Climate Change and New Challenges for Rural Communities: Particulate Matter Matters
Abstract
1. Introduction
2. Materials and Methods
2.1. Dust Sample Collection, Preparation, and Characterization
2.2. Cell Culture and Exposure
2.3. DNA and RNA Extraction
2.4. Gene Expression Analysis
2.5. DNA Methylation Analysis
2.6. Analysis of the Methylation Status of Satellite DNA
2.7. Methyltransferase Activity
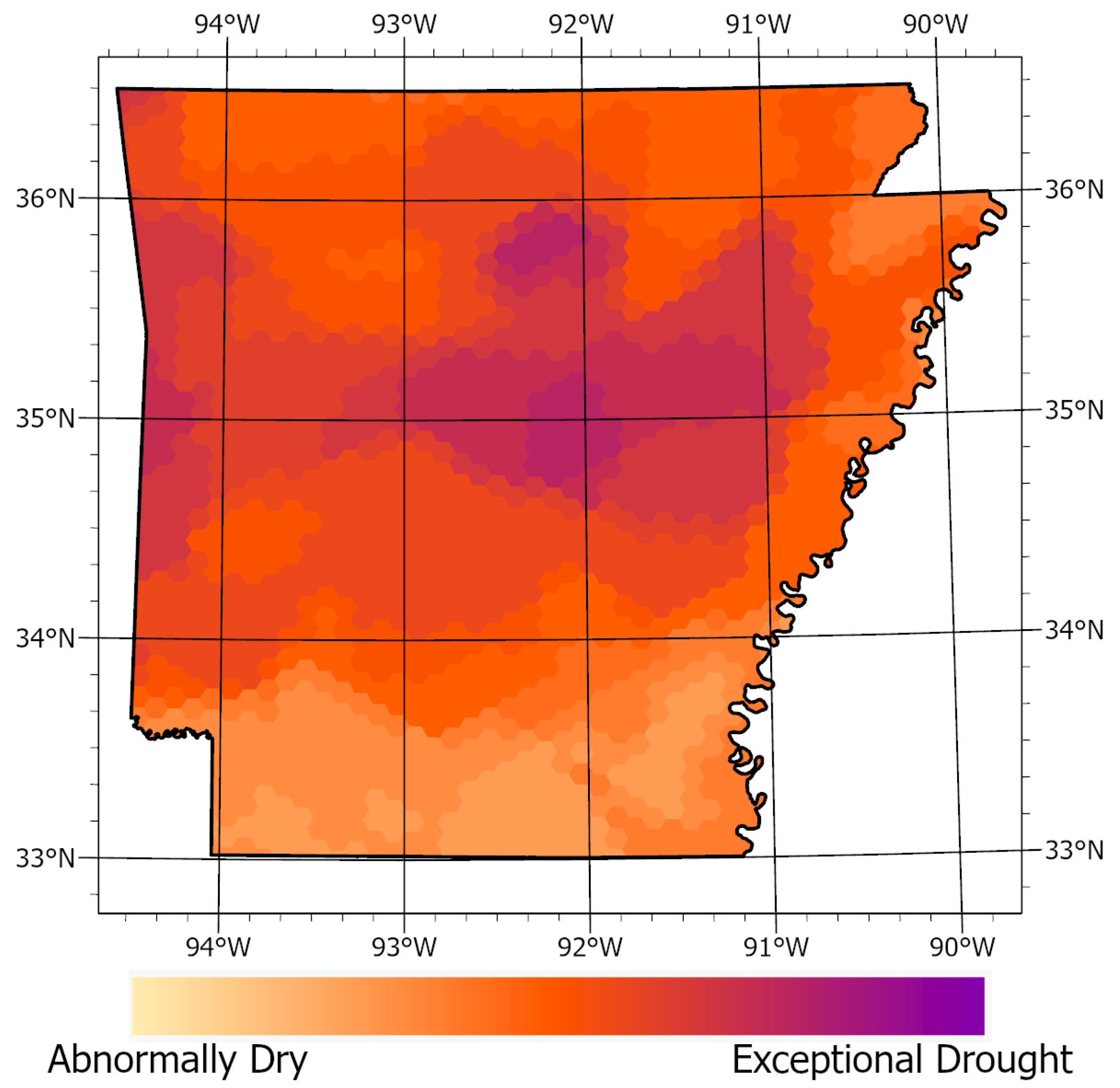
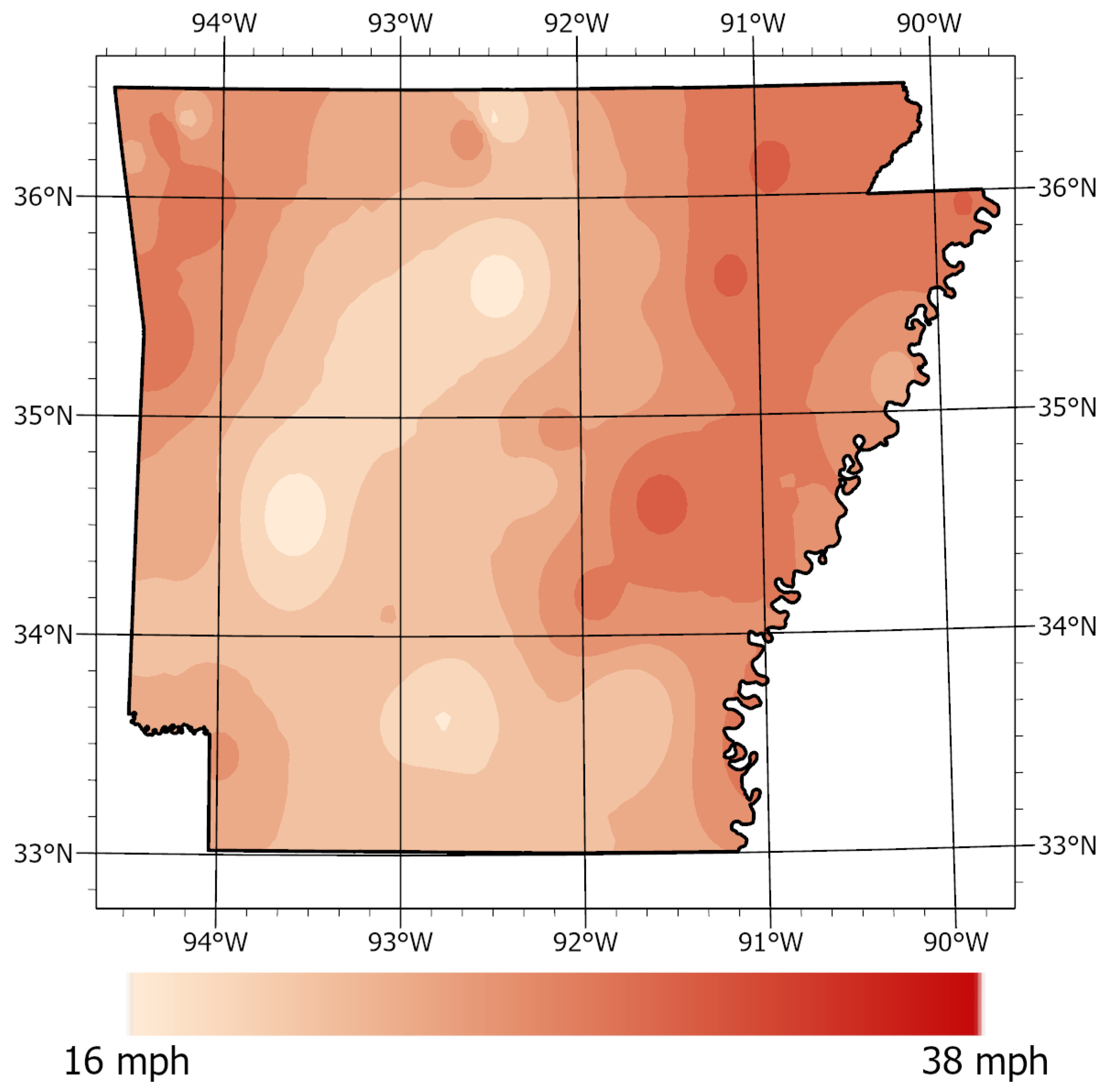
2.8. Geospatial Analysis
2.9. Statistical Analysis
3. Results
3.1. Molecular Effects of Exposure to Dust: Oxidative Stress, Inflammation, and Epigenetic Alterations
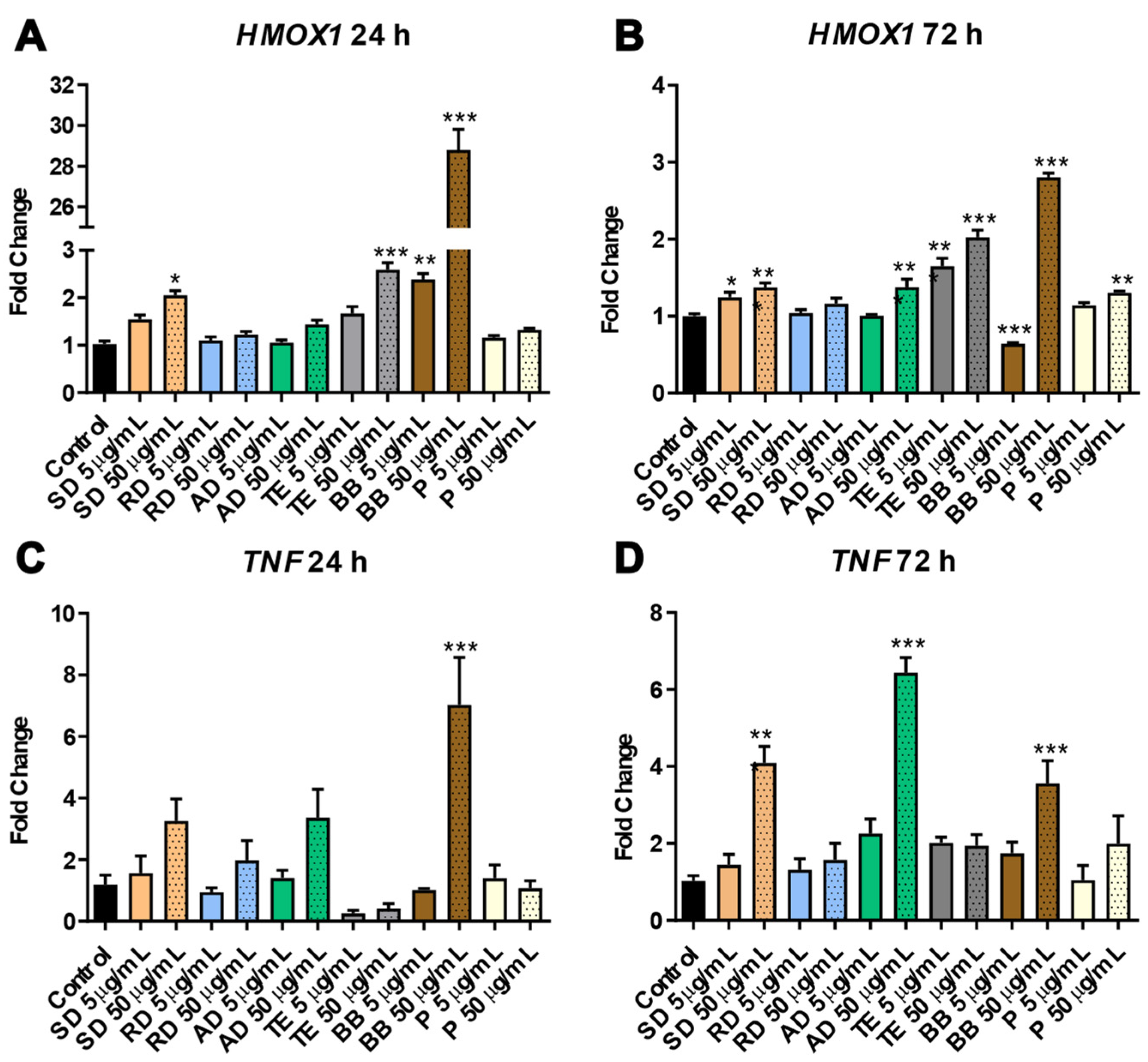
3.1.1. Selective Effects of PM Exposure on Oxidative Stress and Inflammation Markers in SAEC Cells
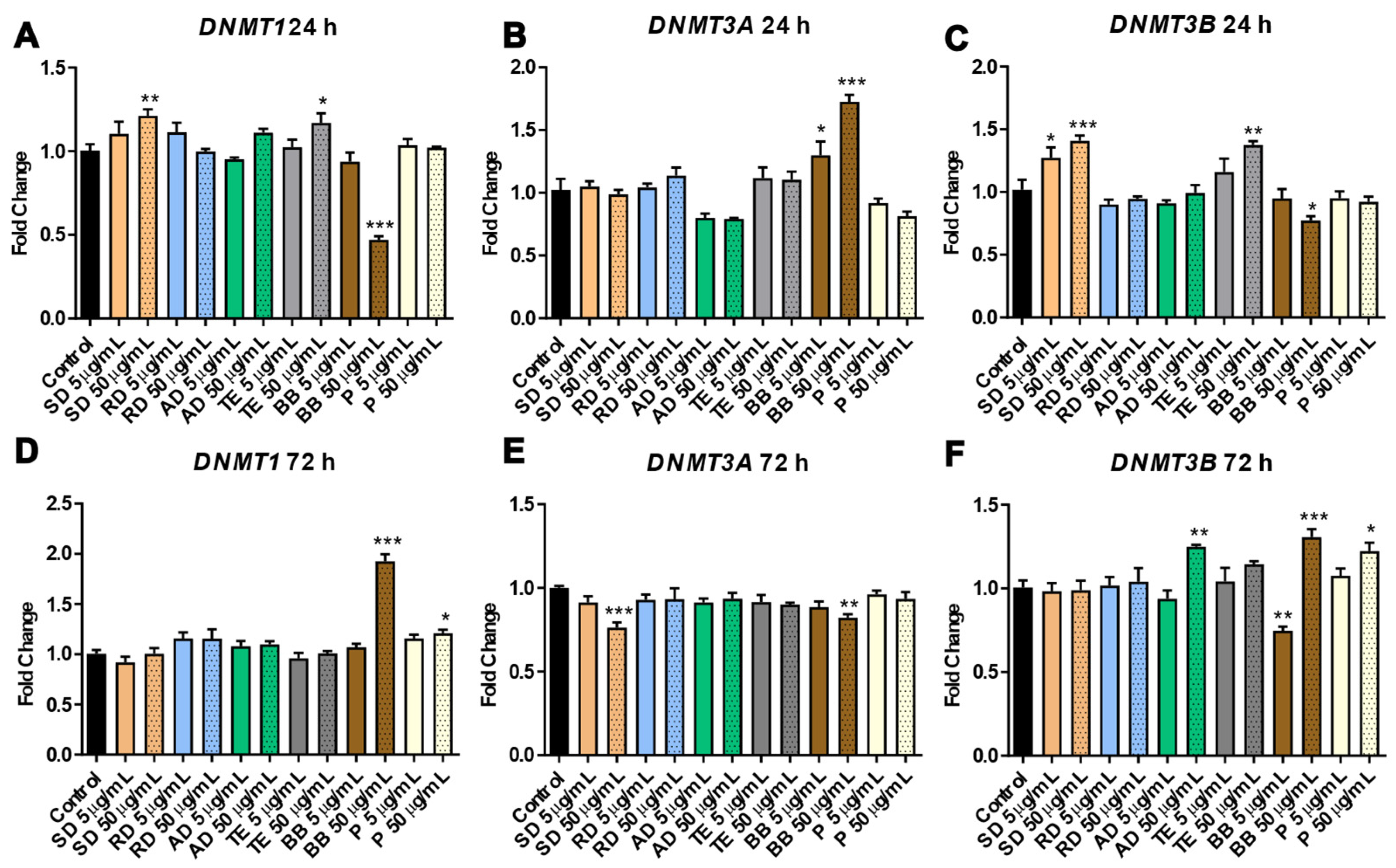
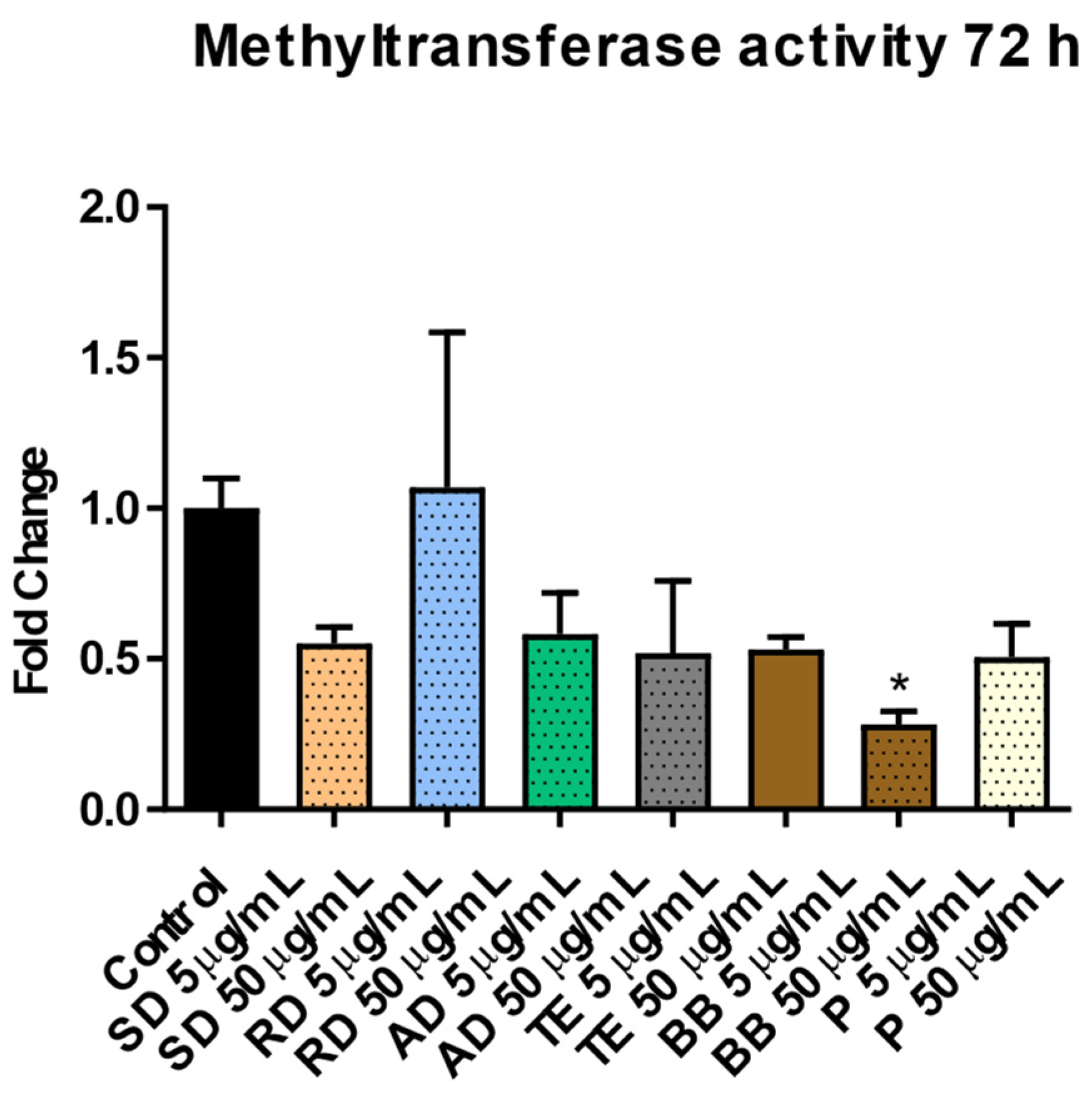
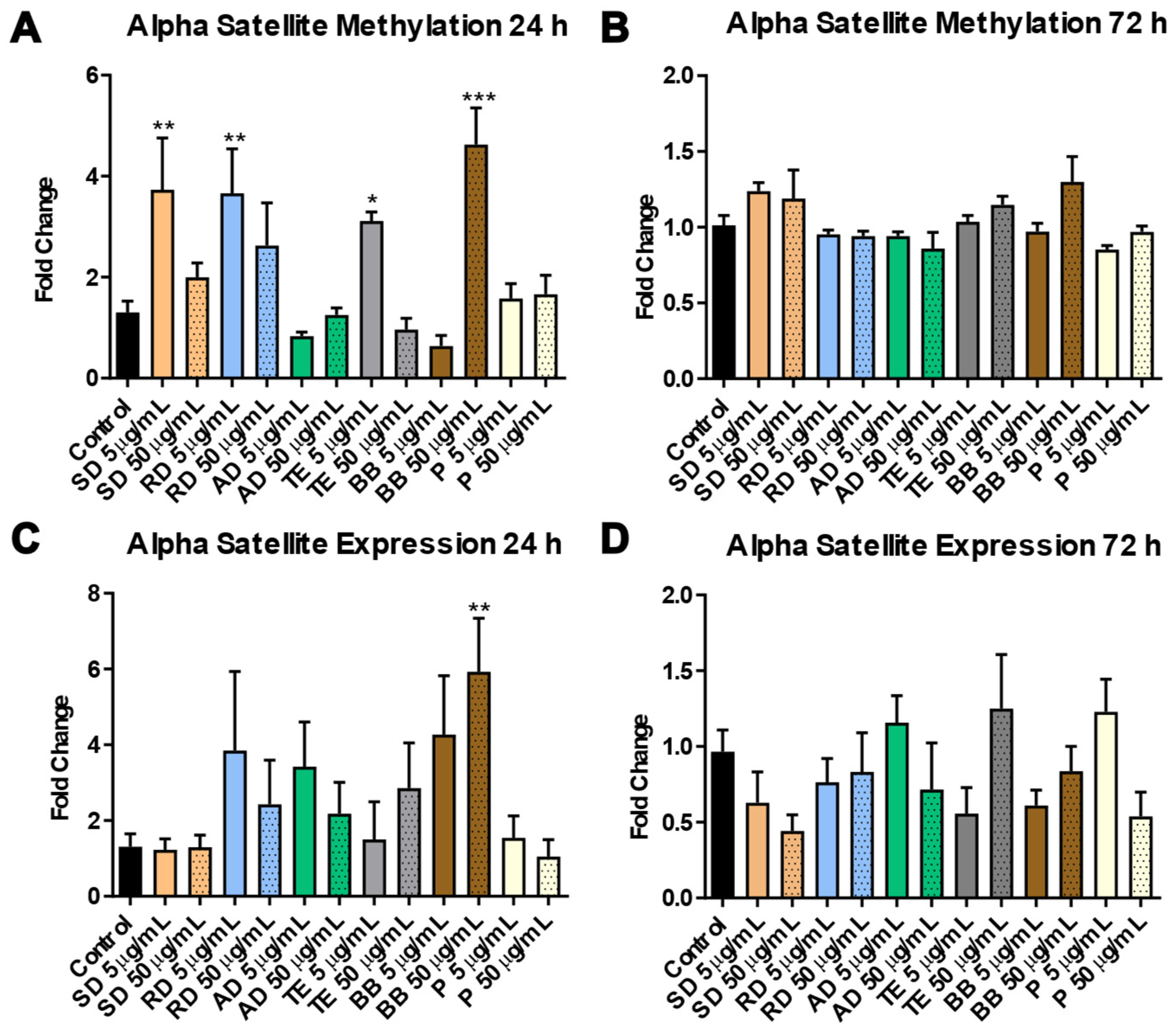
3.1.2. Involvement of Epigenetic Mechanisms in Response to Exposure to PM
