Cultivation of Brackish Water Microalgae for Pig Manure Liquid Digestate Recycling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalage Culture
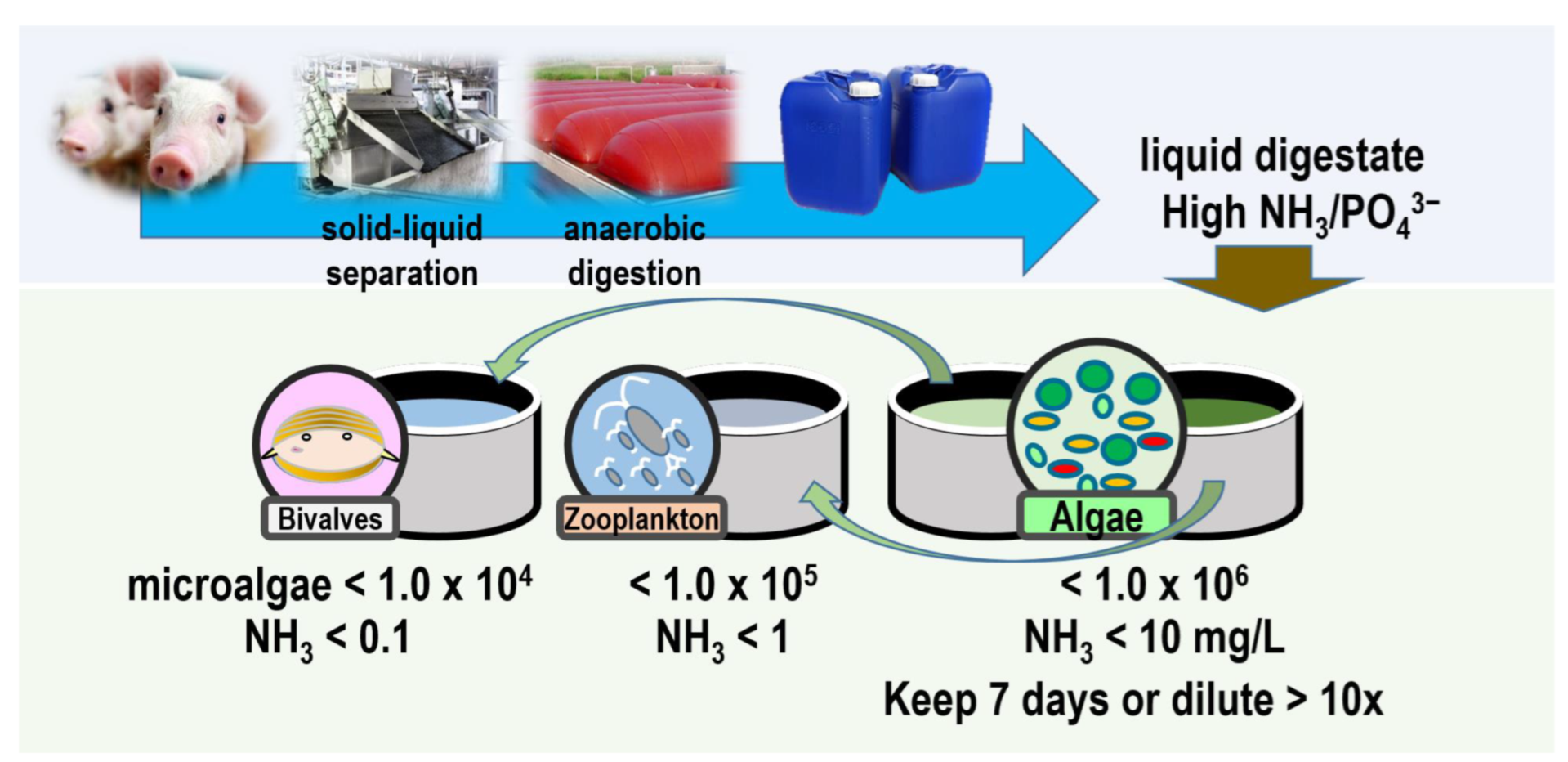
2.2. Collection and Preparation of Pig Manure Liquid Digestate
2.3. Water Quality Parameters
2.4. Digestate Application in Microalgae Cultivation
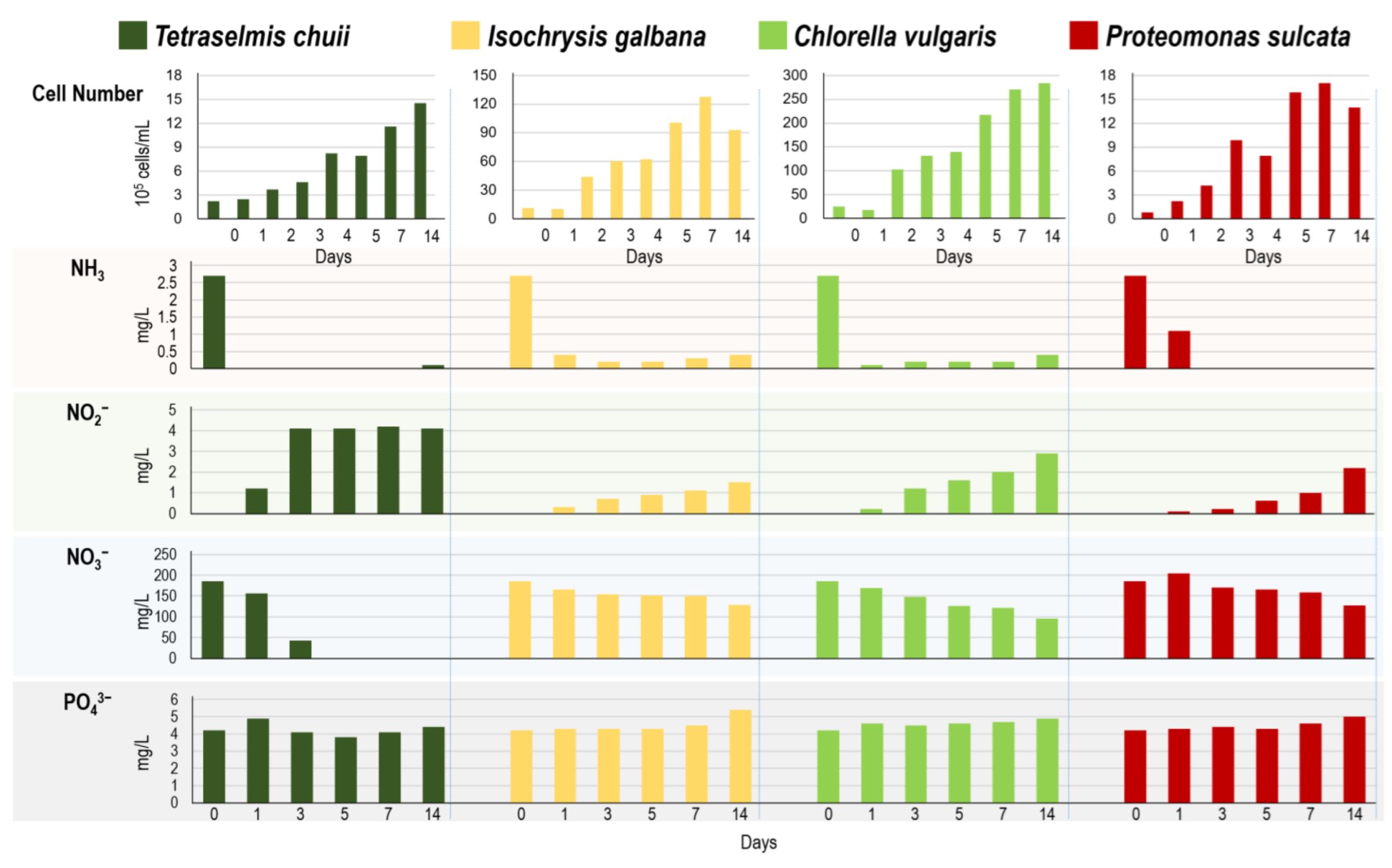
2.4.1. Four Algae Strains in the Same Digestate Medium
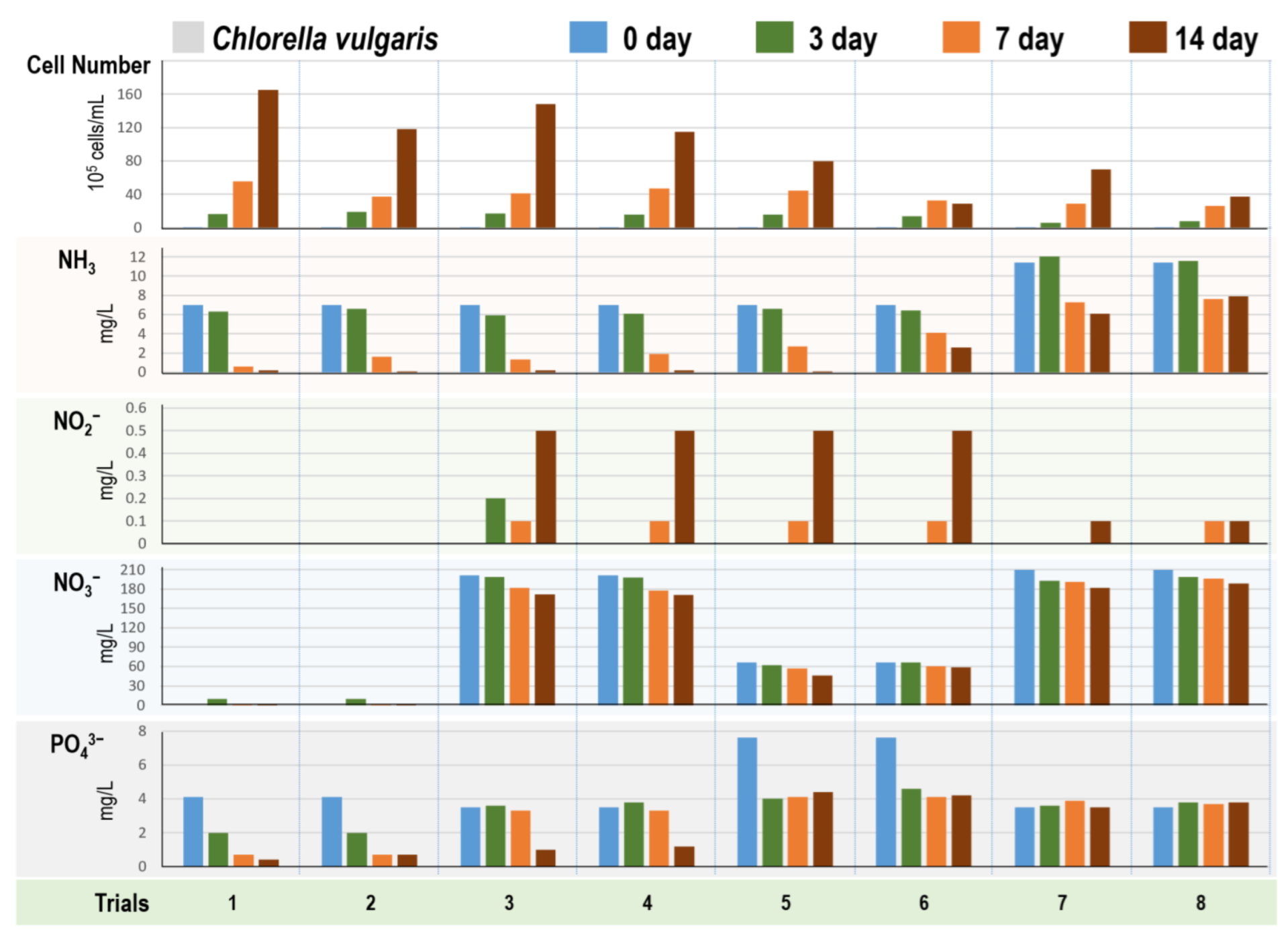
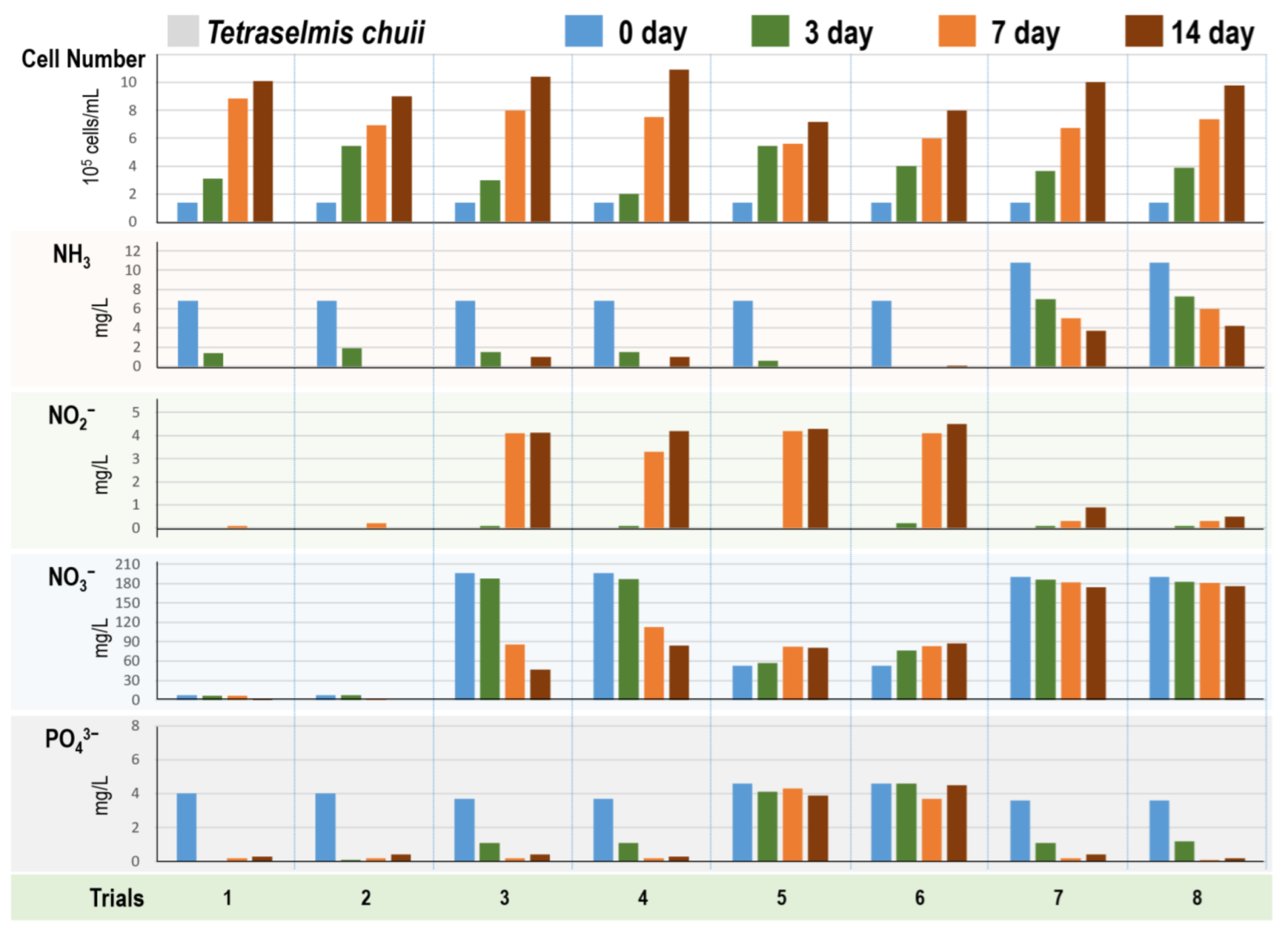
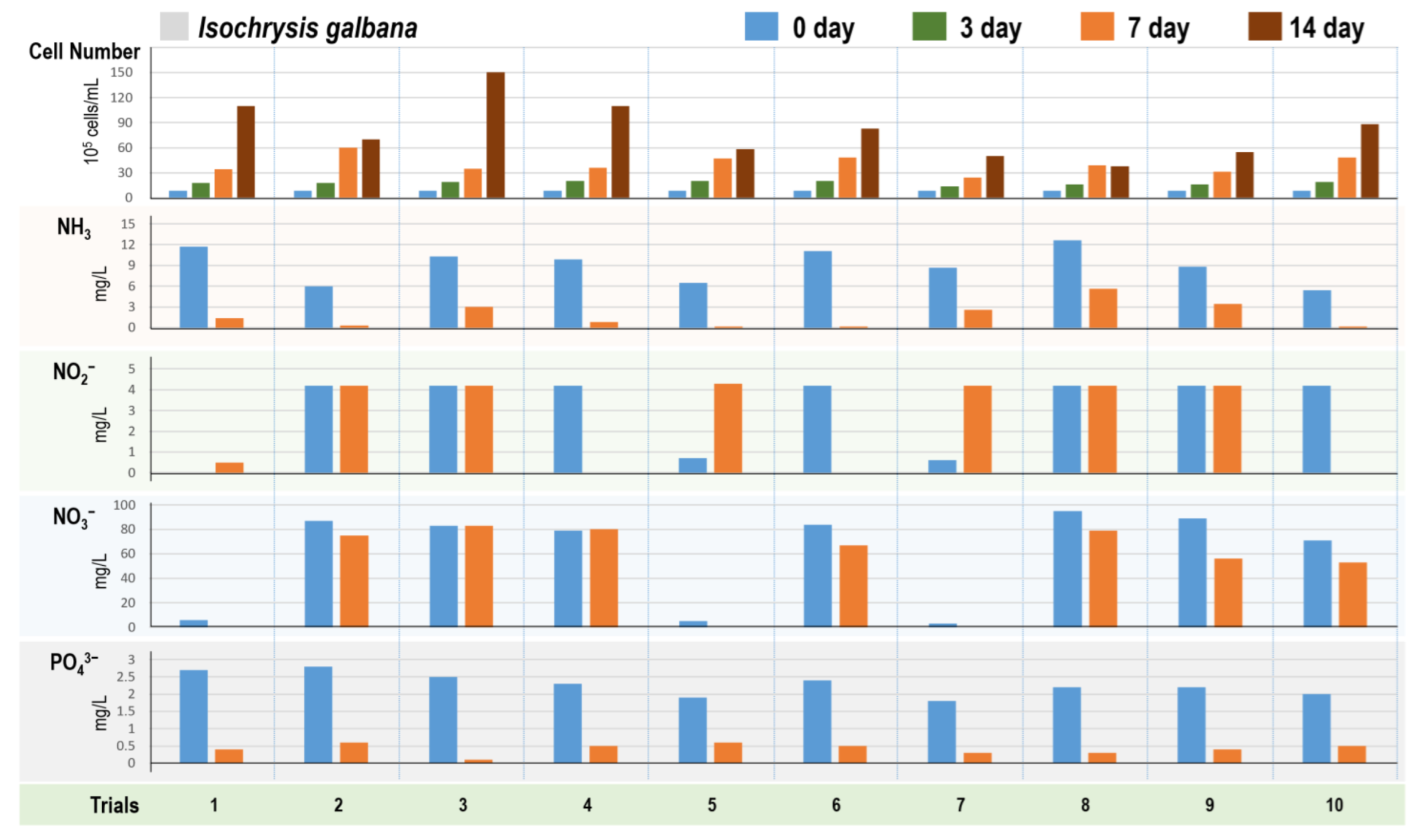
2.4.2. Four Algae Strains in Different Digestate Mediums
2.5. Field Application of Digestate-Cultivated Algae
2.6. Statistical Analysis
3. Results
3.1. Four Algae Strains in the Same Digestate Medium
3.2. Chlorophyta Algae Cultivation in Eight Different Digestate Mediums
3.3. Chromista Algae Cultivation in 10 Different Digestate Mediums
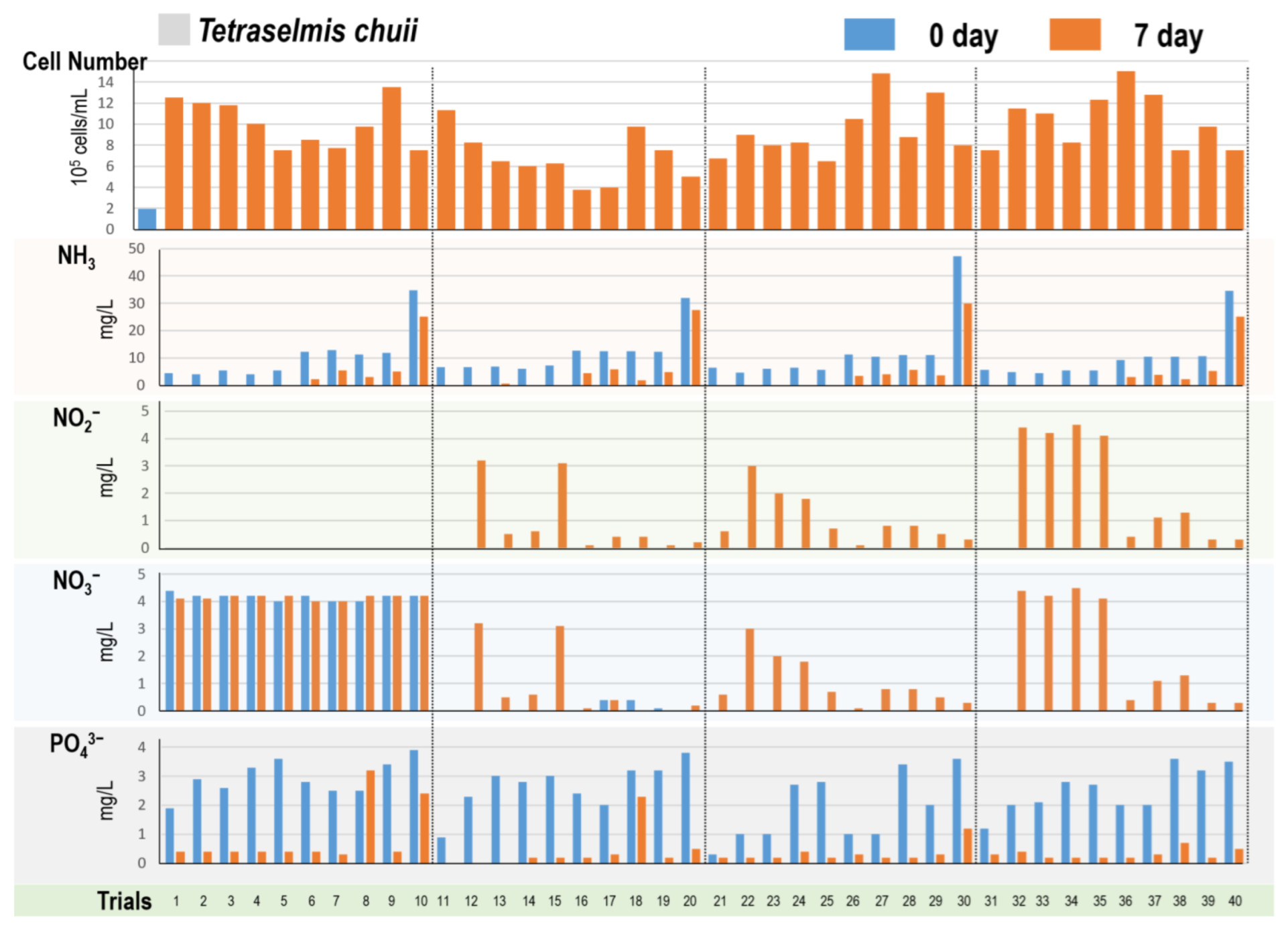
3.4. Tetraselmis Algae Cultivation in 40 Different Digestate Mediums
3.5. Field Application of Digestate-Cultivated Algae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoshnevisan, B.; Duan, N.; Tsapekos, P.; Awasthi, M.K.; Liu, Z.; Mohammadi, A.; Angelidaki, I.; Tsang, D.C.W.; Zhang, Z.; Pan, J.; et al. A Critical Review on Livestock Manure Biorefinery Technologies: Sustainability, Challenges, and Future Perspectives. Renew. Sustain. Energy Rev. 2021, 135, 110033. [Google Scholar] [CrossRef]
- Ronga, D.; Mantovi, P.; Pacchioli, M.T.; Pulvirenti, A.; Bigi, F.; Allesina, G.; Pedrazzi, S.; Tava, A.; Dal Prà, A. Combined Effects of Dewatering, Composting and Pelleting to Valorize and Delocalize Livestock Manure, Improving Agricultural Sustainability. Agronomy 2020, 10, 661. [Google Scholar] [CrossRef]
