1. Introduction
Serious pollution of aboveground water in China has been caused by rapid and concentrated urbanization. Unfortunately, water purification technology has not kept pace with this pollution [
1]. The primary sources of water pollution include industrial wastewater, agricultural and livestock runoff, and domestic sewage. In recent years, there have been strict regulations for the pre-discharge treatment of industrial sewage, and most companies have the financial resources and capabilities to treat their sewage effectively. The treatment of domestic sewage in large cities is fairly standardized. Nevertheless, in rural areas, the treatment of domestic sewage, livestock and poultry waste, and aquaculture waste falls very short of meeting discharge standards. This has led to severe eutrophication in rivers, lakes, and coastal water, characterized by the excessive growth of algae and further deterioration of water quality [
2]. Additionally, due to China’s rapid urbanization, especially in small and medium-sized cities, sewage treatment and other environmental infrastructure have not kept pace. While the sewage outlets in major cities are relatively complete, the sewage network is not ideal, resulting in the majority of residents’ domestic sewage not being able to enter the urban sewage system.
Domestic sewage contains a significant amount of organic matter, such as cellulose, starch, sugar, fat, and protein. Under anaerobic conditions with bacteria, these substances can easily generate odorous compounds, such as hydrogen sulfide and mercaptans [
3]. The effluent from sewage treatment plants is often not allowed to be discharged because it does not meet nitrate concentration standards. Excessive nitrates also affect the water quality of rivers and lakes. High nitrate concentrations lead to excessive algae growth, exacerbating water pollution and sometimes causing the death of fish and shrimps [
4]. Nitrate pollution in aboveground water mainly comes from agricultural fertilization, animal husbandry, and domestic sewage. Excessive nitrate is known to cause methemoglobinemia and cancers [
5,
6].
Commonly used technologies in sewage treatment and water purification include activated charcoal, iron powder or iron-containing compounds, and various bacteria, including photosynthetic bacteria [
7,
8]. Activated charcoal possesses a highly developed pore structure and demonstrates a strong capacity for adsorption on both its surface and interior [
8]. Moreover, it can be regenerated and reused multiple times. Traditionally, activated charcoal has found extensive use as an adsorbent and has been employed since 1932 to treat tap water in Chicago [
9]. Over the years, it has been increasingly utilized in water treatment and has become a research focal point worldwide. Nowadays, the applications of activated charcoal widely span across various industries, including chemical smelting, pharmaceutical engineering, wastewater treatment, gas purification, drinking water purification, and the food industry, primarily for treating waste gas, wastewater, and drinking water [
9]. The Japanese were the first to apply photosynthetic bacteria and
Bacillus spp. in wastewater treatment and water purification [
10]. Subsequently, lactic acid bacteria and yeast were included. The exact mechanisms of action have yet been clearly defined. Under water conditions, the symbiotic proliferation of various bacteria can fully utilize a good microecosystem to effectively complete the complex process of pollutant degradation. Microbial technology has been applied to the purification of wastewater, rivers, and lakes [
11,
12]. Compared to traditional biological methods, compound microbial materials not only select and domesticate microorganisms, but also combine bacteria and enzymes with different functions [
13]. They can incorporate optimal strains for different environments, such as natural water bodies, domestic sewage, and industrial wastewater. These materials can be used in both anaerobic and aerobic states, even in the presence of multiple pollution sources [
12]. The substances and secretions they produce during growth become the substrates for their respective or mutual growth. This symbiotic relationship forms a complex and stable micro-ecosystem that accelerates the degradation of organic matter and performs various functions. Upon activated charcoal, the probiotics are concentrated and able to interact better. This ensures that the compound microbes are securely held in the activated charcoal and not washed away during the sewage purification process.