3.2. Geospatial Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamble, J.L.; Balbus, J.; Berger, M.; Bouye, K.; Campbell, V.; Chief, K.; Conlon, K.; Crimmins, A.; Fiangan, B.; Gonzalez-Maddux, C.; et al. Ch 9 Populations of Concern: The Impacts of Climate Change on Human Health in the United States: A Scientific Assessment. U.S. Global Change Research Program. 2016. Available online: https://health2016.globalchange.gov/low/ClimateHealth2016_09_Populations_small.pdf (accessed on 15 October 2023).
- Dwyer-Lindgren, L.; Bertozzi-Villa, A.; Stubbs, R.W.; Morozoff, C.; Shirude, S.; Naghavi, M.; Mokdad, A.H.; Murray, C.J.L. Trends and Patterns of Differences in Chronic Respiratory Disease Mortality among US Counties, 1980–2014. JAMA 2017, 318, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, A.W.; Himmelstein, D.U.; Christiani, D.C.; Woolhandler, S. Socioeconomic Inequality in Respiratory Health in the US from 1959 to 2018. JAMA Intern. Med. 2021, 181, 968–976, Erratum in JAMA Intern. Med. 2021, 181, 1021. [Google Scholar] [CrossRef] [PubMed]
- Suran, M. Raging Wildfires Are Exposing More People to Smoky Air-Here’s What That Means for Health. JAMA 2023, 330, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- United Nations Organization Report. Number of Wildfires to Rise by 50% by 2100 and Governments are Not Prepared, Experts Warn. 23 February 2022. Available online: https://www.unep.org/news-and-stories/press-release/number-wildfires-rise-50-2100-and-governments-are-not-prepared (accessed on 16 October 2023).
- Chen, K.; Ma, Y.; Bell, M.L.; Yang, W. Canadian Wildfire Smoke and Asthma Syndrome Emergency Department Visits in New York City. JAMA 2023, 330, e2318768. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Feng, M.; Nian, Y.; Huang, L.; Xie, T.; Zhang, K.; Chen, F.; Huang, W.; Chen, J.; et al. High agricultural water consumption led to the continued shrinkage of the Aral Sea during 1992–2015. Sci. Total Environ. 