Short-Term Nitrous Oxide Emissions from Cattle Slurry for Silage Maize: Effects of Placement and the Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. N2O Measurements
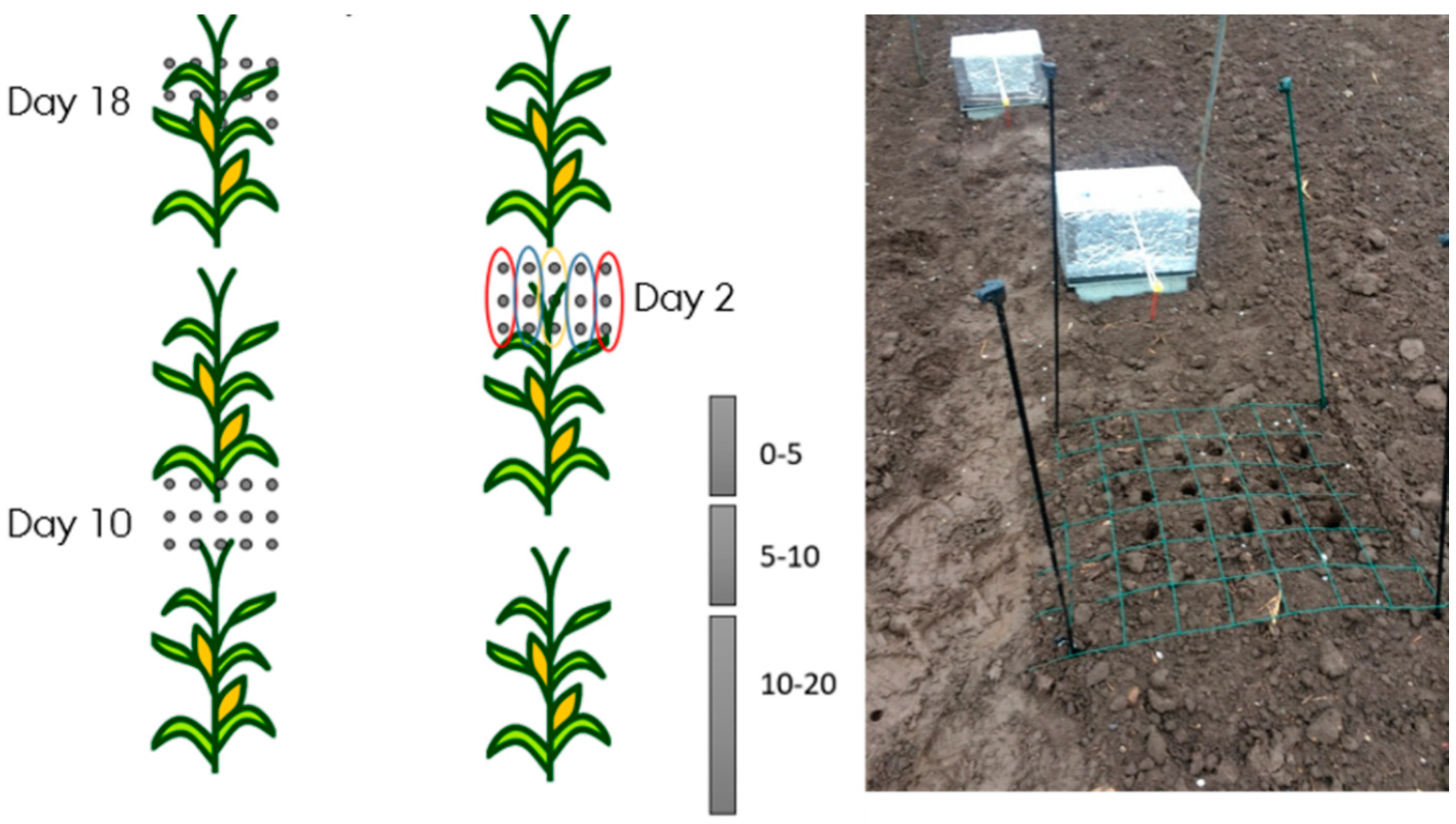
2.3. Soil Sampling
2.4. Distribution of Mineral N after Placement
2.5. Data Analyses
3. Results
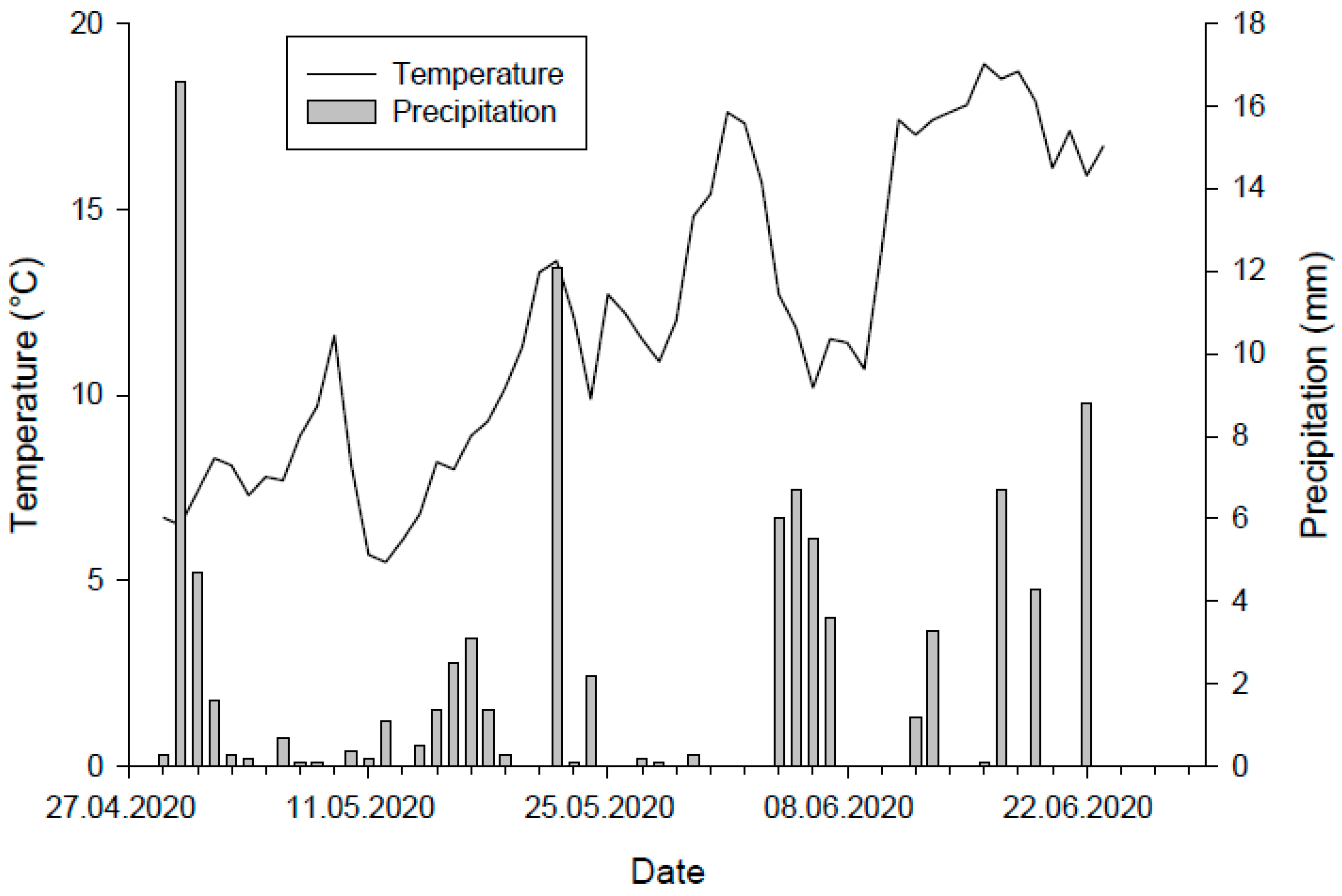
3.1. Environmental Conditions
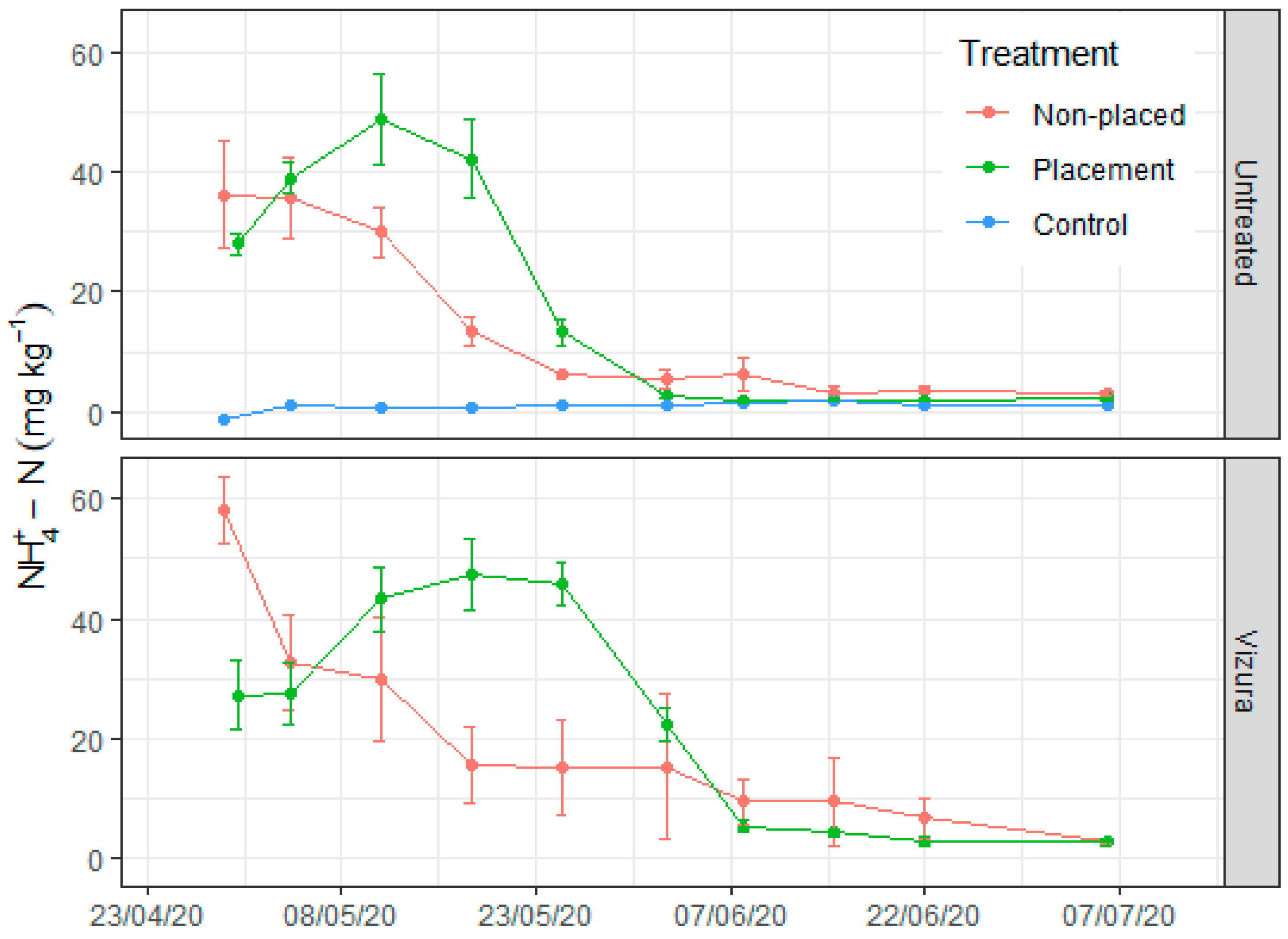
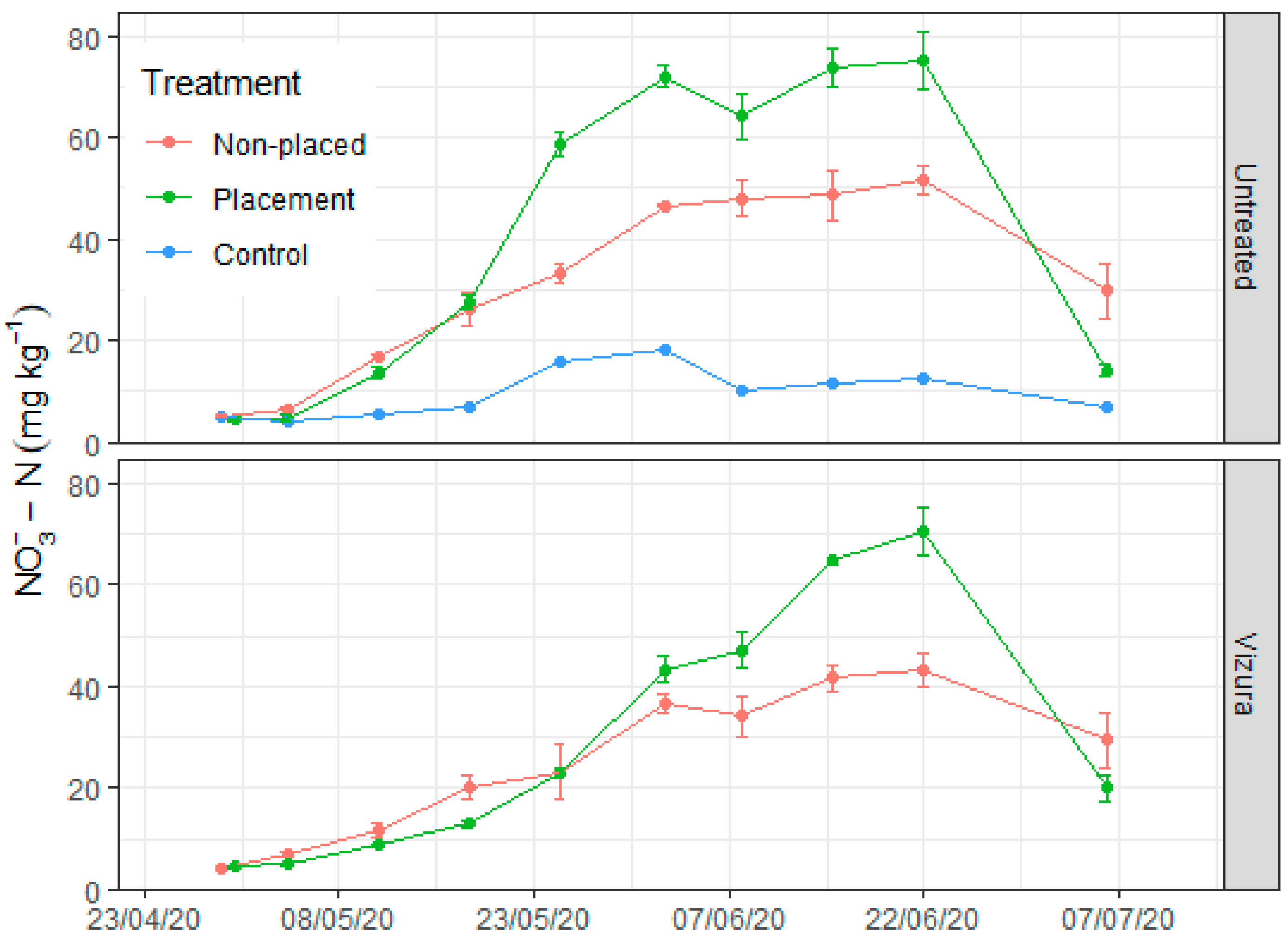