- Finzi, A.; Mattachini, G.; Lovarelli, D.; Riva, E.; Provolo, G. Technical, Economic, and Environmental Assessment of a Collective Integrated Treatment System for Energy Recovery and Nutrient Removal from Livestock Manure. Sustainability 2020, 12, 2756. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Tsai, W.-T. Valorization of Value-Added Resources from the Anaerobic Digestion of Swine-Raising Manure for Circular Economy in Taiwan. Fermentation 2020, 6, 81. [Google Scholar] [CrossRef]
- Hsu, E. Cost-Benefit Analysis for Recycling of Agricultural Wastes in Taiwan. Waste Manag. 2021, 120, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-M.; Liu, X.; Wang, S.-L.; Fang, L.-X.; Sun, J.; Liu, Y.-H.; Liao, X.-P. Distribution Patterns of Antibiotic Resistance Genes and Their Bacterial Hosts in Pig Farm Wastewater Treatment Systems and Soil Fertilized with Pig Manure. Sci. Total Environ. 2021, 758, 143654. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Liu, Y.W.; Zhou, J.L.; Chang, S.W.; Nguyen, D.D.; Bui, X.T.; Zhang, X.B. Bioprocessing for Elimination Antibiotics and Hormones from Swine Wastewater. Sci. Total Environ. 2018, 621, 1664–1682. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, Q.; Wei, D. A Critical Review on Antibiotics and Hormones in Swine Wastewater: Water Pollution Problems and Control Approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef] [PubMed]
- Tigini, V.; Franchino, M.; Bona, F.; Varese, G.C. Is Digestate Safe? A Study on Its Ecotoxicity and Environmental Risk on a Pig Manure. Sci. Total Environ. 2016, 551–552, 127–132. [Google Scholar] [CrossRef]
