Sustainable Use of Organic Seaweed Fertilizer Improves the Metagenomic Function of Microbial Communities in the Soil of Rice Plants
Abstract
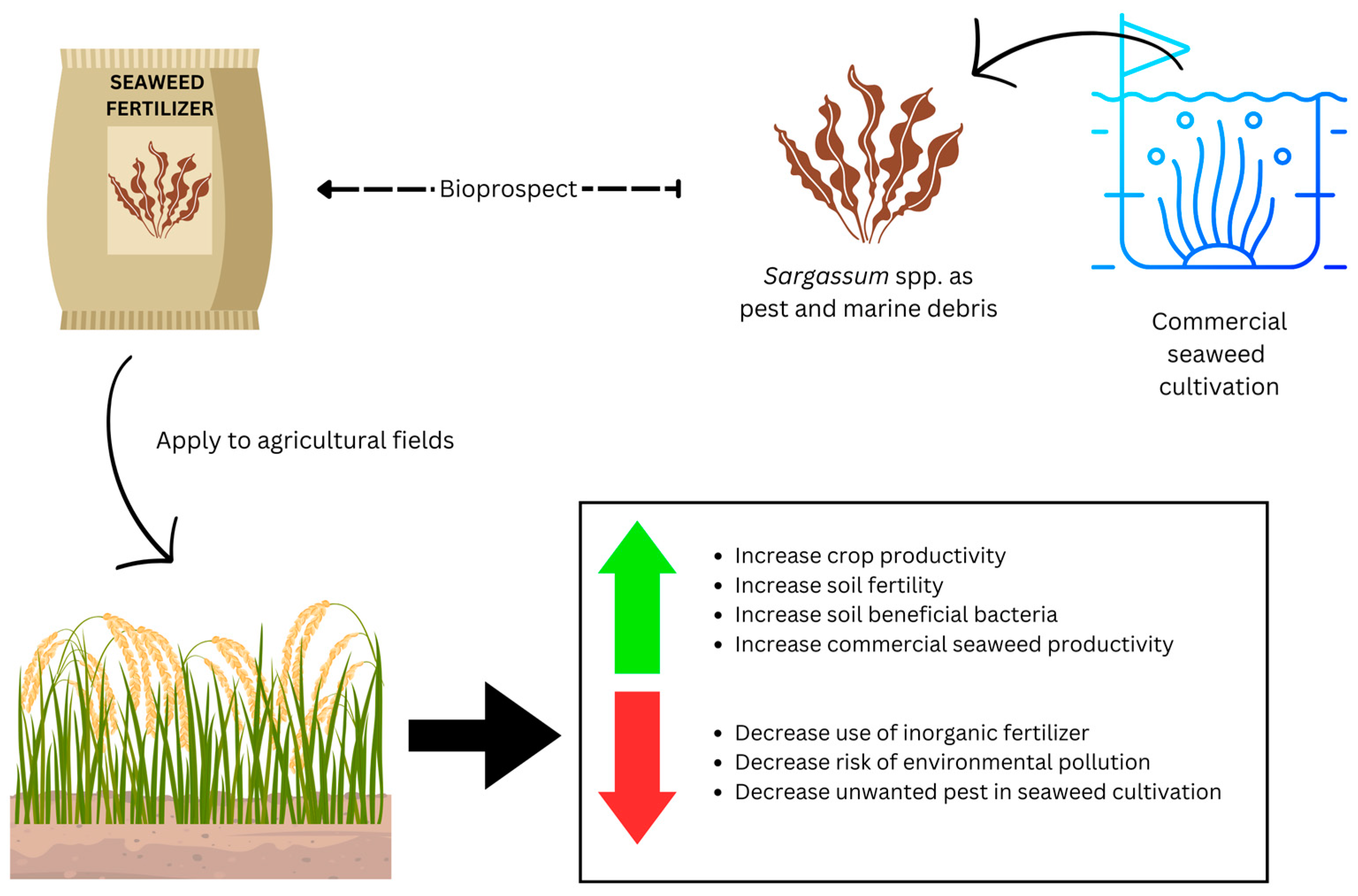
1. Introduction
2. Materials and Methods
2.1. Field Experimental Design
2.2. Determination of Rice Productivity
2.3. Determination of Soil Macronutrient Properties
2.4. Soil DNA Isolation and 16S rRNA Gene Amplification
2.5. Taxonomic Identification and Prediction of Functional Genes
2.6. Statistical Analyses
3. Results and Discussion
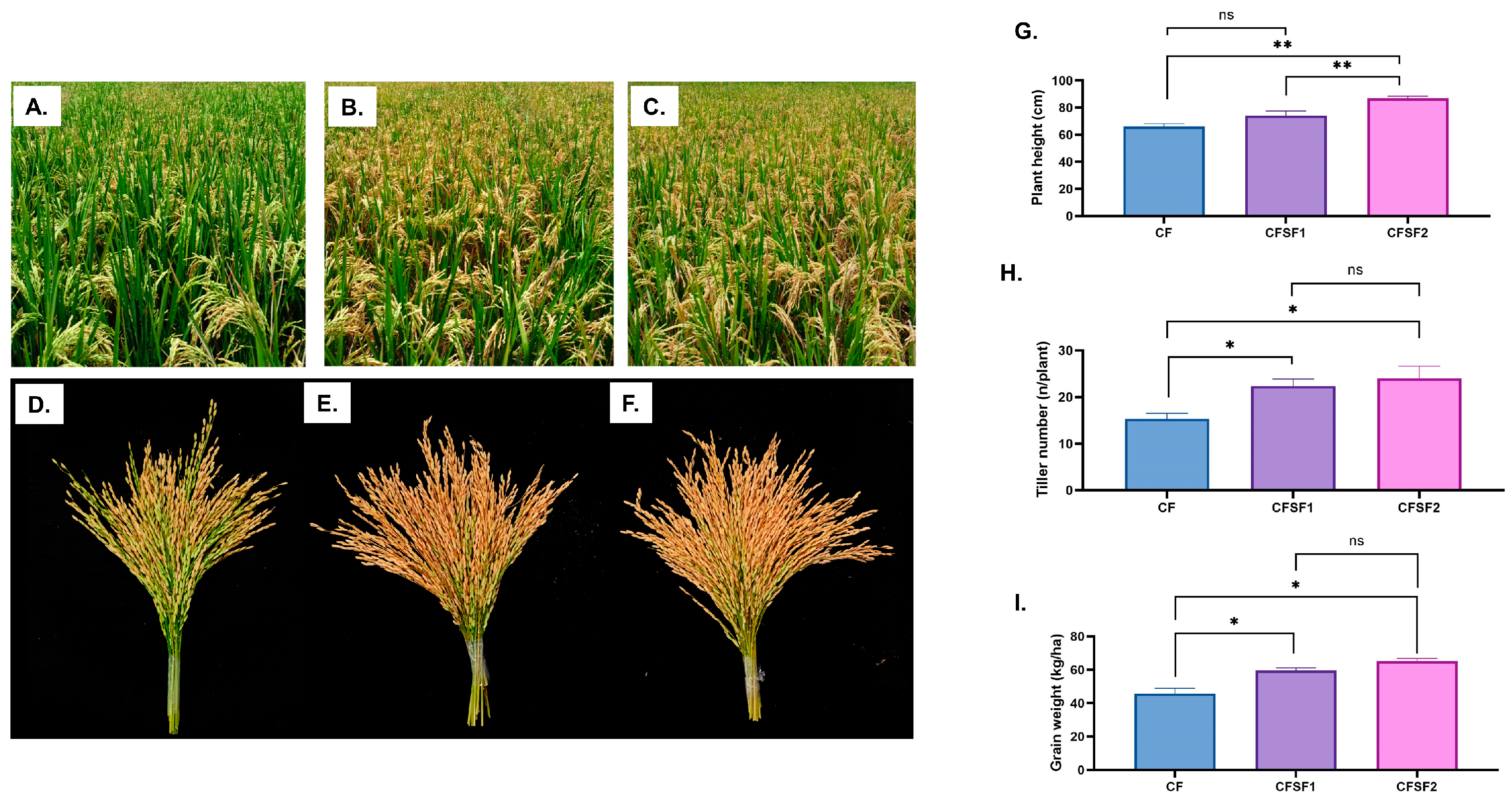
3.1. Effect of SF Supplementation on Rice Plant Growth Field Scale
3.2. Soil Chemical Properties
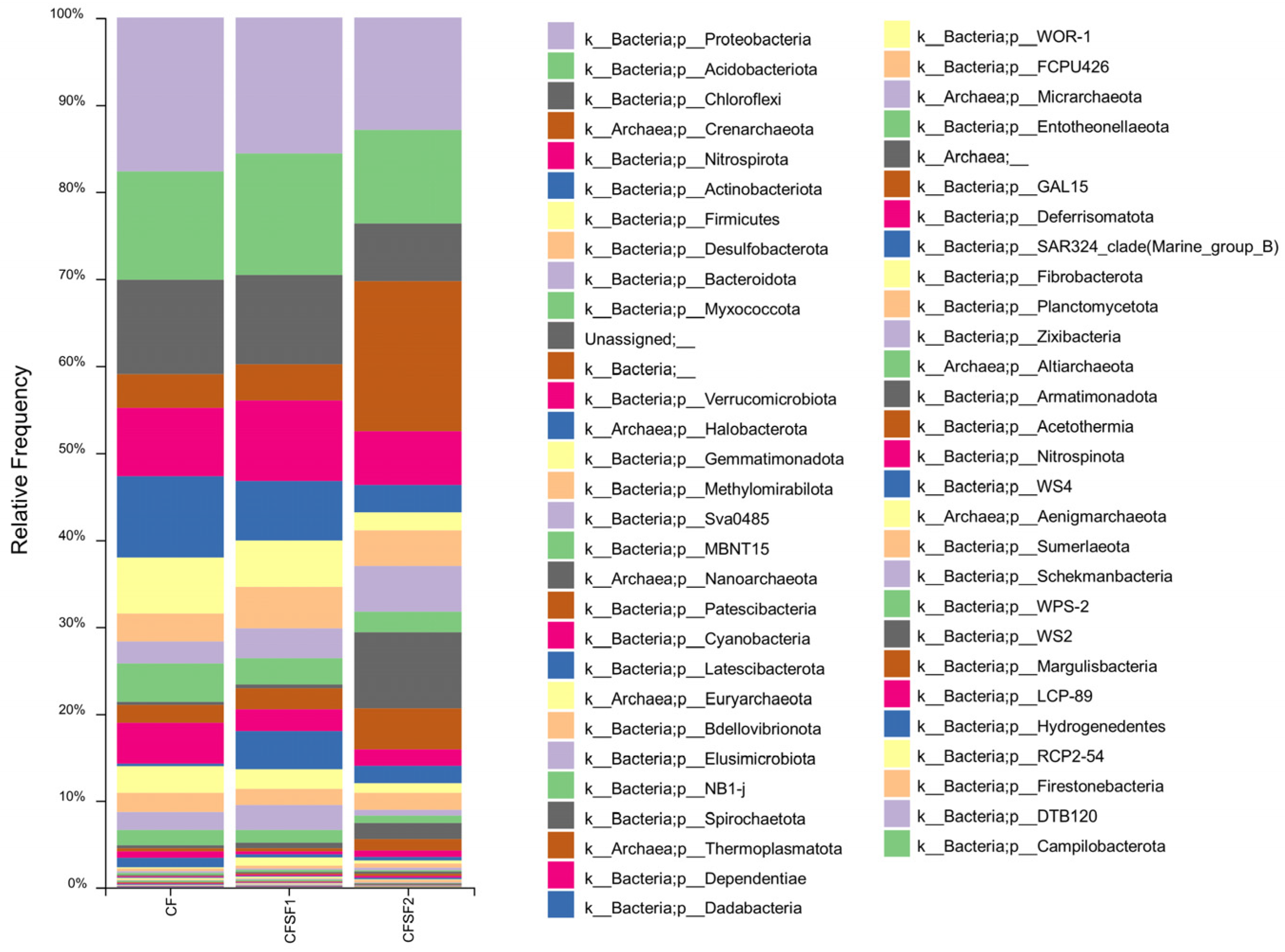
3.3. Microbial Diversity in SF-Supplemented Soils
3.4. Microbial Functional Prediction in SF-Supplemented Soils
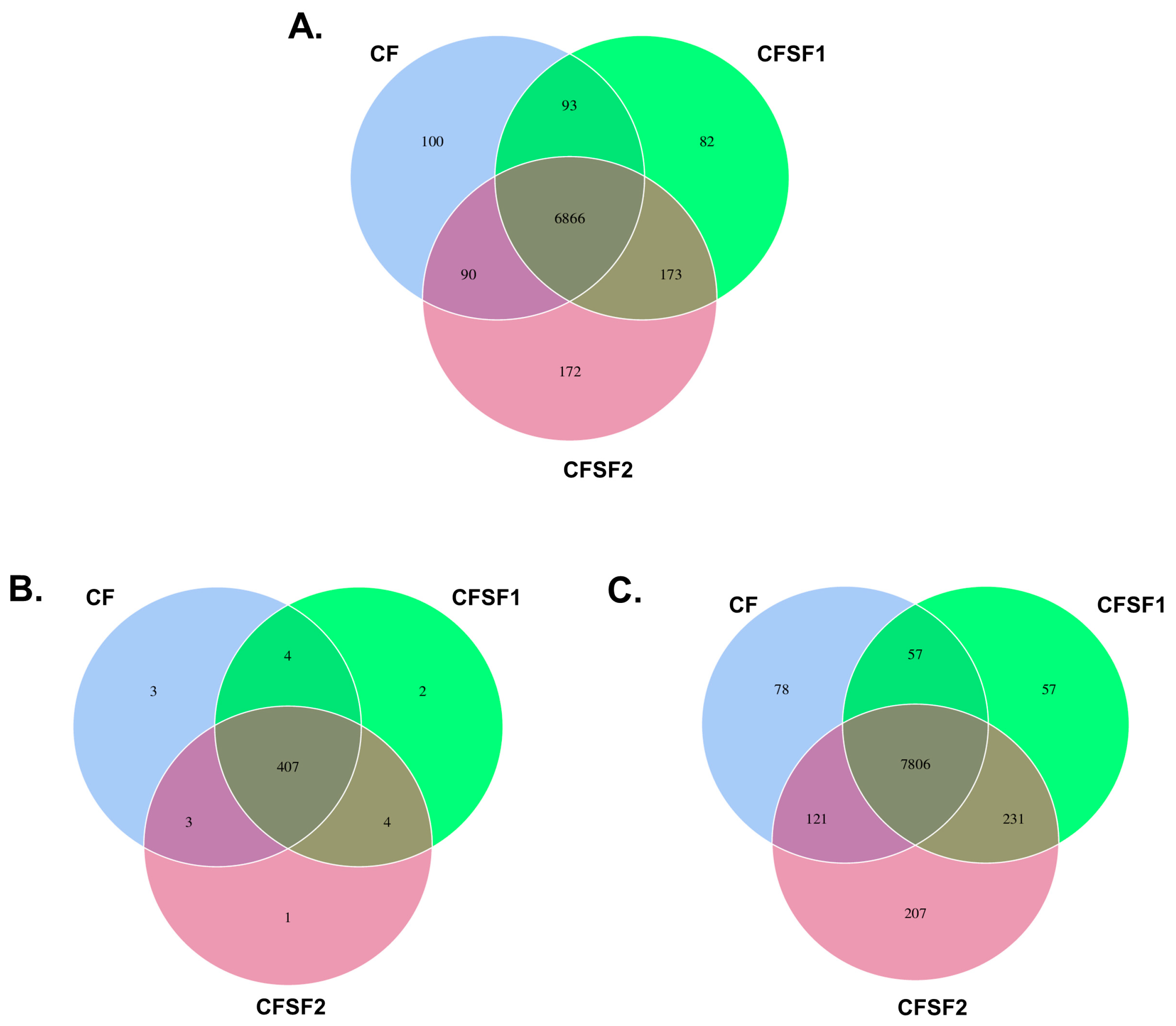
3.5. Unique Operational Taxonomical Units (OTUs) in SF-Supplemented Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khairulbahri, M. Analyzing the Impacts of Climate Change on Rice Supply in West Nusa Tenggara, Indonesia. Heliyon 2021, 7, e08515. [Google Scholar] [CrossRef] [PubMed]
- Fitrawaty; Hermawan, W.; Yusuf, M.; Maipita, I. A Simulation of Increasing Rice Price toward the Disparity of Income Distribution: An Evidence from Indonesia. Heliyon 2023, 9, e13785. [Google Scholar] [CrossRef] [PubMed]
- Krasilnikov, P.; Taboada, M.A. Amanullah Fertilizer Use, Soil Health and Agricultural Sustainability. Agriculture 2022, 12, 462. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Huang, B.; Song, Z.; Ren, L.; Hao, B.; Liu, J.; Zhu, J.; Fang, W.; Yan, D.; et al. Organic Fertilizer Improves Soil Fertility and Restores the Bacterial Community after 1,3-Dichloropropene Fumigation. Sci. Total Environ. 2020, 738, 140345. [Google Scholar] [CrossRef]
- Samuels, L.J.; Setati, M.E.; Blancquaert, E.H. Towards a Better Understanding of the Potential Benefits of Seaweed Based Biostimulants in Vitis vinifera L. Cultivars. Plants 2022, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, X.; Chen, B.; Zhang, M.; Ma, J. Seaweed Extract Improved Yields, Leaf Photosynthesis, Ripening Time, and Net Returns of Tomato (Solanum lycopersicum Mill.). ACS Omega 2020, 5, 4242–4249. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, M.M.; Saad-Allah, K.M.; Gad, D. Potential of Seaweed Extract on Growth, Physiological, Cytological and Biochemical Parameters of Wheat (Triticum aestivum L.) Seedlings. J. Soil Sci. Plant Nutr. 2022, 22, 1818–1831. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Huang, Z.; Su, G.; Li, X.; Sun, Z.; Qin, Y. Impact of Short-Term Application of Seaweed Fertilizer on Bacterial Diversity and Community Structure, Soil Nitrogen Contents, and Plant Growth in Maize Rhizosphere Soil. Folia Microbiol. 2020, 65, 591–603. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, W.; Yang, J.; Ao, J.; Huang, Y.; Shen, D.; Jiang, Y.; Huang, Z.; Shen, H. Effects of Seaweed Extracts on the Growth, Physiological Activity, Cane Yield and Sucrose Content of Sugarcane in China. Front. Plant Sci. 2021, 12, 659130. [Google Scholar] [CrossRef]
