1. Introduction
Municipal solid wastes (MSW) are defined as waste durable goods, nondurable goods, containers and packaging, food scraps, yard trimmings, and miscellaneous inorganic wastes from residential, commercial, and industrial sources [
1]. Landfills are broadly used as waste disposal sites for MSW despite the environmental impacts and risks for human health [
2], and despite being the least favorable option in the waste hierarchy considered by the European Union (Circular Economy Package, Directive, 2008/98/EC) that consider prevention, minimization, re-use, recycling, and energy recovery as preferential options in that order, rather than disposal.
Landfills are sources of groundwater, surface water, and soil pollution due to the production of leachate and gaseous pollutants and their migration through water and air [
3].
Landfills are designed as MSW storage containers, open to air during the filling period and sealed once filled. During the filling period, contamination to the surrounding soils preferentially occur via wind and surface runoff transportation. Landfill deposition has been identified as one of the main anthropogenic activities that release rare earth elements (REEs) into the soil environment via hydrological and wind-driven processes [
4]. Once sealed, the waste is deprived of air and water and organic materials degrade very slowly. The generation of liquid leachates out of the landfill happens in poorly-designed or poorly-managed facilities [
5]. Therefore, landfills are a potential risk to human health and the surrounding environment [
6]. In addition to the engineered barriers system applied, a proper landfill should meet adequate hydrological, geological, and environmental conditions. In order to minimize risks, landfills should be constructed on stable and low permeability terrains, located far from surface waters with a recommendation of at least a 500 m distance, as frequently suggested [
7,
8]. In addition, they must be built on a suggested slope range of 8–12% (higher slopes could impact on larger drainage and potential erosion) and avoid geological faults and locations vulnerable to flooding. Places with minimal wind should be searched to construct landfills, not only to prevent annoying odors from nearing habitable areas, but also to minimize the spread of contaminants to the environment via gases, aerosols, and particulate matter [
9].
Resuspension due to waste discharges, the traffic of heavy vehicles on the surface, the compaction of the layers, or the burning of landfill gas are sources of particulate emissions potentially concentrated in pollutants, and specifically, in heavy metals and REEs. The amount of windblown particles from a landfill depends on the wind speed, rain, and relative air humidity [
10,
11].
Although nowadays the separation of different types of residues are promoted (e.g., organic matter, packaging, glass, paper, and heavily pollutant compounds), to take advantage of them as resources and to ideally exclude their presence in landfills, this growing practice still fails in origin in a significant percentage. In fact, more than a third (36%) of MSW generated in Spain is still disposed of in landfill sites as final storage [
12], which is slightly higher than that of the EU as a whole (31%) [
13]. In the region of Madrid, four large landfills have been in use for the last decades, including the municipal solid waste landfill (MSWLF) of Colmenar Viejo, whose authorization for operation has recently been extended for a few more years.
Landfilled municipal waste include household waste (as well as similar commercial, industrial, and institutional wastes), with or without treatments that imply separately collected fractions; specifically, it includes potentially hazardous fractions as fluorescent tubes and other mercury-containing waste, photochemicals, pesticides, paints, inks, adhesives and resins, wood-containing dangerous substances, batteries and accumulators, discarded electrical and electronic equipment, and metals and metallic packaging [
14]. In this regard, electric and electronic waste deserves special mention, given its unique chemical and physical characteristics compared to other forms of municipal or industrial waste: it contains both valuable and hazardous materials that require special handling and recycling methods to avoid environmental contamination and detrimental effects on human health [
15,
16]. Its components contain numerous elements and materials, such as Pb, Zn, Cu, Sb, Cd, or Hg, which, if discarded improperly, have the potential to contaminate groundwater and soil [
17]. However, difficulties in recycling this waste have led to its disposal in landfills or to its irregular export [
15].
Unlike elements like copper and gold, which usually are found unmixed in electronic products, REE are found extremely dilute in technological products; in this regard, sediments retrieved form stormwater ponds were found to exhibit high leachable REE contents at room temperature and low pH [
16]. REE can be found in catalysts, batteries, and magnets, which are all instrumental in digital and green technologies. Due to their high demand in current technological and industrial production, both light and heavy REE have been listed as critical [
18]. La, Ce, and Nd are the most abundant REEs in soils [
19]. Among REEs, elements with an even atomic number (Ce, Nd, Sm, Gd, Dy, Er, and Yb) are more abundant than elements with an odd atomic number (La, Pr, Eu, Tb, Ho, Tm, and Lu), besides having decreasing contents with an increase in atomic mass [
20]. Cerium is the most abundant REE and the 25th most abundant element in earth’s crust [
21]. The lanthanides found in smaller concentrations—Lu and Tm—are more abundant in the earth’s crust than cadmium (Cd) and selenium (Se) [
22].
Although natural and engineered barriers minimize their transport [
23], the dispersion of contaminants either via gas emissions [
24], the leakage of leaching solutions [
25], or particles dispersion produced by the wind [
26] is inevitable and exerts its influence on the surrounding ecosystems [
27], affecting the atmosphere, surface and ground waters, and soils. The role of organic matter and inorganic colloids is critical in this sense [
28].
This encourages the need to carry out geochemical studies leading to identify the anthropogenic sources of heavy metals and REE.
Numerous studies analyze the influence of controlled and uncontrolled landfills on the quality of surrounding underground and surface waters (e.g., [
29]) and the emissions of volatile organic compounds (VOCs) to the atmosphere [
30,
31]. The spread of toxic elements, such as As and Sb, and heavy metals, such as Cd, Co, Cr, Cu, Pb, Ni, and Zn, that diffuse via air deposition from landfills to the surrounding areas have been shown to increasingly accumulate in soils and plants, even at considerable distances from the source, highlighting the need of reclaiming and remediating contaminated landfill sites [
32,
33].
Uncontrolled, improperly designed, or badly sited liquid storage facilities represent potential sources of pollution due to the vulnerability of groundwater leakage [
34]. An abandoned liquid disposal site in Hungary revealed a large contamination of nitrates, phosphates, ammonium, and organic matter, not only in monitored wells within the disposal basin, but also in the surrounding areas [
35]. Numerical models are able to predict the contaminant-front propagation into groundwaters near liquid disposal sites [
36].
