Abstract
An overview of different adsorbents, based on agricultural and household waste, for chlorophenoxy herbicides removal from water is presented. Several groups of adsorbents are discussed, taking into account the modification method used on the initial material and the type of final product obtained. The adsorbent characteristics and the conditions of the adsorption measurements are given, and a discussion on the obtained results is presented, along with a theoretical description, following the application of various equations and models. A group of the most effective adsorbents is indicated, based on the analysis of the adsorption capacity, towards 2,4-D and/or MCPA, and the adsorption rate. Some important problems connected with adsorbent utility are discussed, taking into account economic and ecological aspects. Moreover, the effectiveness of the analyzed materials is observed through the analysis of its interactions with other components present in real systems.
1. Introduction
Pesticides are a group of chemical compounds that are used to combat plant diseases, remove and destroy weeds, combat pests, and treat seeds. Over the past few decades, there has been a constant increase in plant and animal production around the globe, which has resulted in the growing demand for pesticides. At the global level, in the last decade, a 50% increase in pesticide use compared to the 1990s has been observed. In 2020, the total amount of pesticides used in agriculture was on the level of 2.7 million tons of active ingredients, which corresponds to approximately 7.2 million tons of formulated pesticide products. Taking into account the specific target groups, herbicides accounted for the largest share in the total consumption (52%), followed by fungicides (23%), insecticides (18%), and other pesticides (7%) [1].
The dynamic increase in the use of pesticides poses the risk of water pollution with such compounds. This problem is substantial in countries with a high share of agricultural land and those with low water resources, and in countries where surface water is the main source of drinking water for the inhabitants. According to recent data, on average in the whole of Europe, 37% of the public water supply comes from surface water [2], while in Bulgaria, Ireland, the UK, and Greece it is over 65% [3,4,5,6,7]. The worst situation is in Northern Ireland, where the population uses almost exclusively surface water resources for consumption purposes [8]. The highest concentrations of pesticides have been found in the runoff of meltwater or flood waters and agrochemical treatments [9]. A natural consequence of the presence of pesticides in surface water is their penetration into groundwater. Since surface and underground waters are a source of drinking water, they should not contain harmful substances. However, toxicological and epidemiological studies provide more and more evidence that the quality of some natural water poses a real threat to human health. Among the several thousand organic compounds identified as water pollutants, chlorophenoxy herbicides make a significant contribution. This group of compounds is widely used to control broadleaf weeds in agricultural and non-agricultural areas, while 2,4-dichlorophenoxyacetic acid (2,4-D) and 4-chloro-2-methylphenoxyacetic acid (MCPA) are in the top five most often used herbicides in the US and the EU [10,11].
The chemical structures and the most relevant physicochemical properties of the chlorophenoxy herbicides used are presented in Table 1.

Table 1.
The structure and physicochemical parameters of 2,4-D and MCPA.
The permeation of chlorophenoxy herbicides into the body increases the risk of cancer, such as soft-tissue sarcoma, non-Hodgkin’s lymphoma, and prostate and gastric cancers [12,13,14,15]. Other available data indicate correlations between exposure to these herbicides and Parkinson’s disease and birth defects [16]. The toxicity of 2,4-D and MCPA is evidenced by the fact that the International Agency for Research on Cancer (IARC) has defined them as possibly carcinogenic to humans (Group 2B) [17]. For this reason, both the World Health Organization (WHO) and the United States Environmental Protection Agency (US EPA) have imposed requirements for water intended for human consumption, specifying that it should not contain more than 0.1 μg L−1 of individual chlorophenoxy herbicides, and the total concentration should not exceed 0.5 μg L−1 [18,19]. Such rigorous standards make the water purification process involving these types of compounds extremely important, from a scientific and practical point of view.
The techniques used for this purpose are diverse, and depending on the specificity of the purification process they are classified as physical, chemical, and biological methods, or combined ones. The most used water treatment techniques comprise: adsorption [20], membrane filtration [21], irradiation [22], photocatalytic degradation [23], thermal remediation [24], electrokinetic coagulation [25], oxidation [26], ozonation [27], chemical coagulation–flocculation [28], ion exchange [29], and biodegradation [30]. These techniques have their advantages and drawbacks. Sometimes the latter limits the usefulness of a given technique, especially in technological solutions used on a large scale. The most problematic disadvantages are: (i) high cost, (ii) high energy consumption, (iii) the need for chemical reagents, (iv) the creation of biosolids, sludge, or new harmful compounds as by-products, (v) the pre-treatment stage, (vi) membrane fouling, and (vii) the need for specific instruments and materials [22,26,30,31,32]. Among the above-mentioned techniques for water treatment, adsorption seems to be the most suitable because it is characterized by low cost and low energy consumption; moreover, it is highly selective and effective. There are various groups of adsorbents, i.e., (i) carbonaceous materials (commercial activated carbons, activated carbons from solid waste, carbon blacks, carbon nanotubes, graphene, reduced graphene oxide, and ordered mesoporous carbons); (ii) inorganic materials (silica gels, activated alumina, zeolites, molecular sieves, and metal oxides); (iii) advanced materials (inorganic–organic composites, doped materials); and (iv) low-cost materials (agricultural and household waste-based materials, natural materials, biosorbents, industrial by-products) [33,34]. Due to the low cost of production for most of them, and the renewability and availability of the raw material, adsorbents based on agricultural and household waste are considered to be one of the most promising types. Taking into account these factors, there is no need for the subsequent regeneration and reusability of adsorbents based on agricultural and household waste. Additionally, the idea on the production of this type of adsorbents is also a solution for the proper management of agricultural and household waste. This is extremely important from an economic point of view, because the regeneration process requires energy or the use of expensive reagents. In general, agricultural and household waste can be used as adsorbents in the form of unmodified material, or after physical and/or chemical modification, as modified material, ash, biochar, or activated carbon. The latter, due to its relatively high efficiency in the purification of water from organic pollutants, in the near future, may completely replace commercial activated carbon, which comes from fossil coal and is characterized by a high market price. Straw, bark, and seed husks from various plants, seeds, and fruit peelings are just a few examples of renewable and readily available sources of raw material for activated carbon production. In terms of biochemistry, they mainly consist of biopolymers of cellulose and other polysaccharides (substances that are a source of elemental carbon), which makes them a suitable precursor of activated carbon. However, the production of this type of adsorbent is the most costly compared to other waste-based ones.
In the present paper, an overview of different adsorbents, based on agricultural and household waste, for chlorophenoxy herbicides removal from water is presented. The adsorbents were divided into several groups, depending on the applied method of modification used on the initial material and the type of final product obtained. The characteristics of the adsorbents, the conditions of the adsorption experiments, and the results, along with the theoretical models best describing the adsorption process, were reported. The analysis of the adsorption data, based on the adsorption capacity towards 2,4-D and/or MCPA and the adsorption rate, allowed us to indicate a group of the most effective adsorbents. The reviewed papers were also evaluated for the presence of studies that are crucial for the development of new adsorbents, such as: (i) the selectivity of the adsorbent; (ii) the adsorbent performance in multi-component systems (with heavy metal ions, antibiotics, phenols, dyes, competitive pesticides or metabolites of degradation, natural organic matter); (iii) herbicide interactions with adjuvants as integral components of herbicide formulations; (iv) the regeneration and reusability of the adsorbent; and (v) the cost balance of adsorbent production (collecting, transporting, and processing of waste) and the benefits from its use in the water treatment process.
Because many aspects have not been touched on at the preliminary research stage, the cost effectiveness of the production and applicability of adsorbents based on agricultural and household waste in the water treatment process is a debatable issue. Nevertheless, the authors hope the paper facilitates readers in the optimal selection of raw materials, the modification method particular to a given type of adsorbent, and the adsorption conditions. The paper also indicates the directions for future research that require development in the area of new materials dedicated to adsorption applications.
2. Adsorbents Based on Agricultural and Household Waste
The issue of water purification from chlorophenoxy herbicides is related to the selection of appropriate methods and materials for this purpose, which are effective, economical, and meet the requirements of “Green chemistry” (maximum reduction in the use and generation of harmful substances). The method that mostly meets these expectations is the adsorption of materials based on agricultural and household waste. Due to the biochemical structure, agricultural and household waste can be used as adsorbents in raw form, or after physical and/or chemical modifications, or they can be used as a precursor for the production of activated carbon. The effectiveness of chlorophenoxy herbicide adsorption by these types of adsorbents depends on the pollutant concentration, the physicochemical characteristics of the adsorbent, and the conditions of the adsorption process (solution pH, temperature, ionic strength, and the accompanying substances in the system). In addition to the pollutant concentration, the degree of water purification is determined by the affinity of the herbicide to the adsorbent. Therefore, it is important to select the appropriate raw material and a method of modification/processing to obtain an effective adsorbent. Evaluation of the adsorption properties of the material is carried out based on the adsorption capacity and herbicide adsorption rate. Due to the complex composition of water from natural sources (the presence of other pollutants, i.e., competitive pesticides or metabolites of degradation, heavy metal ions, phenols, dyes, natural organic matter), it is also advisable to assess the selectivity of the adsorbent to a herbicide in multi-component systems and determine the effectiveness of chlorophenoxy herbicides to adsorption under such conditions. Commercial herbicide formulations include active substances and adjuvants that improve the solubility of the preparation, therefore determining the effect of auxiliary substances on the adsorption yield is equally important. With regard to activated carbon, the production of which is relatively expensive compared to other waste-based adsorbents, a study on material regeneration and reusability is recommended. In the final assessment on the potential use of adsorbents based on agricultural or household waste, it is also helpful to make use of the cost balance in adsorbent production (collecting, transporting, and processing of waste) and a list of benefits from the use of adsorbents for the removal of phenoxyacetic herbicides from water. Special attention has been paid to all these issues during the browsing of the cited papers.
2.1. Unmodified, Modified Natural Materials and Ashes
The development of adsorbents using agricultural and household waste provides an interesting alternative to commercial materials intended for environmental applications and at the same time provides a way for proper waste management. So far, these types of waste have been disposed of in landfills or by burning in open agricultural fields. Both methods release greenhouse gases that get into the atmosphere, while field burning is additionally associated with the destruction of the ecosystem and the risk of the uncontrolled spread of fire. When considering agricultural and household waste as raw materials for the preparation of adsorbents, one should emphasize its availability in huge quantities and its little or no economic value. All this means that more and more studies have been focused on the transformation of resource waste into useful and functional adsorbents for the removal of a variety of pollutants from water and wastewater. Excluding waste-based biochars and activated carbons, the remaining types of adsorbents have been obtained through simple physical or chemical modifications, several-stage and more complex modifications, as well as oxidative combustion. There have also been attempts to use unmodified waste materials. The usefulness of adsorbents in polluted water treatments was closely correlated with the method used for its preparation (or modification abandonment) and the type of raw material used.
One such typical agricultural waste is rice husk, which has been tested to remove phenoxy herbicides from water [35,36,37]. Deokar et al. described the liquid-phase removal of 2,4-D [35,38] and MCPA [37] using rice husk ash (RHA). The effects of various parameters, such as the adsorbent dosage, initial concentration, contact time, and particle size of the RHA, were studied. The adsorption kinetics were studied by applying the PFO, PSO, and Elovich equations, and the PSO and Elovich kinetic models were found to be the best models to describe the adsorption. The Langmuir, Freundlich, and Temkin isotherm models were considered to determine the adsorption capacity and adsorbent affinity. The maximum adsorption capacity, based on the Langmuir model, was found to be 1.425 mg g−1 for 2,4-D and 1.681 mg g−1 for MCPA. It was realized that the ash physicochemical parameters, such as a higher surface area and a smaller silica-to-carbon ratio, favored pollutant adsorption.
In other works [36,39], rice husk waste was reduced to nanosized particles via a mechanical method and was evaluated for its effective usage as a 2,4-D adsorbent. The adsorption isotherms and kinetic models were used to describe the experimental data and understand the adsorption mechanism. From the results, the PSO model was found to fit the adsorption best; moreover, the adsorption of 2,4-D onto nanosized rice husk was the result of a combination of film diffusion and intraparticle diffusion processes. The Langmuir, Freundlich, Temkin, and DR isotherms were tested, and it was found that the Langmuir model fitted best with the adsorption data, with the maximum adsorption capacity of 24.75 mg g−1 (in 2,4-D nanoformulation preparation experiments). In the case of the batch adsorption experiment, the Freundlich isotherm model was indicated as the most suitable one, with KF = 8.7 (mg g−1)(L mg−1)1/n.
Okumuş et al. [40] investigated the potential of various bio-waste materials, including apple shell (AS), orange peel (OP), banana peel (BP), and millet waste (MW), as adsorbents for the removal of 2,4-D, 2,4-DP (2,4-dichlorophenoxy propanoic acid), and 2,4-DB (2,4-dichlorophenoxy butyric acid) from aqueous solutions. Batch adsorption experiments were conducted to study the effect of different operational parameters, such as the pH of the solution, the contact time, the adsorbent dosage, and the initial herbicide concentrations. The results showed that the optimum pH for the adsorbent was 6–7 and that the contact time was as short as 60 min for all of the adsorbates. The Langmuir and Freundlich models were applied to describe the adsorption isotherm, and the data fitted well with the Langmuir isotherms. The maximum adsorption capacity was obtained for 2,4-D as OP > MW > BP > AS; 2,4-DP as MW > AS > BP > OP; and 2,4-DB as OP > AS > BP > MW.
A study by Al-Zaben and Mekhamer [41] describes the use of coffee waste to remove MCPA from aqueous solutions. The effects of contact time, pH, and initial herbicide concentration were studied. The adsorption kinetics were well described by the PSO kinetic model, while the equilibrium results were better described by the Langmuir model. The equilibrium adsorption capacity was found to be 340 mg g−1, using the Langmuir equation.