- Banakar, S.N.; PrasannaKumar, M.K.; Mahesh, H.B.; Parivallal, P.B.; Puneeth, M.E.; Gautam, C.; Pramesh, D.; Shiva Kumara, T.N.; Girish, T.R.; Nori, S.; et al. Red-Seaweed Biostimulants Differentially Alleviate the Impact of Fungicidal Stress in Rice (Oryza sativa L.). Sci. Rep. 2022, 12, 5993. [Google Scholar] [CrossRef]
- Nasmia; Rosyida, E.; Masyahoro, A.; Putera, F.H.A.; Natsir, S. The Utilization of Seaweed-Based Liquid Organic Fertilizer to Stimulate Gracilaria verrucosa Growth and Quality. Int. J. Environ. Sci. Technol. 2021, 18, 1637–1644. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Kurniawan, N.S.H.; Kirana, I.A.P.; Ardiana, N.; Abidin, A.S.; Ilhami, B.T.K.; Jupri, A.; Widyastuti, S.; Sunarpi, H.; Nikmatullah, A. Seaweed Fertilizer Prepared by EM-Fermentation Increases Abundance of Beneficial Soil Microbiome in Paddy (Oryzasativa L.) during Vegetative Stage. Fermentation 2022, 8, 46. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Kurniawan, N.S.H.; Ardiana, N.; Ilhami, B.T.K.; Mulyaningsih, T.; Astuti, S.P.; Jupri, A.; Nikmatullah, A.; Jaya, I.K.D.; Widyastuti, S. Effects of Fermented Seaweed Fertilizer Treatment on Paddy Amino Acid Content and Rhizosphere Microbiome Community. Fermentation 2022, 8, 420. [Google Scholar] [CrossRef]
- Sivojiene, D.; Kacergius, A.; Baksiene, E.; Maseviciene, A.; Zickiene, L. The Influence of Organic Fertilizers on the Abundance of Soil Microorganism Communities, Agrochemical Indicators, and Yield in East Lithuanian Light Soils. Plants 2021, 10, 2648. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wu, L.; Wang, Z.; Alabady, M.S.; Parson, D.; Molumo, Z.; Fankhauser, S.C. Characterizing Changes in Soil Microbiome Abundance and Diversity Due to Different Cover Crop Techniques. PLoS ONE 2020, 15, e0232453. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Renaut, S.; Masse, J.; Norrie, J.P.; Blal, B.; Hijri, M. A Commercial Seaweed Extract Structured Microbial Communities Associated with Tomato and Pepper Roots and Significantly Increased Crop Yield. Microb. Biotechnol. 2019, 12, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, T.; Mei, X.; Wang, N.; Li, X.; Yang, Q.; Dong, C.; Jiang, G.; Lin, J.; Xu, Y.; et al. Bio-Organic Fertilizer Promotes Pear Yield by Shaping the Rhizosphere Microbiome Composition and Functions. Microbiol. Spectr. 2022, 10, e0357222. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-Organic Fertilizers Stimulate Indigenous Soil Pseudomonas Populations to Enhance Plant Disease Suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S rRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Bzdyk, R.M.; Olchowik, J.; Studnicki, M.; Oszako, T.; Sikora, K.; Szmidla, H.; Hilszczańska, D. The Impact of Effective Microorganisms (EM) and Organic and Mineral Fertilizers on the Growth and Mycorrhizal Colonization of Fagus Sylvatica and Quercus Robur Seedlings in a Bare-Root Nursery Experiment. Forests 2018, 9, 597. [Google Scholar] [CrossRef]
