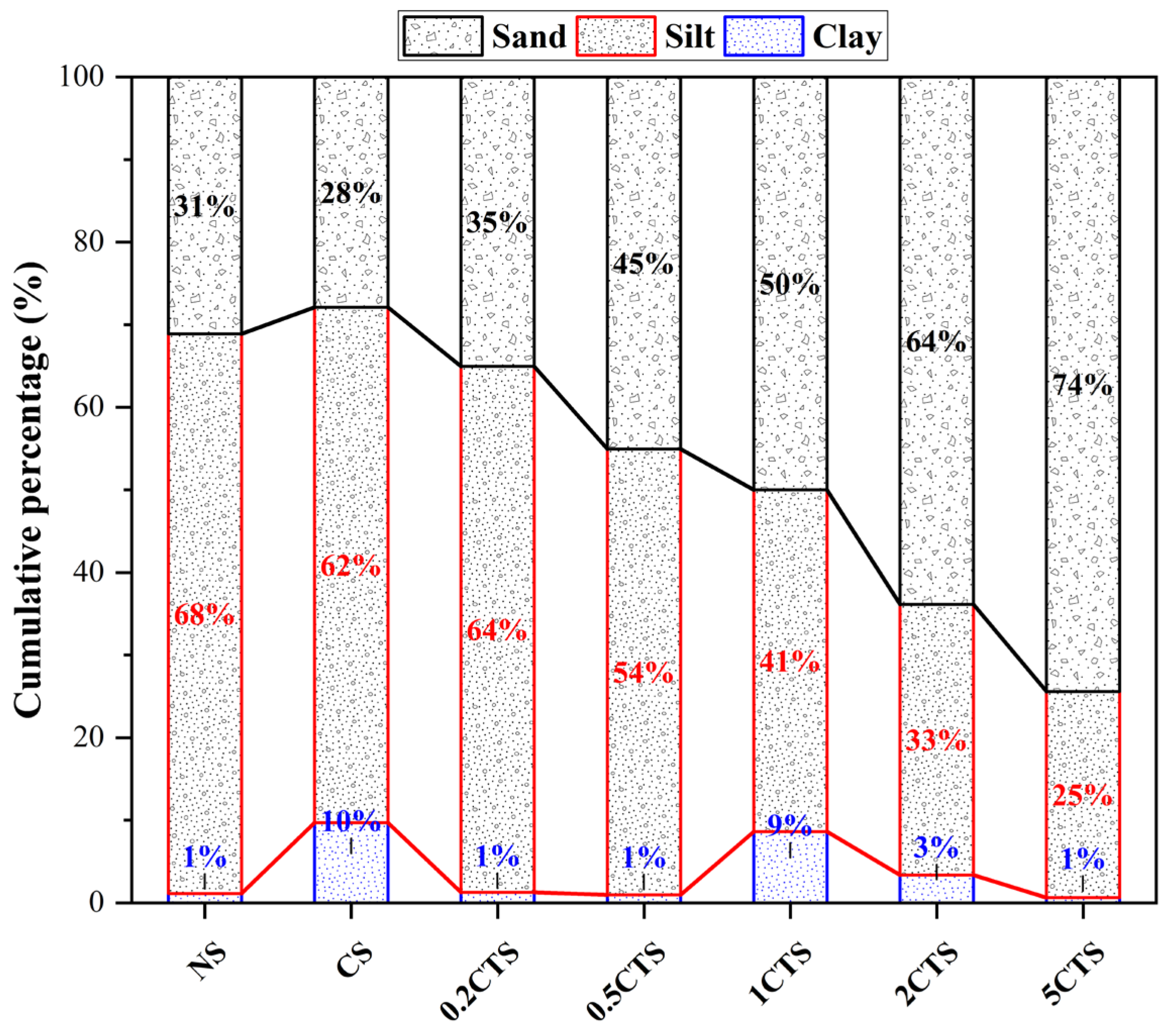
3.3.2. Particle-Size Distribution
4. Conclusions
- (a)
- nZVI efficiently degraded the combined heavy metal ions in the soil, with degradation efficiency exceeding 90% at 2% nZVI after 4 days of a slurry consolidation process. The efficiency showed a positive correlation with a higher nZVI dosage, showing 98%—Pb(II), 85%—Zn(II), and 75% Ni(II) at 1% nZVI. All three heavy metal ions were degraded, exceeding 98% at 5%;
- (b)
- Post-nZVI treatment has a positive effect on soil deformation and permeability by the slurry consolidation process, such as lower soil compressibility, swelling index, and increasing permeability. With rising nZVI dosages, the creep ratio and permeability increased due to the shift from weakened chemical bonds to strengthened physical aggregation structures with the formation of larger aggregates. The presence of three heavy metal ions weakened soil resistance, increased the compression index, decreased the swelling index, and reduced the creep ratio. This was attributed to the weakened interparticle connections, higher clay content, and thinner diffusion layers, allowing easier particle slippage;
- (c)
- The Atterberg limits of nZVI-treated soil raised with nZVI dosages due to the degradation of heavy metal ions and improved plasticity and water retention from the formation of insoluble hydroxides or iron oxide aggregate with soil particles. After contamination with the combined heavy metal ions in the soil, the Atterberg limits decreased due to higher cation concentration in pore fluid, occupying soil particle binding sites, and thinning the diffuse double layer;
- (d)
- The proportion of clay particles dramatically increased in CS, confirming the earlier increase in fragmented particles. In TS, as the nZVI dosage increases, the sand proportion of soil particles gradually rises, especially at 2% and 5%. It should be summarized that unreacted nZVI particles tended to aggregate for larger particles due to their strong magnetism, adsorption, and aggregation, thus stabilizing their chemical effects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.A.; Bardgett, R.D.; Caon, L.; Crowther, T.W.; Delgado-Baquerizo, M.; Montanarella, L. Tracking, targeting, and conserving soil biodiversity. Science 2021, 371, 239–241. [Google Scholar] [CrossRef]