2021, 777, 145993. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Shomurodov, K.; Tian, C. Address the Aral Sea crisis with cooperation. Science 2023, 380, 1114. [Google Scholar] [CrossRef]
- Erkudov, V.O.; Rozumbetov, K.U.; González-Fernández, F.T.; Pugovkin, A.P.; Nazhimov, I.I.; Matchanov, A.T.; Ceylan, H. The Effect of Environmental Disasters on Endocrine Status, Hematology Parameters, Body Composition, and Physical Performance in Young Soccer Players: A Case Study of the Aral Sea Region. Life 2023, 13, 1503. [Google Scholar] [CrossRef]
- Mamyrbayev, A.; Djarkenov, T.; Dosbayev, A.; Dusembayeva, N.; Shpakov, A.; Umarova, G.; Drobchenko, Y.; Kunurkulzhayev, T.; Zhaylybaev, M.; Isayeva, G. The Incidence of Malignant Tumors in Environmentally Disadvantaged Regions of Kazakhstan. Asian Pac. J. Cancer Prev. 2016, 17, 5203–5209. [Google Scholar] [CrossRef]
- Doede, A.L.; DeGuzman, P.B. The Disappearing Lake: A Historical Analysis of Drought and the Salton Sea in the Context of the GeoHealth Framework. Geohealth 2020, 4, e2020GH000271. [Google Scholar] [CrossRef]
- Johnson, T.N. The Dakota Access Pipeline and the Breakdown of Participatory Processes in Environmental Decision-Making. Environ. Commun. 2019, 13, 335–352. [Google Scholar] [CrossRef]
- Goodbred, S.L.; Patiño, R.; Torres, L.; Echols, K.R.; Jenkins, J.A.; Rosen, M.R.; Orsak, E. Are endocrine and reproductive biomarkers altered in contaminant-exposed wild male Largemouth Bass (Micropterus salmoides) of Lake Mead, Nevada/Arizona, USA? Gen. Comp. Endocrinol. 2015, 219, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Rural Development in Arkansas. State of Arkansas, House of Representatives. 17 May 2019. Available online: https://www.arkansashouse.org/news/post/7481/rural-development-in-arkansas#:~:text=In%20Arkansas%2C%2041%25%20of%20the,that%20our%20rural%20communities%20face (accessed on 12 October 2023).