- Aswidinnoor, H.; Listiyanto, R.; Rahim, S.; Holidin; Setiyowati, H.; Nindita, A.; Ritonga, A.W.; Marwiyah, S.; Suwarno, W.B. Stability Analysis, Agronomic Performance, and Grain Quality of Elite New Plant Type Rice Lines (Oryza sativa L.) Developed for Tropical Lowland Ecosystem. Front. Sustain. Food Syst. 2023, 7, 1147611. [Google Scholar] [CrossRef]
- Wang, R.; Han, F.; Wu, W. Estimation of Paddy Rice Maturity Using Digital Imaging. Int. J. Food Prop. 2021, 24, 1403–1415. [Google Scholar] [CrossRef]
- Kekulandara, D.S.; Sirisena, D.N.; Bandaranayake, P.C.G.; Samarasinghe, G.; Wissuwa, M.; Suriyagoda, L.D.B. Variation in Grain Yield, and Nitrogen, Phosphorus and Potassium Nutrition of Irrigated Rice Cultivars Grown at Fertile and Low-Fertile Soils. Plant Soil 2019, 434, 107–123. [Google Scholar] [CrossRef]
- Wapongnungsang, null; Ovung, E.; Upadhyay, K.K.; Tripathi, S.K. Soil Fertility and Rice Productivity in Shifting Cultivation: Impact of Fallow Lengths and Soil Amendments in Lengpui, Mizoram Northeast India. Heliyon 2021, 7, e06834. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Van Staden, J. Chapter Five—Plant Growth Regulators in Seaweeds: Occurrence, Regulation and Functions. In Advances in Botanical Research; Bourgougnon, N., Ed.; Sea Plants; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 125–159. [Google Scholar]
- Liu, Y.; Ding, Y.-F.; Wang, Q.-S.; Li, G.-H.; Xu, J.-X.; Liu, Z.-H.; Wang, S.-H. Effect of Plant Growth Regulators on Growth of Rice Tiller Bud and Changes of Endogenous Hormones. Acta Agron. Sin. 2011, 37, 670–676. [Google Scholar] [CrossRef]
- Sumardi, D.; Bahariawan, M.; Maulani, R.R.; Suhandono, S.; Novia, C.; Harahap, A.F.P.; Gozan, M. The Effect of Concentration of Tobacco (Nicotiana tabacum) Extract on Growth Parameters of Rice (Oryza sativa) Inpari-32. IOP Conf. Ser. Earth Environ. Sci. 2021, 940, 012026. [Google Scholar] [CrossRef]
- Zhao, S.; Jang, S.; Lee, Y.K.; Kim, D.-G.; Jin, Z.; Koh, H.-J. Genetic Basis of Tiller Dynamics of Rice Revealed by Genome-Wide Association Studies. Plants 2020, 9, 1695. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Ren, T.; Hussain, S.; Guo, C.; Wang, S.; Cong, R.; Li, X. Effects of Nitrogen and Tiller Type on Grain Yield and Physiological Responses in Rice. AoB Plants 2017, 9, plx012. [Google Scholar] [CrossRef]
- Huang, M.; Shan, S.; Cao, J.; Fang, S.; Tian, A.; Liu, Y.; Cao, F.; Yin, X.; Zou, Y. Primary-Tiller Panicle Number Is Critical to Achieving High Grain Yields in Machine-Transplanted Hybrid Rice. Sci. Rep. 2020, 10, 2811. [Google Scholar] [CrossRef] [PubMed]