In this context, the hypothesis raised in this study is that the presence of a controlled landfill can generate quantitatively and spatially heterogeneous geochemical anomalies for different heavy elements and REEs in soils and waters in its surroundings. This research is based on an area adjacent to a municipal landfill near Madrid (Central Spain), within the Jarama river basin. The area is characterized by being topographically lower from this landfill as well as leeward of the dominant winds, constituting the head of two small temporary channels. Such conditions are optimal to evaluate the effect of environmental factors on the spatial distribution of proximal geochemical anomalies. This type of study is proposed as a model to be applied in the surrounding areas of long-operated landfills of similar characteristics worldwide.
2. Materials and Methods
2.1. The Colmenar Viejo Landfill
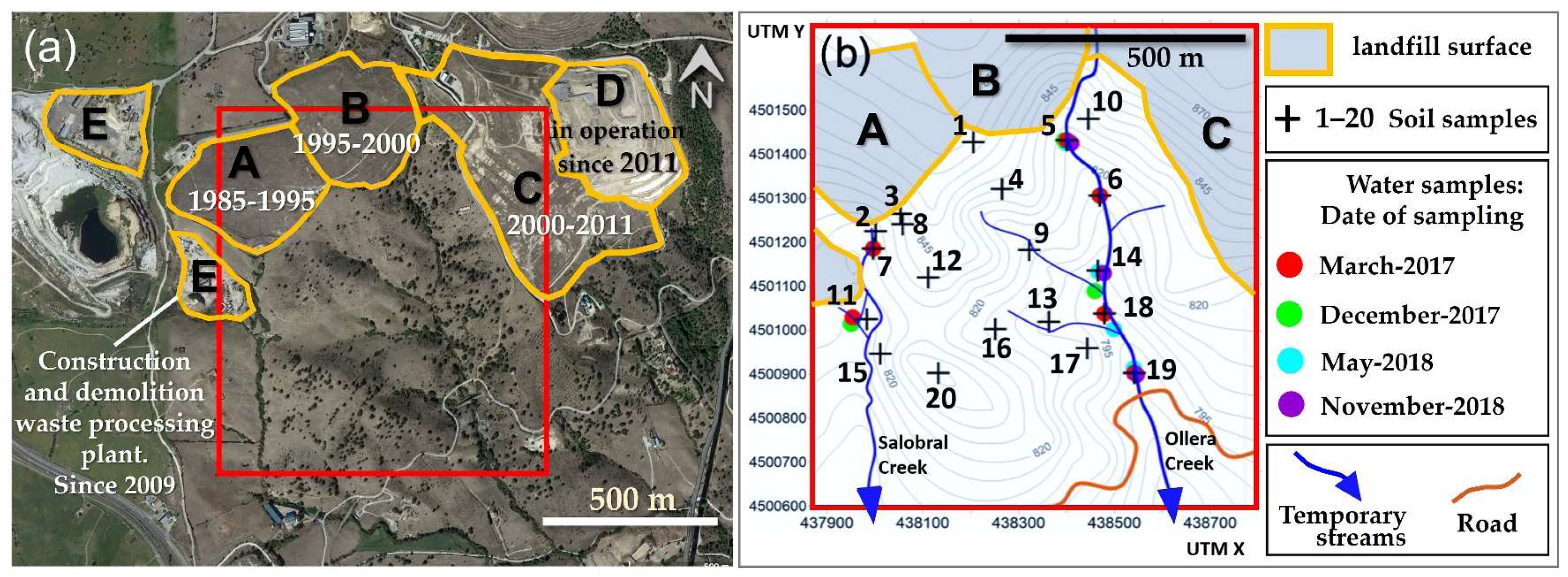
The Colmenar Viejo controlled landfill is located 3 km to the East from Colmenar Viejo, a municipality of almost 50,000 inhabitants which, in turn, is located 30 km to the North from Madrid city (central Spain,
Figure 1).
The landfill, that hosts MSW from more than 80 municipalities of the North and West zones of the Autonomous Community of Madrid, was constructed and exploited in several phases (
Figure 2). The first phase (A in
Figure 2) started in 1985 and closed down in 1995. A second phase was operated in a new adjacent vessel (B in
Figure 2) from 1995 to 2000. The high rate of leachates, in addition to the lack of an adequate waterproofing of the vessel, required an installation in 1998 of a network of leachate extraction wells, automatic piezometers to control further possible leaks, the addition of geomembranes, the enlargement of the bentonite barriers, and the drainage of perimeter ditches. These two phases contain a waste volume of 2.3 × 10
6 m
3 and the upper cover was sealed in 1997 and 2000, respectively.
There was also a third phase (C in
Figure 2), which operated from 2000 to 2011 and contained a volume of 3.0 × 10
6 m
3, and currently, a fourth phase, which is presently in operation since 2011 (D in
Figure 2). The 3rd and 4th phases were constructed under the European Council Directive 1999/31/EC that demands more exhaustive operational and technical requirements to prevent or reduce, as far as possible, negative effects on the environment. The landfill only accepts non-hazardous and inert waste from household origin. The zone E in
Figure 2 corresponds to a construction and demolition waste processing plant of 22,000 m
2, in operation since 2009, that mostly treat inert materials such as sand, bricks, and concrete.
2.2. Area of Study
The selected area of study is adjacent, by the South face, to the Colmenar Viejo landfill, which is constituted of natural soils and has an approximate extension of 40 ha. Near 75% of the surface is covered by grassland and used for extensive cattle grazing. In some areas, the soil surface is devoid of vegetation, favoring erosion. The rest of the surface corresponds to dense sclerophyllous bushes and scattered trees, associated with the main drainage network. The geological substrate is made up mostly of high-grade metamorphic rocks, gneiss, and schists, framed to the north, west, and south by granites. Locally, there are quartz dikes that generate smooth ridges in the terrain [
37]. Substrate materials are hydrogeologically impermeable, so there are no significant aquifers in the area, which is related to a dense surface drainage network.