- Duan, N.; Khoshnevisan, B.; Lin, C.; Liu, Z.; Liu, H. Life Cycle Assessment of Anaerobic Digestion of Pig Manure Coupled with Different Digestate Treatment Technologies. Environ. Int. 2020, 137, 105522. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural Benefits and Environmental Risks of Soil Fertilization with Anaerobic Digestates: A Review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Walsh, J.J.; Jones, D.L.; Edwards-Jones, G.; Williams, A.P. Replacing Inorganic Fertilizer with Anaerobic Digestate May Maintain Agricultural Productivity at Less Environmental Cost. J. Plant Nutr. Soil Sci. 2012, 175, 840–845. [Google Scholar] [CrossRef]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakas, K.; Chojnacka, K. The Challenges and Perspectives for Anaerobic Digestion of Animal Waste and Fertilizer Application of the Digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Hong, Y.; Liu, X.; Wang, Q.; Zhai, Q.; Zhang, H. Attached Cultivation of Microalgae on Rational Carriers for Swine Wastewater Treatment and Biomass Harvesting. Bioresour. Technol. 2022, 351, 127014. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Y.; Pan, J.; Zhang, Y.; Liu, Z.; Lu, H.; Duan, N. Pretreatment of Pig Manure Liquid Digestate for Microalgae Cultivation via Innovative Flocculation-Biological Contact Oxidation Approach. Sci. Total Environ. 2019, 694, 133720. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Zhao, G.; Zhang, H. Nutrient Removal and Biogas Upgrading by Integrating Freshwater Algae Cultivation with Piggery Anaerobic Digestate Liquid Treatment. Appl. Microbiol. Biotechnol. 2015, 99, 6493–6501. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Ranglová, K.; Masojídek, J.; Drosg, B.; Meixner, K. Digestate as Sustainable Nutrient Source for Microalgae—Challenges and Prospects. Appl. Sci. 2021, 11, 1056. [Google Scholar] [CrossRef]