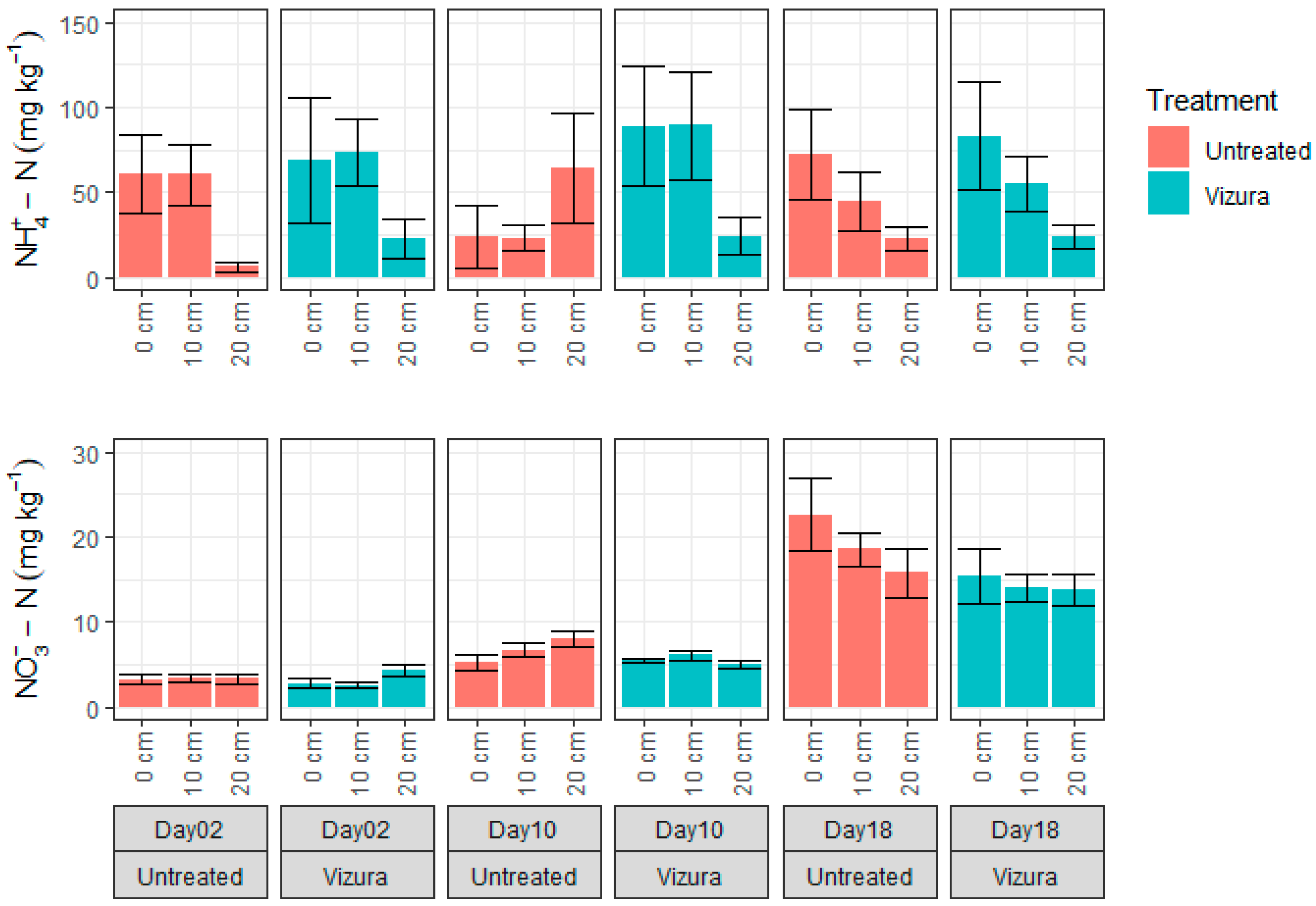
3.2. Soil Moisture and Mineral N Dynamics
3.3. Mineral N Distribution after Placement of Slurry
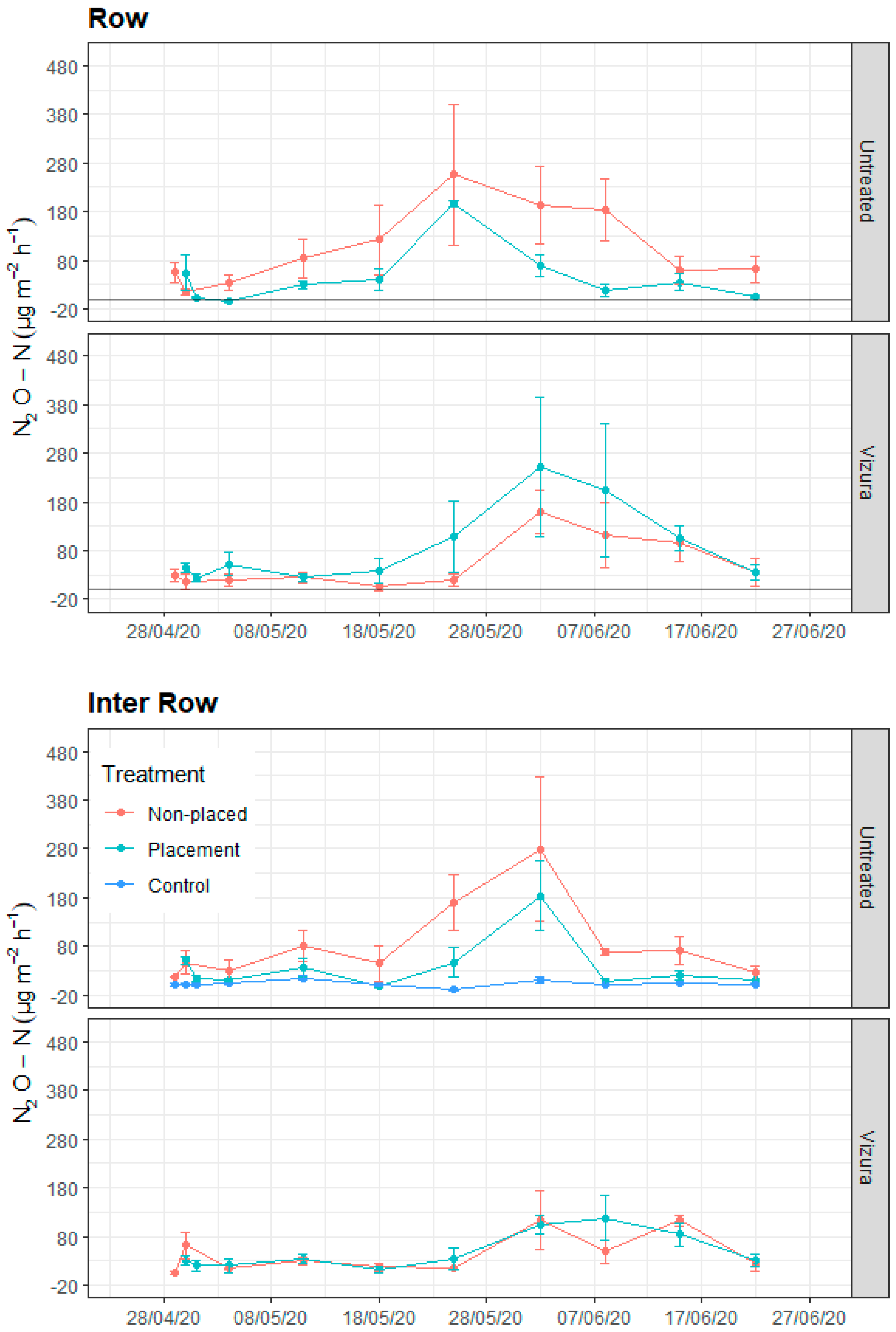
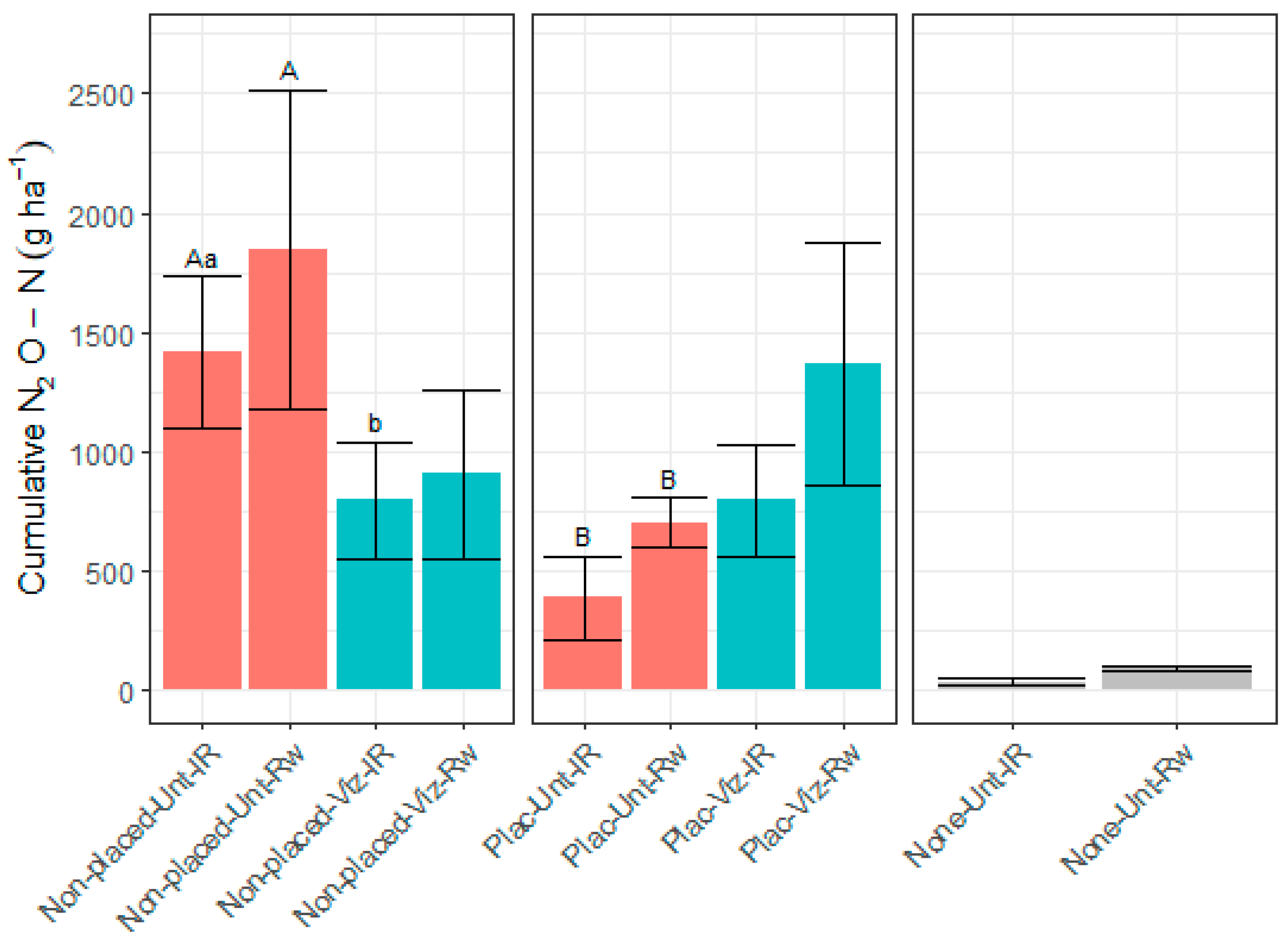
3.4. N2O Emissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiertz, J.H.J. Nitrogen, sustainable agriculture, and food security. A review. Agron. Sustain. Dev. 2010, 30, 43–55. [Google Scholar] [CrossRef]
