A Study on the Hydrothermal Synthesis of Calcium Silicate Products by Calcination of Full-Component Waste Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Composition
2.2. Materials Activation
2.3. Samples and Preparation
2.4. Strength Gained and Water Resistance
2.5. XRD Analysis
2.6. Microstructural Analysis
3. Results
3.1. Materials Activation
3.2. Compressive Strength
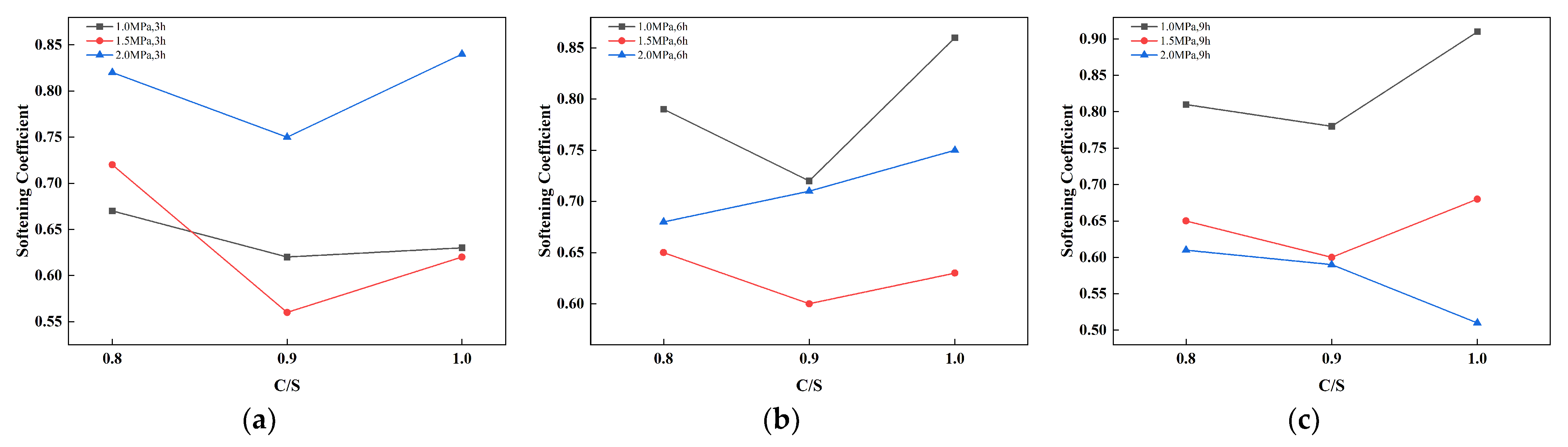
3.3. Water Resistance Analysis
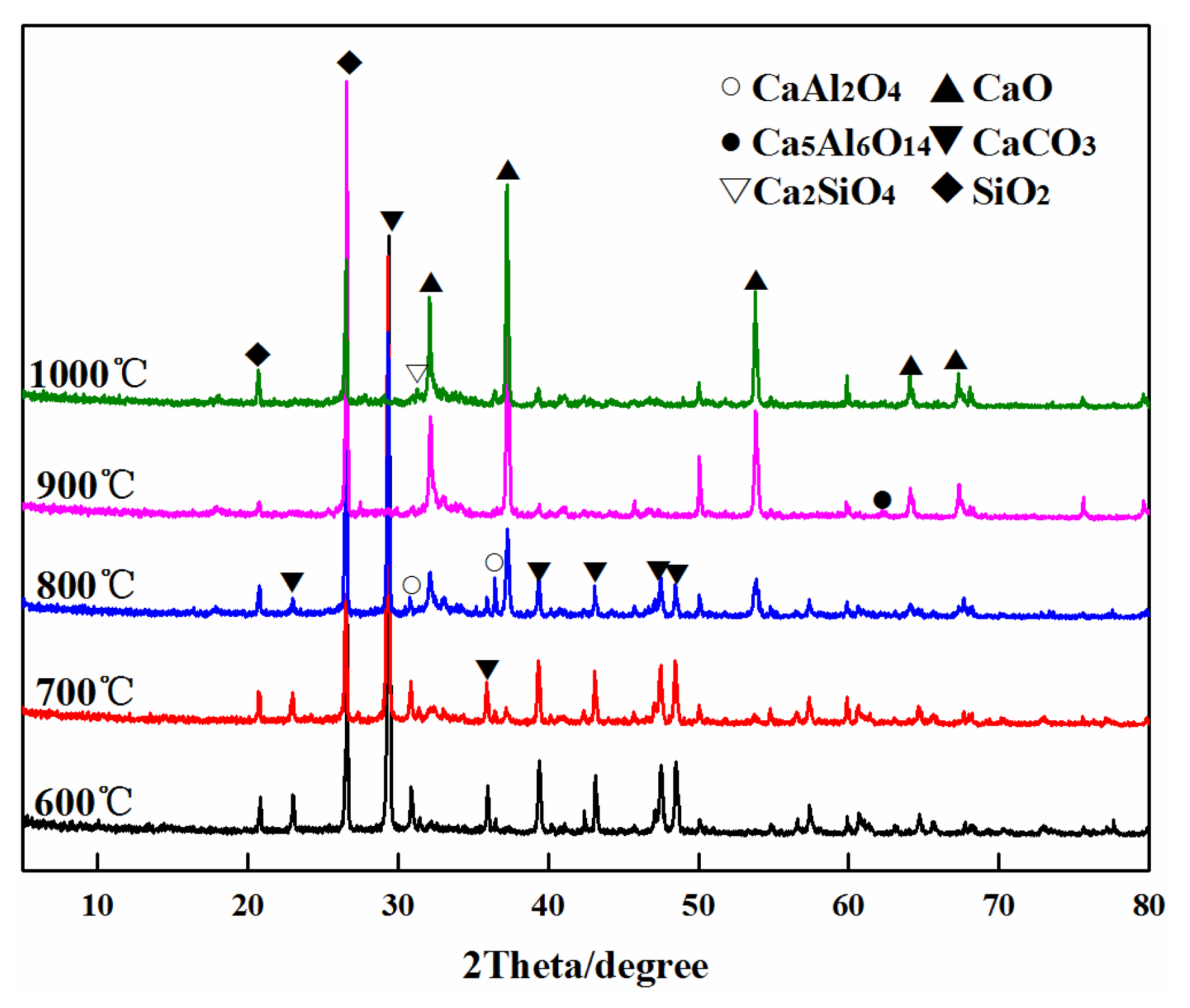
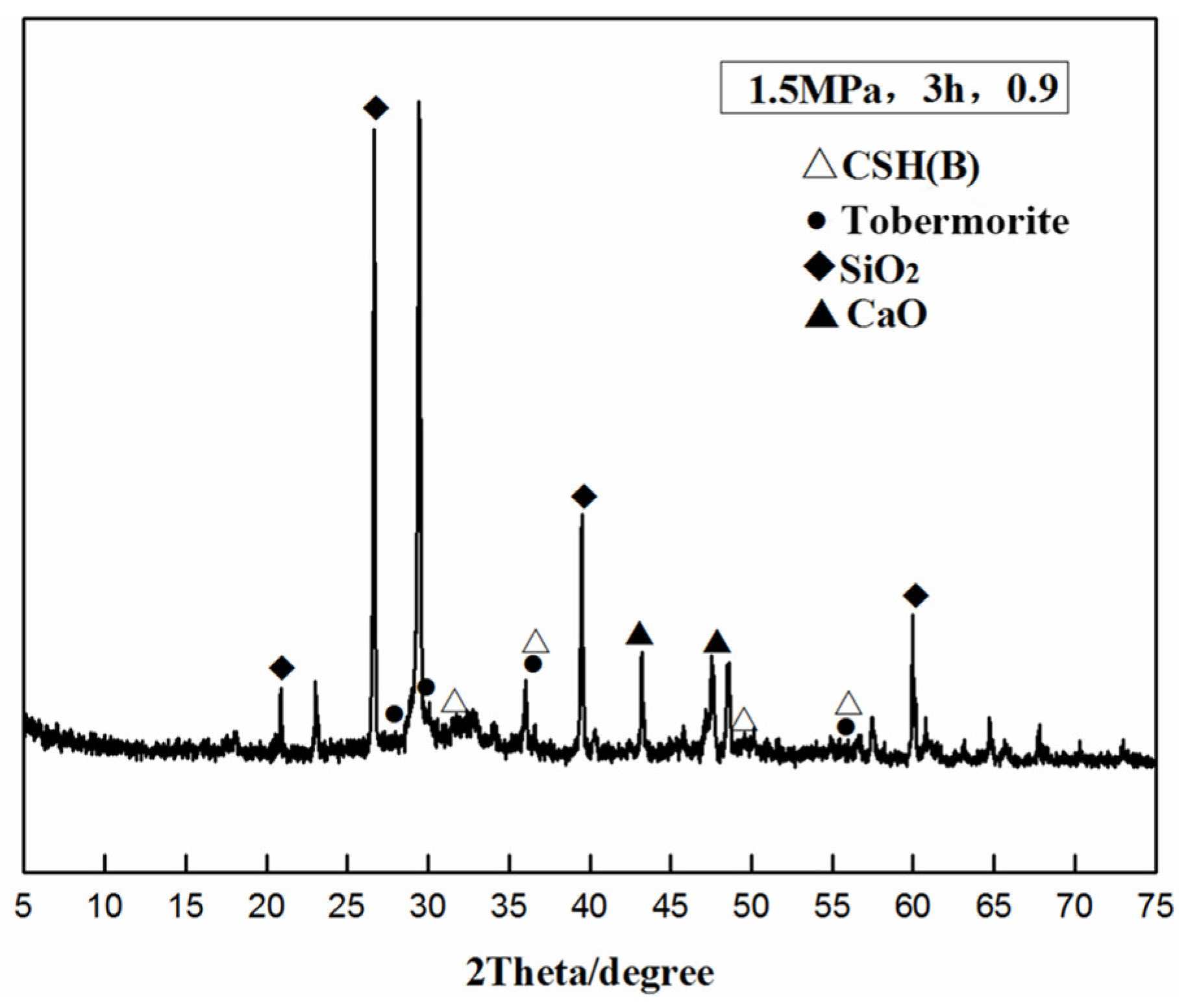
3.4. XRD Phase Analysis
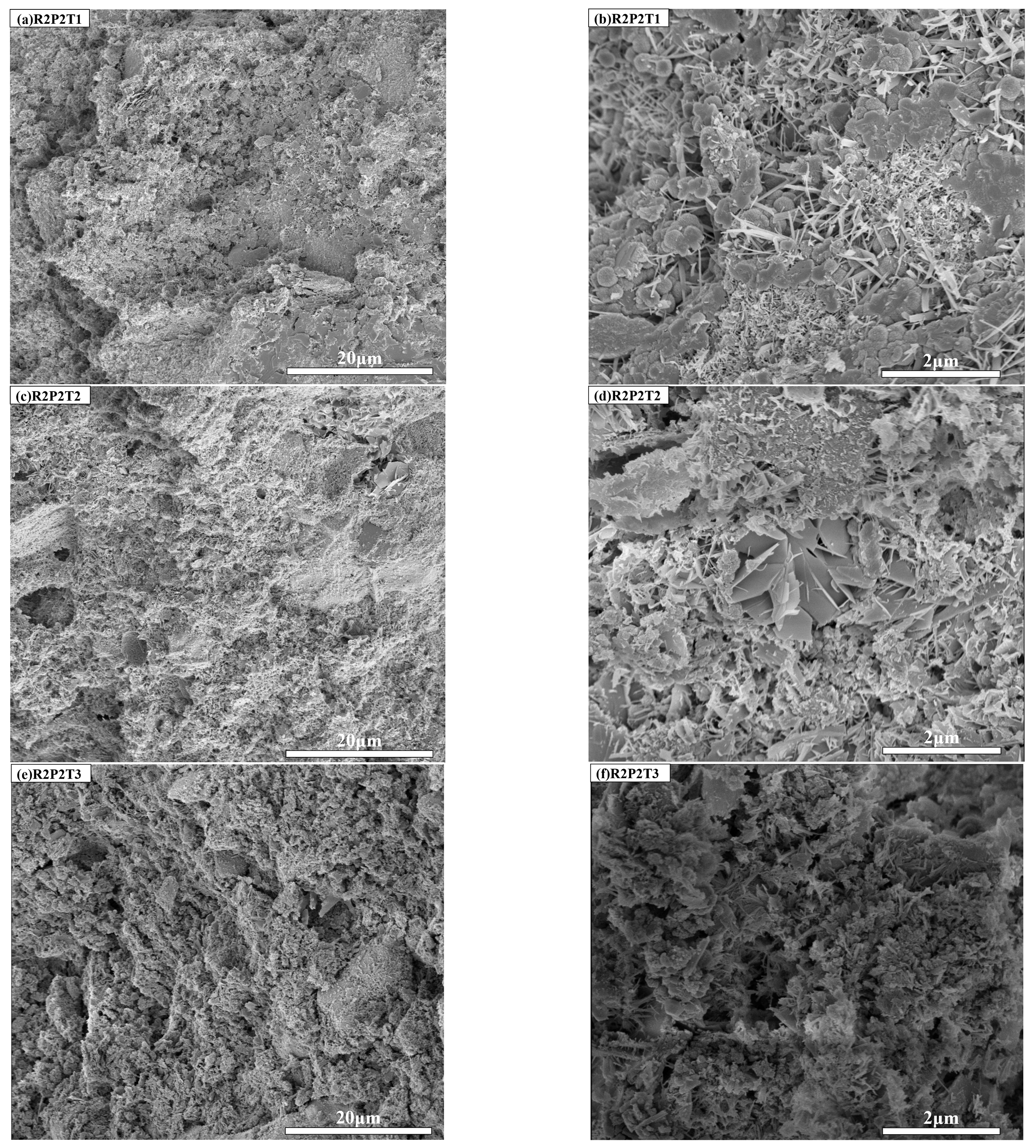
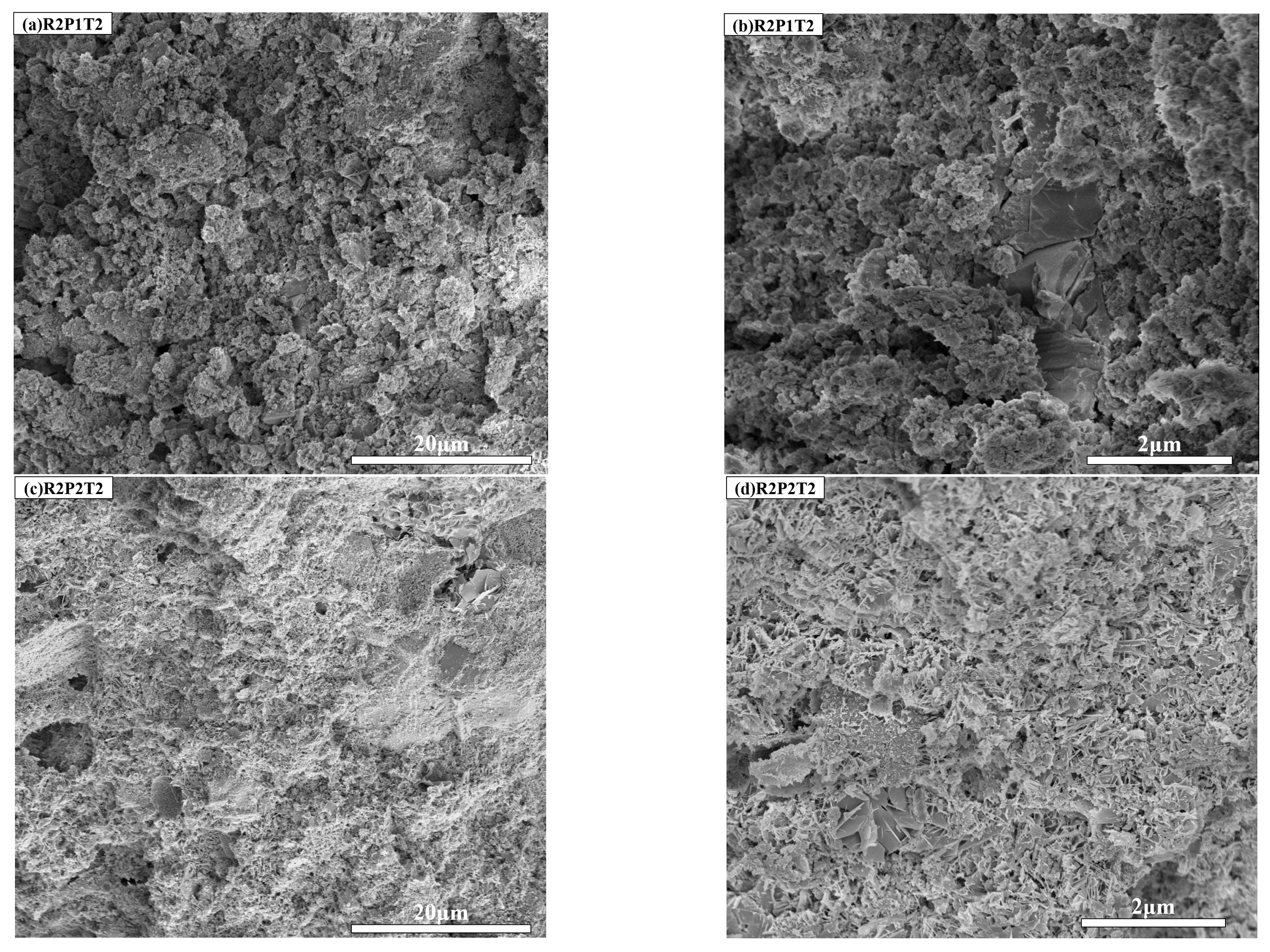
3.5. SEM Analysis
3.5.1. Influence of Steaming Holding Time
3.5.2. Influence of Autoclave Pressure
3.5.3. Influence of Calcium–Silica Ratio
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Zhang, Y.X. Recycling fresh concrete waste: A review. Struct. Concr. 2018, 19, 1939–1955. [Google Scholar] [CrossRef]
- Hao, J.L.; Yu, S.; Tang, X.; Wu, W. Determinants of workers’ pro-environmental behaviour towards enhancing construction waste management: Contributing to China’s circular economy. J. Clean. Prod. 2022, 369, 133265. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, Z.; Ding, T. Reclamation chain of waste concrete: A case study of Shanghai. Waste Manag. 2016, 48, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Zhong, X.; Guo, S.; Liu, G.; Shen, G.Q.; Yu, T. Water-energy nexus and its efficiency in China’s construction industry: Evidence from province-level data. Sustain. Cities Soc. 2019, 48, 101557. [Google Scholar] [CrossRef]
- Jebelli, H.; Choi, B.; Lee, S. Application of Wearable Biosensors to Construction Sites. I: Assessing Workers’ Stress. J. Constr. Eng. Manag. 2019, 145, 04019079. [Google Scholar] [CrossRef]
- Du, Q.; Zhao, L.; Zhao, L.; Guo, Y. Technical and economic feasibility of construction and demolition waste used in road construction. In Proceedings of the 2011 International Symposium on Water Resource and Environmental Protection (ISWREP), Xi’an, China, 20–22 May 2011; Wang, W., Ed.; IEEE: Piscataway, NJ, USA, 2011; p. 8, ISBN 978-1-61284-339-1. [Google Scholar]
- Wang, Y.; Zheng, J.; You, F. Review on Enhancement Methods of Recycled Aggregate. Mater. Rev. 2021, 35, 5053–5061. [Google Scholar]
- Kim, J. Influence of quality of recycled aggregates on the mechanical properties of recycled aggregate concretes: An overview. Constr. Build. Mater. 2022, 328, 127071. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Huang, Q.; Shui, Z.; Liu, Y.; Zhao, H.; Zhang, Z. Basic performance, heavy metal leaching mechanism and risk assessment analysis of waste concrete. Arch. Civ. Mech. Eng. 2023, 23, 1–24. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, Z.; Wu, H.; Wang, W. The utilization of eco-friendly recycled powder from concrete and brick waste in new concrete: A critical review. Cem. Concr. Compos. 2020, 114, 103807. [Google Scholar] [CrossRef]
- Shandilya, M.; Rai, R.; Singh, J. Review: Hydrothermal technology for smart materials. Adv. Appl. Ceram. 2016, 115, 354–376. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Z.; Xu, X.; Cui, C.; Zhang, S.; Ding, X. Research Status of Structures, Thermal Behavior and Synthesis of Tobermorite. J. Chin. Ceram. Soc. 2020, 48, 1536–1551. [Google Scholar]
- Yang, X.; Cui, X.; Cui, C.; Ma, H.; Yang, Q. Study on High-Temperature Phase Change of Tobermorite. Spectrosc. Spectr. Anal. 2013, 33, 2227–2230. [Google Scholar]