- Pulgarin, A.; Kapeller, A.G.; Tarik, M.; Egloff, S.; Mariotto, M.; Ludwig, C.; Refardt, D. Cultivation of Microalgae at High-Density with Pretreated Liquid Digestate as a Nitrogen Source: Fate of Nitrogen and Improvements on Growth Limitations. J. Clean. Prod. 2021, 324, 129238. [Google Scholar] [CrossRef]
- Zou, X.; Xu, K.; Chang, W.; Qu, Y.; Li, Y. A Novel Microalgal Biofilm Reactor Using Walnut Shell as Substratum for Microalgae Biofilm Cultivation and Lipid Accumulation. Renew. Energy 2021, 175, 676–685. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Z.; Jin, S.; Liu, C.; Li, Y.; Guo, D.; Hu, M.; Ruan, R.; Liu, Y. Role of Surface Roughness in the Algal Short-Term Cell Adhesion and Long-Term Biofilm Cultivation under Dynamic Flow Condition. Algal Res. 2020, 46, 101787. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, H.; Cheng, P.; Chang, T.; Chen, P.; Zhou, C.; Ruan, R. Development of Integrated Culture Systems and Harvesting Methods for Improved Algal Biomass Productivity and Wastewater Resource Recovery—A Review. Sci. Total Environ. 2020, 746, 141039. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, Composition, Production, Processing and Applications of Chlorella vulgaris: A Review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Development and Applications of Attached Growth System for Microalgae Biomass Production. BioEnergy Res. 2021, 14, 709–722. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Microalgae Biofuels: A Critical Review of Issues, Problems and the Way Forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Mantzorou, A.; Ververidis, F. Microalgal Biofilms: A Further Step over Current Microalgal Cultivation Techniques. Sci. Total Environ. 