The altitude of the studied area ranges from 790 to 860 m, while the highest altitude in the landfill is 890 m and the lowest, 820 m. Moderate to steep slopes favor the surface runoff after precipitations from the disposal area, concentrated in two temporal main streams that run in an approximate N-S direction, draining to the South. Both streams form the headwater of the Salobral creek, which is mainly dry in summer but maintain certain humidity in some sections. The entire drainage network corresponds to the Guadalix river sub-basin that belongs to the Jarama basin.
According to the Köppen climate classification, the area of study corresponds to a Csa Mediterranean subtype, characterized by hot and dry summers and mild, wet winters, with certain Continental influence due to both its location in the center of the Iberian Peninsula and the altitude (meteorological station of Colmenar Viejo, period 1981–2010, AEMET, web online). According to the data for the aforementioned station, the situations with the highest average wind speed correspond to two dominant directions: N-NE and W-SW.
The area of study is made up of natural soils, whose characteristics are notably conditioned by livestock use. Soils are conditioned by the geomorphological, lithological, and climatic factors. According to the USDA classification [
38], soils within Entisols Order are dominant in this area of study. Entisols (within Typic and Lithic Xerorthents subgroups) correspond to poorly developed soils in the Mediterranean climate. The Lithic subgroup is associated with erosive surfaces, as convex slopes and summits, while the Typic subgroup dominates in areas of active sedimentation, as concave slopes and valley bottoms; the latter are associated with the areas with the highest relative moisture accumulation. Both soils are characterized by a poor organic matter surface horizon, located directly either on altered rocks or sediments. These types of soils are normally characterized by a moderate acid reaction (pH 5.5–6.0), low electrical conductivity, and a lack of calcium carbonate [
39,
40].
2.3. Soil Sampling
Twenty soil samples were collected using sampling criteria that involve the whole topographic area with the objective to differentiate the zones where runoff processes predictably dominated (convex slopes and summits) from deposition zones (concave slopes and valley bottoms), understanding that a wind deposition would initially take place homogeneously over the area of study. A random sampling was subsequently applied to each stratum. The collected samples are included in two main types of existing lithologies, schist-gneisses, and granites. The sampling strategy permitted to study the geochemical distribution of elements and physicochemical properties on the soil surface. The soil sampling campaign was carried out in December 2016 and January 2017, collecting representative samples at a depth of 0–20 cm. The soils samples were collected in sealed plastic bags from the field and air-dried in the laboratory for further analysis.
2.4. Water Sampling
Eighteen water samples were collected in the studied zone in four different sampling campaigns (six samples in March 2017, three samples in December 2017, six samples in May 2018, three samples in November 2018, and some of them in the same location). The samples were collected in the two parallel streams facing South from the landfill; both of them are oriented along the N-S direction (
Figure 2) and are named Salobral (West) and Ollera creek (East). Both water streams are seasonal, being mostly dry in summer, and their estimated water discharge is lower than 500 m
3 year
−1. Such seasonality largely conditions the water sampling dates. Additionally, in May 2019, two reference water samples were collected in creeks out of the area of study: one sample was approximately 1.5 km to the North and the other was 1 km to the East of the area of study, but far enough away to be considered not directly influenced by the landfill.
Water temperature, pH, electric conductivity, and dissolved oxygen were measured on site with field equipment. The water samples were collected in polyethylene bottles for further laboratory analysis.
2.5. Laboratory Methods
The soil samples were sieved below 2 mm and this fraction was studied using several analytical techniques. The samples with soil/water ratios of 1:2.5 and 1:5 were prepared for the determination of pH and electric conductivity, respectively. Water content was measured by heating a <2 mm fraction sample for 24 h at 105 °C and calculated in wt.% of dry material. The organic matter content (OM) was determined using the loss-on-ignition (LOI) procedure, with heating at 400 °C for 2 h [
41]. Calcium carbonate content was measured with the Bernard calcimeter. Cation exchange capacity (CEC) was determined via ammonium displacement of exchangeable cations at pH 7, followed by a sequential sodium displacement at pH 8.2 and the determination of ammonium in the aqueous extract using a selective ammonium electrode. All physic-chemical analyses were determined in duplicate.
Elemental analyses of the soil samples were performed in the fraction <50 µm. The samples were treated via acid digestion with a CEM MARS 5 microwave system using the conditions described by the EPA (US Environmental Protection Agency) method 3151A. Multi-elemental semi-quantitative analyses (screening analyses that determine the concentration for each sample for more than 70 elements) were conducted in the aqueous samples via inductively coupled plasma mass spectrometry (ICP-MS) with a NexION 300XX Perkin Elmer Spectrometer. The detection limit of this equipment reaches the ng/l scale for most elements, although the precision error for the semi-quantitative analyses is only below 30%. Maps of spatial distribution were performed with the Surfer® software 13.6 version (Golden Software LLC, Golden, CO, USA) using the kriging regression method.
Six powder samples representative of the whole mesh were grinded manually on an agate mortar and analyzed via X-ray diffraction (XRD) in order to identify and quantify the main minerals. The diffractometer used was a Siemens D-5000 and the quantification was performed with the software High Score Expert plus 2.2 (Malvern Panalytical Ltd., London, UK).
The determination of pH, temperature, electrical conductivity (EC), redox potential, and dissolved oxygen was performed in situ in the water samples with field electrodes. Major cations and anions were determined in the laboratory via ionic chromatography with a Compact IC plus-882 device by Metrohm. Alkalinity was determined with a Titrando-888 device by Metrohm. The aqueous chemical analyses were determined in duplicate. Again, multi-elemental semi-quantitative analyses were performed on the selected water samples and filtered below 0.45 µm with the already described ICP-MS equipment. Chemical oxygen demand (COD) and biochemical oxygen demand (BOD5) were also performed on the selected samples. COD was performed using the potassium dichromate method using HACH reagents and measuring the Cr3+ produced at 420 nm with a DR/2100 spectrophotometer via HACH. BOD5 was performed via the change in pressure resulting from the consumption of oxygen in the flasks, after 5 days of incubation in the dark, at 20 °C, and continuous stirring, using electronic Lovibond Oxidirect pressure sensors.