Trivedi et al. [42] published a research article that presented data about the use of cotton plant ash as a cheap adsorbent for the removal of 2,4-D. The BET surface area of the material was found to be 2 m2 g−1. The PFO, PSO, and Weber–Morris kinetic models were applied to the experimental data, and the PSO equation showed the best fit for the adsorption of 2,4-D. The equilibrium data were analyzed using Freundlich and Langmuir adsorption isotherms, and these fit well with the Langmuir model. Langmuir’s adsorption capacity was found to be 0.64 mg g−1.
The same authors [43] evaluated mustard plant ash as an adsorbent for the removal of 2,4-D from water. Optimum adsorption conditions were determined as a function of the adsorbent dose, the initial 2,4-D concentration, the contact time, and the temperature. Data from the experiments were fitted to various kinetic (PFO and PSO) and isothermal (Langmuir, Freundlich, and Temkin) models. The PSO kinetic model was found to show the best fit with the experimental data. The equilibrium data fitted best with the Langmuir adsorption isotherm, which confirmed the monolayer adsorption (qm = 0.76 mg g−1). Thermodynamic constants indicated the spontaneity, as well as the exothermic nature, and irreversibility of the 2,4-D adsorption.
The next papers by this research team deal with the investigation of 2,4-D adsorption by groundnut shell ash [44,45] and wheat straw ash [46]. The first of these works [44] describes the herbicide adsorption of groundnut shell ash, with a BET surface area of 22 m2 g−1. Batch adsorption experiments were conducted to study the effect of different operational parameters, such as the adsorbent dosage, the contact time, and the initial adsorbate concentration, on the adsorption process. The PSO model, as well as the Langmuir adsorption isotherm model, showed the best fit with the experimental data. The maximum uptake capacity of the ash was determined as 0.87 mg g−1. The paper [45] describes the preparation of three types of adsorbents produced using groundnut shells, namely ash, biochar, and activated carbon, and their adsorption performance.
Wheat straw ash was characterized and tested as a 2,4-D adsorbent [46]. The BET surface area of the material was found to be 37 m2 g−1. The effects of different operational parameters, such as the adsorbent dose, initial 2,4-D concentration, contact time, and pH, were investigated. The experimental data were analyzed using the PFO and PSO kinetic models, as well as the Freundlich, Langmuir, and Temkin isotherm models. The PSO and Langmuir models showed the best fit with the experimental data. The adsorption capacity was found to be 1.89 mg g−1.
Recently, the adsorption of 2,4-D and ketoprofen by Physalis peruviana fruit residue functionalized with sulfuric acid was reported by Dhaouadi et al. [47]. Adsorption isotherms were determined at 298–328 K and pH 2. It was found that the adsorption of 2,4-D by this biosorbent was multi-anchoring.
Peanut (Arachis hypogaea) skins treated with sulfuric acid were employed as an adsorbent for the removal of 2,4-D from aqueous solutions [48]. The results revealed that the adsorption was favored at acid pH = 2, and the optimal adsorbent dosage was 0.9 g L−1. The equilibrium was reached quickly in the first 30 min, and the kinetics were well represented by the general order model. The Langmuir model was the one that obtained the best fit with the experimental data. The adsorption capacity of the acid-treated peanut skin towards 2,4-D was 246.72 mg g−1.
In another work, wheat husks (Fagopyrum esculentum) modified using a treatment with H2SO4 were applied as an adsorbent to remove the 2,4-D herbicide from water [49]. The kinetics, equilibrium, and thermodynamic behavior, as well as the pH effects and adsorbent dosage, on the adsorption capacity were investigated. It was found that the Bangham kinetic model better represented the experimental data, and that the maximum adsorption capacity was 161.1 mg g−1 at 298 K. The thermodynamic studies indicated spontaneous, favorable, and exothermic adsorption.
Recently, N-cetylpyridinium-modified tiger nut residue (TNR-CPC) as an adsorbent was investigated by Kani et al. [50]. The results revealed that the solution pH, adsorbent dose, temperature, and solution ionic strength affected the adsorptive capacity of TNR-CPC. The adsorption kinetics followed the PSO model, while the equilibrium data were best fitted to the Langmuir isotherm equation. The maximum monolayer adsorption capacity at 298 K was 79.3 mg g−1.
Bartczak et al. [51] studied the use of saw-sedge Cladium mariscus (C. mariscus) for the adsorption of 2,4-D from aqueous systems. The C. mariscus consisted of carbon (48%), hydrogen (7%), nitrogen (0.95%), and sulfur (0.4%), and was characterized by a low surface area (0.6 m2 g−1). It was found that the adsorption performance was dependent on the following parameters: contact time, adsorbent mass, pH, and temperature of the adsorption system. Maximum adsorption was attained at pH = 3 and T = 25 °C, and equaled 65.58 mg g−1. The experimental data corresponded to the pseudo-second-order kinetic model and the Langmuir isothermal model.
Recently, reports on the development and application of more advanced waste-based materials with magnetic properties for the removal of 2,4-D were published [52,53]. According to the authors of the paper [52], the forming process of a biomass-MOF composite was based on the loading of NH2-MIL-101 (Fe) onto magnetized peanut husk. The adsorption capacity of the composite for 2,4-D removal was found to be 79.2 mg g−1 according to the Langmuir isotherm model, while the kinetics were very rapid (attaining equilibrium within 5 min) and followed the PFO and PSO models. The second advanced magnetic material, a ferrospinel composite [53], was prepared based on activated carbon from lentil residues. For the synthesis of activated carbon, microwave-assisted K2CO3 chemical activation was applied instead of conventional heating, as a faster and more economical method. The analysis of the 2,4-D adsorption data indicated that the Langmuir model, with the maximum adsorption capacity of 400 mg g−1 at 45 °C, and the pseudo-second-order model were the most suitable models. The magnetic properties of both the MOF and ferrospinel composites facilitated their separation from a purified medium.
Various unmodified and modified agricultural and household waste materials and ashes, and their adsorption performance in regard to chlorophenoxy herbicides removal, are summarized in Table 2. Additionally, the cost–benefit of the given adsorbents was evaluated based on a comparison between their adsorption capacity towards chlorophenoxy herbicides and the synthesis cost, the impact of their production on the natural environment (energy consumption, harmfulness of the reagents used, secondary waste), and their recyclability. Such subjective evaluation is widely accepted and practiced by many scientists [54,55].

Table 2.
The results from chlorophenoxy herbicides adsorption studies on unmodified adsorbents, modified adsorbents, and ashes based on agricultural and household waste.
To sum up, due to the variety of materials (i.e., pure agricultural and household waste, physically or chemically modified materials, ashes, and advanced composite materials), they show diversity in their biochemical composition, texture, chemical structure, and surface chemistry. The majority of adsorbents have a non-porous structure and their ability to bind herbicides from aqueous solutions is closely correlated with the presence of various functional groups of natural origin, introduced on their surface during the modification process, or resulting from the oxidation of mineral compounds during raw material combustion. Ashes are characterized by the lowest adsorption capacity, ranging from 0.64 to 1.89 mg g−1 and, therefore, their adsorption potential is considered secondary to other functions they can perform in the economy and agriculture. First of all, the generation of ash involves the use of waste with low moisture content as feedstock for industrial boilers or home heating furnaces. Thus, the product obtained is not an end in itself, but is a consequence of the functioning of the fuel economy. Due to its rich composition of micronutrients, such as Ca, Mg, K, P, and Si, its high water capacity, and its carbon sequestration capability, ash is considered a useful addition to soil, improving its fertility. Moreover, ash after being spread on fields plays a protective role for the soil, as it adsorbs herbicides. The adsorption is relatively low, but equilibrium is reached almost instantly.
The adsorption capacities of unmodified, physically modified, and chemically modified materials are in the range of 22.71–340 mg g−1, 24.75–76.92 mg g−1, and 79.3–246.7 mg g−1, respectively. Taking into account the simplicity and low cost of production of these adsorbents, or even the possibility of using some waste in an unprocessed form, these results seem to be promising.
Regarding magnetic composite materials, their adsorption capacities are equal to 79.2–400 mg g−1. The great advantage of these adsorbents is the ease of their separation, from the medium subjected to purification, using an external magnet.
2.2. Biochars
Biochar is a solid product that can be obtained from the thermal degradation of agricultural and household waste under oxygen-limited conditions (pyrolysis process). Biochar is generated from the part of biomass that has already been used in human activities and is problematic for society due to the need for storage and the putrefaction that occurs within it, which may lead to the uncontrolled emission of greenhouse gases. The physicochemical properties of biochar greatly depend on various parameters, such as the biomass type, pyrolysis temperature and duration, pyrolysis procedure, and post-treatment processes. It is generally assumed that biochar is produced at low temperatures, but in practice a wide temperature range of 200–800 °C has been used. In the course of biochar production, the volatile constituents and tar existing in the biomass are emitted and, as a result, a porous structure in the final product is formed. The development of the porous structure is visible in the specific increase in the surface area of the biochar by up to several folds compared to that of ashes. The physicochemical properties of the final product determine its suitability for specific applications. From the perspective of the utilization of biochar as an adsorbent, a high capacity towards pollutants, fast adsorption kinetics, and low reversibility of the adsorption process are the most desirable features. In recent years, several studies in the scientific literature have dealt with the production of designed biochars suitable for the adsorption of various organic pollutants, including phenoxyacetic herbicides.
Mandal et al. [56] studied the adsorption process of 2,4-D by biochars produced from various green wastes, namely tea (TW), oak wood (OW), bamboo (B), and bur cucumber (BU), at two pyrolytic temperatures (400 and 700 °C). The specific surface area of the TW, OW, BU, and B biochars was 421.3, 270.7, 475.6, and 2.3 m2 g−1, respectively. The experimental data were fitted using the Langmuir and Freundlich isotherm models, as well as the PFO, PSO, and Elovich kinetic models. The kinetics of the 2,4-D adsorption was best described by the PSO kinetic model, and the adsorption equilibrium data were successfully fitted to both the Langmuir and Freundlich equations. The maximum adsorption capacity was 10.05, 42.67, 26.66, and 28.92 mg g−1 for TW, BU, OW, and B biochars, respectively.
A carbonized chestnut shell was used for the removal of 2,4-D from water [57]. The surface area of the carbonized adsorbent was 280.4 m2 g−1. The adsorption equilibrium was achieved in 250 min, and the kinetics followed the PFO model. The experimental data were modeled using the Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich equations. The Temkin isotherm model represented the adsorption phenomenon very well, at all the temperatures studied (at 35, 45, and 55 °C). Langmuir’s maximum adsorption of 2,4-D was 0.93 mg g−1.
Biochars generated from corncobs, bamboo, wood chips, and rice straw were tested as adsorbents for the removal of 2,4-D from aqueous solutions [58]. Various pyrolysis conditions, including the pyrolysis time and temperature (400–700 °C), were investigated. The highest BET surface area was 389 m2 g−1 (700 °C) for the wood chips biochar, 510 m2 g−1 (700 °C) for the bamboo biochar, 128 m2 g−1 (500 °C) for the rice straw biochar, and 2.3 m2 g−1 (600 °C) for the corncobs biochar. The Freundlich KF adsorption parameters were 8.72, 19.5, 1.23, and 1.06 (mg g−1)(L mg−1)1/n, respectively.
The corncob biochar was synthesized at 600 °C for 4 h, as a low-cost adsorbent for the removal of 2,4-D from water [59]. The specific surface area of the biochar was 298 m2 g−1. It was found that the pH was a key factor for 2,4-D removal by the corncob biochar. The experimental adsorption data followed the PSO model and the Langmuir isotherm, with a maximum adsorption capacity of 37.40 mg g−1.
A study by Essandoh et al. [60] describes the preparation of biochar from switchgrass (Panicum virgatum). The BET surface area of the biochar was found to be 1.1 m2 g−1. Kinetic and adsorption isotherm studies for 2,4-D and MCPA were conducted. The PSO model was superior to the PFO kinetic model for both 2,4-D and MCPA. The adsorption isotherms were described by the Freundlich, Langmuir, Toth, and Redlich–Peterson models. In general, all the theoretical isotherm equations provided reasonable fits with the experimental data; however, the highest overall regression coefficients belonged to the data fitted to the Freundlich model (in the case of 2,4-D) and the Redlich–Peterson model (in the case of MCPA). The maximum adsorption capacity for 2,4-D and MCPA was 134 mg g−1 and 50 mg g−1, respectively.
A series of biochars were produced from softwood shavings (WS), pig manure (PM), and sewage sludge (SS), under various conditions (with an initial heating rate of 25 °C min−1 up to 200 °C, 350 °C, and 500 °C, maintained for 4 h) [61]. The specific surface area ranged from 4 to 347 m2 g−1 for the WS, from 2 to 27 m2 g−1 for the PM, and 11 to 43 m2 g−1 for the SS. The biochars were examined as potential adsorbents for the removal of 2,4-D, MCPA, 2,4-DB (4-(2,4-dichlorophenoxy)-butanoic acid), and triclosan (5-chloro-2-(2,4-dichlorophenoxy)phenol) from an aqueous solution. The Freundlich model fitted the adsorption isotherm of the biochars very well. The results revealed that the adsorption increased in the order: 2,4-D < MCPA < 2,4-DB < triclosan.
Biochars produced by slow pyrolysis from a mixture of about 80% birchwood (Betula sp.) and 20% Norway spruce wood (Picea abies) were tested in batch [62] and column [63] studies. The wood-based biochar was additionally modified by a heat treatment and by an iron salts treatment [62]. The BET surface area of the untreated biochar, heated biochar, and biochar treated with iron salts was 74, 116, and 59 m2 g−1, respectively. These materials were used for the adsorptive removal of five pesticides, including bentazone, chlorpyrifos, diuron, glyphosate, and MCPA, from aqueous solutions. The experimental data were modelled using the Freundlich isotherm model, and the KF values decreased in the order: diuron > chlorpyrifos > MCPA > bentazone > glyphosate. The results showed that heat treatment increased the adsorption of bentazone and MCPA, while iron salts treatment increased the adsorption of glyphosate.