- Robert Wood Johnson Foundation Program. County Health Rankings and Road Maps. 2023. Available online: http://www.countyhealthrankings.org/ (accessed on 17 October 2023).
- Miousse, I.R.; Chalbot, M.C.; Aykin-Burns, N.; Wang, X.; Basnakian, A.; Kavouras, I.G.; Koturbash, I. Epigenetic alterations induced by ambient particulate matter in mouse macrophages. Environ. Mol. Mutagen. 2014, 55, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Miousse, I.R.; Chalbot, M.C.; Pathak, R.; Lu, X.; Nzabarushimana, E.; Krager, K.; Aykin-Burns, N.; Hauer-Jensen, M.; Demokritou, P.; Kavouras, I.G.; et al. In Vitro Toxicity and Epigenotoxicity of Different Types of Ambient Particulate Matter. Toxicol. Sci. 2015, 148, 473–487. [Google Scholar] [CrossRef]
- Cline, S. Louisiana Plagued by Unprecedented Wildfires, as Largest Active Blaze Grows. Associated Press, 29 August 2023. Available online: https://apnews.com/article/louisiana-wildfire-f396f586d0404e4ceefa194ffbff8a82 (accessed on 17 October 2023).
- Cline, S. In a State Used to Hurricanes and Flooding, Louisiana is Battling an Unprecedented Wildfire Season. Associated Press, 18 September 2023. Available online: https://apnews.com/article/louisiana-wildfire-b9d8968c1ce98b009c3ce95fa08a8f40 (accessed on 17 October 2023).
- Marlon, J.; Neyens, L.; Jefferson, M.; Howe, P.; Mildenberger, M.; Leiserowitz, A.; Yale Climate Opinion Maps 2021. Yale Program on Climate Change Communication. 23 February 2022. Available online: https://climatecommunication.yale.edu/visualizations-data/ycom-us/ (accessed on 17 October 2023).
- Gunnar, I.; Koturbash, B.; (UAMS, Dermott, AR, USA). Personal Communication. Tanya Broadnax (Rural Community Alliance) and Chicot County Community member meeting. 27 August 2023. [Google Scholar]
- Marple, V.A.; Rubow, K.L.; Turner, W.; Spengler, J.D. Low flow rate sharp cut impactors for indoor air sampling: Design and calibration. JAPCA 1987, 37, 1303–1307. [Google Scholar] [CrossRef]
- Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A.; Cass, G.R.; Simoneit, B.R.T. Sources of fine organic aerosol. 3. Road dust, tire debris, and organometallic brake lining dust: Roads as sources and sinks. Environ. Sci. Technol. 1993, 27, 1892–1904. [Google Scholar] [CrossRef]
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential—The RAPTES project. Particle Fibre Toxicol. 2011, 8, 26. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Ewing, L.E.; Pathak, R.; Landes, R.D.; Skinner, C.M.; Binz, R.; Young, S.G.; Riklon, S.; Stahr, S.; Su, J.; Boerma, M.; et al. Cytogenetic and epigenetic aberrations in peripheral lymphocytes of northwest Arkansas Marshallese. Int. J. Radiat. Biol. 2023, 99, 644–655. [Google Scholar] [CrossRef]
- Martens, J.H.; O’Sullivan, R.J.; Braunschweig, U.; Opravil, S.; Radolf, M.; Steinlein, P.; Jenuwein, T. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J. 2005, 24, 800–812. [Google Scholar] [CrossRef]
- Koturbash, I.; Scherhag, A.; Sorrentino, J.; Sexton, K.; Bodnar, W.; Tryndyak, V.; Latendresse, J.R.; Swenberg, J.A.; Beland, F.A.; Pogribny, I.P.; et al. Epigenetic alterations in liver of C57BL/6J mice after short-term inhalational exposure to 1,3-butadiene. Environ. Health Perspect. 2011, 119, 635–640. [Google Scholar] [CrossRef][Green Version]
- National Drought Mitigation Center (NDMC); U.S. Department of Agriculture (USDA); National Oceanic and Atmospheric Administration (NOAA). GIS Data. U.S. Drought Monitor. Available online: https://droughtmonitor.unl.edu/DmData/GISData.aspx (accessed on 19 October 2023).