The water treatment industry has shown great interest in using iron powder, especially zero-valent iron, for sewage treatment [
14]. Zero-valent iron is a cost-effective and efficient treatment agent because its standard oxidation electrode has low potential, enabling it to reduce various contaminants, including nitrate. It is also more economical and does not require additional treatment. Previous studies have extensively studied the use of zero-valent iron in wastewater treatment, showcasing its effectiveness in treating difficult wastewater, such as that contaminated with arsenic, phenol, dyes, and nitrate-eutrophicated water [
8,
15,
16]. In recent years, nanometer zero-valent iron (nZVI) has gained attention due to its finer particle size, larger specific surface area, higher reactivity, and easy oxidization. Studies have demonstrated that nZVI eventually oxidizes to a complex of iron oxide and fibrous iron ore, especially when in an acidic environment [
17]. Several mechanisms, including activated charcoal, nano-iron powder, and microbes, have been described for water purification [
8,
14,
17]. However, there are certain disadvantages to using zero-valent iron powder for reducing nitrate in water. Firstly, the pH of the reaction must be strictly regulated. Secondly, after the reaction, there is an increase in ammonia nitrogen and nitrite nitrogen in the water, which requires additional treatment. Despite these drawbacks, the iron reduction method remains a popular choice as it does not have any specific environmental requirements other than pH regulation. Currently, there are several improvement measures that have been proposed, suggesting that using zero-valent iron with auxiliary methods to remove nitrate from water is an emerging research area [