- Adderley, A.; Wallace, S.; Stubbs, D.; Bowen-O’Connor, C.; Ferguson, J.; Watson, C.; Gustave, W. Sargassum sp. as a Biofertilizer: Is It Really a Key towards Sustainable Agriculture for The Bahamas? Bull. Natl. Res. Cent. 2023, 47, 112. [Google Scholar] [CrossRef]
- Mohammed, S.; El-Sheekh, M.M.; Hamed Aly, S.; Al-Harbi, M.; Elkelish, A.; Nagah, A. Inductive Role of the Brown Alga Sargassum Polycystum on Growth and Biosynthesis of Imperative Metabolites and Antioxidants of Two Crop Plants. Front. Plant Sci. 2023, 14, 1136325. [Google Scholar] [CrossRef] [PubMed]
- Adekiya, A.O.; Ejue, W.S.; Olayanju, A.; Dunsin, O.; Aboyeji, C.M.; Aremu, C.; Adegbite, K.; Akinpelu, O. Different Organic Manure Sources and NPK Fertilizer on Soil Chemical Properties, Growth, Yield and Quality of Okra. Sci. Rep. 2020, 10, 16083. [Google Scholar] [CrossRef]
- Moe, K.; Htwe, A.Z.; Thu, T.T.P.; Kajihara, Y.; Yamakawa, T. Effects on NPK Status, Growth, Dry Matter and Yield of Rice (Oryza sativa) by Organic Fertilizers Applied in Field Condition. Agriculture 2019, 9, 109. [Google Scholar] [CrossRef]
- Tursun, A.O. Effect of Foliar Application of Seaweed (Organic Fertilizer) on Yield, Essential Oil and Chemical Composition of Coriander. PLoS ONE 2022, 17, e0269067. [Google Scholar] [CrossRef]
- Ashour, M.; Hassan, S.M.; Elshobary, M.E.; Ammar, G.A.G.; Gaber, A.; Alsanie, W.F.; Mansour, A.T.; El-Shenody, R. Impact of Commercial Seaweed Liquid Extract (TAM®) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum). Plants 2021, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, E.S.; West, P.C.; Gerber, J.S.; Foley, J.A. Redefining Agricultural Yields: From Tonnes to People Nourished per Hectare. Environ. Res. Lett. 2013, 8, 034015. [Google Scholar] [CrossRef]
- Gojon, A. Nitrogen Nutrition in Plants: Rapid Progress and New Challenges. J. Exp. Bot. 2017, 68, 2457–2462. [Google Scholar] [CrossRef]
- Silver, W.L.; Perez, T.; Mayer, A.; Jones, A.R. The Role of Soil in the Contribution of Food and Feed. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20200181. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Lu, H.; Liu, Y.; Mao, C. Phosphate Uptake and Transport in Plants: An Elaborate Regulatory System. Plant Cell Physiol. 2021, 62, 564–572. [Google Scholar] [CrossRef] [PubMed]
- de Sosa, L.L.; Navarro-Fernández, C.M.; Panettieri, M.; Madejón, P.; Pérez-de-Mora, A.; Madejón, E. Application of Seaweed and Pruning Residue as Organic Fertilizer to Increase Soil Fertility and Vine Productivity. Soil Use Manag. 2023, 39, 794–804. [Google Scholar] [CrossRef]