The Liu research group [64] studied the adsorption performance of hydrochars (HCs) prepared by the hydrothermal carbonization (HTC) of lettuce waste (LHC), taro waste (THC), and watermelon peel (WHC) in low-temperature conditions (180–240 °C). The determined adsorption capacities of the HCs were hydrochar precursor and HTC temperature dependent. The greatest capacities of LHC180 (88.4 mg g−1), THC180 (90.2 mg g−1), and WHC240 (88.4 mg g−1) for 2,4-D removal were achieved at HTC temperatures of 180, 180, and 240 °C, respectively. It was found that such hydrochar properties as a well-developed surface area with small mesopores, low aromaticity, and a high amount of C–O functional groups, favored herbicide adsorption via intensive partitioning and/or chemisorption. The pseudo-second-order model fitted the 2,4-D adsorption data better than the pseudo-first-order model.
A series of biochars based on water lettuce (WL), water hyacinth (WH), wheat bran (WB), pinewood sawdust (PS), and oak tree wood biomass (OTW), was fabricated via pyrolysis at 500 °C [65]. The adsorption performance was found to be in the following order: PS (62.53%) > OTW (53.24%) > WB (46.56%) > WH (33.54%) > WL (28.25%).
Ma et al. [66] and Almahri et al. [67] studied spent coffee ground biochars as adsorbents for the removal of 2,4-D from aqueous solutions. Ma et al. [66] obtained the char via low-temperature pyrolysis (300 °C) and impregnation with H3PO4 as a pre-treatment stage. The best kinetic and isotherm fits were found for the Elovich and Freundlich equations, respectively. It was shown that the adsorption efficiency diminishes with a rising pH, and the highest efficiency was achieved at a pH of 2. The maximum adsorption capacity of biochar was reported as 323.76 mg g−1. The thermodynamic studies pointed out that the adsorption process was exothermic. Almahri et al. [67] synthetized biochar by biomass pyrolysis at 700 °C in a neutral atmosphere, followed by the transfer to a steam atmosphere. The surface area and pore volume of the biochar reached 422.4 m2 g−1 and 0.46 cm2 g−1, respectively. The results showed that the adsorption behavior was significantly impacted by changes in the solution pH. The pH value of 6 was indicated as the most beneficial to the adsorption process (qm = 276.3 mg g−1). The thermodynamic parameters were determined and revealed that the process was endothermic, chemisorptive, and spontaneous. The adsorption process was best fitted to the Langmuir isotherm model and the pseudo-second-order kinetic model.
In the subsequent works [68,69], the adsorption of 2,4-D by rice husk biochar from synthetic wastewater and drainage water was studied. Of the various operating parameters, the initial adsorbate concentration and adsorbent dosage were indicated as those that had the greatest impact on the adsorption capacity. The highest efficiency of biochar in 2,4-D removal was achieved at a pH of 5.5, a contact time of 80 min, a temperature of 60 °C, an adsorbent dose of 0.2 g, and an initial solution concentration of 60 mg L−1. The PFO and PSO kinetic models, as well as the Langmuir, Freundlich, Langmuir–Freundlich, and Redlich–Peterson isotherms, were tested. The pseudo-first-order model and Freundlich equations were chosen as those that best described the adsorption process. The maximum adsorption capacity of 2,4-D by rice husk biochar equaled 246 mg g−1. Regarding thermodynamics, the adsorption process was exothermic and spontaneous. The adsorption potential of the biochar was also evaluated with other materials, e.g., granular activated carbon (GAC) and multi-walled carbon nanotubes (MWCNTs). The maximum adsorption capacities of the chars were lower than the GAC, but higher than the MWCNTs. However, in terms of the herbicide removal dynamics, the adsorption process required the longest time to reach an equilibrium state.
A study by Zhu et al. [70] describes the effect of various modifications of corn stalk biochar (BC) on the adsorption properties of the obtained materials. The applied modifications involved activation of the BC with potassium carbonate (KBC), followed by surface oxidation (OKBC), surface amination (NKBC), loading nano-zero valent iron (nZVI@KBC), and loading nano-iron oxyhydroxide (nHIO@KBC), respectively. The biochars exhibited the following order towards 2,4-D adsorption capacity: NKBC > KBC > OKBC > BC > nHIO@KBC > nZVI@KBC. It was shown that a well-developed specific surface area of the adsorbent was not the only factor determining the adsorption capacity. The surface properties of the adsorbent, such as the polarity, hydrophobicity, and surface functional groups, are equally important factors. The activation of the biochar structure with potassium carbonate, surface amination, or surface oxidation, made it more suitable for 2,4-D adsorption in comparison with unmodified char, while the surface loading of nanometal and nanometal oxyhydroxide made the biochar worse regarding its adsorbency.
Kearns et al. [71,72] tested the usefulness of biochars generated from updraft gasifiers, under conditions of simultaneous co-pyrolysis thermal air activation (CPTA), on the adsorption of 2,4-D and simazine from surface water containing dissolved organic matter. Cherry pits, Jatropha press-cake waste, sugarcane bagasse pellets, pecan shells, bamboo, pine, eucalyptus, and longan wood, were used as raw materials. Enhanced adsorption capabilities of the as-received chars by about one order of magnitude, compared to conventional anoxic pyrolysis (CAP) biochars, were observed. Applying a high-temperature synthesis mode combined with CPTA conditions resulted in biochars with a specific surface area and mesopore surface area of 330 and 110 m2 g−1, respectively. Such characteristics of the final product were related to the thermochemical widening of the pores and/or the removal of the pyrolysis tars from its structure. Increasing the pyrolysis temperature or process duration during the biochar synthesis procedure, conducted by using a horizontal drum kiln, increased the 2,4-D adsorption capacity of the biochar, whereas the type of feedstock did not affect it.
The removal of 2,4-D and carbofuran from water by biochars obtained from agricultural by-products, i.e., rice husk (RH), corn stover (CS), corncob (CC), and sorghum stems (SS), was studied as a function of pH [73]. It was shown that the greatest efficiency in herbicide removal was obtained at pH = 6. The equilibrium adsorption capacities of these biochars for 2,4-D were found to be 0.64, 0.76, 0.81, and 0.71 mg g−1 for RH, CS, CC, and SS, respectively.
Evaluation of the impact of the physicochemical properties of a high-temperature wheat straw biochar on its adsorption behavior related to various pesticides (2,4-D, MCPA, metolachlor, carbaryl, and carbofuran) was the research theme of Ćwieląg-Piasecka et al. [74]. The obtained adsorbent was characterized by a surface area of 237.39 m2 g−1. The adsorption affinity of the biochar to the pesticides decreased in the order: carbofuran > carbaryl > metolachlor > 2,4-D > MCPA. The relatively low adsorption capability of the biochar for chlorophenoxy acids was ascribed to the electrostatic attraction between the herbicides and the polar functional groups on the BC surface, or to a nonspecific interaction with the adsorbate. Hydrophobic interaction was postulated as the main mechanism of pesticide adsorption by the BC.
Lü et al. [75] tested the use of rice straw biochars made by slow pyrolysis at 200 °C (RS200), 350 °C (RS350), and 500 °C (RS500), for the regulation on the release and leaching of acetochlor and 2,4-D in soils. Part of the research concerned the equilibrium adsorption of herbicides by biochars differentiated in structure (SBET = 2.1, 20.6, and 128 m2 g−1, respectively), the elemental composition, and ash residue. The Freundlich KF adsorption parameters for the adsorption of 2,4-D were 0.206, 4.48 and 3.24 (mg−1)(L mg−1)1/n by RS200, RS350, and RS500 biochars, respectively. The lower adsorption capacity of RS500 than RS350, despite its more developed surface area, was explained by the high content of stable minerals (ash), accounting for nearly one-half of the mass of the sample.
Trivedi et al. [42,45,46] used the cotton plant biochar (SBET = 109 m2 g−1), groundnut shells biochar (SBET = 43 m2 g−1), and wheat straw biochar (SBET = 96 m2 g−1), for the removal of 2,4-D. The experimental adsorption data for the studied biochars followed the PSO model and the Langmuir isotherm, with a qm that equaled 3.93, 3.02, and 2.02 mg g−1.
Table 3 shows some of the adsorption capacities of the biochars reported in the literature.

Table 3.
The results from chlorophenoxy herbicides adsorption studies on biochars.
Most of the pristine biochars synthetized through pyrolysis under a neutral atmosphere are characterized by a relatively small surface area (~1–400 m2 g−1), an undeveloped pores structure, and a limited amount of surface functional groups. Only a few biochars have a more extensive network of internal pores, translating into surface areas in the range of 400–523 m2 g−1. However, the more developed pores’ structure in a solid does not always guarantee its ability to adsorb chlorophenoxy acids from aqueous solutions. There are also biochars with a poor pores’ structure that exhibit relatively high adsorption capacities for these pollutants. Noteworthy are the hydrochars whose adsorption capacity towards 2,4-D results mainly from the surface chemistry of the solid and to a lesser extent from its porosity. It has been found that the greater the aromaticity, the oxygen-containing surface functional groups (e.g., phenolic, carboxyl, hydroxyls), and the lower the content of ash in the biochar, improves its adsorption ability. These solid features, in turn, depend on various factors, such as the type of raw material, the synthesis parameters, the furnace characteristics, and the post-synthesis treatments.
To enhance the applicability of biochars in removing chlorophenoxy herbicides from aqueous solutions, they were subjected to structural activation or surface modification. Structural activation was carried out using: (i) specialized equipment, e.g., an updraft gasifier working under conditions of simultaneous co-pyrolysis and thermal air activation, (ii) gases, or (iii) chemicals. Regarding surface modification of biochars, the processes of amination and oxidation brought satisfactory results. In our opinion, the structural activation of biochar using a steam atmosphere or potassium carbonate leads to a change in the classification of the final product from biochar to activated carbon, but they have been left in this subsection based on the nomenclature given by the researchers.
Some biochars exhibit adsorption capacities comparable to waste-based activated carbons (see the subsection below), but their production is low cost and does not use harmful chemicals, so it can be concluded that these materials are competitors to activated carbons in regard to the adsorption techniques for removing phenoxyacetic herbicides from water and wastewater.
In the case of those biochars that show relatively low adsorption capacity towards herbicides, they may be suitable materials for the prevention of herbicide leaking and mobility in soil profiles if they are applied as a soil amendment. Moreover, biochars have great potential for the improvement of soil properties, such as increasing the pH, nutrient availability, and water retention. These filter materials could be applied in farm fields, as well as in agricultural ditches, drainage systems, and places accidentally contaminated with chlorophenoxy herbicides. Mitigating climate change is the secondary environmental benefit of biochar soil amendment associated with carbon sequestration.
2.3. Activated Carbons
Activated carbons are another group of adsorbents that can be obtained from agricultural and household waste and used for the removal of phenoxyacetic herbicides from water. Activated carbon synthesis involves two main stages: the carbonization of the raw material at temperatures below 800 °C in an inert atmosphere and the activation of the carbonized product (char). The latter stage is carried out by physical and/or chemical activation. Physical activation is based on the char treatment, with oxidizing gases at elevated temperatures. During this process, the char is partially gasified leading to the development of the internal surface of the solid. Chemical activation is based on the mixing of the raw material with a chemical reagent, i.e., oxidizing acid, salt solution, or alkali followed by heat treatment. The mechanism of chemical activation depends on the activating agent used and, in some cases, is very complex. Depending on the type of raw material, the activating agent, and the conditions of carbonization and activation processes, activated carbons may be characterized by various pore structures, surface chemistry, and adsorption properties towards specific pollutants.
The agricultural and household waste-based activated carbons presented in this subsection, as potential adsorbents for the water treatment of phenoxyacetic herbicides, were divided into groups depending on the type of activation process used. Among the physical activating agents, steam (ST) and carbon dioxide (CD) are the most popular; however, mixtures of these agents and microwave radiation (MV) are occasionally used. In one case, the activation process followed the self-activation mode, where various gases like CO, CH4, H2, CO2, and H2O, emitted during the biomass pyrolysis, were activating agents. Regarding chemical activation, the leading activating agents were zinc chloride (ZnCl2), followed by potassium hydroxide (KOH), orthophosphoric acid (H3PO4), sulfuric acid (H2SO4), a combination of different chemical activating agents (H2SO4 and KOH; KOH and Fe3O4), or a combination of chemical and physical activating agents (KOH and CO2).
2.3.1. Activated Carbons Based on Physical Activation
Activated carbon produced from Lagenaria vulgaris shell by thermo-chemical carbonization and steam activation was applied to the removal of 2,4-D from aqueous solutions by Bojić et al. [76]. The BET of the as-prepared AC was found to be 665 m2 g−1. The equilibrium data were fitted to the Langmuir, Freundlich, Sips, and Brouers–Sotolongo models, and the maximum adsorption capacity was 333.3 mg g−1. The thermodynamic data showed that the adsorption was endothermic, spontaneous, and physical.
Brito et al. [77] evaluated the removal efficiency of 2,4-D from water by three ACs from agricultural biomass waste. These materials were prepared using the one-step carbonization/physical activation route, using steam at 800 °C under an argon atmosphere, from sugarcane bagasse, coconut shell, and endocarp from babassu coconut. The BET surface areas of the ACs were 547, 991, and 1068 m2 g−1, respectively. The adsorption process for 2,4-D was investigated using the Langmuir, Freundlich, Redlich–Peterson, Temkin, and Dubinin–Radushkevich isotherm models. The results revealed that the Langmuir and R-P equations best described the adsorption process. The adsorption kinetics were described well by the PSO model, and the thermodynamic data indicated the spontaneous nature of the adsorption process.
Amiri et al. [78] investigated the potential of activated carbon derived from canola stalk as an adsorbent for 2,4-D removal from water. The AC was synthesized via the physical activation of the canola stalk biochar by steam, and its BET surface area was 556.8 m2 g−1. According to the authors, the maximum amount of 2,4-D removal was achieved as 135.8 mg g−1 under a pH of 2 and an initial herbicide concentration of 150 mg g−1. The particle diffusion model was the best-fitting kinetic model for the 2,4-D adsorption.