- Liao, J.; Wen, Z.; Ru, X.; Chen, J.; Wu, H.; Wei, C. Distribution and migration of heavy metals in soil and crops affected by acid mine drainage: Public health implications in Guangdong Province, China. Ecotoxicol. Environ. Saf. 2016, 124, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yuan, Z.; Li, D.; Zheng, M.; Nie, X.; Liao, Y. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal (loid) s in soil: A review. Environ. Sci. Process. Impacts 2020, 22, 1596–1615. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, S.; Zeng, G.; Li, F.; Gu, Y.; Shi, Y.; Shi, L.; Liu, W.; Peng, S. A new exploration of health risk assessment quantification from sources of soil heavy metals under different land use. Environ. Pollut. 2018, 243, 49–58. [Google Scholar] [CrossRef] [PubMed]
- World’s Worst Pollution Problems. The New Top Six Toxic Threats: A Priority List for Remediation; Pure Earth: Zurich, Switzerland, 2016. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Song, W.E.; Chen, S.B.; Liu, J.F.; Chen, L.; Song, N.N.; Li, N.; Liu, B. Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J. Integr. Agric. 2015, 14, 1845–1854. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mansour, S.N.; Najafi, M.L.; Toolabi, A.; Abdolahnejad, A.; Faraji, M.; Miri, M. Probabilistic risk assessment of soil contamination related to agricultural and industrial activities. Environ. Res. 2022, 203, 111837. [Google Scholar] [CrossRef]
- Sabet Aghlidi, P.; Cheraghi, M.; Lorestani, B.; Sobhanardakani, S.; Merrikhpour, H. Analysis, spatial distribution and ecological risk assessment of arsenic and some heavy metals of agricultural soils, case study: South of Iran. J. Environ. Health Sci. Eng. 2020, 18, 665–676. [Google Scholar] [CrossRef]
- European Environment Agency. Air Quality in Europe—2018 Report; European Environment Agency: Copenhagen, Denmark, 2018.
- EPA US. Cleaning up the Nation’s Waste Sites: Markets and Technology Trends; National Technical Information; United States Environmental Protection Agency: Washington, DC, USA, 2004.
- The Ministry of Environmental Protection (MEP). The Ministry of Land and Resources Report on the National Soil Contamination Survey; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2014.