2019, 651, 3187–3201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Z.; Jin, S.; Zhu, L.; Liu, C.; Zheng, H.; Zhou, T.; Liu, Y.; Ruan, R. Lignocellulosic Residue as Bio-Carrier for Algal Biofilm Growth: Effects of Carrier Physicochemical Proprieties and Toxicity on Algal Biomass Production and Composition. Bioresour. Technol. 2019, 293, 122091. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Patidar, S.K. Microalgae Harvesting Techniques: A Review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Thornton, P.K.; Gerber, P.J. Climate Change and the Growth of the Livestock Sector in Developing Countries. Mitig. Adapt. Strateg. Glob. Chang. 2010, 15, 169–184. [Google Scholar] [CrossRef]
- Harrison, M.T.; Cullen, B.R.; Mayberry, D.E.; Cowie, A.L.; Bilotto, F.; Badgery, W.B.; Liu, K.; Davison, T.; Christie, K.M.; Muleke, A.; et al. Carbon Myopia: The Urgent Need for Integrated Social, Economic and Environmental Action in the Livestock Sector. Glob. Chang. Biol. 2021, 27, 5726–5761. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lin, C.-I. Overview Analysis of Bioenergy from Livestock Manure Management in Taiwan. Renew. Sustain. Energy Rev. 2009, 13, 2682–2688. [Google Scholar] [CrossRef]
- Markou, G.; Diamantis, A.; Arapoglou, D.; Mitrogiannis, D.; González-Fernández, C.; Unc, A. Growing Spirulina (Arthrospira Platensis) in Seawater Supplemented with Digestate: Trade-Offs between Increased Salinity, Nutrient and Light Availability. Biochem. Eng. J. 2021, 165, 107815. [Google Scholar] [CrossRef]