- Karthik, T.; Jayasri, M.A. Systematic Study on the Effect of Seaweed Fertilizer on the Growth and Yield of Vigna radiata (L.) R. Wilczek (Mung Bean). J. Agric. Food Res. 2023, 14, 100748. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Li, Y.; Chen, L.; Liu, Z.; Wang, X.; Yan, P.; Qin, S. Responses of Soil Microbial Communities to a Short-Term Application of Seaweed Fertilizer Revealed by Deep Amplicon Sequencing. Appl. Soil Ecol. 2018, 125, 288–296. [Google Scholar] [CrossRef]
- Ren, C.; Liu, Z.; Wang, X.; Qin, S. The Seaweed Holobiont: From Microecology to Biotechnological Applications. Microb. Biotechnol. 2022, 15, 738–754. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.G.A.; Hartmann, M. Networking in the Plant Microbiome. PLOS Biol. 2016, 14, e1002378. [Google Scholar] [CrossRef] [PubMed]
- John, N.; Salim, R. A Study on Major Plant Growth Promoting Traits of Bacteria Isolated from Marine Environment. Mater. Today Proc. 2020, 25, 269–273. [Google Scholar] [CrossRef]
- Mostarda, E.; Campo, D.; Castriota, L.; Esposito, V.; Scarabello, M.P.; Andaloro, F. Feeding Habits of the Bullet Tuna Auxis Rochei in the Southern Tyrrhenian Sea. J. Mar. Biol. Assoc. 2007, 87, 1007–1012. [Google Scholar] [CrossRef]
- Abbasi, S. Plant–Microbe Interactions Ameliorate Phosphate-Mediated Responses in the Rhizosphere: A Review. Front. Plant Sci. 2023, 14, 1074279. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-Term Nitrogen Fertilization Decreases Bacterial Diversity and Favors the Growth of Actinobacteria and Proteobacteria in Agro-Ecosystems across the Globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Li, S.; Fan, W.; Xu, G.; Cao, Y.; Zhao, X.; Hao, S.; Deng, B.; Ren, S.; Hu, S. Bio-Organic Fertilizers Improve Dendrocalamus Farinosus Growth by Remolding the Soil Microbiome and Metabolome. Front. Microbiol. 2023, 14, 1117355. [Google Scholar] [CrossRef] [PubMed]
- Wuchter, C.; Abbas, B.; Coolen, M.J.L.; Herfort, L.; van Bleijswijk, J.; Timmers, P.; Strous, M.; Teira, E.; Herndl, G.J.; Middelburg, J.J.; et al. Archaeal Nitrification in the Ocean. Proc. Natl. Acad. Sci. USA 2006, 103, 12317–12322. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Shibata, S.; Miyaki, T.; Aono, T.; Oyaizu, H. Peculiar Archaea Found in Japanese Paddy Soils. Biosci. Biotechnol. Biochem. 1997, 61, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Pesaro, M.; Widmer, F. Identification of Novel Crenarchaeota and Euryarchaeota Clusters Associated with Different Depth Layers of a Forest Soil. FEMS Microbiol. Ecol. 2002, 42, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.W.; Schleper, C. Ammonia-Oxidising Crenarchaeota: Important Players in the Nitrogen Cycle? Trends Microbiol. 2006, 14, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.R.; McKew, B.A.; Dong, L.F.; Leung, G.; Dumbrell, A.J.; Stott, A.; Grant, H.; Nedwell, D.B.; Trimmer, M.; Whitby, C. Mineralization and Nitrification: Archaea Dominate Ammonia-Oxidising Communities in Grassland Soils. Soil Biol. Biochem. 2020, 143, 107725. [Google Scholar] [CrossRef]