- Xi, Y.; Mallavarapu, M.; Naidu, R. Reduction and adsorption of Pb2+ in aqueous solution by nano-zero-valent iron—A SEM, TEM and XPS study. Mater. Res. Bull. 2010, 45, 1361–1367. [Google Scholar] [CrossRef]
- Efecan, N.; Shahwan, T.; Eroğlu, A.E.; Lieberwirth, I. Characterization of the uptake of aqueous Ni2+ ions on nanoparticles of zero-valent iron (nZVI). Desalination 2009, 249, 1048–1054. [Google Scholar] [CrossRef]
- Rathor, G.; Chopra, N.; Adhikari, T. Remediation of nickel ion from soil and water using Nano particles of zero-valent Iron (nZVI). Orient. J. Chem. 2016, 33, 1025. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Ortiz, L.T.; Costa, G.; Alonso, J.; Rodríguez-Membibre, M.L.; Sánchez-Fortún, S. Immobilization and leaching of Pb and Zn in an acidic soil treated with zerovalent iron nanoparticles (nZVI): Physicochemical and toxicological analysis of leachates. Water Air Soil Pollut. 2014, 225, 1990. [Google Scholar] [CrossRef]
- Vasarevičius, S.; Danila, V.; Januševičius, T. Immobilisation of cadmium, copper, lead, and nickel in soil using nano zerovalent iron particles: Ageing effect on heavy metal retention. Water Air Soil Pollut. 2020, 231, 496. [Google Scholar] [CrossRef]
- He, M.; Shi, H.; Zhao, X.; Yu, Y.; Qu, B. Immobilization of Pb and Cd in contaminated soil using nano-crystallite hydroxyapatite. Procedia Environ. Sci. 2013, 18, 657–665. [Google Scholar] [CrossRef]
- Arabyarmohammadi, H.; Darban, A.K.; Abdollahy, M.; Yong, R.; Ayati, B.; Zirakjou, A.; Van der Zee, S.E. Utilization of a novel chitosan/clay/biochar nanobiocomposite for immobilization of heavy metals in acid soil environment. J. Polym. Environ. 2018, 26, 2107–2119. [Google Scholar] [CrossRef]
- Lian, M.; Wang, L.; Feng, Q.; Niu, L.; Zhao, Z.; Wang, P.; Song, C.; Li, X.; Zhang, Z. Thiol-functionalized nano-silica for in-situ remediation of Pb, Cd, Cu contaminated soils and improving soil environment. Environ. Pollut. 2021, 280, 116879. [Google Scholar] [CrossRef]
- Mohammed, S.A.S.; Sanaulla, P.F.; Alnuaim, A.M.; Moghal, A.A.B. Role of Different Leaching Methods to Arrest the Transport of Ni2+ in Soil and Soil Amended with Nano Calcium Silicate. Geo-China 2016, 2016, 49–56. [Google Scholar]
- Wang, J.; Liu, G.; Li, T.; Zhou, C.; Qi, C. Zero-valent iron nanoparticles (NZVI) supported by kaolinite for CuII and NiII ion removal by adsorption: Kinetics, thermodynamics, and mechanism. Aust. J. Chem. 2015, 68, 1305–1315. [Google Scholar] [CrossRef]
- Sang, L.; Wang, G.; Liu, L.; Bian, H.; Jiang, L.; Wang, H.; Zhang, Y.; Zhang, W.; Peng, C.; Wang, X. Immobilization of Ni (II) at three levels of contaminated soil by rhamnolipids modified nano zero valent iron (RL@ nZVI): Effects and mechanisms. Chemosphere 2021, 276, 130139. [Google Scholar] [CrossRef] [PubMed]
- Karabelli, D.; Üzüm, C.; Shahwan, T.; Eroglu, A.E.; Scott, T.B.; Hallam, K.R.; Lieberwirth, I. Batch removal of aqueous Cu2+ ions using nanoparticles of zero-valent iron: A study of the capacity and mechanism of uptake. Ind. Eng. Chem. Res. 2008, 47, 4758–4764. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Y.; Zhou, X.; Dai, C.; Keller, A.A. A new insight on the core–shell structure of zerovalent iron nanoparticles and its application for Pb (II) sequestration. J. Hazard. Mater. 2013, 263, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef] [PubMed]