- Silva, G.H.R.; Sueitt, A.P.E.; Haimes, S.; Tripidaki, A.; van Zwieten, R.; Fernandes, T.V. Feasibility of Closing Nutrient Cycles from Black Water by Microalgae-Based Technology. Algal Res. 2019, 44, 101715. [Google Scholar] [CrossRef]
- Figler, A.; Márton, K.; B-Béres, V.; Bácsi, I. Effects of Nutrient Content and Nitrogen to Phosphorous Ratio on the Growth, Nutrient Removal and Desalination Properties of the Green Alga Coelastrum Morus on a Laboratory Scale. Energies 2021, 14, 2112. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Guo, D.; Ye, T.; Xiong, M.; Zhu, L.; Liu, C.; Jin, S.; Hu, Z. Operation of a Vertical Algal Biofilm Enhanced Raceway Pond for Nutrient Removal and Microalgae-Based Byproducts Production under Different Wastewater Loadings. Bioresour. Technol. 2018, 253, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pei, H.; Li, Y.; Yang, Z.; Xie, Z.; Hou, Q.; Nie, C. Inclined Algal Biofilm Photobioreactor (IABPBR) for Cost-Effective Cultivation of Lipid-Rich Microalgae and Treatment of Seawater-Diluted Anaerobically Digested Effluent from Kitchen Waste with the Aid of Phytohormones. Bioresour. Technol. 2020, 315, 123761. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of Lakes Cannot Be Controlled by Reducing Nitrogen Input: Results of a 37-Year Whole-Ecosystem Experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Hillebrand, H.; Steinert, G.; Boersma, M.; Malzahn, A.; Meunier, C.L.; Plum, C.; Ptacnik, R. Goldman Revisited: Faster-Growing Phytoplankton Has Lower N : P and Lower Stoichiometric Flexibility. Limnol. Oceanogr. 2013, 58, 2076–2088. [Google Scholar] [CrossRef]
- Xiao, M.; Burford, M.A.; Wood, S.A.; Aubriot, L.; Ibelings, B.W.; Prentice, M.J.; Galvanese, E.F.; Harris, T.D.; Hamilton, D.P. Schindler’s Legacy: From Eutrophic Lakes to the Phosphorus Utilization Strategies of Cyanobacteria. FEMS Microbiol. Rev. 2022, 46, fuac029. [Google Scholar] [CrossRef]