14,
15,
16].
This research promotes the concept of using waste materials, such as activated charcoal, to conserve resources and protect the environment. Meanwhile, we aim to develop a composite material using activated charcoal, nano-iron powder, and compound microbes, and investigate its effectiveness in removing nitrate from water. Our focus is on the reduction in nitrate ion concentration as an index of water purification; however, the impact on COD (chemical oxygen demand), phosphorus, and heavy metals will be explored in future research projects.
2. Materials and Methods
2.1. Preparation of Compound Microbial Liquid
The raw materials listed in
Table 1 were mixed and added to a stirring tank. The raw material liquid was aerated and thoroughly stirred to ensure saturation of dissolved oxygen. The stirred fermentation liquid was then transferred to a fermenter, closed, and fermented at 36.8 °C for 72 h. During the first half of the fermentation period, aerobic fermentation took place to consume dissolved oxygen. In the second half, the fermentation automatically transitioned to the anaerobic stage. After fermentation was completed, the fermentation liquid was transferred to a ripening tank, which was then closed for an additional 4 weeks to complete residual fermentation of remaining sugar and prevent gas release, thus avoiding container expansion.
2.2. Preparation of Activated Charcoal Containing Microbes and Chelated Iron
Bamboo-derived activated charcoal (BAC, referred to as “activated charcoal” or “charcoal” in this article) was purchased from Dingcheng-Ou-Bao Charcoal Corporation in Changde, China. The activated charcoal was processed into blocks or rods of various sizes. The process involved sealing the bamboo-derived activated charcoal in a plastic vacuum bag, removing the air from the micropores of the charcoal, submerging the bag in fermentation liquid, breaking the bag, and allowing the liquid to be absorbed into the activated charcoal under negative pressure. This resulted in activated charcoal with microbes and chelated iron soaked in, known as ACMI.
2.3. Water Purification Experiment in the Laboratory
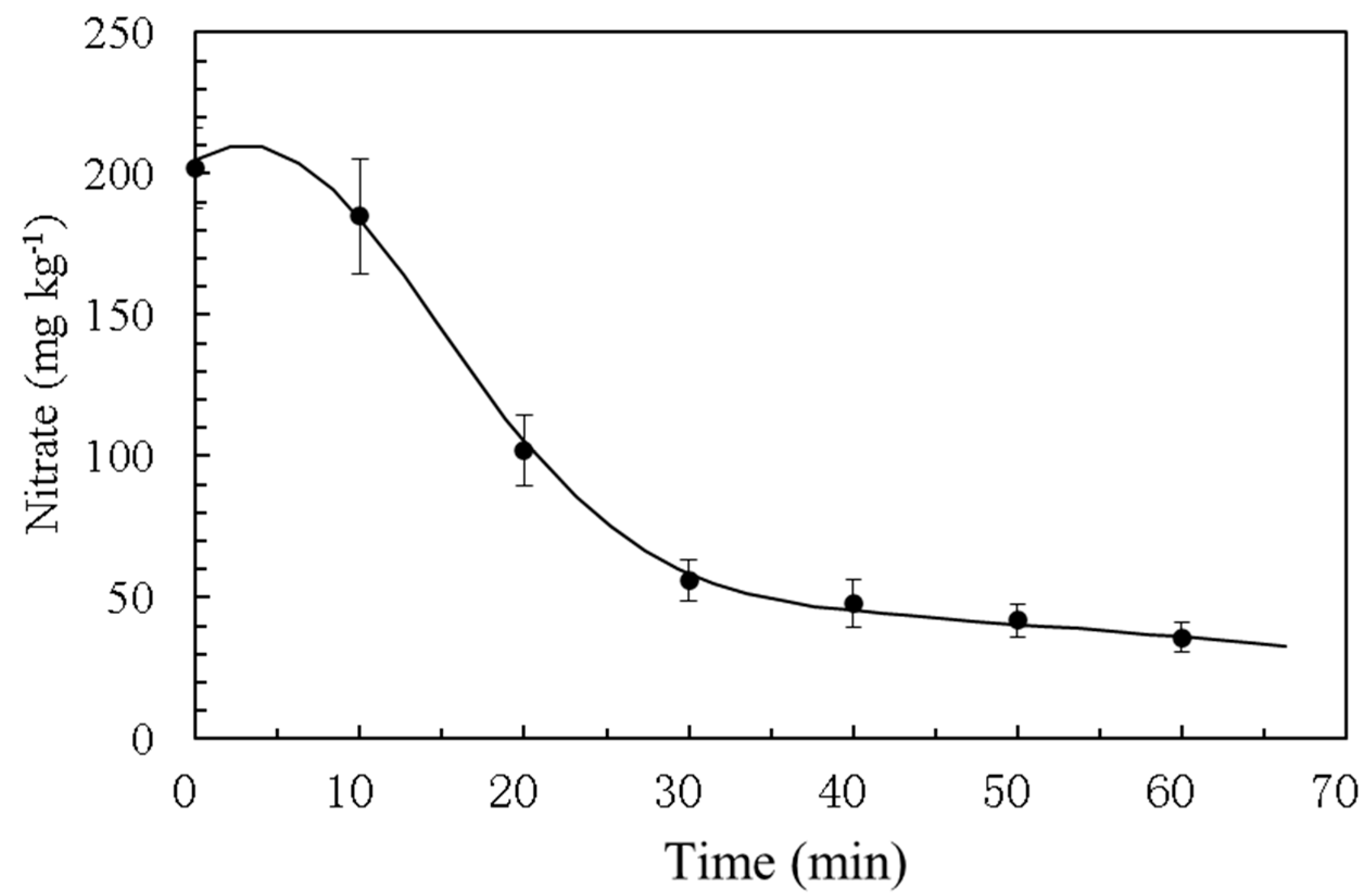
In the laboratory water purification experiment, contaminated water with 200 ppm of nitrate ions was placed in a 1000 mL beaker. A gauze pad absorbing 100 cm3 of ACMI was suspended in the water. The beaker was positioned on a magnetic stirrer, which stirred the water, while maintaining a temperature of 22 °C. Water samples were taken every 10 min to determine the nitrate concentration.
2.4. Purification Experiment of Polluted River Water
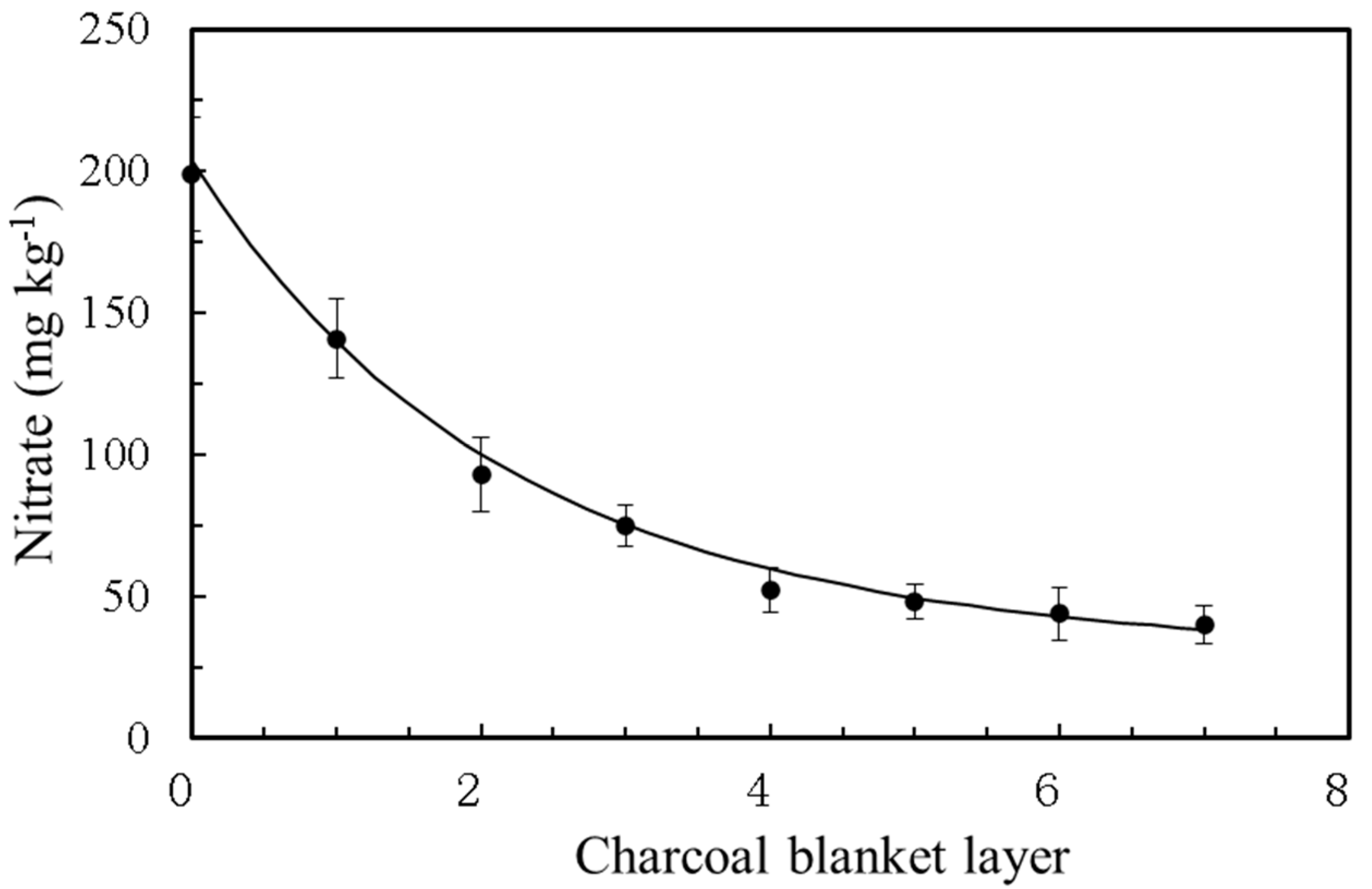
An ACMI-soaked gauze pad was sewn into two layers of chemical fiber cloth to create a blanket. The blanket was equipped with fishing net foot lead drops on one side and tied to floating balls on the other side. In a 5 m wide and 100 m2 test area of a small, slow-moving river, ACMI blankets were set every 5 m. After 24 h, the nitrate ion concentration was measured at each interval, using the water sample upstream of the ACMI blanket as a control.