- Lehtovirta-Morley, L.E. Ammonia Oxidation: Ecology, Physiology, Biochemistry and Why They Must All Come Together. FEMS Microbiol. Lett. 2018, 365, fny058. [Google Scholar] [CrossRef]
- Zhou, G.; Qiu, X.; Zhang, J.; Tao, C. Effects of Seaweed Fertilizer on Enzyme Activities, Metabolic Characteristics, and Bacterial Communities during Maize Straw Composting. Bioresour. Technol. 2019, 286, 121375. [Google Scholar] [CrossRef]
- Escalas, A.; Hale, L.; Voordeckers, J.W.; Yang, Y.; Firestone, M.K.; Alvarez-Cohen, L.; Zhou, J. Microbial Functional Diversity: From Concepts to Applications. Ecol. Evol. 2019, 9, 12000–12016. [Google Scholar] [CrossRef]
- Kanehisa, M. The KEGG Database. Novartis Found. Symp. 2002, 247, 91–101; Discussion 101–103, 119–128, 244–252. [Google Scholar]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Punta, M.; Coggill, P.C.; Eberhardt, R.Y.; Mistry, J.; Tate, J.; Boursnell, C.; Pang, N.; Forslund, K.; Ceric, G.; Clements, J.; et al. The Pfam Protein Families Database. Nucleic Acids Res. 2012, 40, D290–D301. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Disoma, C.; Zhou, Y.; Chen, Z.; Zhang, L.; Shen, Y.; Zhou, M.; Du, A.; Zheng, R.; Li, S.; et al. Protein Interactome of the Deamidase Phosphoribosylformylglycinamidine Synthetase (PFAS) by LC-MS/MS. Biochem. Biophys. Res. Commun. 2019, 513, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Sahu, U.; Villa, E.; O’Hara, B.P.; Gao, P.; Beaudet, C.; Wood, A.W.; Asara, J.M.; Ben-Sahra, I. ERK2 Phosphorylates PFAS to Mediate Posttranslational Control of De Novo Purine Synthesis. Mol. Cell 2020, 78, 1178–1191.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Linnemann, T.V.; Schreiber, L.; Bartels, D. The Role of Transketolase and Octulose in the Resurrection Plant Craterostigma plantagineum. J. Exp. Bot. 2016, 67, 3551–3559. [Google Scholar] [CrossRef] [PubMed]
- Guevara, D.R.; El-Kereamy, A.; Yaish, M.W.; Mei-Bi, Y.; Rothstein, S.J. Functional Characterization of the Rice UDP-Glucose 4-Epimerase 1, OsUGE1: A Potential Role in Cell Wall Carbohydrate Partitioning during Limiting Nitrogen Conditions. PLoS ONE 2014, 9, e96158. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B.J.; Habash, D.Z. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Nitrogen Assimilation and Possibilities for Improvement in the Nitrogen Utilization of Crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.-Q.; Zhang, W.-B.; Zhang, C.; Song, Y.-L.; Liao, Y.-L.; Ma, J.-C.; Yu, Y.-H.; Wang, H.-H. Characterization of 3-Oxacyl-Acyl Carrier Protein Reductase Homolog Genes in Pseudomonas Aeruginosa PAO1. Front. Microbiol. 2019, 10, 1028. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, D.; Zhao, L.; Liu, J.; Shang, B.; Yang, W.; Duan, X.; Sun, H. Metabolomic Analysis Reveals Insights into Deterioration of Rice Quality during Storage. Foods 2022, 11, 1729. [Google Scholar] [CrossRef]