- Fernandes, T.V.; Suárez-Muñoz, M.; Trebuch, L.M.; Verbraak, P.J.; Van de Waal, D.B. Toward an Ecologically Optimized N:P Recovery from Wastewater by Microalgae. Front. Microbiol. 2017, 8, 1742. [Google Scholar] [CrossRef]
- Purkhold, U.; Pommerening-Röser, A.; Juretschko, S.; Schmid, M.C.; Koops, H.-P.; Wagner, M. Phylogeny of All Recognized Species of Ammonia Oxidizers Based on Comparative 16S rRNA and amoA Sequence Analysis: Implications for Molecular Diversity Surveys. Appl. Environ. Microbiol. 2000, 66, 5368–5382. [Google Scholar] [CrossRef]
- Risgaard-Petersen, N.; Nicolaisen, M.H.; Revsbech, N.P.; Lomstein, B.A. Competition between Ammonia-Oxidizing Bacteria and Benthic Microalgae. Appl. Environ. Microbiol. 2004, 70, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Chang, Y.-C.; Lin, Y.-Y.; Hsu, T.-H.; Pan, Y.-J. Effects of Temperature and Microalgal Diet on the Fecundity, Population Growth and Fatty Acid Composition of Pseudodiaptomus Ishigakiensis (Calanoid, Copepoda). Aquac. Rep. 2023, 32, 101715. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ma, C.-H.; Lee, H.-T.; Hsu, T.-H. Polyculture of Juvenile Dog Conch Laevistrombus Canarium Reveals High Potentiality in Integrated Multitrophic Aquaculture (IMTA). Biology 2021, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Dassey, A.J.; Hall, S.G.; Theegala, C.S. An Analysis of Energy Consumption for Algal Biodiesel Production: Comparing the Literature with Current Estimates. Algal Res. 2014, 4, 89–95. [Google Scholar] [CrossRef]
- Quines, O.D. Microorganisms: Indicators of Pollution in Integrated Livestock-Fish Farming Systems. Environ. Int. 1988, 14, 531–534. [Google Scholar] [CrossRef]