- Leip, A.; Weiss, F.; Wassenaar, T.; Perez, I.; Fellmann, T.; Loudjani, P.; Tubiello, F.; Grandgirard, D.; Monni, S.; Biala, K. Evaluation of the Livestock Sector’s Contribution to the EU Greenhouse Gas Emissions (GGELS)—Final Report; European Commission, Joint Research Centre: Ispra, Italy, 2010. [Google Scholar]
- Webb, J.; Pain, B.; Bittman, S.; Morgan, J. The impacts of manure application methods on emissions of ammonia, nitrous oxide and on crop response—A review. Agric. Ecosyst. Environ. 2010, 137, 39–46. [Google Scholar] [CrossRef]
- Hafner, S.D.; Pacholski, A.; Bittman, S.; Burchill, W.; Bussink, W.; Chantigny, M.; Carozzi, M.; Génermont, S.; Häni, C.; Hansen, M.N.; et al. The ALFAM2 database on ammonia emission from field-applied manure: Description and illustrative analysis. Agric. For. Meteorol. 2018, 258, 66–79. [Google Scholar] [CrossRef]
- Lemaire, G.; Charrier, X.; Hébert, Y. Nitrogen uptake capacities of maize and sorghum crops in different nitrogen and water supply conditions. Agronomie 1996, 16, 231–246. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portman, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Ibraim, E.; Denk, T.; Wolf, B.; Barthel, M.; Gasche, R.; Wanek, W.; Zhang, S.; Kiese, R.; Butterbach-Bahl, K.; Eggleston, S.; et al. Denitrification is the main nitrous oxide source process in grassland soils according to quasi-continuous isotopocule analysis and biogeochemical