- Bao, M.; Guo, X.; Shi, H. Research progress on hydrothermal synthesis of tobermorite whisker and its high temperature resistance. J. Funct. Mater. 2017, 48, 11075–11080, 11085. [Google Scholar]
- Galvankova, L.; Masilko, J.; Solny, T.; Stepankova, E. Tobermorite synthesis under hydrothermal conditions. Procedia Eng. 2016, 151, 100–107. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Xu, L.; Zhu, Z.; Zhou, Y.; Pan, F.; Wu, K. Insight into the local C-S-H structure and its evolution mechanism controlled by curing regime and Ca/Si ratio. Constr. Build. Mater. 2022, 333, 127388. [Google Scholar] [CrossRef]
- Yi, L.; Wang, H.; Wang, X.; Peng, J. Research Progress of Utilizing Fly Ash as Resource of Building Material. Bull. Chin. Ceram. Soc. 2012, 31, 88–91. [Google Scholar]
- Qian, J.; Ji, X.; Fan, Y.; Wang, Z. The Formation and Effect of Calcic Minerals in Fly Ash. J. Chongqing Jianzhu Univ. 2008, 30, 148–152. [Google Scholar]
- Wang, Y.; Burris, L.; Hooton, R.D.; Shearer, C.R.; Suraneni, P. Effects of unconventional fly ashes on cementitious paste properties. Cem. Concr. Compos. 2022, 125, 104291. [Google Scholar] [CrossRef]