- Adusei-Gyamfi, J.; Acha, V. Carriers for nano zerovalent iron (nZVI): Synthesis, application and efficiency. RSC Adv. 2016, 6, 91025–91044. [Google Scholar] [CrossRef]
- Lu, H.J.; Wang, J.K.; Ferguson, S.; Wang, T.; Bao, Y.; Hao, H. Mechanism, synthesis and modification of nano zerovalent iron in water treatment. Nanoscale 2016, 8, 9962–9975. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Zhou, W.H.; Liu, F.; Yi, S. Exploring the effects of nanoscale zero-valent iron (nZVI) on the mechanical properties of lead-contaminated clay. Can. Geotech. J. 2019, 56, 1395–1405. [Google Scholar] [CrossRef]
- Yang, Z.; Elgamal, A. Influence of permeability on liquefaction-induced shear deformation. J. Eng. Mech. 2002, 128, 720–729. [Google Scholar] [CrossRef]
- Eggers, C.G.; Berli, M.; Accorsi, M.L.; Or, D. Deformation and permeability of aggregated soft earth materials. J. Geophys. Res. Solid Earth 2006, 111, B10204. [Google Scholar] [CrossRef]
- Sadiq, A.S.; Fattah, M.Y.; Aswad, M.F. Enhancement of Clay Compressibility and Strength Using Nano Magnesium Oxide. E3S Web Conf. 2023, 427, 01013. [Google Scholar] [CrossRef]
- Luo, X.; Kong, L.; Bai, W. Effect of Superhydrophobic Nano-SiO2 on the Hydraulic Conductivity of Expansive Soil and Analysis of Its Mechanism. Appl. Sci. 2023, 13, 8198. [Google Scholar] [CrossRef]
- Ouhadi, V.R.; Noori, A. Impact of Nano-Clay on Consolidation and Permeability Behaviour of Bentonite in the Presence of Heavy Metal Contaminant. Modares Civ. Eng. J. 2013, 13, 1–10. [Google Scholar]
- Chen, Y.Z.; Zhou, W.H.; Liu, F.; Yi, S.; Geng, X. Microstructure and morphological characterization of lead-contaminated clay with nanoscale zero-valent iron (nZVI) treatment. Eng. Geol. 2019, 256, 84–92. [Google Scholar] [CrossRef]
- Antia, D.D.J. Hydrodynamic Decontamination of Groundwater and Soils Using ZVI. Water 2023, 15, 540. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.P.; Zhu, L. Remediation of soil contaminated with organic compounds by nanoscale zero-valent iron: A review. Sci. Total Environ. 2021, 760, 143413. [Google Scholar] [CrossRef]
- ASTM D4318-17; Standard Test Method for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D698-12; Standard Test Method for Laboratory Compaction Characteristics of Soil Using Standard Effort (12400 ft-lbf/ft3 (600 kN-m/m3)). ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D2487-17; Standard Test Method for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2017.
- Huang, P.; Ye, Z.; Xie, W.; Chen, Q.; Li, J.; Xu, Z.; Yao, M. Rapid magnetic removal of aqueous heavy metals and their relevant mechanisms using nanoscale zero valent iron (nZVI) particles. Water Res. 2013, 47, 4050–4058. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Liang, F.; Zhang, W.X. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. J. Hazard. Mater. 2017, 322, 163–171. [Google Scholar] [CrossRef]
- ASTM D2435/D2435M−11; Standard Test Method for One-Dimensional Consolidation Properties of Soils Using Incremental Loading. ASTM International: West Conshohocken, PA, USA, 2020.
- U.S. Environmental Protection Agency. Test Methods for Evaluating Solid Waste (SW-846); U.S. Environmental Protection Agency: Washington, DC, USA, 2015.