2.5. Repurification of Tailwater in Sewage Treatment Plant
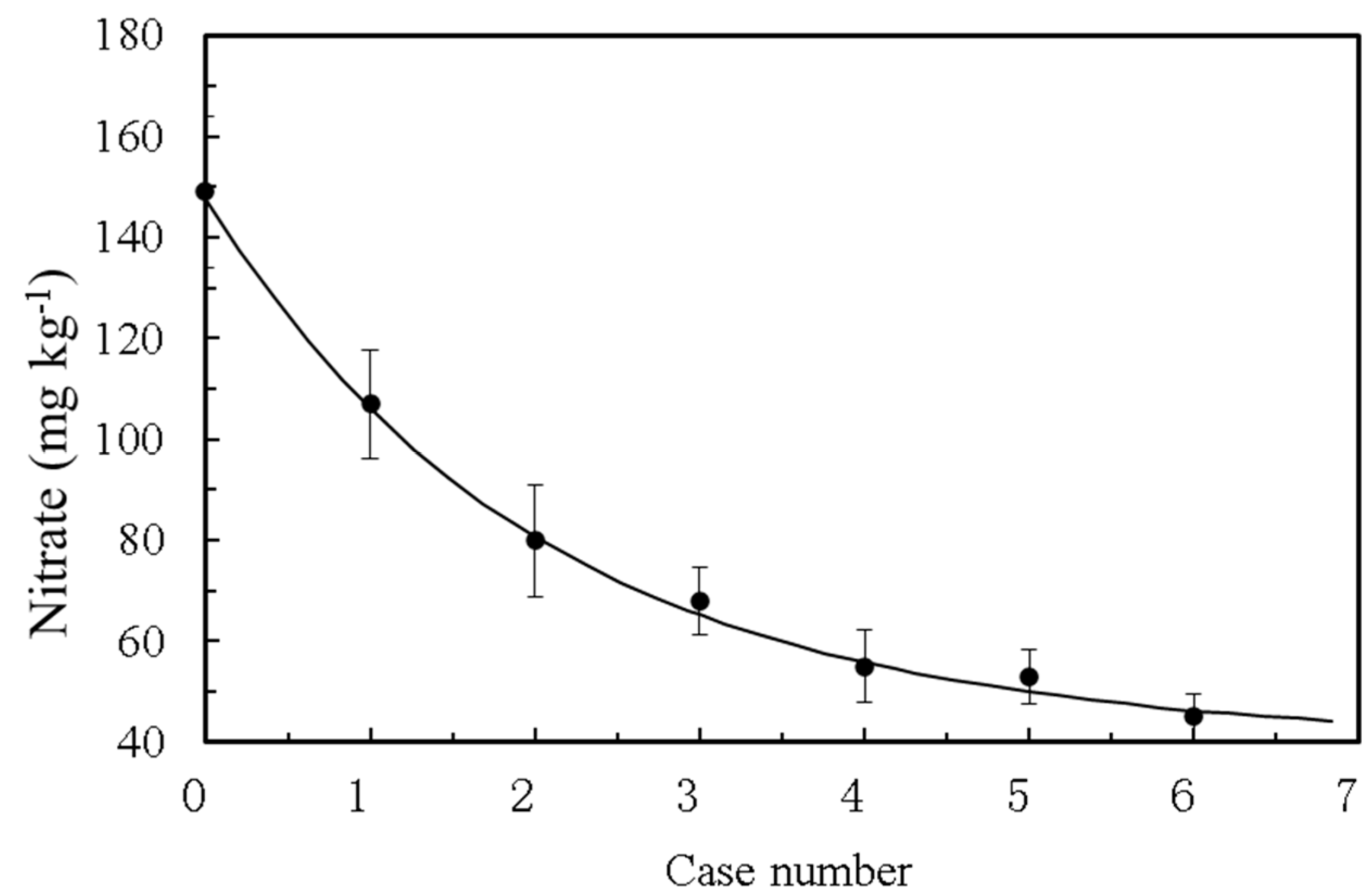
The tailwater from a wastewater treatment plant has a nitrate ion concentration of 200 ppm and needs to be re-treated. In the repurification of tailwater in this sewage treatment plant, a wire mesh container measuring 4 m long, 2 m wide, and 1 m high was used. ACMI blankets covered the bottom and four walls of the container, with longitudinal ACMI blankets arranged every 0.5 m along the long side, creating 6 compartments. The container was placed at a 15° tilt, and the tailwater was injected into the first compartment, flowing down the slope through 7 ACMI blankets. Water samples were taken after each blanket to determine nitrate concentration.