- National Centers for Environmental Information (NCEI). Climate Data Online. Climate Data Online (CDO)—The National Climatic Data Center’s (NCDC) Climate Data Online (CDO) Provides Free Access to NCDC’s Archive of Historical Weather and Climate Data in Addition to Station History Information. National Climatic Data Center (NCDC). Available online: https://www.ncei.noaa.gov/cdo-web/ (accessed on 19 October 2023).
- Kurosaki, Y.; Mikami, M. Threshold wind speed for dust emission in East Asia and its seasonal variations. J. Geophys. Res. Atmos. 2007, 112, D17. [Google Scholar] [CrossRef]
- Mandal, A.; Sarkar, B.; Mandal, S.; Vithanage, M.; Patra, A.K.; Manna, M.C. Chapter 7—Impact of agrochemicals on soil health. In Agrochemicals Detection, Treatment and Remediation: Pesticides and Chemical Fertilizers; Butterworth-Heinemann: Oxford, UK, 2020; pp. 161–187. [Google Scholar]
- US EPA. Persistent Bioaccumulative and Toxic (PBT) Chemical Program. 2000. Available online: http://www.epa.gov/pbt/pubs/pestaction.htm#Introduction (accessed on 15 October 2023).
- Vasileva, D.; Greenwood, C.M.T.; Daley, D. A Review of the Epigenetic Clock: Emerging Biomarkers for Asthma and Allergic Disease. Genes 2023, 14, 1724. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.D.; Liu, Y.; Kelly, T.K.; Witt, H.; Farnham, P.J.; Jones, P.A.; Berman, B.P. The role of DNA methylation in directing the functional organization of the cancer epigenome. Genome Res. 2015, 25, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Lieberman-Cribbin, W.; Domingo-Relloso, A.; Navas-Acien, A.; Cole, S.; Haack, K.; Umans, J.; Tellez-Plaza, M.; Colicino, E.; Baccarelli, A.A.; Gao, X.; et al. Epigenetic Biomarkers of Lead Exposure and Cardiovascular Disease: Prospective Evidence in the Strong Heart Study. J. Am. Heart Assoc. 2022, 11, e026934. [Google Scholar] [CrossRef]
- Bozack, A.K.; Rifas-Shiman, S.L.; Coull, B.A.; Baccarelli, A.A.; Wright, R.O.; Amarasiriwardena, C.; Gold, D.R.; Oken, E.; Hivert, M.F.; Cardenas, A. Prenatal metal exposure, cord blood DNA methylation and persistence in childhood: An epigenome-wide association study of 12 metals. Clin. Epigenetics 2021, 13, 208. [Google Scholar] [CrossRef]
- Holliday, K.M.; Gondalia, R.; Baldassari, A.; Justice, A.E.; Stewart, J.D.; Liao, D.; Yanosky, J.D.; Jordahl, K.M.; Bhatti, P.; Assimes, T.L.; et al. Gaseous air pollutants and DNA methylation in a methylome-wide association study of an ethnically and environmentally diverse population of U.S. adults. Environ Res. 2022, 212 Pt C, 113360. [Google Scholar] [CrossRef]
- Gao, X.; Huang, J.; Cardenas, A.; Zhao, Y.; Sun, Y.; Wang, J.; Xue, L.; Baccarelli, A.A.; Guo, X.; Zhang, L.; et al. Short-Term Exposure of PM2.5 and Epigenetic Aging: A Quasi-Experimental Study. Environ. Sci. Technol. 2022, 56, 14690–14700. [Google Scholar] [CrossRef]
- Gondalia, R.; Baldassari, A.; Holliday, K.M.; Justice, A.E.; Stewart, J.D.; Liao, D.; Yanosky, J.D.; Engel, S.M.; Sheps, D.; Jordahl, K.M.; et al. Epigenetically mediated electrocardiographic manifestations of sub-chronic exposures to ambient particulate matter air pollution in the Women’s Health Initiative and Atherosclerosis Risk in Communities Study. Environ Res. 2021, 198, 111211. [Google Scholar] [CrossRef]
- Alam, M.N.; Shapla, U.M.; Shen, H.; Huang, Q. Linking emerging contaminants exposure to adverse health effects: Crosstalk between epigenome and environment. J. Appl. Toxicol. 2021, 41, 878–897. [Google Scholar] [CrossRef]
- Wu, H.; Eckhardt, C.M.; Baccarelli, A.A. Molecular mechanisms of environmental exposures and human disease. Nat. Rev. Genet. 2023, 24, 332–344. [Google Scholar] [CrossRef]
- Cardenas, A.; Fadadu, R.; Bunyavanich, S. Climate change and epigenetic biomarkers in allergic and airway diseases. J. Allergy Clin. Immunol. 2023, 152, 1060–1072. [Google Scholar] [CrossRef]
- De Koning, A.P.; Gu, W.; Castoe, T.A.; Batzer, M.A.; Pollock, D.D. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011, 7, e1002384. [Google Scholar] [CrossRef] [PubMed]
- Miousse, I.R.; Chalbot, M.C.; Lumen, A.; Ferguson, A.; Kavouras, I.G.; Koturbash, I. Response of transposable elements to environmental stressors. Mutat. Res. Rev. Mutat. Res. 2015, 765, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Reddam, A.; Bollati, V.; Wu, H.; Favero, C.; Tarantini, L.; Hoxha, M.; Comfort, N.; Gold, D.R.; Phipatanakul, W.; Baccarelli, A.A. Air pollution and human endogenous retrovirus methylation in the school inner-city asthma intervention study. Toxicol Sci. 2023, 193, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.; Miousse, I.R.; Nzabarushimana, E.; Pathak, R.; Skinner, C.; Kutanzi, K.R.; Allen, A.R.; Raber, J.; Tackett, A.J.; Hauer-Jensen, M.; et al. Densely ionizing radiation affects DNA methylation of selective LINE-1 elements. Environ. Res. 2016, 150, 470–481. [Google Scholar] [CrossRef]
- Feliciello, I.; Pezer, Ž.; Kordiš, D.; Bruvo Mađarić, B.; Ugarković, Đ. Evolutionary History of Alpha Satellite DNA Repeats Dispersed within Human Genome Euchromatin. Genome Biol. Evol. 2020, 12, 2125–2138. [Google Scholar] [CrossRef]
- Arkansas Department of Environmental Quality, 1999. Water Quality Assessment of Arkansas’ Significant Publicly-Owned Lakes, Summer 1999. WQ-00-06.1. Available online: https://www.adeq.state.ar.us/water/planning/pdfs/publications/WQ00-06-1.pdf (accessed on 15 October 2023).