Mandal et al. [56] studied the adsorption process for 2,4-D using activated carbon produced from tea biochar, through activation with steam. The specific surface area of the material was 576 m2 g−1. The herbicide adsorption followed the PSO kinetic model, and the adsorption equilibrium data were successfully fitted to the Langmuir equation, with a maximum adsorption capacity of 58.8 mg g−1.
Orduz et al. [79] investigated the 2,4-D adsorption by activated carbon from peanut shells (steam activation, SBET = 1240 m2 g−1) in aqueous and organic solvents. The pollutant desorption kinetics and the application of the AC as a filler in a solid-phase extraction procedure (SPE) were presented. It was stated that the pollutant affinity towards the AC was higher in aqueous solvents than in organic ones (acetonitrile and methanol), with q = 549 mg g−1 (F). The 2,4-D desorption process was the most effective in methanol (regarding the pollutant amount and desorption rate); mini columns filled with AC were characterized by high reuse capacity.
Cansado et al. [80] obtained activated carbons based on Tectona grandis tree sawdust using physical activation with carbon dioxide at 973 K and different activation times, e.g., 360 and 480 min for Teak-7360 and Teak-7480, respectively. The adsorbents were characterized by high values for the specific surface area (787 and 910 m2 g−1) and were applied for the removal of MCPA during the liquid phase. The adsorption capacity of Teak-7360 and Teak-7480 was found to be 212.7 and 313 mg g−1, respectively. The herbicide adsorption experimental data were optimized using the Langmuir and Freundlich equations.
The same gaseous activating agent in the production of activated carbons, based on wood waste from Angola, was used by Tchikuala et al. [81]. The activation time and carbon precursor were variable synthesis factors. It was stated that the values of MCPA adsorption (85–295 mg g−1) correlated with the degree of development of the activated carbons’ specific surface area (603–838 m2 g−1).
Blachnio et al. [82] used pistachio shells for the preparation of activated carbons through physical activation using carbon dioxide; steam and carbon dioxide; and carbon dioxide and microwave radiation. The obtained activated carbons were characterized by high values for the specific surface area (556–685 m2 g−1) and were utilized for the removal of the model organic pollutants from the single- and multi-component systems. The adsorption capacities for MCPA removal were equal to 314–288.9 mg g−1 (the general Langmuir isotherm model). A slight effect of the crystal violet presence on the MCPA adsorption and the inverse of this was noticed as a result of the adsorption by different types of pores. For similar herbicides (MCPA and 2,4-D), strong competition in the capacity and adsorption rate was observed.
The paper [83] describes the results from the conversion of soybean residues via one-step microwave irradiation (SBET = 1696 m2 g−1) and its application for the removal of 2,4-D from aqueous solutions. The equilibrium uptake was analyzed using the Langmuir, Freundlich, and Temkin isotherms, with the Freundlich equation indicated as the best one (q = 253.17 mg g−1). In addition to the adsorptive properties, the adsorbent showed antibacterial and antifungal activity.
Gurav et al. [65] fabricated a self-activated carbon from Pinus taeda wood by utilizing gases released during the biomass pyrolysis process. The as-received adsorbent with a well-developed surface area (SBET = 1450 m2 g−1) was applied for 2,4-D removal during the aqueous phase. At pH = 2, the maximum adsorption capacity reached 471.70 mg g−1 (the Langmuir model). The adsorption kinetics obeyed the pseudo-second-order model. The effect of natural organic matter and salinity on 2,4-D removal was also tested. The reusability studies on self-activated carbon revealed outstanding performance in up to seven adsorption–regeneration cycles.
2.3.2. Activated Carbons Based on Chemical Activation
Activated carbon from sesame seeds was prepared by ZnCl2 activation and its adsorption capacity for 2,4-D in aqueous solutions was investigated [84]. The effect of varying parameters (pH, temperature, adsorbent dose, and contact time) was also studied. The adsorption kinetics followed the PSO kinetic model. The Freundlich and DR models described the adsorption at equilibrium well.
In a study by Angin and Ilci [85], activated carbon was prepared from olive-waste cake by chemical activation using ZnCl2 and used for 2,4-D removal. The results revealed that the adsorption kinetics and adsorption isotherms were described well by the PSO kinetic model and the Langmuir isotherm equation, respectively. The maximum adsorption capacity, based on the Langmuir model, was found to be 129.9 mg g−1. The thermodynamic data indicated that the adsorption process was feasible, spontaneous, and exothermic.
The removal of 2,4-D from water using AC prepared from queen palm endocarp (Syagrus romanzoffiana) by pyrolysis with ZnCl2 activation was also reported [86]. In that investigation, the influence of the initial pH, the adsorbent dose, the initial 2,4-D concentration, and the temperature on the adsorption was evaluated. The adsorption kinetic study indicated that the process reached equilibrium at 180 min and followed the PSO model. The equilibrium data were analyzed using the Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherm equations. The results showed that the adsorption isotherms were well described by the Langmuir equilibrium isotherm equation, and the calculated monolayer adsorption capacity was 367.8 mg g−1. The thermodynamic data indicated that the adsorption process was spontaneous and endothermic.
A recent study by Angın and Güneş [87] describes the preparation of activated carbon from orange (Citrus sinensis L.) pulp by chemical activation with zinc chloride. The AC was characterized and then used as an adsorbent for 2,4-D removal from an aqueous solution. The effects of the adsorbent dosage, the contact time, the initial concentration of 2,4-D, the temperature, and the pH, on the adsorption were studied. The PSO, PFO, and intra-particle diffusion kinetic models were tested with the experimental data, and the PSO kinetics model was the best fit for the adsorption of 2,4-D by the orange pulp-derived AC. The experimental data were analyzed by the Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherm models. The equilibrium data fitted well with the Langmuir model, with a maximum adsorption capacity of 71.9 mg g−1. The thermodynamic parameters indicated feasible, spontaneous, and exothermic adsorption.
Boumaraf et al. [88] reported the synthesis, characterization, and applicability of activated carbon from Elaeagnus angustifolia seeds using ZnCl2 activation. The influence of the operating parameters (solid dose, contact time, stirring speed, and pH) on 2,4-D adsorption was investigated. The adsorption process was spontaneous and exothermic, and obeyed the Langmuir and the pseudo-second-order models. The maximum adsorption capacity equaled 189 mg g−1. Satisfactory results from the application of AC for the elimination of 2,4-D from environmental waters (Algerian dam waters) synthetically polluted with 2,4-D were obtained (efficiency of 96%).
The same chemical activating agent (zinc chloride) was used to develop mushroom residues-based activated carbons for the removal of 2,4-D [89]. The differentiation in the pyrolysis temperature (400 and 500 °C) resulted in various values of SBET of the adsorbents (1067 and 799 m2 g−1) and of the adsorption capacity for the herbicide (105.4 and 118.2 mg g−1, respectively). The maximum adsorption capacity of carbon pyrolyzed in higher temperatures equaled 241.7 mg g−1 (pH = 4). The adsorption data were best described using the Langmuir and the linear driving force (LDF) models. It was shown that the use of the adsorbent resulted in a high degree of herbicide removal (70%) from the river water solution and, after the regeneration process, it was retained with high efficiency until the ninth cycle of reuse.
The elimination of 2,4-D and MCPA from an aqueous solution by activated carbons from rice straw in a batch system was investigated [90]. The ACs were prepared by chemical activation with ZnCl2 and H3PO4, and their surface area was 771 and 613 m2 g−1, respectively. The adsorption kinetics was described well by the PSO model, while the adsorption isotherms were well fitted to the Langmuir model. The adsorption capacity of both the ACs for MCPA removal was greater than for 2,4-D.
Activated carbon from an oil palm trunk was fabricated via a chemical activation route using H3PO4 to test its ability to remove 2,4-D from aqueous solutions [91]. The effects of the pH, the temperature, the initial adsorbate concentration, and the contact time, on the adsorption process were investigated. The highest removal efficiency was obtained at pH 2. The adsorption efficiency decreased as the temperature increased. The Langmuir isotherm model and the PSO kinetic model showed a better correlation with the experimental data. The monolayer maximum adsorption capacity was found to be 420.4 mg g−1 at 25 °C.
The activated carbon prepared from peanut shells by chemical activation with H3PO4 was tested as an adsorbent for 2,4-D removal from water [92]. Adsorption experiments for 2,4-D removal were performed via a batch system, in which the effects of the initial 2,4-D concentration, the adsorbent dose, the contact time, the pH, and the temperature were studied. Moreover, the influence of the simultaneous presence of inorganic salts and humic acid on the adsorption process was also investigated. The kinetic results yielded well to the PSO model. The adsorption equilibrium was best fitted to the Langmuir model, and the maximum adsorption capacity of the AC for 2,4-D removal was 281.2 mg g−1 at 30 °C.
Mesoporous activated carbon derived from Peltophorum pterocarpum pods was obtained by Samanth et al. [93] through chemical activation with H3PO4, followed by low-temperature carbonization. The surface area of the adsorbent was found to be 1078 m2 g−1 and its adsorption capacity for 2,4-D removal was 255.1 mg g−1. The adsorption data were fitted using the Langmuir and pseudo-second-order models. The values on the adsorption energy and thermodynamic functions revealed physisorption and exothermicity.
Naboulsi et al. [94] generated activated carbon from Ritama-Monosperma (L.) Boiss powder through carbonization at 500 °C and activation with H3PO4. The influence of various experimental parameters (pH, temperature, adsorbate dose) on the equilibrium, kinetics, and thermodynamics of 2,4-D and 2,4,5-T adsorption from a bicomponent solution was evaluated. A maximum adsorption capacity of 69.44 mg g−1 was found for 2,4-D and 13.81 mg g−1 for 2,4,5-T. The Freundlich and the pseudo-second-order kinetic equations were indicated as working well for the theoretical description of the adsorption process.
A paper by Doczekalska et al. [95] reports the efficient removal of 2,4-D and MCPA during an aqueous phase, using activated carbons derived from various lignocellulosic materials. In that investigation, the ACs were prepared from willow, miscanthus, flax, and hemp shives, via KOH activation. The adsorption was pH-dependent, and the removal of both herbicides decreased with an increase in the initial pH of the solution. The adsorption kinetics of both herbicides was better represented by the PSO model, while the equilibrium data followed the Langmuir isotherm.
Trivedi et al. [45] proposed activated carbon synthesis from groundnut shells via chemical activation with KOH. The as-received AC was characterized by SBET = 709 m2 g−1 and an adsorption capacity for 2,4-D removal of 250 mg g−1.
Activated carbon obtained by KOH activation of the biochar, obtained as a result of the pyrolysis of safflower press cake, was prepared and tested as an adsorbent for the removal of 2,4-D from aqueous solutions [96]. The effects of the initial pH value, the adsorbent dosage, the initial concentration, the contact time, and the temperature were evaluated. The results showed that the PSO model was more compatible with the experimental kinetic data and that the Langmuir isotherm model provided a better correlation for the adsorption of 2,4-D. The maximum adsorption capacity was found to be 344.8 mg g−1 at 318 K. The thermodynamic studies revealed that the adsorption process was spontaneous and endothermic.
Koyuncu et al. [97], Rambabu et al. [98], and Pandiarajan et al. [99] prepared activated carbons from capsicum industrial processing pulp, date palm coir, and orange peel, respectively, via single-step KOH-catalyzed pyrolysis. The first research group demonstrated that the isotherm data were described well by the Langmuir isotherm model, and the maximum adsorption capacity for 2,4-D removal was 385 mg g−1. The date palm coir-based activated carbon [98] was characterized by a flaky morphology, a well-graphitized porous structure (947 m2 g−1), and oxygen-rich surface moieties. The Langmuir and the pseudo-second-order models best described the equilibrium (with qm = 50.3 mg g−1) and the kinetics of the adsorption process. An evaluation of the costs of the adsorbent production and regeneration was made, and cyclic reusability tests were also conducted. Pandiarajan’s group extended an adsorption study to other chlorophenoxy acid herbicides (2,4-DP, MCPA, 2,4,5-T, MCPP). The Langmuir model fitted all the herbicide adsorption isotherms very well. The results showed that the adsorption increased in the order: 2,4-DP < 2,4,5-T < MCPA < 2,4-D < MCPP. The pseudo-second-order kinetic model was indicated as the one with the highest fitting quality to the experimental data for all the adsorption systems.
Tefera and Tulu [100] tested activated carbon prepared from wheat straw treated with sulfuric acid and potassium hydroxide for the removal of 2,4-D from aqueous solutions. The removal efficiency of 2,4-D was evaluated using varying parameters, such as the adsorbent dose, temperature, pH, and contact time. It was observed that the adsorption efficiency of the herbicide increased with the increasing temperature, contact time, and adsorbent dose, and decreased with an increase in the solution pH. The equilibrium data were analyzed using the Langmuir and Freundlich isotherms and the Langmuir model best described the experimental data.
Herrera-García et al. [101] prepared activated carbon from agricultural waste made of yam peel (Dioscorea rotundata) by chemical activation with KOH. The as-prepared AC was chemically modified using magnetite nanoparticles. Both the ACs were characterized and tested as adsorbents for the removal of 2,4-D from aqueous solutions. The effects of the solution pH, the initial concentrations of 2,4-D, and the temperature, were investigated. The PFO, PSO, Elovich, and Webber–Morris kinetic equations, as well as the Langmuir, Freundlich, and Elovich isotherms, were used for the description of the experimental data. The results showed that the adsorption kinetics and isotherms were better described through the PSO and Freundlich models, respectively. Langmuir’s maximum adsorption capacity values at 25 °C were 99.0 and 86.2 mg g−1 for non-modified and Fe3O4-modified ACs, respectively.
Salman in the paper [102] dealt with the optimization of the conditions of activated carbon preparation from palm oil fronds using the physiochemical activation method (KOH and CO2 as activating agents), and its application for the removal of various types of pesticides (e.g., bentazon, carbofuran, and 2,4-D). Of the variable synthesis parameters (activation time and chemical impregnation ratios KOH/biochar, activation temperature), the latter one was found to have the greatest effect on the activated carbon yield in regard to pesticides adsorption. The surface area of the activated carbon, prepared under optimum conditions, equaled 1237 m2 g−1. The optimizing analysis of variance was carried out using the response surface methodology (RSM).