2.6. Artificially Constructed Five-Step Wetlands
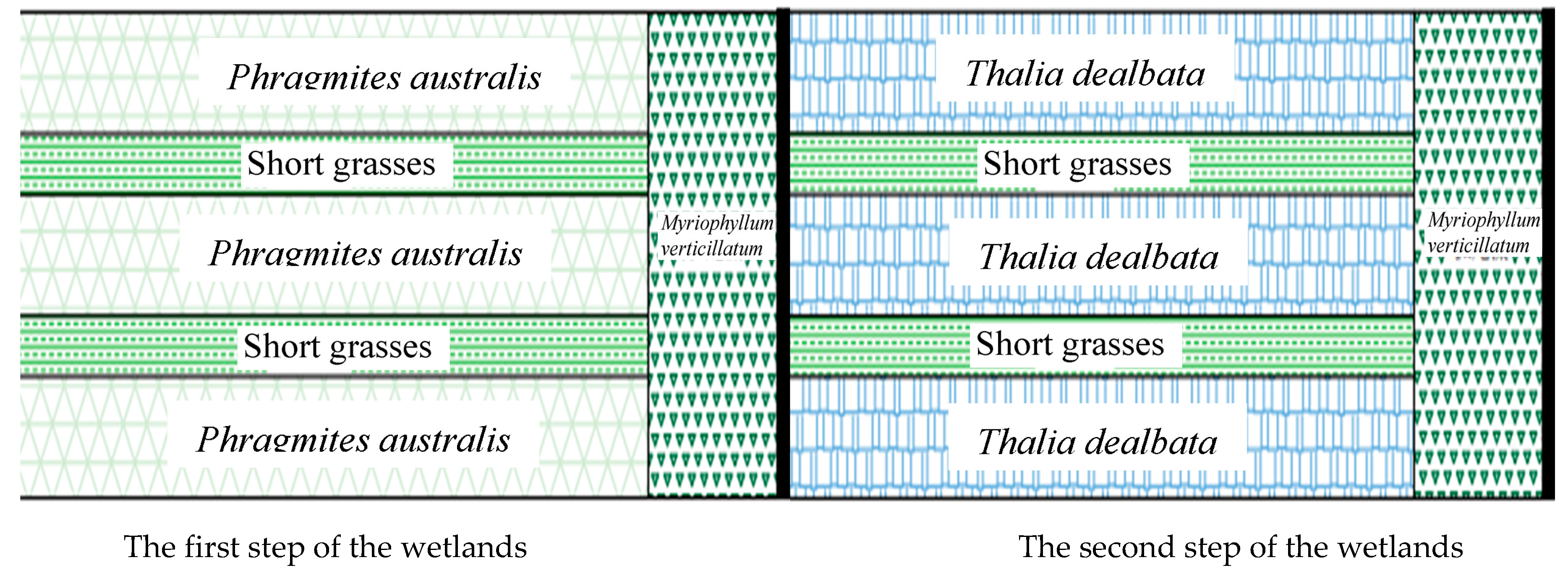
The water purification experiment took place in artificially constructed wetlands located along a small river in the suburb of Haikou City, China.
Figure 1 illustrates how the river water was directed into the constructed wetlands for purification, and then, returned to the river, similar to hemodialysis treatment for kidney patients. The first-step wetland featured
Phragmites australis (Cav.) Trin. ex Steud. as the dominant plant.
Thalia dealbata Fraser was the main plant in the second-step wetland, and rice (
Oryza sativa L.) was used in the third-step wetland.
Eleocharis dulcis (Burm. f.) Trin. ex Hensch.) was the plant in the fourth-step wetland, followed by
Oenanthe javanica (Blume) DC in the fifth step. Each plant band had a width of 2 m and was 30 m long. A 0.5 m walkway separated the adjacent plant bands, and a mix of aquatic dwarf water plants, including
Cynodon dactylon (L.) Persoon,
Vallisneria natans (Lour.) Hara, and
Euphrasia pectinata Ten., were planted in these areas. The reed belt and the dwarf water grass belt were repeated eight times, with each wetland being 20 m wide and 30 m long. At the end of each wetland step, there was an ACMI filter dam standing 0.5 m above the ground level. These dams consisted of two layers of barbed wire placed 0.3 m apart, sandwiched between bamboo-derived activated charcoal bars measuring 0.5 m. As mentioned earlier, the bamboo-derived activated charcoal was soaked with microbial liquid containing chelated iron. The water discharged from the ACMI filter dam entered a stabilization pond measuring 7 m by 3 m. This pond was planted with
Myriophyllum verticillatum L. The purified water from the previous wetland flowed into the next wetland. Finally, the water from the fifth-step wetland was discharged into a pond containing foxtail algae (
Myriophyllum verticillatum L.). Aquatic grasses were grown on the bottom of the pond, while slope-protecting grass (
Cynodon dactylon (L.) Persoon) was cultivated on the slope of the stream bank. The purified water was then released into the river. After the fifth-step wetland, there was a
Myriophyllum pond, which remained filled with water for 24 h. Subsequently, 300 m
3 of river water was added to the water inlet of the first-step wetland every day. Before refilling the water, samples were collected at five locations within each step of the wetland using the diagonal sampling method.