- Liang, Z.; Peng, X.; Luan, Z. Immobilization of Cd, Zn and Pb in sewage sludge using red mud. Environ. Earth Sci. 2012, 66, 1321–1328. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Zheng, W. Copyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain. Chem. Eng. 2016, 4, 1630–1636. [Google Scholar] [CrossRef]
- Diao, Z.H.; Du, J.J.; Jiang, D.; Kong, L.J.; Huo, W.Y.; Liu, C.M.; Wu, Q.H.; Xu, X.R. Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron: Coexistence effect and mechanism. Sci. Total Environ. 2018, 642, 505–515. [Google Scholar] [CrossRef]
- Kržišnik, N.; Mladenovič, A.; Škapin, A.S.; Škrlep, L.; Ščančar, J.; Milačič, R. Nanoscale zero-valent iron for the removal of Zn2+, Zn(II)–EDTA and Zn(II)–citrate from aqueous solutions. Sci. Total Environ. 2014, 476, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Apriliani, N. Green Synthesis of Nanoscale Zero-Valent Iron and Its Activity as an Adsorbent for Ni(II) and Cr(VI). Chem. Mater. 2022, 1, 71–76. [Google Scholar] [CrossRef]
- Fan, R.D.; Liu, S.Y.; Du, Y.J.; Reddy, K.R.; Yang, Y.L. Impacts of presence of lead contamination on settling behavior and microstructure of clayey soil-calcium bentonite blends. Appl. Clay Sci. 2017, 142, 109–119. [Google Scholar] [CrossRef]
- Caporale, A.G.; Violante, A. Chemical processes affecting the mobility of heavy metals and metalloids in soil environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, X.; Han, L.; Yang, L.; Gu, M.; Li, J.; Chen, M. The evolution of stable nanohybrids to complex heteroaggregates between nZVI and soil nanoparticles: The influence of ionic strength and soil components. J. Hazard. Mater. 2022, 436, 129155. [Google Scholar] [CrossRef] [PubMed]
- Van Koetsem, F.; Van Havere, L.; Du Laing, G. Impact of carboxymethyl cellulose coating on iron sulphide nanoparticles stability, transport, and mobilization potential of trace metals present in soils and sediment. J. Environ. Manag. 2016, 168, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chakma, S.; Birke, V. Performance of field-scale permeable reactive barriers: An overview on potentials and possible implications for in-situ groundwater remediation applications. Sci. Total Environ. 2023, 858, 158838. [Google Scholar] [CrossRef]
- Zekkos, D.; Kabalan, M.; Syal, S.M.; Hambright, M.; Sahadewa, A. Geotechnical characterization of a municipal solid waste incineration ash from a Michigan monofil. Waste Manag. 2013, 33, 1442–1450. [Google Scholar] [CrossRef]
- Polidori, E. Relationship between the Atterberg limits and clay content. Soils Found. 2007, 47, 887–896. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, N. Correlation between Atterberg limits and soil adsorptive water. J. Geotech. Geoenviron. Eng. 2021, 147, 04020162. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Ashfaq, M.; Al-Shamrani, M.A.; Al-Mahbashi, A. Effect of heavy metal contamination on the compressibility and strength characteristics of chemically modified semiarid soils. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020029. [Google Scholar] [CrossRef]
- Mar Gil-Díaz, M.; Pérez-Sanz, A.; Angeles Vicente, M.; Carmen Lobo, M. Immobilisation of Pb and Zn in soils using stabilised zero-valent iron nanoparticles: Effects on soil properties. Clean–Soil Air Water 2014, 42, 1776–1784. [Google Scholar] [CrossRef]
- Li, J.S.; Xue, Q.; Wang, P.; Li, Z.Z. Effect of lead (II) on the mechanical behavior and microstructure development of a Chinese clay. Appl. Clay Sci. 2015, 105, 192–199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, C.; Chen, Y.; Dong, Q.; Wei, J.; Zou, M. Deformation Characteristics of Combined Heavy Metals-Contaminated Soil Treated with nZVI through the Modified Slurry Consolidation Method. Sustainability 2023, 15, 16959. https://doi.org/10.3390/su152416959
Fan C, Chen Y, Dong Q, Wei J, Zou M. Deformation Characteristics of Combined Heavy Metals-Contaminated Soil Treated with nZVI through the Modified Slurry Consolidation Method. Sustainability. 2023; 15(24):16959. https://doi.org/10.3390/su152416959
Chicago/Turabian StyleFan, Chen, Yongzhan Chen, Qinxi Dong, Jing Wei, and Meng Zou. 2023. "Deformation Characteristics of Combined Heavy Metals-Contaminated Soil Treated with nZVI through the Modified Slurry Consolidation Method" Sustainability 15, no. 24: 16959. https://doi.org/10.3390/su152416959
APA StyleFan, C., Chen, Y., Dong, Q., Wei, J., & Zou, M. (2023). Deformation Characteristics of Combined Heavy Metals-Contaminated Soil Treated with nZVI through the Modified Slurry Consolidation Method. Sustainability, 15(24), 16959. https://doi.org/10.3390/su152416959