Table 4 presents a compilation of the results of various activated carbons in the removal of 2,4-D and MCPA and their adsorption capacity.

Table 4.
The results from chlorophenoxy herbicides adsorption studies on activated carbons produced from agricultural and household waste.
The collected adsorption results showed that activated carbons derived from agricultural and household waste were very good adsorbents for the removal of chlorophenoxy herbicides from an aqueous solution. The adsorption capacities of activated carbons towards 2,4-D and MCPA were in the range of 1.015–592.7 mg g−1 and 85–546.7 mg g−1, respectively. The observed differences resulted mainly from the degree of development of the porous structure of the solids, the chemical characteristics of the surface, and the experimental conditions of the adsorption process. The high surface area of activated carbon, the basic nature of its surface, and the acidic conditions of the adsorption experiment favored chlorophenoxy herbicides adsorption. In general, the adsorption capacities of the activated carbons were very high when compared with other waste-based adsorbents, i.e., unmodified/modified materials, ashes, and biochars. Additionally, their adsorption capacities were not inferior to commercial activated carbons derived from fossil coals, for which the values of the parameter qm towards 2,4-D and MCPA were in the range of 8.11–555.5 mg g−1 and 105.3–599.9 mg g−1, respectively [33]. A significant share of micropores in the pores’ structure caused the overall adsorption rate to be pore volume diffusion-dependent.
3. Mechanism of Adsorption
Adsorption of chlorophenoxy herbicides by the different adsorbents, based on agricultural and household waste, is the result of various specific and nonspecific interactions. The adsorption mechanism of the same compound may change depending on the different properties of the adsorbent. The same situation occurs, for example, with a change in pH because the pH of the solution affects both the degree of dissociation and ionization of the adsorbate molecules, as well as the charge that will be present on the surface of the adsorbent. Thus, depending on the pH of the solution, adsorption can occur via electrostatic interactions (attractive or repulsive) between the adsorbate and the adsorbent.
The most typical and important forces driving adsorption by biochars and activated carbons include π-π interactions, electrostatic attraction and repulsion, hydrophobic interactions, covalent bonding and hydrogen bonding interactions between the herbicide molecules and the adsorbent surface functional groups. A significant share of the micro- or small mesopores in the structure of these materials causes the adsorption to proceed mainly through the pore-filling mechanism. Figure 1a presents possible supramolecular systems created during the adsorption of the 2,4-D molecule on the exemplary biochar [66]. Impregnation of the raw material with H3PO4 at the pre-treatment stage of synthesis determined the occurrence of typical interactions between the adsorbate and the adsorbent, as well as specific ones resulting from the activating agent used (i.e., with metaphosphate groups). The high value of the maximum adsorption capacity for this biochar towards 2,4-D (323.76 mg g−1) at pH = 2 and T = 318 K was found. Additionally, the morphological structure of the used adsorbent and the herbicide adsorption isotherms are illustrated in Figure 1b–d, respectively.
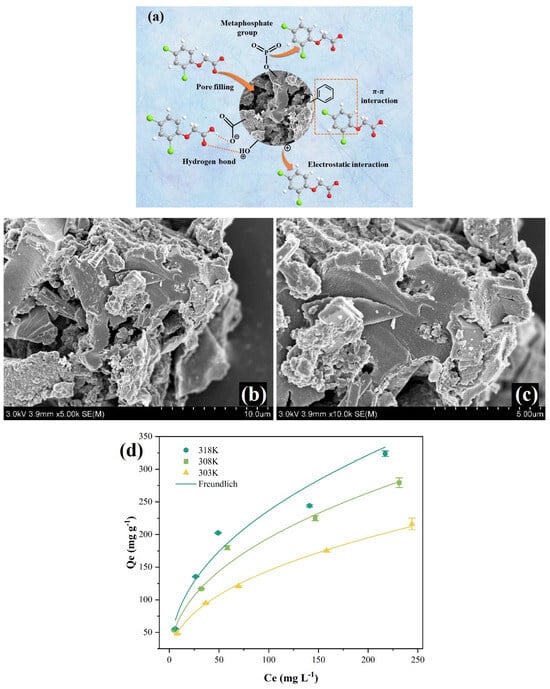
Figure 1.
The possible interactions in the 2,4-D adsorptive process using coffee ground char (a). SEM images of the adsorbent (b,c). The herbicide adsorption isotherms on the biochar (d) Reprinted with permission from Ref. [66]. Copyright 2023, copyright Elsevier.
Regarding unmodified and modified natural materials and ashes, the mechanism of the 2,4-D or MCPA adsorption must be considered individually in conjunction with the unique (individual) physicochemical properties of the adsorbent. According to the authors of some works [35,36,37,39], the adsorption of chlorophenoxy herbicides by rice husks with a positive surface charge, follows a chemical ion-exchange mechanism or occurs via a chemical process (involving valency force sharing). Chemical reactions (through free electron pairs of surface functional groups, e.g., carbonyl, amide, ether), cation–dipole interactions (due to the occurrence of hydroxyl, amine, and amide groups), as well as hydrogen bonds are proposed for the adsorption of 2,4-D by saw-sedge Cladium mariscus (C. mariscus). In turn, for bio-waste materials, such as apple shell, orange peel, banana peel, and millet residue, the discussed process results from molecular interactions (when the molecular form of the pesticide occurs) and repulsive electrostatic interactions (at a solution pH where both the adsorbent surface moieties, i.e., carboxyl and sulphate, and 2,4-D are negatively charged) [40]. Al-Zaben and Mekhamer [41] reported no relationship between the MCPA removal efficiency and the pH solution using coffee waste as the adsorbent. The existence of an electrostatic interaction was excluded in this system, and the responsibility for the adsorption was assigned to the hydrogen bonds between the OH moieties on the adsorbent surface and the acidic group of the adsorbate molecules.
According to the authors of papers [47,48,49], the oxidation of various biomass (Physalis peruviana fruit, Arachis hypogaea skins and wheat husks) with sulfuric acid resulted in oxygenated functionalities on their surface, which participated in the adsorption of the chlorophenoxy herbicides via electrostatic forces, hydrogen bonding, and van der Waals forces. The authors of the latter paper [49] also indicated halogen bonds and π–π interactions as responsible for adsorption. Noteworthy is the relatively high adsorption efficiency of acid-modified wheat husks, with the capacity of 161.1 mg g−1 (pH = 2, T = 298 K). For this reason, the possible supramolecular systems of this adsorbent with the 2,4-D molecule (Figure 2a), along with the morphological structure of the adsorbent (Figure 2b–d), and the equilibrium isotherms at various experimental temperatures (Figure 2e), are presented.
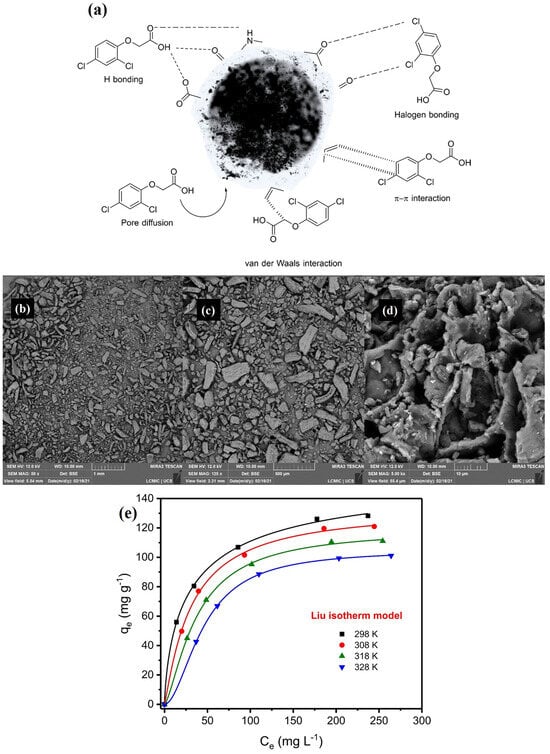
Figure 2.
The possible interactions in the 2,4-D adsorptive process using acid-modified wheat husks (a). SEM images of the adsorbent (magnitudes: (b) ×500, (c) ×1000, (d) ×3000), (b–d). Equilibrium isotherms of the 2,4-D adsorption by modified wheat husks (pH = 2) (e) Reprinted with permission from Ref. [49]. Copyright 2021, copyright Elsevier.
The modification of tiger nut residue using N-cetylpyridinium [50] resulted in obtaining the adsorbent, which interacted with 2,4-D through electrostatic attraction, hydrogen bonding, and triggered π-π stacking. Trivedi’s comprehensive research [42,43,44,45,46] on ashes originating from various waste materials showed a close relationship between their chemical composition and the mechanism of the chlorophenoxy herbicides adsorption. The presence of metallic oxides (CaO, K2O) in ash, which in water take the form of metallic hydroxides, resulted in electrostatic repulsion forces between them and adsorbate anions. In turn, for Al2O3, which constitutes positive centers on the adsorbent surface, the triggering of attractive electrostatic interactions was observed. Thus, the mineral composition of ash determines the mechanism and effectiveness of the adsorption process. For hydrochars (HCs), the mechanism of the 2,4-D adsorption via intensive partitioning and/or chemisorption was postulated [64]. Regarding the interactions of pollutants with more advanced magnetic waste-based materials [52,53] with various functional groups (hydroxyl, amine, carboxyl), the hydrogen bonding, electrostatic complexation, and π-π mechanisms were suggested. Moreover, some of these interactions were additionally enhanced by the occurrence of iron. The high efficiency of the ferrospinel composite (FLPWAC) [53] (qm = 400 mg g−1, T = 318 K) was found. The possible supramolecular adsorbate–adsorbent systems (Figure 3a), the morphological structure of the adsorbent (Figure 3b–f), and the equilibrium isotherms at various experimental temperatures (Figure 3g), are presented.
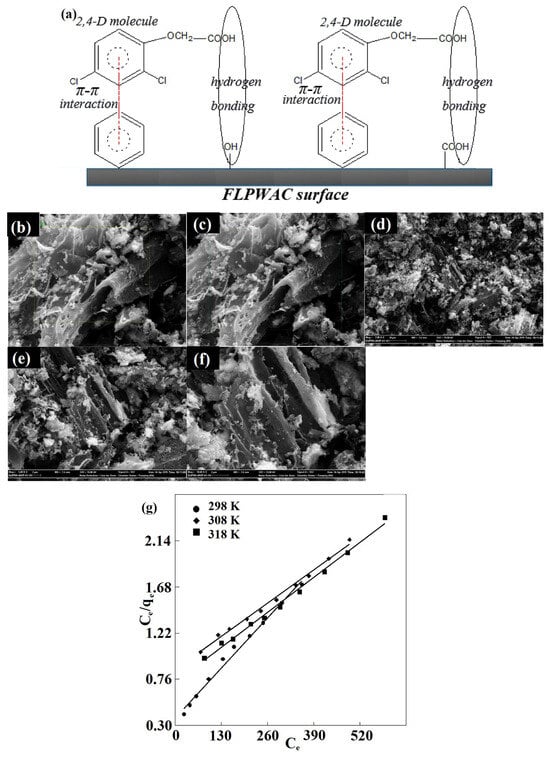
Figure 3.
The possible interactions in the 2,4-D adsorptive process using the ferrospinel composite (a). SEM images of the adsorbent at different expansion ratios (b–f). Langmuir plots of the 2,4-D adsorption on the composite (g). Reprinted with permission from Ref. [53]. Copyright 2020, copyright Elsevier.
Summing up, the variety of types of adsorbents and their different characteristics translate into a multitude of mechanisms leading to the adsorption of chlorophenoxy herbicides.
4. Effect of Co-Substances on Chlorophenoxy Herbicides Adsorption by Waste-Based Adsorbents
Environmental waters are complex biological systems, containing several compounds of natural and anthropogenic origin, so research on the assessment of the impact of various co-substances on chlorophenoxy herbicide removal by waste-based adsorbents is enormously important. This issue has been addressed in only a few papers out of all the ones cited. The studies concerned the co-existence of mineral salts, organic matter, dyes, and other herbicides, but there was no information on the co-existence of adjuvants, which are auxiliary substances in commercial formulations of herbicide preparations.
Fatombi et al. [92] and Almahri [67] reported that 2,4-D removal efficiency by activated carbon and biochar in the presence of NaCl, CaCl2, and humic acid (HA) decreased, wherein the influence of CaCl2 was lower than NaCl. Such a difference was explained by the higher polarizability effect of the Ca2+ ion and the lower thickness of the double layer in the solid with the participation of this ion. The adsorption capacities were found to be 281.22, 86.12, 160.32, and 154.96 mg g–1 for adsorption of 2,4-D only, and with the co-existence of NaCl, CaCl2, and HA, respectively. It was also noticed that an increase in the salt concentration negatively affected herbicide adsorption, due to a higher dissociation degree of the 2,4-D molecules and the forming of pairs between the salt cations and herbicide anions. The slighter impact of CaCl2 on 2,4-D adsorption was related to the screening effect of the negative charge of 2,4-D by the formation of complexes based on intermolecular bridges (R–COO––Ca2+––OOC–R).
A similar trend in the influence of the presence of natural organic matter on 2,4-D adsorption by AC was also noticed by Gurav’s research group [65]. Here, the adsorption capacities equaled 491.76 and 466.73 mg g–1 for 2,4-D adsorbed from single- and bicomponent solutions, respectively. However, the effect of salinity on 2,4-D removal was reversed compared to that observed by Fatombi et al. [92]. The occurrence of NaCl in water in a certain concentration range (1–4 g L–1) slightly enhanced the 2,4-D adsorption (from 493.86 to 496.39 mg g–1), but a further increase in salt concentration caused a decrease in adsorption (to 489.08 mg g–1). The enhancement of the adsorption with the rise in the ionic strength of the solution was connected with the screening effect of the carbon surface charge by NaCl.