3. Results
3.1. Soil Properties in the Area of Study
Spatial distribution of general soil properties (pH, OM, EC, carbonates, and CEC) is presented in
Figure 3. pH (
Figure 3a) has a high relevancy as it incises in the behavior of diverse factors such as the soil degradation, the availability of nutrients, and the solubility of heavy metals and their mobilization. It is mainly the original siliceous material which control the pH in soils, with incidence from the organic matter. pH variations are quite large (2.5 units from the lower to upper value; 5.7 to 8.3), considering the apparent homogeneity of the soil in the studied area. Median, mean, and standard deviation values in pH measurements are 6.3 and 6.6 ± 0.7, respectively. Higher pH values are observed as associated to sediments in both creeks.
The organic matter content (LOI procedure) varies from 2.8 to 21.7 wt.%, with an averaged value of 9.3 ± 6.5% (
Figure 3b). The range is wide, but the median value is 6.9%, so just a few high values correspond to the soil samples near the creeks or zones of denser vegetation. In general, these values are higher compared to the same type of soils in the neighboring areas. This increase could be partially attributed to the determination method since the LOI procedure eliminates not only readily oxidable OM, but also includes fresh and non-humified plant debris.
The EC varies from 13 to 123 μS cm
−1 with a median value of 36.5 and a mean value of 43.5 ± 26.8 μS cm
−1 (
Figure 3c). In general, the values are low and typical of this type of soil; however, an increase is observed at the accumulation zone in the Ollera creek.
Calcium carbonate content is low, in agreement with soil type. The median, mean, and standard deviation values are 0.9 and 1.0 ± 0.6% (
Figure 3d). The only significant factor is the increase in carbonates observed at the West side, close to the construction and demolition waste processing plant, so it seems very plausible that the dust originated from the demolition of construction materials, with certain content in carbonates, which could have been transported by the wind to the neighboring deposition area.
The median, mean, and standard deviation values of the CEC are 13.1 and 14.9 ± 6.5 cmol
(+) kg
−1 (
Figure 3e). CEC is property exhibited, mainly by clay minerals and OM that contributes to the retention of cationic pollutants. As expected, a linear correlation is observed between the CEC and OM (R
2 = 0.58).
The soil samples presented a general brown color (10YR 4/3 to 6/3, according to soil Munsell Color Charts), similar moderately coarse textures (sandy loam to sandy clay loam) and an average loss of water of 1.6 wt.% after air drying (n = 20). Water content (at 105 °C) varies from 1.3 to 8.3 wt.% with an average value of 3.3 wt.%.
Most samples analyzed via XRD show a high content in quartz, feldspars, and plagioclases and a low content in clay minerals (below 5 wt.%) and carbonates (below 1 wt.%). Only two samples show a higher clay minerals’ content (14 and 19 wt.%), accompanied by a relatively high content in calcite (4 and 14 wt.%). One of these two samples is located at the creek basin and the other near the construction and demolition waste processing plant, so either are exposed to the accumulation of sediments or influenced by the dust accumulated near the plant.
3.2. Spatial Distribution of Heavy Elements
In order to evaluate potential anomalies in the geochemical distribution of heavy elements in the surface soil samples, a direct comparison has been made with the background and reference values established in the soils of the Autonomous Community of Madrid [
39], for selected elements (
Table 1). Background values are defined as systematic concentrations found in natural soils, which are not influenced by anthropogenic activities, while reference values are defined as statistical limits with a low probability to be exceeded by a population of samples. The values of the percentile, 90%, are used as reference values (VR90).
The area of study corresponds to the Type 1 unit, characterized by soils mainly developed on metamorphic materials with occurrences in some areas of clays, arkoses, and carbonates [
39]. Background and reference values are considered for soils of this Type 1 unit, which are typically higher than those generic reference levels expressed in the Orden 2770/2006 that legislates at the Madrid regional level for contaminated soils by heavy metals and trace elements.
As observed in
Table 1, the median values for Ag, Cd, Cu, Mn, Ni, Pb, Tl, and Zn, in the area of study, exceed the median background values established for the same type of lithology, while the maximum values for Ag, Cd, Cu, Mn, Mo, Ni, Pb, Tl, and V exceed the reference values VR90. Out of the 15 tabulated elements, only As, Co, and Cr lie within the background and reference limits [
39]. Conversely, none of the concentrations determined reach the intervention levels established for the agricultural soils, residential, or recreational areas at the Spanish national or the international level, which are typically one order of magnitude higher (e.g., [
42,
43]). Hg and Sb have not been determined in the present study due to their volatility during the preparation method for ICP-MS determination.