- Liu, B.; Yang, L.; Shi, J.; Zhang, S.; Yalcinkaya, C.; Alshalif, A.F. Effect of curing regime on the immobilization of municipal solid waste incineration fly ash in sustainable cement mortar. Environ. Pollut. 2023, 317, 120839. [Google Scholar] [CrossRef]
- GB/T4111-2013; Test Methods for the Concrete Block and Brick. National Standards of People’s Republic of China: Beijing, China, 2013.
- Calvo, E.G.; Arranz, M.A.; Leton, P. Effects of impurities in the kinetics of calcite decomposition. Thermochim. Acta 1990, 170, 7–11. [Google Scholar] [CrossRef]
- Liu, C.; Han, K.; Wang, D. An In Situ Decarbonation Kinetic Study of Calcite Using Synchrotron Radiation XRD. Can. Mineral. 2022, 60, 687–698. [Google Scholar] [CrossRef]
- Myers, R.; L’Hôpital, E.; Provis, J.; Lothenbach, B. Effect of temperature and aluminium on calcium (alumino)silicate hydrate chemistry under equilibrium conditions. Cem. Concr. Res. 2015, 68, 83–93. [Google Scholar] [CrossRef]
- Palubinskaite, D.; Kantautas, A. Influence of tribomechanical milling and activation of primary mixtures on the synthesis of calcium silicate hydrates. Mater. Sci.-Pol. 2006, 24, 395–403. [Google Scholar]