In the paper [82], the effect of competing substances was analyzed for MCPA adsorption by two activated carbons, differentiated in terms of their porous structure and surface functionality. A slight effect of crystal violet co-existence on the MCPA adsorption was revealed, which was explained by the affinity of both pollutants to different adsorption sites. The large dye molecules were adsorbed in the mesopores, while the small MCPA ones were mainly located in the micropores. Thus, the authors stated that the adsorption of both compounds was mostly independent. However, a significant influence of the competing substance was observed when in the solution there were two substances belonging to the same group of herbicides (MCPA and 2,4-D). Here, a decrease in MCPA adsorption with the co-existing substance was a consequence of the occupation of the same adsorption sites (qm equals 314 and 144.43 mg g–1 for the MCPA adsorbed from single- and bicomponent solutions, respectively). Relatively similar physicochemical properties and structures of the herbicides caused strong competition between each other in the adsorption process by activated carbon. It was also noticed that 2,4-D showed greater affinity to the carbon surface than MCPA due to the greater adsorption strength, which favored keeping 2,4-D in the adsorption active sites or replacing it after MCPA desorption. The adsorption capacities were found to be 242.58 and 144.43 mg g–1 for 2,4-D and MCPA adsorbed from the bicomponent solution, respectively.
Naboulsi et al. [94] studied the competition effect on the adsorption of 2,4-D and 2,4,5-T by waste-based ACs. The maximum adsorption capacity of 69.44 mg g–1 for 2,4-D and 13.81 mg g–1 for 2,4,5-T for the bicomponent system was determined. The application of density functional theory (DFT) enabled the identification of 2,4-D as a more selective pollutant due to the greater reactivity of its molecules (more active sites, either nucleophilic or electrophilic in nature).
A higher selectivity of the biomass-MOF composite towards 2,4-D compared to tetracycline (antibiotic) in binary systems was also observed [52]. Such behavior by the adsorbent was attributed to the relatively smaller size of the herbicide, which reduced the steric hindrance. However, it was found that an increase in the adsorbent dose reduced the adsorption efficiency due to grains agglomeration (limiting accessibility to the surface area).
In the paper [69], promising results from 2,4-D adsorption studies on biochar in multi-component systems were presented. A 100% biochar performance for pollutants from drainage water and 96.8% from simulated one-component wastewater was noted. This was due to the synergistic effect of the coexisting cations and anions in the drainage water on herbicide adsorption.
The 2,4-D removal in the range of 86.5–95.6% by biochar from real water (river effluent, drinking water, and wastewater) was achieved by Almahri et al. [67]. Only slightly worse results on 2,4-D removal efficiencies (72–76.30%) by sulfuric acid-treated peanut skins and wheat husks were found in the treatment of synthetic wastewater, consisting of a mixture of 2,4-D, atrazine, NaCl, KCl, or the simulated effluent from two Brazilian rivers [48,49].
The studies on various co-existing substances and their impact on the chlorophenoxy herbicides adsorption by waste-based adsorbents showed the complexity of the interactions between the components of adsorption systems and highlighted the significance of taking them into account in the development and optimization of new adsorbents for environmental applications. However, taking into account the complex composition of natural water for a better understanding of the interactions between other co-substances, herbicides, and adsorbents, further research in this direction is required.
5. Regeneration and Reusability of Waste-Based Activated Carbons
The evaluation of the recycling and reuse potential of waste-based ACs is another crucial factor in determining the implementation of these materials for adsorption applications. This is related to cost reduction for new adsorbents and actions to prevent possible secondary pollution of the environment with herbicides and other co-adsorbates, as a result of their leaching due to environmental factors from used activated carbon deposits at storage sites. The proper management of spent adsorbents seems to be such an important issue that many research groups have initiated studies in this direction. Generally, chemical, thermal, physical, and biological methods are used to regenerate activated carbons. Regarding the regeneration of waste-based activated carbons with adsorbed chlorophenoxy herbicides, the commonly used chemical method was based on the desorption of the adsorbate using NaOH solution as an eluent [50,65,84,86,89,92,93,98], and less frequently using other specific solvents or solutions, such as acetone [78], ethanol [78], water [78,98], and NaCl. The performance of this method depended on the interactions between the herbicide–activated carbon and between the herbicide–regenerating chemical agent. The adsorption efficiency of the regenerated adsorbent usually decreased with the number of adsorption–desorption cycles (maximum number of cycles 3–5), but some adsorbents could be reused even 7–9 times, with the maintenance of the adsorption effectiveness close to the initial use [65,86,89,98]. The decrease in the adsorption capacity in the following regeneration stages resulted from various factors: (i) the decrease in the surface sites’ adsorption potential, (ii) steric effects, (iii) the disintegration of the pore structure, and (iv) the inability of the herbicide to desorb from the carbon structure. Of the solvents, e.g., acetone, ethanol, and water, the first one appeared to be the superior agent for AC regeneration. The thermal method of regeneration was applied by Bojić et al. [76] and Herrera-García et al. [101], with 49–60% keeping their initial adsorption capacities after five cycles. More satisfying results (70–79% initial efficiency) were obtained for the physical method of regeneration using a microwave heating oven. All these findings showed that ACs successfully passed the regeneration and reusability tests. However, for the application of adsorbents on an industrial scale, it is advisable to perform additional adsorption studies within a column or a continuous flow system (fluidized-bed column) for the treatment of water polluted with chlorophenoxy herbicides.
Research in the field of regeneration and reusability of adsorbents makes economic and practical sense if a given material shows high adsorption capacity towards pollutants. Therefore, this issue has been described in only a few papers devoted to materials other than activated carbon. More precisely, they concerned coffee waste-based biochar and acid-modified wheat husk, with adsorption capacities for 2,4-D of 276.3 and 161.1 mg g–1, respectively [49,67]. As in the case of many active carbons, the best results (retaining 45–84% removal efficiency after five cycles of adsorption–desorption) were obtained using sodium hydroxide as a desorbing agent. Nitric acid, calcium chloride, hydrochloric acid, ethylenediamine tetraacetic acid (EDTA), and ultrasonic cleaning with alcohol, were found not to be effective enough in the desorption of pollutants from biochars [67,70]. There were also materials that were treated with NaOH after use, but their subsequent efficiency was negligible. An example of such a material is a peanut husk-based MOF composite with magnetic properties [52]. Nevertheless, the regeneration process used allowed the recovery and reuse of the adsorbate, while the adsorbent disposed into soil acted as organic amendment due to the slow release of its major elements (i.e., Fe, C, N, and O).
6. Cost of Production of Waste-Based Activated Carbons
A review of the relevant literature revealed the selectivity and satisfying adsorption capability of waste-based activated carbons towards chlorophenoxy herbicides in model experiments both in single-component and multi-component systems. Importantly, these adsorbents could be regenerated using simple methods, which enables multiple use at a high level of efficiency. Another issue to analyze is the cost effectiveness of waste-based AC production compared to commercial materials. In a paper from 2022 [97], the authors estimated the production cost of a Capsicum pulp-based AC at approximately USD 1.75 per kg (summing up the costs of the raw material transport; the reagents, namely nitrogen gas, KOH, and hydrochloric acid; and the media, namely power, water), which is lower than that for commercial ACs, e.g., USD 2–5 per kg. In other works from 2021, the fabrication of ACs from mushroom residues [89] and date palm coir waste [98] was assessed to be USD 2.39 per kg (the total costs of reagents, namely nitrogen gas, hydrochloric acid, and zinc chloride; and media, namely power) and USD 3 per kg (the total costs of the capital expenses, namely the installation equipment, land, buildings, service; and the operating ones, namely the precursor collection, water, electricity, and labor), respectively. According to [98], the cost of commercial activated carbon may even reach USD 15 per kg. Actually, only a few cited works contained cost estimations, but based on the available data, the undertaking to expand the production of these adsorbents from a laboratory scale to an industrial one seems to be economically viable. Moreover, this project is in line with the trend of sustainable waste management, which is beneficial for the natural environment.
7. Conclusions
This paper presents a wide range of agricultural and household wastes as low-cost alternative adsorbents for the removal of two common herbicides, 2,4-D and MCPA, from water. The characteristics of the adsorbents, the conditions of the adsorption experiments and their results, and the theoretical models that best describe the adsorption process have been reported. Based on the available literature, mainly from the last decade, the use of waste materials as low-cost adsorbents, both in their original form and after modification, mainly in the form of waste-based biochar and activated carbon, was shown to be very attractive. Such adsorbents are not only effective, but are also very interesting from an economic point of view. The use of waste itself as an adsorbent reduces the cost of waste disposal and, thus, promotes environmental protection, and the low cost of these materials means that they can be disposed of after use without costly regeneration. However, the problems concerning the regeneration and reusability of waste-based activated carbons and the costs involved have been discussed. The waste materials presented in this paper showed a satisfactory affinity with 2,4-D and MCPA, and since they are relatively low- or no-cost, readily available, and easily modified, e.g., by pyrolysis and activation, their use as alternative adsorbents seems very interesting and promising. The development of adsorbents using agricultural and household waste offers an interesting alternative to commercial materials for environmental applications and at the same time offers a way forward for proper waste management.
At the same time, it should be added that the presented review on the use of adsorbents based on waste materials for water purification, also showed the need to intensify the research towards extending it to more complex systems corresponding to real ones. Only a few studies concern this problem, while water and sewage contain many toxic substances with very diverse properties, originating from various sources, including those used as pesticide additives. The aim of adsorption techniques is to remove substances to the highest possible extent, which is difficult to achieve in such complex systems, in which a large part of the components may compete for access to the adsorption centers. Therefore, an important direction of future research is the analysis of adsorption in model and real multi-component systems. Taking into account the diversity of the physicochemical properties of the toxic substances present in water and sewage, including the molecular size, hydrophobic or hydrophilic nature, and molecular form, it is also necessary to analyze materials or systems of materials with a different porosity and chemical nature of the surface in order to achieve the maximum possible level of purification. On the other hand, in many cases the selective adsorbents should be applied; thus, also the studies concerning the mutual interactions between the adsorbates in complex systems, which can decrease the adsorption effectiveness of a given substance, are of great importance.
Author Contributions
Conceptualization, M.B., K.K., A.S. and A.D.-M.; data curation, M.B. and K.K.; visualization, M.B. and K.K.; formal analysis, K.K., A.S. and A.D.-M.; supervision, K.K., A.S. and A.D.-M.; writing—original draft, M.B., K.K. and A.S.; writing—review and editing, K.K., A.S. and A.D.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AC | Activated carbon |
| A | Avrami kinetic model |
| Ba | Bangham kinetic model |
| DR | Dubinin–Radushkevich isotherm |
| E | Elovich isotherm model |
| F | Freundlich isotherm model |
| GL | The generalized Langmuir isotherm |
| Hi | Hill isotherm model |
| IDM | Intraparticle diffusion model |
| ITM | Theoretical adsorption model |
| J | Jovanovic equilibrium model |
| K | Khan equilibrium model |
| K-C | Koble–Corrigan equilibrium model |
| ki | Intraparticle diffusion rate in W-M kinetic model, |
| k1 | The rate constant for the PFO kinetic model, min−1 |
| k2 | The rate constant for the PSO kinetic model, g mg−1 min−1 |
| L | Langmuir isotherm |
| LDF | Linear driving force kinetic model |
| L-F | Langmuir–Freundlich isotherm |
| m-exp | Multi-exponential kinetic equation |
| MOE | Mixed-order equation |
| PFO | Pseudo-first-order kinetic model/equation (PFOE) |
| PSO | Pseudo-second-order kinetic model/equation (PSOE) |
| qm | The maximum adsorption capacity, mg g−1 |
| R-P | Redlich–Peterson isotherm |
| S | Sips isotherm |
| Te | Temkin isotherm |
| To | Toth isotherm |
| W-M | Weber–Morris kinetic model |
References
- U.S. FAO Food and Agriculture Organization of the United Nations. Pesticides Use, Pesticides Trade and Pesticides Indicators. Global, Regional and Country Trends, 1990–2020. 2022. Available online: http://www.fao.org/faostat/en/#data/RP (accessed on 1 July 2023).
- Schröter, M.; Bonn, A.; Klotz, S.; Seppelt, R.; Baessler, C. Atlas of Ecosystem Services: Drivers, Risks, and Societal Responses; Springer: Cham, Switzerland, 2019. [Google Scholar]
- EPA. Drinking Water Report for Public Water Supplies 2016; Environmental Protection Agency, Johnstown Castle: Wexford, Ireland, 2017.
- European Topic Centre. Overview of the Drinking Water Quality in Bulgaria. Results of the Reporting 2011–2013 under the Drinking Water Directive 98/83/EC, European Topic Centre: Inland, Coastal and Marine Waters; European Topic Centre: Copenhagen, Denmark, 2016. [Google Scholar]
- European Topic Centre. Overview of the Drinking Water Quality in Greece. Results of the Reporting 2011–2013 under the Drinking Water Directive 98/83/EC, European Topic Centre: Inland, Coastal and Marine Waters; European Topic Centre: Copenhagen, Denmark, 2016. [Google Scholar]
- European Topic Centre. Overview of the Drinking Water Quality in Ireland. Results of the Reporting 2011–2013 under the Drinking Water Directive 98/83/EC, European Topic Centre: Inland, Coastal and Marine Waters; European Topic Centre: Copenhagen, Denmark, 2016. [Google Scholar]
- European Topic Centre. Overview of the Drinking Water Quality in United Kingdom. Results of the Reporting 2011–2013 under the Drinking Water Directive 98/83/EC, European Topic Centre: Inland, Coastal and Marine Waters; European Topic Centre: Copenhagen, Denmark, 2016. [Google Scholar]
- NI Water. Drinking Water Quality Annual Report 2018; Northern Ireland Water: Belfast, UK, 2018. [Google Scholar]
- Glozier, N.E.; Struger, J.; Cessna, A.J.; Gledhill, M.; Rondeau, M.; Ernst, W.R.; Sekela, M.A.; Cagampan, S.J.; Sverko, E.; Murphy, C.; et al. Occurrence of glyphosate and acidic herbicides in select urban rivers and streams in Canada, 2007. Environ. Sci. Pollut. Res. 2012, 19, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Atwood, D.; Paisley-Jones, C. Pesticides Industry Sales and Usage 2008–2012 Market Estimates. Available online: https://www.epa.gov/sites/production/files/2017-01/documents/pesticides-industry-sales-usage-2016_0.pdf (accessed on 29 October 2023).