- Bergman, C.; Pandhi, M. Organic Rice Production Practices: Effects on Grain End-Use Quality, Healthfulness, and Safety. Foods 2022, 12, 73. [Google Scholar] [CrossRef]
- Vroon, D.H.; Israili, Z. Aminotransferases. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; ISBN 978-0-409-90077-4. [Google Scholar]
- Hwang, B.-Y.; Lee, H.-J.; Yang, Y.-H.; Joo, H.-S.; Kim, B.-G. Characterization and Investigation of Substrate Specificity of the Sugar Aminotransferase WecE from E. Coli K12. Chem. Biol. 2004, 11, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, C.; Suglo, P.; Sun, S.; Wang, M.; Su, T. L-Aspartate: An Essential Metabolite for Plant Growth and Stress Acclimation. Molecules 2021, 26, 1887. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New Insights into the Shikimate and Aromatic Amino Acids Biosynthesis Pathways in Plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Evolutionary Trails of Plant Group II Pyridoxal Phosphate-Dependent Decarboxylase Genes. Front. Plant Sci. 2016, 7, 1268. [Google Scholar] [CrossRef] [PubMed]
- Facchini, P.J.; Huber-Allanach, K.L.; Tari, L.W. Plant Aromatic L-Amino Acid Decarboxylases: Evolution, Biochemistry, Regulation, and Metabolic Engineering Applications. Phytochemistry 2000, 54, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Cellini, B.; Zelante, T.; Dindo, M.; Bellet, M.M.; Renga, G.; Romani, L.; Costantini, C. Pyridoxal 5′-Phosphate-Dependent Enzymes at the Crossroads of Host–Microbe Tryptophan Metabolism. Int. J. Mol. Sci. 2020, 21, 5823. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Hajko, L.; Franczuk, J.; Zaniewicz-Bajkowska, A.; Andrejiová, A.; Mezeyová, I. Effect of L-Tryptophan and L-Glutamic Acid on Carrot Yield and Its Quality. Agronomy 2023, 13, 562. [Google Scholar] [CrossRef]
- Blok, C.; Jackson, B.E.; Guo, X.; de Visser, P.H.B.; Marcelis, L.F.M. Maximum Plant Uptakes for Water, Nutrients, and Oxygen Are Not Always Met by Irrigation Rate and Distribution in Water-Based Cultivation Systems. Front. Plant Sci. 2017, 8, 562. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Liu, C.; Lin, S.; Zuo, Q.; Butterbach-Bahl, K. Improving Rice Production Sustainability by Reducing Water Demand and Greenhouse Gas Emissions with Biodegradable Films. Sci. Rep. 2017, 7, 39855. [Google Scholar] [CrossRef]
- Saito, J.; Yamada, M.; Watanabe, T.; Iida, M.; Kitagawa, H.; Takahata, S.; Ozawa, T.; Takeuchi, Y.; Ohsawa, F. Crystal Structure of Enoyl–Acyl Carrier Protein Reductase (FabK) from Streptococcus Pneumoniae Reveals the Binding Mode of an Inhibitor. Protein Sci. 2008, 17, 691–699. [Google Scholar] [CrossRef]
- Green, M.L.; Karp, P.D. The Outcomes of Pathway Database Computations Depend on Pathway Ontology. Nucleic Acids Res. 2006, 34, 3687–3697. [Google Scholar] [CrossRef] [PubMed]