- Mostafa, N.Y.; Shaltout, A.A.; Omar, H.; Abo-El-Enein, S.A. Hydrothermal synthesis and characterization of aluminium and sulfate substituted 1.1nm tobermorites. J. Alloys Compd. 2009, 467, 332–337. [Google Scholar] [CrossRef]
- Tränkle, S.; Jahn, D.; Neumann, T.; Nicoleau, L.; Hüsing, N.; Volkmer, D. Conventional and microwave assisted hydrothermal syntheses of 11 Å tobermorite. J. Mater. Chem. A 2013, 1, 10318–10326. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, D.; Tan, S. Novel hydrothermal synthesis of tobermorite fibers using Ca(II)-EDTA complex precursor. J. Eur. Ceram. Soc. 2003, 23, 123–126. [Google Scholar] [CrossRef]
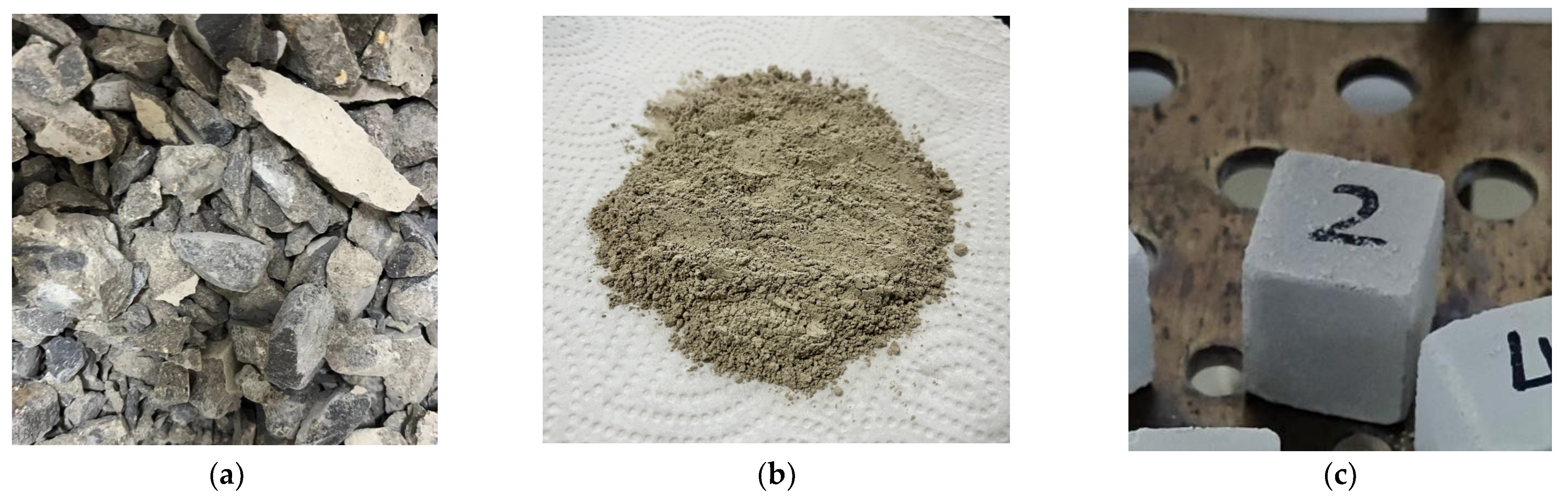



| Components | Cement | Crushed Stone | River Sand | Water |
|---|---|---|---|---|
| Weight (Kg) | 440 | 1108 | 638 | 215 |
| Matching ratio | 1 | 2.52 | 1.45 | 0.49 |
| Basic material parameters | P.O 42.5 | Dmax = 20 mm | — | — |
| Components | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | K2O | SrO | LOI |
|---|---|---|---|---|---|---|---|---|---|
| WC | 47.39 | 19.02 | 1.61 | 0.97 | 1.27 | 0.78 | 0.49 | 0.10 | 28.24 |
| Fly ash | 9.8 | 51.49 | 24.36 | 5.49 | 1.2 | 2.14 | \ | \ | 2.34 |