- Benfeito, S.; Garrido, J.; Sottomayor, B.M.J.; Fernanda, G. Pesticides and Cancer: Studies on the Interaction of Phenoxy Acid Herbicides with DNA. In Handbook on Herbicides; Kobayashi, D., Watanabe, E., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013. [Google Scholar]
- Knopper, L.D.; Lean, D.R.S. Carcinogenic and genotoxic potential of turf pesticides commonly used on golf courses. J. Toxicol. Environ. Health Part B Crit. Rev. 2004, 7, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Hardell, L.; Carlberg, M.; Akerman, M. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int. J. Cancer 2008, 123, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Miligi, L.; Costantini, A.S.; Bolejack, V.; Veraldi, A.; Benvenuti, A.; Nanni, O.; Ramazzotti, V.; Tumino, R.; Stagnaro, E.; Rodella, S.; et al. Non-Hodgkin’s lymphoma, leukemia, and exposures in agriculture: Results from the Italian multicenter case-control study. Am. J. Ind. Med. 2003, 44, 627–636. [Google Scholar] [CrossRef]
- Mills, P.K.; Yang, R.C. Agricultural exposures and gastric cancer risk in Hispanic farm workers in California. Environ. Res. 2007, 104, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Ross, G.W.; Jewell, S.A.; Hauser, R.A.; Jankovic, J.; Factor, S.A.; Bressman, S.; Deligtisch, A.; Marras, C.; Lyons, K.E.; et al. Occupation and risk of parkinsonism: A multicenter case-control study. Arch. Neurol. 2009, 66, 1106–1113. [Google Scholar] [CrossRef]
- IARC. DDT, Lindane, and 2,4-D. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC publications: Lyon, France, 2018. [Google Scholar]
- World Health Organization. Chlorophenoxy Herbicides (Excluding 2,4-D and MCPA) in Drinking-Water. Background Document for Preparation of WHO Guidelines for Drinking-Water Quality (WHO/SDE/WSH/03.04/44); World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- U.S. EPA. R.E.D. Facts: MCPA. Prevention, Pesticides and Toxic Substances (7508C); U.S. EPA: Washington, DC, USA, 2004.
- Ighalo, J.O.; Ojukwu, V.E.; Umeh, C.T.; Aniagor, C.O.; Chinyelu, C.E.; Ajala, O.J.; Dulta, K.; Adeola, A.O.; Rangabhashiyam, S. Recent advances in the adsorptive removal of 2,4-dichlorophenoxyacetic acid from water. J. Water Process. Eng. 2023, 56, 104514. [Google Scholar] [CrossRef]
- Zhang, J.; Weston, G.; Yang, X.; Gray, S.; Duke, M. Removal of herbicide 2-methyl-4-chlorophenoxyacetic acid (MCPA) from saline industrial wastewater by reverse osmosis and nanofiltration. Desalination 2020, 496, 114691. [Google Scholar] [CrossRef]
- Muszyński, P.; Brodowska, M.S.; Paszko, T. Occurrence and transformation of phenoxy acids in aquatic environment and photochemical methods of their removal: A review. Environ. Sci. Pollut. Res. 2020, 27, 1276–1293. [Google Scholar] [CrossRef]
- Aziz, F.; Ouazzani, N.; Mandi, L.; Muhammad, M.; Uheida, A. Composite nanofibers of polyacrylonitrile/natural clay for decontamination of water containing Pb(II), Cu(II), Zn(II) and pesticides. Sep. Sci. Technol. 2017, 52, 58–70. [Google Scholar] [CrossRef]
- Phorostianenko, O.; Petruk, H. Thermal Treatment as a Method of Remediation of Soil Contaminated with Pesticides. Personal. Environ. Issues 2023, 3, 38–42. [Google Scholar] [CrossRef]
- Brillas, E.; Boye, B.; Baños, M.; Calpe, J.; Garrido Ponce, J.A. Electrochemical degradation of chlorophenoxy and chlorobenzoic herbicides in acidic aqueous medium by the peroxi-coagulation method. Chemosphere 2003, 51, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Girón-Navarro, R.; Linares-Hernández, I.; Teutli-Sequeira, E.A.; Martínez-Miranda, V.; Santoyo-Tepole, F. Evaluation and comparison of advanced oxidation processes for the degradation of 2,4-dichlorophenoxyacetic acid (2,4-D): A review. Environ. Sci. Pollut. Res. 2021, 28, 26325–26358. [Google Scholar] [CrossRef] [PubMed]
- Bradu, C.; Magureanu, M.; Parvulescu, V.I. Degradation of the chlorophenoxyacetic herbicide 2,4-D by plasma-ozonation system. J. Hazard. Mater. 2017, 336, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Natural-based coagulants/flocculants as sustainable market-valued products for industrial wastewater treatment: A review of recent developments. RSC Adv. 2023, 13, 19335–19355. [Google Scholar] [CrossRef]
- Mourid, E.H.; Lakraimi, M.; Legrouri, A. Preparation of well-structured hybrid material through ion exchange of chloride by 2,4,5-trichlorophenoxyacetic herbicide in a layered double hydroxide. Mater. Chem. Phys. 2022, 278, 125570. [Google Scholar] [CrossRef]
- Magnoli, K.; Carranza, C.; Aluffi, M.; Magnoli, C.; Barberis, C. Fungal biodegradation of chlorinated herbicides: An overview with an emphasis on 2,4-D in Argentina. Biodegradation 2023, 34, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, K.; Carranza, C.S.; Aluffi, M.E.; Magnoli, C.E.; Barberis, C.L. Herbicides based on 2,4-D: Its behavior in agricultural environments and microbial biodegradation aspects. A review. Environ. Sci. Pollut. Res. 2020, 27, 38501–38512. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Mandavgane, S.A. Fundamentals of 2, 4 Dichlorophenoxyacetic Acid Removal from Aqueous Solutions. Sep. Purif. Rev. 2018, 47, 337–354. [Google Scholar] [CrossRef]
- Blachnio, M.; Kusmierek, K.; Swiatkowski, A.; Derylo-Marczewska, A. Adsorption of Phenoxyacetic Herbicides from Water on Carbonaceous and Non-Carbonaceous Adsorbents. Molecules 2023, 28, 5404. [Google Scholar] [CrossRef]
- Aziz, K.; Aziz, F.; Mamouni, R.; Aziz, L.; Saffaj, N. Engineering of highly Brachychiton populneus shells@polyaniline bio-sorbent for efficient removal of pesticides from wastewater: Optimization using BBD-RSM approach. J. Mol. Liq. 2022, 346, 117092. [Google Scholar] [CrossRef]
- Deokar, S.K.; Mandavgane, S.A. Rice husk ash for fast removal of 2,4-dichlorophenoxyacetic acid from aqueous solution. Adsorpt. Sci. Technol. 2015, 33, 429–440. [Google Scholar] [CrossRef]
- Abigail, M.E.A.; Samuel, S.M.; Chidambaram, R. Application of rice husk nanosorbents containing 2,4-dichlorophenoxyacetic acid herbicide to control weeds and reduce leaching from soil. J. Taiwan Inst. Chem. Eng. 2016, 63, 318–326. [Google Scholar] [CrossRef]
- Deokar, S.K.; Mandavgane, S.A.; Kulkarni, B.D. Agro-industrial waste: A low cost adsorbent for effective removal of 4-chloro-2-methylphenoxyacetic acid herbicide in batch and packed bed modes. Environ. Sci. Pollut. Res. 2016, 23, 16164–16175. [Google Scholar] [CrossRef] [PubMed]
- Deokar, S.K.; Mandavgane, S.A. Estimation of packed-bed parameters and prediction of breakthrough curves for adsorptive removal of 2,4-dichlorophenoxyacetic acid using rice husk ash. J. Environ. Chem. Eng. 2015, 3, 1827–1836. [Google Scholar] [CrossRef]
- Abigail, M.E.A.; Chidambaram, R. Rice husk as a low cost nanosorbent for 2,4-dichlorophenoxyacetic acid removal from aqueous solutions. Ecol. Eng. 2016, 92, 97–105. [Google Scholar] [CrossRef]
- Okumuş, V.; Çelik, K.S.; Özdemir, S.; Dündar, A.; Kılınç, E. Biosorption of chlorophenoxy acid herbicides from aqueous solution by using low-cost agricultural wastes. Desalination Water Treat. 2015, 56, 1898–1907. [Google Scholar] [CrossRef]
- Al-Zaben, M.I.; Mekhamer, W.K. Removal of 4-chloro-2-methyl phenoxy acetic acid pesticide using coffee wastes from aqueous solution. Arab. J. Chem. 2017, 10, S1523–S1529. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Rhushikesh, A.K.; Mandavgane, A.S. Utilization of cotton plant ash and char for removal of 2, 4-dichlorophenoxyacetic acid. Resour. Effic. Technol. 2016, 2, S39–S46. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Mandavgane, A.S.; Kulkarni, D.B. Mustard plant ash: A source of micronutrient and an adsorbent for removal of 2,4-dichlorophenoxyacetic acid. Environ. Sci. Pollut. Res. 2016, 23, 20087–20099. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Mandavgane, S.A. Utilization of an Agro Waste, Groundnut Shell Ash, for Removal of 2,4-Dichlorophenoxyacetic Acid. In Recent Advances in Chemical Engineering; Springer: Singapore, 2016; pp. 165–173. [Google Scholar]
- Trivedi, N.S.; Kharkar, R.A.; Mandavgane, S.A. 2,4-Dichlorophenoxyacetic acid adsorption on adsorbent prepared from groundnut shell: Effect of preparation conditions on equilibrium adsorption capacity. Arab. J. Chem. 2019, 12, 4541–4549. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Kharkar, R.A.; Mandavgane, S.A. Use of wheat straw combustion residues for removal of chlorinated herbicide (2,4-Dichlorophenoxyacetic acid). Waste Biomass Valorization 2019, 10, 1323–1331. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Taamalli, S.; Louis, F.; El Bakali, A.; Badawi, M.; Georgin, J.; Franco, D.S.P.; Silva, L.F.O.; Bonilla-Petriciolet, A.; et al. Enhanced adsorption of ketoprofen and 2,4-dichlorophenoxyactic acid on Physalis peruviana fruit residue functionalized with H2SO4: Adsorption properties and statistical physics modeling. Chem. Eng. J. 2022, 445, 136773. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Foletto, E.L.; Dotto, G.L. Adsorption investigation of 2,4-D herbicide on acid-treated peanut (Arachis hypogaea) skins. Environ. Sci. Pollut. Res. 2021, 28, 36453–36463. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Silva, L.F.O.; da Boit Martinello, K.; Diel, J.C.; Georgin, J.; Netto, M.S.; Pereira, H.A.; Lima, E.C.; Dotto, G.L. Transforming agricultural waste into adsorbent: Application of Fagopyrum esculentum wheat husks treated with H2SO4 to adsorption of the 2,4-D herbicide. J. Environ. Chem. Eng. 2021, 9, 106872. [Google Scholar] [CrossRef]
- Kani, A.N.; Dovi, E.; Aryee, A.A.; Han, R.; Qu, L. Efficient removal of 2,4-D from solution using a novel antibacterial adsorbent based on tiger nut residues: Adsorption and antibacterial study. Environ. Sci. Pollut. Res. 2022, 29, 64177–64191. [Google Scholar] [CrossRef]
- Bartczak, P.; Żółtowska, S.; Norman, M.; Klapiszewski, Ł.; Zdarta, J.; Komosa, A.; Kitowski, I.; Ciesielczyk, F.; Jesionowski, T. Saw-sedge Cladium mariscus as a functional low-cost adsorbent for effective removal of 2,4-dichlorophenoxyacetic acid from aqueous systems. Adsorption 2016, 22, 517–529. [Google Scholar] [CrossRef]
- Aryee, A.A.; Xiao, Y.; Han, R.; Qu, L. Uptake of 2,4-dichlorophenoxyacetic acid and tetracycline in single and binary systems onto a biomass-MOF composite: Adsorption and mechanism study. Biomass Convers. Bioref. 2023. [Google Scholar] [CrossRef]
- Sayğılı, G.A. Conversion of a renewable bio-resource to a functional composite material: Product design, comprehensive characterization and adsorption of 2,4-D herbicide. Sustain. Chem. Pharm. 2020, 18, 100338. [Google Scholar] [CrossRef]
- Li, X.-G.; Huang, M.-R.; Tao, T.; Ren, Z.; Zeng, J.; Yu, J.; Umeyama, T.; Ohara, T.; Imahori, H. Highly cost-efficient sorption and desorption of mercury ions onto regenerable poly(m-phenylenediamine) microspheres with many active groups. Chem. Eng. J. 2020, 391, 123515. [Google Scholar] [CrossRef]
- Li, X.-G.; Huang, M.-R.; Jiang, Y.-B.; Yu, J.; He, Z. Synthesis of poly(1,5-diaminonaphthalene) microparticles with abundant amino and imino groups as strong adsorbers for heavy metal ions. Microchim. Acta 2019, 186, 208. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Sarkar, B.; Igalavithana, A.D.; Ok, Y.S.; Yang, X.; Lombi, E.; Bolan, N. Mechanistic insights of 2,4-D sorption onto biochar: Influence of feedstock materials and biochar properties. Bioresour. Technol. 2017, 246, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Gulen, J.; Aslan, S. Adsorption of 2,4-dichlorophenoxyacetic acid from aqueous solution using carbonized chest nut as low cost adsorbent: Kinetic and thermodynamic. Z. Phys. Chem. 2020, 234, 461–484. [Google Scholar] [CrossRef]