- Cooper, C.M.; Knight, S.S. Residual pesticides in fishes from Lake Chicot, Arkansas. J. Ark. Acad. Sci. 1987, 41, 9. [Google Scholar]
- Indoitu, R.; Kozhoridze, G.; Batyrbaeva, M.; Vitkovskaya, I.; Orlovsky, N.; Blumberg, D.; Orlovsky, L. Dust emission and environmental changes in the dried bottom of the Aral Sea. Aeolian Res. 2015, 17, 101–115. [Google Scholar] [CrossRef]
- Hamilton, J.D. Competing and converging values of public participation: A case study of participant views in Department of Energy nuclear weapons cleanup. In Communication and Public Participation in Environmental Decision Making; Depoe, S.P., Delicath, J.W., Elsenbeer, M.-F.A., Eds.; SUNY Press: Albany, NY, USA, 2004; pp. 58–81. [Google Scholar]
- Hamilton, J.D. Convergence and divergence in the public dialogue on nuclear weapons cleanup. In Nuclear Legacies: Communication, Controversy, and the U.S. Nuclear Weapons Complex; Taylor, B.C., Kinsella, W.J., Depoe, S.P., Metzler, M.S., Eds.; Lexington Book: Lanham, MD, USA, 2007; pp. 41–72. [Google Scholar]
- Fung, A. Putting the Public Back into Governance: The Challenges of Citizen Participation and Its Future. Public Adm. Rev. 2015, 75, 513–522. [Google Scholar] [CrossRef]
- Johnston, J.E.; Razafy, M.; Lugo, H.; Olmedo, L.; Farzan, S.F. The disappearing Salton Sea: A critical reflection on the emerging environmental threat of disappearing saline lakes and potential impacts on children’s health. Sci. Total Environ. 2019, 663, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Kastelic, S.L.; Wallerstein, N.; Duran, B.; Oetzel, J.G. Chapter 6—Socio-ecologic framework for CBPR: Development and testing of a model. In Community-Based Participatory Research for Health: Advancing Social and Health Equity; Jossey-Bass: Hoboken, NJ, USA, 2018; pp. 175–201. [Google Scholar]
- Den Broeder, L.; Devilee, J.; Van Oers, H.; Schuit, A.J.; Wagemakers, A. Citizen science for public health. Health Promot. Int. 2018, 33, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.; Hambly, J.; Kelley, A.; Lees, W.; Miller, S. Coastal heritage, global climate change, public engagement, and citizen science. Proc. Natl. Acad. Sci. USA 2020, 117, 8280–8286. [Google Scholar] [CrossRef]
- Rathnayake, C.; Joshi, S.; Cerratto-Pargman, T. Mapping the current landscape of citizen-driven environmental monitoring: A systematic literature review. Sustain. Sci. Pract. Policy 2020, 16, 326–334. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miousse, I.R.; Hale, R.B.; Alsbrook, S.; Boysen, G.; Broadnax, T.; Murry, C.; Williams, C.; Park, C.H.; Richards, R.; Reedy, J.; et al. Climate Change and New Challenges for Rural Communities: Particulate Matter Matters. Sustainability 2023, 15, 16192. https://doi.org/10.3390/su152316192
Miousse IR, Hale RB, Alsbrook S, Boysen G, Broadnax T, Murry C, Williams C, Park CH, Richards R, Reedy J, et al. Climate Change and New Challenges for Rural Communities: Particulate Matter Matters. Sustainability. 2023; 15(23):16192. https://doi.org/10.3390/su152316192
Chicago/Turabian StyleMiousse, Isabelle Racine, Rachel B. Hale, Scott Alsbrook, Gunnar Boysen, Tanya Broadnax, Carleisha Murry, Candace Williams, Chul Hyun Park, Robert Richards, Justin Reedy, and et al. 2023. "Climate Change and New Challenges for Rural Communities: Particulate Matter Matters" Sustainability 15, no. 23: 16192. https://doi.org/10.3390/su152316192
APA StyleMiousse, I. R., Hale, R. B., Alsbrook, S., Boysen, G., Broadnax, T., Murry, C., Williams, C., Park, C. H., Richards, R., Reedy, J., Chalbot, M.-C., Kavouras, I. G., & Koturbash, I. (2023). Climate Change and New Challenges for Rural Communities: Particulate Matter Matters. Sustainability, 15(23), 16192. https://doi.org/10.3390/su152316192