- Langa-Lomba, N.; Grimplet, J.; Sánchez-Hernández, E.; Martín-Ramos, P.; Casanova-Gascón, J.; Julián-Lagunas, C.; González-García, V. Metagenomic Study of Fungal Microbial Communities in Two PDO Somontano Vineyards (Huesca, Spain): Effects of Age, Plant Genotype, and Initial Phytosanitary Status on the Priming and Selection of Their Associated Microorganisms. Plants 2023, 12, 2251. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, K.; Kumar, R.; Vijay Anand, K.G.; Bhojani, G.; Kubavat, D.; Ghosh, A. Structural and Functional Changes in Soil Bacterial Communities by Drifting Spray Application of a Commercial Red Seaweed Extract as Revealed by Metagenomics. Arch. Microbiol. 2021, 204, 72. [Google Scholar] [CrossRef] [PubMed]

| Physical Properties | Chemical Properties (g/kg) | |||
|---|---|---|---|---|
| pH | Texture | Total N | Total P | Total K |
| 7.63 ± 0.17 | Sandy | 2.13 ± 0.15 | 0.54 ± 0.03 | 0.83 ± 0.02 |
| Treatment Group Name | Inorganic Fertilizer | Seaweed Based Organic Fertilizer | ||
|---|---|---|---|---|
| CON2H4 | Na3PO4 | KCl | ||
| CF | 300 | 100 | 100 | - |
| CFSF1 | 150 | 50 | 50 | 1000 |
| CFSF2 | 150 | 50 | 50 | 2000 |
| Treatment | Total N (g/kg) | Total P (g/kg) | Total K (g/kg) |
|---|---|---|---|
| Before fertilization | 2.13 ± 0.15 a | 0.34 ± 0.03 a | 0.43 ± 0.02 a |
| CF | 1.18 ± 0.15 b | 0.23 ± 0.01 a | 0.48 ± 0.03 a |
| CFSF1 | 2.67 ± 0.15 c | 0.61 ± 0.03 b | 0.97 ± 0.03 b |
| CFSF2 | 2.77 ± 0.12 c | 0.60 ± 0.02 b | 1.07 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasedya, E.S.; Kurniawan, N.S.H.; Fitriani, F.; Saraswati, P.B.A.; Qoriasmadillah, W.; Ilhami, B.T.K.; Hernawan, A.; Widyastuti, S. Sustainable Use of Organic Seaweed Fertilizer Improves the Metagenomic Function of Microbial Communities in the Soil of Rice Plants. Sustainability 2023, 15, 16328. https://doi.org/10.3390/su152316328
Prasedya ES, Kurniawan NSH, Fitriani F, Saraswati PBA, Qoriasmadillah W, Ilhami BTK, Hernawan A, Widyastuti S. Sustainable Use of Organic Seaweed Fertilizer Improves the Metagenomic Function of Microbial Communities in the Soil of Rice Plants. Sustainability. 2023; 15(23):16328. https://doi.org/10.3390/su152316328
Chicago/Turabian StylePrasedya, Eka Sunarwidhi, Nanda Sofian Hadi Kurniawan, Fitriani Fitriani, Putu Bella Aprillia Saraswati, Wanda Qoriasmadillah, Bq Tri Khairina Ilhami, Ari Hernawan, and Sri Widyastuti. 2023. "Sustainable Use of Organic Seaweed Fertilizer Improves the Metagenomic Function of Microbial Communities in the Soil of Rice Plants" Sustainability 15, no. 23: 16328. https://doi.org/10.3390/su152316328
APA StylePrasedya, E. S., Kurniawan, N. S. H., Fitriani, F., Saraswati, P. B. A., Qoriasmadillah, W., Ilhami, B. T. K., Hernawan, A., & Widyastuti, S. (2023). Sustainable Use of Organic Seaweed Fertilizer Improves the Metagenomic Function of Microbial Communities in the Soil of Rice Plants. Sustainability, 15(23), 16328. https://doi.org/10.3390/su152316328