- Kearns, J.P.; Wellborn, L.S.; Summers, R.S.; Knappe, D.R.U. 2,4-D adsorption to biochars: Effect of preparation conditions on equilibrium adsorption capacity and comparison with commercial activated carbon literature data. Water Res. 2014, 62, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Binh, Q.A.; Nguyen, H.-H. Investigation the isotherm and kinetics of adsorption mechanism of herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) on corn cob biochar. Bioresour. Technol. Rep. 2020, 11, 100520. [Google Scholar] [CrossRef]
- Essandoh, M.; Wolgemuth, D.; Pittman, C.U.; Mohan, D.; Mlsna, T. Phenoxy herbicide removal from aqueous solutions using fast pyrolysis switchgrass biochar. Chemosphere 2017, 174, 49–57. [Google Scholar] [CrossRef]
- Sigmund, G.; Sun, H.; Hofmann, T.; Kah, M. Predicting the sorption of aromatic acids to noncarbonized and carbonized sorbents. Environ. Sci. Technol. 2016, 50, 3641–3648. [Google Scholar] [CrossRef]
- Cederlund, H.; Borjesson, E.; Lundberg, D.; Stenstrom, J. Adsorption of pesticides with different chemical properties to a wood biochar treated with heat and iron. Water Air Soil Pollut. 2016, 227, 203. [Google Scholar] [CrossRef]
- Cederlund, H.; Borjesson, E.; Stenstrom, J. Effects of a wood-based biochar on the leaching of pesticides chlorpyrifos, diuron, glyphosate and MCPA. J. Environ. Manag. 2017, 191, 28–34. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Wan, Z.; Jing, F.; Li, Z.; Chen, J.; Tsang, D.C.W. Tailored design of food waste hydrochar for efficient adsorption and catalytic degradation of refractory organic contaminant. J. Clean. Prod. 2021, 310, 127482. [Google Scholar] [CrossRef]
- Gurav, R.; Mandal, S.; Smith, L.M.; Shi, S.Q.; Hwang, S. The potential of self-activated carbon for adsorptive removal of toxic phenoxyacetic acid herbicide from water. Chemosphere 2023, 339, 139715. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Fan, J.; Cui, X.; Wang, Y.; Yan, Y.; Meng, Z.; Gao, H.; Lu, R.; Zhou, W. Pyrolyzing spent coffee ground to biochar treated with H3PO4 for the efficient removal of 2,4-dichlorophenoxyacetic acid herbicide: Adsorptive behaviors and mechanism. J. Environ. Chem. Eng. 2023, 11, 109165. [Google Scholar] [CrossRef]
- Almahri, A.; Abou-Melha, K.S.; Katouah, H.A.; Al-bonayan, A.M.; Saad, F.A.; El-Desouky, M.G.; El-Bindary, A.A. Adsorption and removal of the harmful pesticide 2,4-dichlorophenylacetic acid from an aqueous environment via coffee waste biochar: Synthesis, characterization, adsorption study and optimization via Box-Behnken design. J. Mol. Struct. 2023, 1293, 136238. [Google Scholar] [CrossRef]
- Beigzadeh, B.; Bahrami, M.; Amiri, M.J.; Mahmoudi, M.R. A new approach in adsorption modeling using random forest regression, Bayesian multiple linear regression, and multiple linear regression: 2,4-D adsorption by a green adsorbent. Water Sci. Technol. 2020, 82, 1586–1602. [Google Scholar] [CrossRef]
- Amiri, M.J.; Bahrami, M.; Beigzadeh, B.; Gil, A. A response surface methodology for optimization of 2,4-dichlorophenoxyacetic acid removal from synthetic and drainage water: A comparative study. Environ. Sci. Pollut. Res. 2018, 25, 34277–34293. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, N.; Tong, L.; Lv, Y.; Li, G. Characterization and evaluation of surface modified materials based on porous biochar and its adsorption properties for 2,4-dichlorophenoxyacetic acid. Chemosphere 2018, 210, 734–744. [Google Scholar] [CrossRef]
- Kearns, J.; Shimabuku, K.; Knappe, D.; Summers, R. High Temperature Co-pyrolysis Thermal Air Activation Enhances Biochar Adsorption of Herbicides from Surface Water. Environ. Eng. Sci. 2019, 36, 710–723. [Google Scholar] [CrossRef]
- Kearns, J.P.; Knappe, D.R.U.; Summers, R.S. Feasibility of Using Traditional Kiln Charcoals in Low-Cost Water Treatment: Role of Pyrolysis Conditions on 2,4-D Herbicide Adsorption. Environ. Eng. Sci. 2015, 32, 912–921. [Google Scholar] [CrossRef]
- Vimal, V. Aqueous removal of carbofuran and 2,4-D using sustainable biochars developed from agricultural byproducts. Creat. Crit. 2019, 9, 28–37. [Google Scholar]
- Ćwieląg-Piasecka, I.; Medyńska-Juraszek, A.; Jerzykiewicz, M.; Dębicka, M.; Bekier, J.; Jamroz, E.; Kawałko, D. Humic acid and biochar as specific sorbents of pesticides. J. Soils Sediments 2018, 18, 2692–2702. [Google Scholar] [CrossRef]
- Lü, J.; Li, J.; Li, Y.; Chen, B.; Bao, Z. Use of Rice Straw Biochar Simultaneously as the Sustained Release Carrier of Herbicides and Soil Amendment for Their Reduced Leaching. J. Agric. Food Chem. 2012, 60, 6463–6470. [Google Scholar] [CrossRef] [PubMed]
- Bojic, D.V.; Kostic, M.M.; Vucic, M.D.R.; Velinov, N.D.; Najdanovic, S.M.; Petrovic, M.M.; Bojic, A.L. Removal of the herbicide 2,4-dichlorophenoxyacetic acid from water by using an ultrahighly efficient thermochemically activated carbon. Hem. Ind. 2019, 73, 223–237. [Google Scholar] [CrossRef]
- Brito, G.M.; Roldi, L.L.; Schetino, M.A.; Freitas, J.C.C.; Coelho, E.R.C. High-performance of activated biocarbon based on agricultural biomass waste applied for 2,4-D herbicide removing from water: Adsorption, kinetic and thermodynamic assessments. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2020, 55, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.J.; Roohi, R.; Arshadi, M.; Abbaspourrad, A. 2,4-D adsorption from agricultural subsurface drainage by canola stalk-derived activated carbon: Insight into the adsorption kinetics models under batch and column conditions. Environ. Sci. Pollut. Res. 2020, 27, 16983–16997. [Google Scholar] [CrossRef]
- Orduz, A.E.; Acebal, C.; Zanini, G. Activated carbon from peanut shells: 2,4-D desorption kinetics study for application as a green material for analytical purposes. J. Environ. Chem. Eng. 2021, 9, 104601. [Google Scholar] [CrossRef]
- Cansado, I.P.d.P.; Belo, C.R.; Mourão, P.A.M. Valorisation of Tectona Grandis tree sawdust through the production of high activated carbon for environment applications. Bioresour. Technol. 2018, 249, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Tchikuala, E.F.; Mourão, P.A.M.; Nabais, J.M.V. Activated Carbons from Angolan Wood Wastes for the Adsorption of MCPA Pesticide. In WASTES–Solutions, Treatments and Opportunities II, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–6. [Google Scholar]
- Blachnio, M.; Derylo-Marczewska, A.; Charmas, B.; Zienkiewicz-Strzalka, M.; Bogatyrov, V.; Galaburda, M. Activated Carbon from Agricultural Wastes for Adsorption of Organic Pollutants. Molecules 2020, 25, 5105. [Google Scholar] [CrossRef]
- Lim, K.Y.; Foo, K.Y. One-step synthesis of carbonaceous adsorbent from soybean bio-residue by microwave heating: Adsorptive, antimicrobial and antifungal behavior. Environ. Res. 2022, 204, 112044. [Google Scholar] [CrossRef]
- Kirbiyik, C.; Putun, A.E.; Putun, E. Equilibrium, kinetic, and thermodynamic studies of the adsorption of Fe(III) metal ions and 2,4-dichlorophenoxyacetic acid onto biomass-based activated carbon by ZnCl2 activation. Surf. Interfaces 2017, 8, 182–192. [Google Scholar] [CrossRef]
- Angin, D.; Ilci, A. Removal of 2,4-dichlorophenoxy acetic acid from aqueous solutions by using activated carbon derived from olive-waste cake. Desalination Water Treat. 2017, 82, 282–291. [Google Scholar] [CrossRef]
- Salomon, Y.L.D.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. High-performance removal of 2,4-dichlorophenoxyacetic acid herbicide in water using activated carbon derived from Queen palm fruit endocarp (Syagrus romanzoffiana). J. Environ. Chem. Eng. 2021, 9, 104911. [Google Scholar] [CrossRef]
- Angin, D.; Gunes, S. The usage of orange pulp activated carbon in the adsorption of 2,4-dichlorophenoxy acetic acid from aqueous solutions. Int. J. Phytoremediation 2021, 23, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Boumaraf, R.; Khettaf, S.; Benmahdi, F.; Masmoudi, R.; Ferhati, A. Removal of 2,4-dichlorophenoxyacetic acid from aqueous solutions by nanofiltration and activated carbon. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; da Boit Martinello, K.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Silva, L.F.O.; Lima, E.C.; Dotto, G.L. Preparation of activated carbon from the residues of the mushroom (Agaricus bisporus) production chain for the adsorption of the 2,4-dichlorophenoxyacetic herbicide. J. Environ. Chem. Eng. 2021, 9, 106843. [Google Scholar] [CrossRef]
- Daiem, M.M.A.; Sanchez-Polo, M.; Rashed, A.S.; Kamal, N.; Said, N. Adsorption mechanism and modelling of hydrocarbon contaminants onto rice straw activated carbons. Pol. J. Chem. Technol. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Mohd Noor Hazrin, H.M.; Lim, A.; Li, C.; Chew, J.J.; Sunarso, J. Adsorption of 2,4-dichlorophenoxyacetic acid onto oil palm trunk-derived activated carbon: Isotherm and kinetic studies at acidic, ambient condition. Mater. Today Proc. 2022, 64, 1557–1562. [Google Scholar] [CrossRef]
- Fatombi, J.K.; Agani, I.; Osseni, S.A.; Idohou, E.A.; Neumeyer, D.; Verelst, M.; Mauricot, R.; Aminou, T. Influence of salts and humic acid on 2,4-dichlorophenoxyacetic acid removing from aqueous solution by peanut shell activated carbon. Desalination Water Treat. 2020, 189, 250–263. [Google Scholar] [CrossRef]
- Samanth, A.; Vinayagam, R.; Murugesan, G.; Varadavenkatesan, T.; Selvaraj, R.; Pugazhendhi, A. Enhanced adsorption of 2,4-dichlorophenoxyacetic acid using low-temperature carbonized Peltophorum pterocarpum pods and its statistical physics modeling. Chemosphere 2023, 336, 139143. [Google Scholar] [CrossRef]
- Naboulsi, A.; El Himri, M.; El Haddad, M. Adsorption mechanism of a pesticide/herbicide mixture on a new eco-friendly activated carbon of Ritama-monosperma (L.) Boiss used DFT/B3LYP. Biomass Convers. Bioref. 2023, 15, 13715–13728. [Google Scholar] [CrossRef]
- Doczekalska, B.; Kusmierek, K.; Swiatkowski, A.; Bartkowiak, M. Adsorption of 2,4-dichlorophenoxyacetic acid and 4-chloro-2-metylphenoxyacetic acid onto activated carbons derived from various lignocellulosic materials. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2018, 53, 290–297. [Google Scholar] [CrossRef]
- Angin, D.; Gunes, S.; Ates, A.; Selengil, U.; Altıntıg, E.; Tan, B.; Demirel, H. Effect of activated carbon produced from biochar on removal of 2,4-dichlorophenoxy acetic acid from aqueous solutions. Indian J. Chem. Technol. 2021, 28, 701–708. [Google Scholar]
- Koyuncu, F.; Guzel, F.; Inal, I.I.G. High surface area and supermicroporous activated carbon from capsicum (Capsicum annuum L.) industrial processing pulp via single-step KOH-catalyzed pyrolysis: Production optimization, characterization and its some water pollutants removal and supercapacitor performance. Diam. Relat. Mater. 2022, 124, 108920. [Google Scholar] [CrossRef]
- Rambabu, K.; AlYammahi, J.; Bharath, G.; Thanigaivelan, A.; Sivarajasekar, N.; Banat, F. Nano-activated carbon derived from date palm coir waste for efficient sequestration of noxious 2,4-dichlorophenoxyacetic acid herbicide. Chemosphere 2021, 282, 131103. [Google Scholar] [CrossRef] [PubMed]
- Pandiarajan, A.; Kamaraj, R.; Vasudevan, S.; Vasudevan, S. OPAC (orange peel activated carbon) derived from waste orange peel for the adsorption of chlorophenoxyacetic acid herbicides from water: Adsorption isotherm, kinetic modelling and thermodynamic studies. Bioresour. Technol. 2018, 261, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Tefera, M.; Tulu, M. Preparation and characterization of activated carbon from wheat straw to remove 2,4-dichlorophenoxy acetic acid from aqueous solutions. Curr. Chem. Lett. 2021, 10, 175–186. [Google Scholar] [CrossRef]
- Herrera-Garcia, U.; Castillo, J.; Patino-Ruiz, D.; Solano, R.; Herrera, A. Activated carbon from yam peels modified with Fe3O4 for removal of 2,4-dichlorophenoxyacetic acid in aqueous solution. Water 2019, 11, 2342. [Google Scholar] [CrossRef]
- Salman, J.M. Optimization of preparation conditions for activated carbon from palm oil fronds using response surface methodology on removal of pesticides from aqueous solution. Arab. J. Chem. 2014, 7, 101–108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).