- Li, S.; Show, P.L.; Ngo, H.H.; Ho, S.-H. Algae-Mediated Antibiotic Wastewater Treatment: A Critical Review. Environ. Sci. Ecotechnology 2022, 9, 100145. [Google Scholar] [CrossRef]
- Lee, S.-A.; Lee, N.; Oh, H.-M.; Ahn, C.-Y. Stepwise Treatment of Undiluted Raw Piggery Wastewater, Using Three Microalgal Species Adapted to High Ammonia. Chemosphere 2021, 263, 127934. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Vadiveloo, A.; Bahri, P.B.; McKirdy, S.J.; Moheimani, N.R. Understanding Photochemical Adaptations of Microalgal Ammonium Extremophiles to Elevated Ammonium Concentrations in Abattoir Anaerobic Digestate. Algal Res. 2023, 75, 103272. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Pan, Y.-J.; Huang, T.-H.; Hsiao, T.-H.; Wei, L.-Y.; Hsu, T.-H. Cultivation of Brackish Water Microalgae for Pig Manure Liquid Digestate Recycling. Sustainability 2023, 15, 16278. https://doi.org/10.3390/su152316278
Chang Y-C, Pan Y-J, Huang T-H, Hsiao T-H, Wei L-Y, Hsu T-H. Cultivation of Brackish Water Microalgae for Pig Manure Liquid Digestate Recycling. Sustainability. 2023; 15(23):16278. https://doi.org/10.3390/su152316278
Chicago/Turabian StyleChang, Yung-Cheng, Yen-Ju Pan, Tzu-Hsuan Huang, Ting-Hsun Hsiao, Liang-Yu Wei, and Te-Hua Hsu. 2023. "Cultivation of Brackish Water Microalgae for Pig Manure Liquid Digestate Recycling" Sustainability 15, no. 23: 16278. https://doi.org/10.3390/su152316278
APA StyleChang, Y.-C., Pan, Y.-J., Huang, T.-H., Hsiao, T.-H., Wei, L.-Y., & Hsu, T.-H. (2023). Cultivation of Brackish Water Microalgae for Pig Manure Liquid Digestate Recycling. Sustainability, 15(23), 16278. https://doi.org/10.3390/su152316278