| C/S | 1.0 MPa | 1.5 MPa | 2.0 MPa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 h | 6 h | 9 h | 3 h | 6 h | 9 h | 3 h | 6 h | 9 h | |
| 0.8 | R1P1T1 | R1P1T2 | R1P1T3 | R1P2T1 | R1P2T2 | R1P2T3 | R1P3T1 | R1P3T2 | R1P3T3 |
| 0.9 | R2P1T1 | R2P1T2 | R2P1T3 | R2P2T1 | R2P2T2 | R2P2T3 | R2P3T1 | R2P3T2 | R2P3T3 |
| 1.0 | R3P1T1 | R3P1T2 | R3P1T3 | R3P2T1 | R3P2T2 | R3P2T3 | R3P3T1 | R3P3T2 | R3P3T3 |
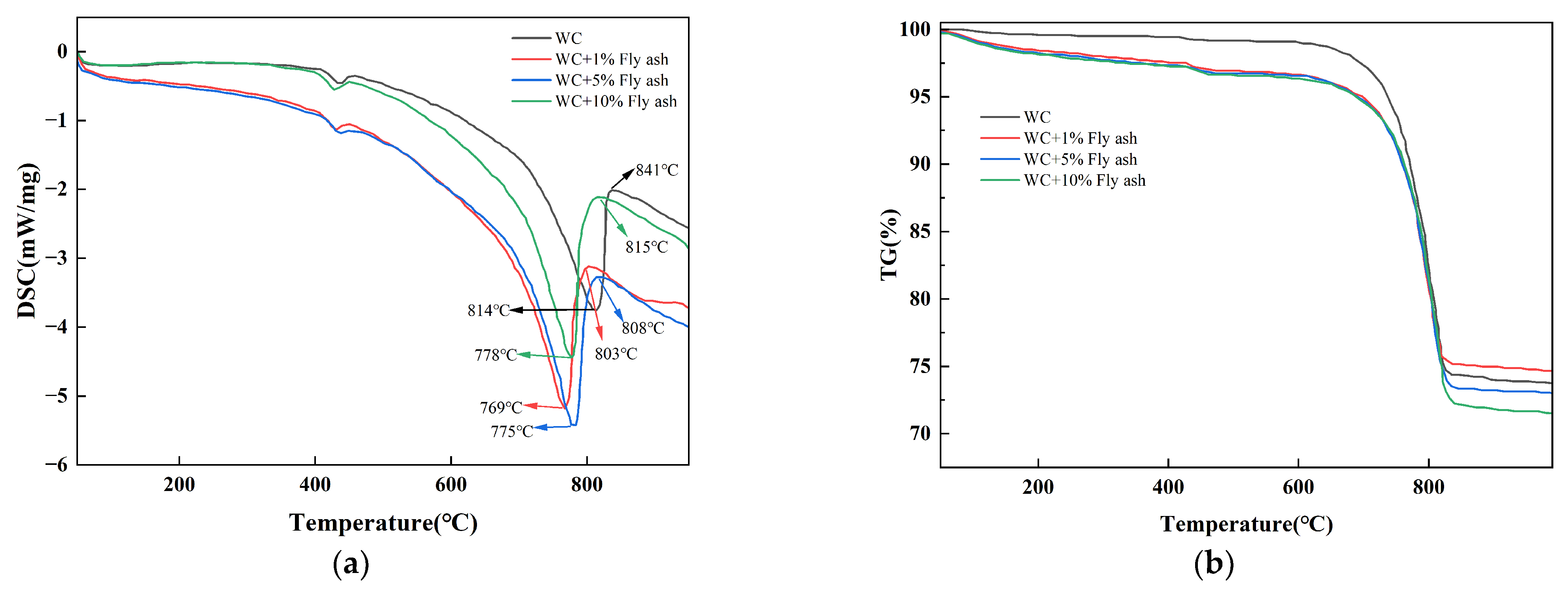
| Components | Starting Temperature (°C) | Peak Temperature (°C) | End Temperature (°C) |
|---|---|---|---|
| WC | 647 | 814 | 841 |
| WC + 1% Fly ash | 616 | 769 | 803 |
| WC + 5% Fly ash | 602 | 775 | 808 |
| WC + 10% Fly ash | 604 | 778 | 815 |
| C/S | 1.0 Mpa | 1.5 Mpa | 2.0 Mpa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 h | 6 h | 9 h | 3 h | 6 h | 9 h | 3 h | 6 h | 9 h | |
| 0.8 | 18.62 | 23.77 | 34.02 | 28.92 | 20.62 | 22.13 | 33.59 | 27.04 | 21.71 |
| 0.9 | 26.91 | 28.11 | 34.64 | 42.53 | 22.81 | 25.89 | 33.01 | 31.78 | 23.63 |
| 1.0 | 31.94 | 33.39 | 32.69 | 38.74 | 23.17 | 34.62 | 43.98 | 36.67 | 26.51 |
| C/S | 1.0 Mpa | 1.5 Mpa | 2.0 Mpa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 h | 6 h | 9 h | 3 h | 6 h | 9 h | 3 h | 6 h | 9 h | |