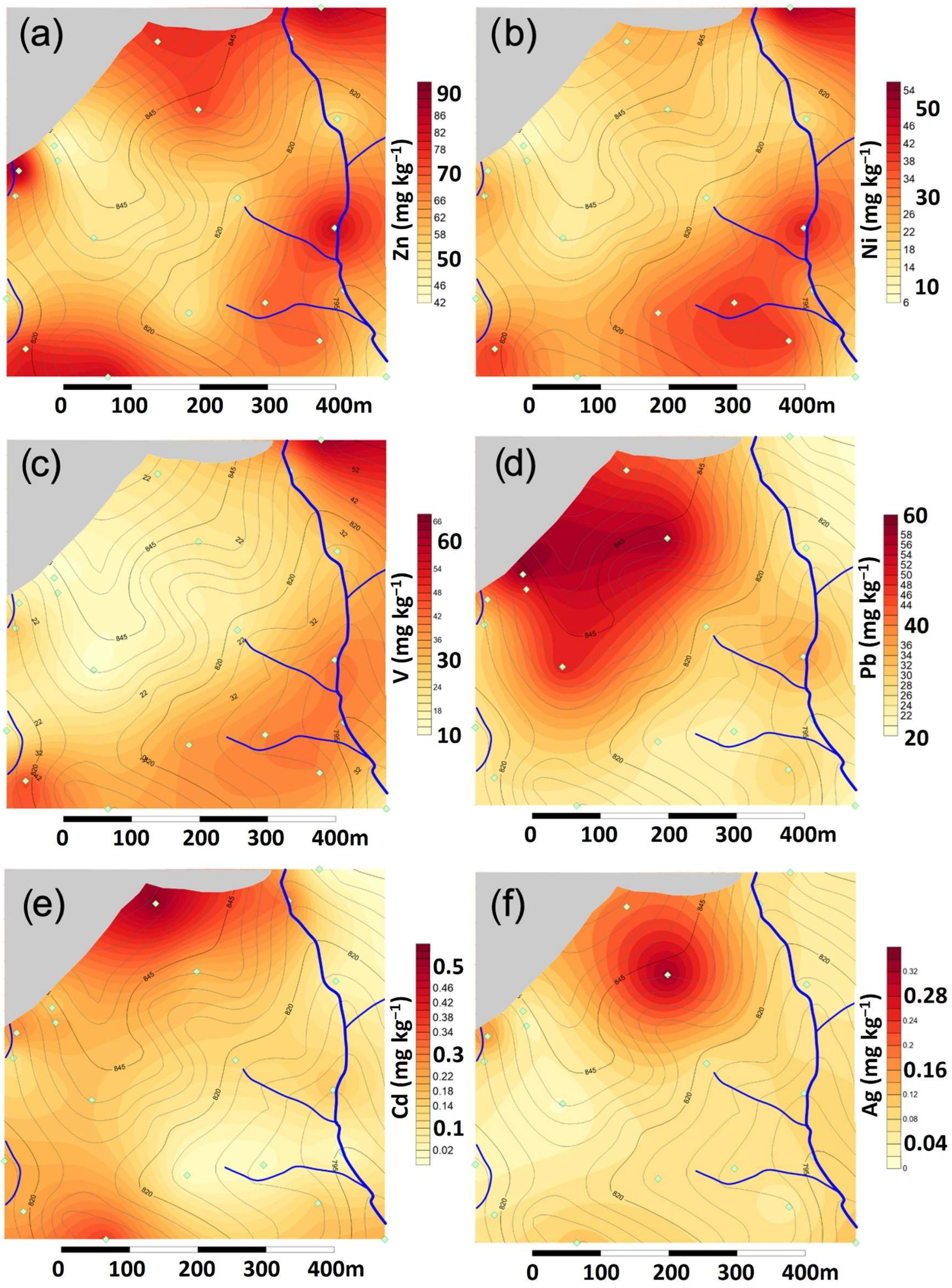
The distribution of Zn is heterogeneous in the area of study (
Figure 4a). All concentrations are comprised between the background and the V90 values; however, the spatial distribution shows that higher concentrations are found near both creeks and at the North face, close to the limit with the older vessels of the landfill. High concentrations of Ni and V are associated to the samples collected near the creeks (
Figure 4b,c), while concentrations in the central area are in a range close to the background values.
The soil anomalies of Pb, Zn, Cd, and Ag show a distribution notably linked to the oldest vessels of the landfill (phases A and, to a lesser extent, B), corresponding to the decades of the 80s and 90s (
Figure 4d–f). The extension of the highest concentrations of Cd and Ag are limited to a distance of 100–150 m from the border with the landfill, while for Pb, the extension is larger, up to 200 m.
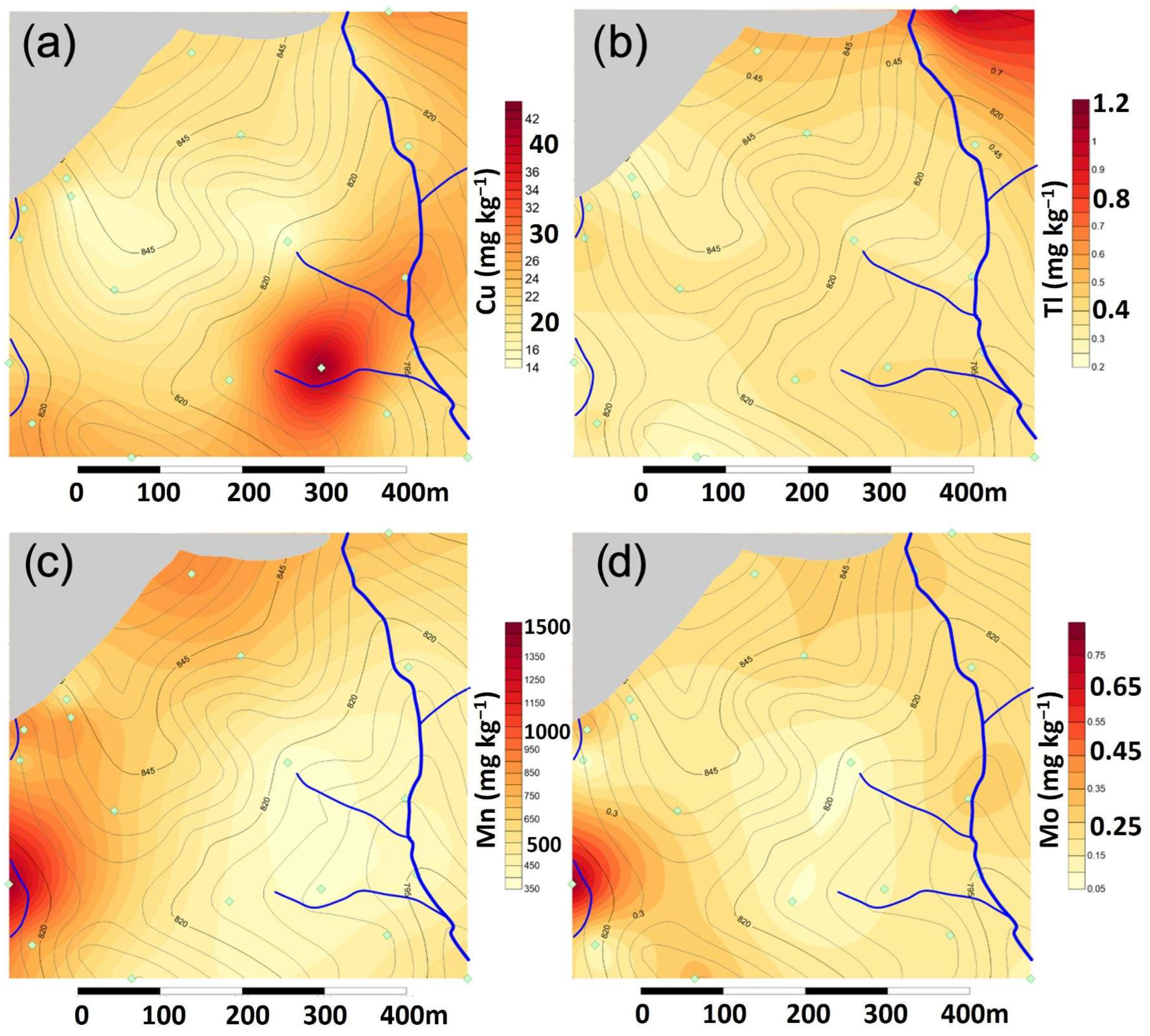
The spatial distributions of Cu, Tl, Mn, and Mo (
Figure 5) show punctual high concentrations near any of the two creeks. Other than that, the concentrations remain above the background limits, except for Mo, but within a homogeneous spatial distribution.