2.7. Determination of Nitrate Concentration
Nitrate concentration was measured in rivers, in wetlands, and in the laboratory using Attenuated Total Reflectance Fourier Transform Infrared spectroscopy (ATR-FTIR) coupled with a deconvolution algorithm [
18].
4. Discussion
The Chinese government has implemented strict environmental protection policies to improve industrial sustainability through cleaner production and a circular economy. Small chemical businesses struggle to meet national wastewater discharge standards due to funding and technology limitations, resulting in closures. However, in China, more than 1/3 of rivers and a higher proportion of urban water are seriously polluted, affecting drinking water quality and public health [
19,
20]. Water pollution is mainly caused by inadequate sewage treatment with low technology. Activated charcoal is often considered an ideal material for sewage treatment due to its abundant pore structure and strong adsorption capacity on its inner surface [
21]. Additionally, it can be reused multiple times.
In this study, a new material called ACMI (activated charcoal soaked with microbial fermented liquid and chelated iron ions) was developed. We conducted a comprehensive study on the purification of wastewater from various sources using ACMI.
ACMI contains organic acids that effectively chelate iron ions and prevent their oxidation. By soaking activated charcoal in microbial fermented liquid, compound microbes, chelated iron, and organic acids attach to the inner micropores of ACMI. ACMI acts as a barrier, preventing chelated iron, microbes, and their secretions from being washed away by flowing water.
In the laboratory water purification experiments, the nitrate concentration decreased significantly within 60 min, indicating that most of the nitrate ions were degraded in the absence of chelated irons.
Afterwards, ACMI was used to create an ACMI blanket, which consisted of two layers of chemical fibers and was suspended in polluted river water. The nitrate ion concentration dropped significantly with the increment of the blankets, indicating that ACMI blankets were able to effectively reduce nitrate.
ACMI was also found to be effective in repurifying the tailwater of a sewage treatment plant. The nitrate concentration sharply decreased after passing through ACMI layers, which indicates the effectiveness of ACMI in degrading nitrate in the tailwater.
Furthermore, the ACMI material was applied in a five-step wetlands system, which significantly reduced nitrate levels in river water. In the wetlands experiment, ACMI was sandwiched between two pieces of wire net to form a wall or dam. Water was first purified by aquatic plants, and then, further purified by the ACMI dam or wall. The purification effects were attributed to the factors within the ACMI material and the combination of ACMI and wetland aquatic plants.
The key component of ACMI was the chelated iron ion, which served as the electron donor in redox reactions. Previous studies have focused on the use of zero-valent iron in water purification, but it requires a high pH for nitrate removal. ACMI, on the other hand, does not change the pH of river water, but its internal pH is stable, ensuring nitrate reduction. The components and conditions within the micropores of ACMI are not easily affected by external water, allowing iron ions and microbes to attach to the inner surface of the micropores. The compound microbial fermented liquid used in this study stabilizes iron ions and serves as an electron donor for redox enzymes. This liquid enters the micropores of activated charcoal, avoiding removal by running water and providing long-term effects.
China, being rich in bamboo resources with a short growth cycle, can utilize bamboo charcoal as a functional and environmentally friendly biological adsorption material. Bamboo charcoal has a wide range of sources, low production costs, high mechanical strength, and significant adsorption effects. By combining microbial technology with bamboo charcoal material, the quality of water can be effectively improved, greatly enhancing the purification efficiency of bamboo charcoal water. Particularly, our results show that ACMI has a significant effect on nitrate removal and is cost-effective. This approach has broad application prospects and significant potential for development in the field of sewage treatment [
9].
Currently, bamboo charcoal material is widely used in the treatment of industrial and agricultural sewage as well as domestic sewage [
8]. It has become a substitute for relatively expensive wood-activated carbon, addressing the development limitations of the wood-activated carbon industry caused by wood shortages. The development and utilization of bamboo charcoal material can deepen the theoretical research on water quality purification, thereby protecting the environment, promoting local agricultural and forestry economies, and driving the industrial development and sustainable utilization of bamboo resources.