| 0.8 | 0.67 | 0.79 | 0.81 | 0.72 | 0.65 | 0.65 | 0.82 | 0.68 | 0.61 |
| 0.9 | 0.62 | 0.72 | 0.78 | 0.56 | 0.6 | 0.6 | 0.75 | 0.71 | 0.59 |
| 1.0 | 0.63 | 0.86 | 0.91 | 0.62 | 0.63 | 0.68 | 0.84 | 0.75 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, F.; Ai, H. A Study on the Hydrothermal Synthesis of Calcium Silicate Products by Calcination of Full-Component Waste Concrete. Sustainability 2023, 15, 16341. https://doi.org/10.3390/su152316341
Mao F, Ai H. A Study on the Hydrothermal Synthesis of Calcium Silicate Products by Calcination of Full-Component Waste Concrete. Sustainability. 2023; 15(23):16341. https://doi.org/10.3390/su152316341
Chicago/Turabian StyleMao, Famao, and Hongmei Ai. 2023. "A Study on the Hydrothermal Synthesis of Calcium Silicate Products by Calcination of Full-Component Waste Concrete" Sustainability 15, no. 23: 16341. https://doi.org/10.3390/su152316341
APA StyleMao, F., & Ai, H. (2023). A Study on the Hydrothermal Synthesis of Calcium Silicate Products by Calcination of Full-Component Waste Concrete. Sustainability, 15(23), 16341. https://doi.org/10.3390/su152316341