The Mn and Mo anomalies are the only ones clearly associated with the presence of the construction waste treatment plant, similar to the behavior of high pH and calcium carbonate values (
Figure 3).
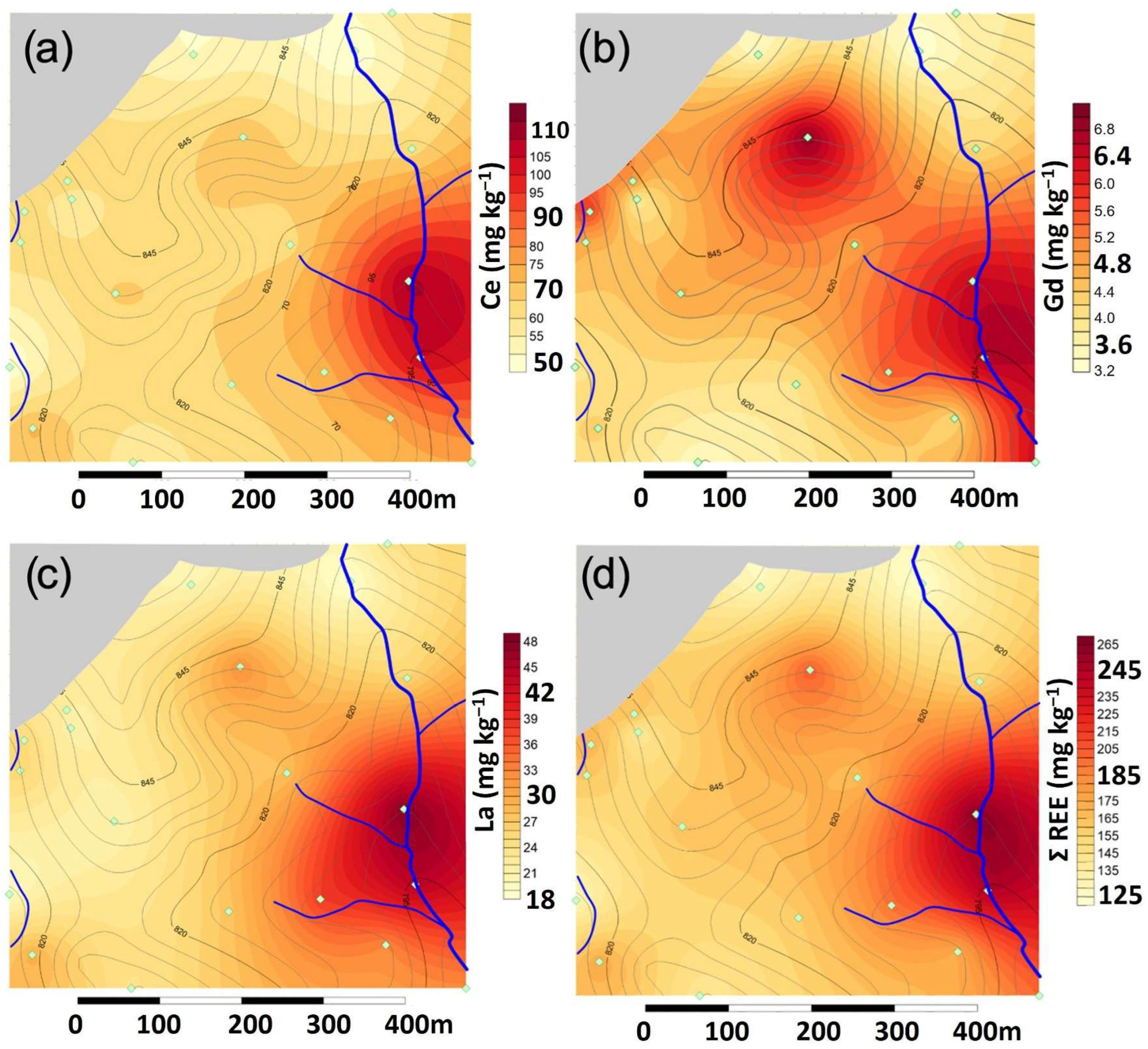
The spatial distribution of the most studied REEs (Ce, Gd, La, and the sum of all REEs) has been analyzed in order to evaluate their possible transference from the landfill to the surrounding area. As observed in
Figure 6, Ce, Gd, La, as well as the sum of REEs, are mostly concentrated in sediments at the accumulation zone of the Ollera creek once the slope becomes less pronounced. In addition, high concentrations of Gd are also observed to the North of the Salobral creek, near the border with the oldest vessel of the landfill and at the central upper part of the area of study.
In order to establish local background values for REEs in soils, seven samples collected in the vicinity of the area of study, in a radial distance up to 9 km, were selected from the data provided in the Geochemical Atlas of Spain [
44].
Table 2 shows the mean, standard deviation, and minimum and maximum values for the REEs of these seven samples with labels 509T14, 509T23, 509T24, 534T1, 534T2, 534T3, and 534T4. The samples are representative of the upper part of the soil, from 2–4 cm to 20 cm in depth. Their coordinates and additional data on these samples can be consulted in the Geochemical Atlas of Spain.
The comparison of concentrations between the samples from the Geochemical Atlas of Spain and the samples of the present study is not straightforward, as the quantitative analyses were performed on different size fractions, using different extraction methods, and measured with different analytical equipment. We observe lower concentrations in the samples that correspond to non-accumulation zones than the minimum values of the reference samples; therefore, it might be wise to consider that the methodology used in the present study provide, in general, lower concentrations than in the reference method, although they are in the same order of magnitude.
Ce is often found in trace amounts in most of soils and geological materials with similar concentrations as those found for Cu and Zn [
45], which agrees with the reference values (
Table 2). However, the large increase observed at the accumulation zone of the Ollera creek confirms the enrichment of Ce in cumulative soils associated to this stream (
Figure 6a).
Similarly, the mean soil concentration of La, considering several worldwide studies at the surface horizon, might be around 35 mg kg
−1 of dry sample, which is also in agreement with the reference values presented in
Table 2. Extensive collection of La concentrations in soils worldwide are reported within a range of 6–44 mg kg
−1 of dry sample [
22], with an average value of 30 mg kg
−1 [
19]. Higher concentrations are usually attributed to human pollution. Again, an accumulation effect is observed for La in sediments of the Ollera creek (
Figure 6c).
The concentrations of Gd and the sum of REEs shown in
Table 2 are also in an averaged range as in the background values within the area of study, except in the accumulation zones in the sediment samples collected near the Ollera creek, but also in the samples collected in the North, within the area of study (
Figure 6b,c).
3.3. Water Properties in the Area of Study
The water samples of the studied area are characterized by relatively high electrical conductivity, high alkalinity, high salinity, high ammonium content, and a significant chemical contamination that should not correspond to the expected water quality of natural creeks in these lithological types (
Table 3). There are no significant groundwater aquifers in the zone that could make any ionic contribution to the temporal creeks and the amount of ammonium is excessive for the potential contribution of the cattle present in the area. Some of the samples presented color and odor.
The reference water samples present electrical conductivities in the order of 0.1 to 0.4 mS cm−1, which might be predictable for natural surface waters influenced by igneous and metamorphic rocks; however, most of the samples in the area of study exhibit values one order of magnitude higher.
The analyses of the BOD5 and COD determinations in the Ollera creek, in comparison with the reference samples, reveal a considerable concentration of non-biodegradable organic compounds.
The percentage of error in the charge balance is acceptable for the reference samples (3–4%) and for the samples from the Salobral creek. However, the balance for some samples of the Ollera creek exceed a 10% error, even when the standard deviations obtained via the duplicate analyses were low. It might be assumed that some contribution of either non-analytically measured charged aqueous organic species and/or inorganic ions (normally not considered significant in natural aquifers) have occurred.
The Piper diagram shown in
Figure 7 indicates different water qualities as a function of the water source, independent of the sampling date. In general, the samples from the Ollera creek exhibit a lower content in Ca
2+ and a higher content in Na
+ + K
+ than the samples from the Salobral creek. The content in Mg
2+ does not exceed 20% in any case. With respect to the anions, the samples from the Ollera creek exhibit a lower content in SO
42− and CO
32− + HCO
3 and a higher content in Cl
− than the samples from the Salobral creek. It is also remarkable that the samples from both creeks, in general, exhibit an averaged lower content in CO
32− + HCO
3− than the reference samples and a much higher content in Cl
−.
Therefore, the water samples from the Ollera creek could be mainly classified as a sodium chloride type, while the samples from the Salobral are more disperse but could be mostly classified as a magnesium bicarbonate type.
The concentrations of heavy elements obtained via ICP-Ms are dispersed and may vary over time, even from one location to another in the same creek and in the same sampling campaign, but again, the comparison against the reference samples reveal much larger concentrations of several elements (
Table 3). In addition to the REEs, the concentration of Mn, Co, Ni, Sr, and W for most of the samples surpass, in several orders of magnitude, the concentration of the reference samples and the recommendations for drinking water (see that Sc and Pm are not included in the table as part of the REEs: concentrations of Sc were low in all samples, as in the reference samples, and Pm was not measured via IPC-MS).
At the local scale, the contrast of concentrations of REEs is remarkable in the studied samples in both creeks in comparison with the reference samples, where none or negligible concentrations were measured (
Table 4).
Although Mn is an essential element for plants and animals, it is also considered an undesirable impurity in water supplies and in concentrations higher than 50 µg L−1, which is not recommended for human consumption. Concentrations of Mn were found up to 852 µg L−1 in the Ollera creek and up to 3211 µg L−1 in the Salobral creek, highly surpassing the recommended quality for drinking water and exceeding the concentration found in the reference samples (≤1 µg L−1).
In the present study, W (tungsten or wolfram) concentrations in the range of 0.7 to 3.6 µg L
−1 have been measured in the Ollera creek. These concentrations might still be far below from the drinking recommendations but reach concerning values and contrast with the absence of W found in the samples from the Salobral creek and both reference samples (
Table 4). Tungsten usually occurs in low concentrations in natural waters, although it is considered one of the metals less regulated and studied. In river waters and groundwaters, the concentration of W is highly influenced by the geology of the catchment. The W averaged concentration for river waters is 0.54 nmol L
−1, which is ten times higher than the average seawater concentration [
46,
47]. High concentrations of W, ranging from 0 to 610 µg L
−1 (median 2 µg L
−1), were detected in households’ tap water from private wells in a rural county in Nevada, USA [
48]. The World Health Organization (WHO) has not issued any drinking water guidelines for W, but the Russian Federation regulates tungsten in drinking water below the limit of 50 µg L
−1 [
49].
Co and Ni have some similar characteristics and may substitute for iron in ferromagnesian igneous-rock minerals, tending to coprecipitate with manganese oxides. The cobalt activity in aqueous systems is predicted to be 10
−1 to 10
−2 times lower than Mn in oxygenated waters at pH between 5 and 8, according to the model proposed by [
50]. Nickel is somewhat more abundant than cobalt in most natural freshwaters. The concentrations of Co and Ni found in the studied samples are in an acceptable range compared to other averaged mineralized waters worldwide, but contrast with the absence (or concentrations below the detection limits) of these elements in the reference samples.
4. Discussion
The potential soil contamination studied in the selected area, adjacent to the landfill, is considered to have been produced during the filling period of the different vessels that have lasted for more than 30 years. The observed distribution of elements is compatible with polluted soil particles detached and transported by raindrops, wind-driven rain, and wind [
51]. In this regard, climatic conditions are favorable for dust dispersion at least 4–6 months a year (combination of high temperatures, little or no precipitation, low relative air humidity, and wind). The dominant wind directions (W, N) are also compatible with the saltation and deposition of particles from the landfill towards the area of study. In the other half of the year (October to April), frequent rainfall and situations of high soil moisture occur, which limit dust emissions but favor surface runoff.
As observed by the distribution maps performed in this study (
Figure 3,
Figure 4,
Figure 5 and
Figure 6), the accumulation of heavy elements has mainly occurred in the North, close to border with the oldest vessels of the landfill, and in the S-E, where the slope is less pronounced and sediments are accumulated at the West side of the Ollera creek (
Figure 4,
Figure 5 and
Figure 6). This observation may agree with the general relationship between the increasing release of heavy metals and REEs as pH decreases [
52]. In the studied area, although no extreme pH values were measured, higher pHs were measured in the East and West, indicating that the release of heavy elements and REEs at lower pH could have been transported via the water flow of both creeks, deposited with sediments, then immobilized once the pH has increased. The higher content in clay minerals in these zones could contribute to retaining the heavy elements and REEs. The content of OM or carbonates were not significant either in the areas where heavy elements accumulated; hence, a natural environmental distribution cannot be attributed.
Therefore, two different physical processes are inferred for the accumulation of heavy elements and REEs in the identified zones: the higher concentrations’ distribution in the North, observed for Zn, Ni, V, Pb, Cd, Ag, Tl, and Gd, might have occurred via the wind and wind-driven rain transport from the landfill during the filling periods, as well as further deposition on the soils, while the higher concentrations’ distribution in the S-E, observed for Zn, Ni, and the sum of REEs, might be linked to water transport through the Ollera creek, as well as sediment accumulation. The distribution of other elements, such as Mn and Mo, show punctual enrichment that could be linked to water transport through the Salobral creek and sedimentation, but the increase in concentration is just observed in one soil sample, so this attribution could be premature.
In terms of soil contamination risks, heavy elements and REEs concentrations have not reached trigger values to show concern at regulatory levels, although they locally exceed the influence from the regional origin of the parent materials.
The chemical analyses on surface waters, on the contrary, indicate concerning levels of contamination. The concentrations of all REEs are obviously low and may be considered near the confidence limit, not due to the characteristics of the ICP-MS equipment that could measure with high precision, but because of the semiquantitative method applied, that provides, in a single analysis, the concentration of most elements of the periodic table without specific accuracy for each element. Assuming the validity of the data, we observe that concentrations of most REEs are at least one order of magnitude higher than those measured in rivers from different continents characterized by large discharge areas, such as the Amazon and the Mississippi, and other published data for the rivers of the world [
53,
54].
Again, at the local scale, the contrast of concentrations of REEs is remarkable in the studied samples in both creeks in comparison with the reference samples, where none or negligible concentrations were measured (
Table 4).
The characterization performed on the water samples was not focused on the identification and quantification of organic compounds, water odor in some samples, and concentrations of COD that exceed, in all measured samples, the maximum admissible concentration for the discharge of urban wastewater treatment plants in Spain [
55], in addition to the high concentrations of inorganic species such, with special concern of nitrates and ammonia, and high electrical conductivity, that also highly exceeded the expected range according to the parent rock materials and reveal failure in the sealing of water from the landfill. The concentrations of Ni, NO
2−, and NO
3− in several water samples surpassed the concentration limits adopted by the Drinking Water Directive of the European Union (Council Directive 98/83/EC), that was revised in 2020 (Directive 2020/2184), and also exceeded the concentrations of the indicator parameters for Mn, Na
+, Cl
−, and EC.
The BOD
5/COD ratio in all determined samples is much below 0.2, indicating that the type of contamination is produced by non-biodegradable organic pollutants. Similar results were obtained by [
56], who attributed the high total alkalinity to the concentration of dissolved organic materials and ionically charged organic acids. The pH was measured in a neutral to alkaline range (7.1 to 8.3), which was compatible with the start of the aerobic and methanogenic phase of the OM degradation [
57]. In a previous work, the state of polluted soils and the surface water in their discharge areas of 15 landfills was studied in the Madrid region [
58]. In agreement with the results presented in this study, these authors found high concentrations of inorganic ions in surface waters, high electrical conductivity, and high COD concentration. Additionally, they identified several organic compounds including a variety of environmentally persistent chemicals, like phthalates. Their study indicated that the poor quality of the water in the creeks occurred over many years and verified that the results presented here are not limited to a temporal contamination during 2017–2018, when the samples were collected. Presumably, the contamination is caused by a continuous leakage from the landfill, as observed in other international studies reported worldwide where heavy metals (e.g., Cd, Cu, As, Pb, and Cr) and inorganic ions, such as ammonium and nitrate, migrate from uncontrolled landfills to groundwater [
59].
Although the water discharge of both creeks is relatively low and intermittent, the surface runoff has both a negative impact on the local ecosystem and a negative influence on the quality of the water received downstream at the Jarama river.
The study confirms the initial hypothesis showing the heterogeneous distribution of geochemical anomalies observed for heavy elements and REEs in soils and waters in a local surrounding area near to an MSW landfill.