modeling. Glob. Biogeochem. Cycles 2020, 33, e2019GB006505. [Google Scholar] [CrossRef]
- Rice, C.W.; Tiedje, J.M.; Sierzega, P.E.; Jacobs, L.W. Stimulated denitrification in the microenvironment of a biodegradable organic waste injected into soil. Soil Sci. Soc. Am. J. 1988, 52, 102–108. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Nielsen, L.P.; Revsbech, N.P. Nitrification and coupled nitrification-denitrification associated with a soil-manure interface. Soil Sci. Soc. Am. J. 1996, 60, 1829–1840. [Google Scholar] [CrossRef]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Chang. Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- VanderZaag, A.C.; Jayasundara, S.; Wagner-Riddle, C. Strategies to mitigate nitrous oxide emissions from land applied manure. Anim. Feed. Sci. Technol. 2011, 166–167, 464–479. [Google Scholar] [CrossRef]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; Horchler von Locquenghien, K.; Pasda, G.; Rädle, M.; Wissemeier, A. 3,4-Dimethylpyrazole phosphate (DMPP)—A new nitrification inhibitor for agriculture and horticulture. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Peixoto, L.; Petersen, S.O. Efficacy of three nitrification inhibitors to reduce nitrous oxide emissions from pig slurry and mineral fertilizers applied to spring barley and winter wheat in Denmark. Geoderma Reg. 2023, 32, e00597. [Google Scholar] [CrossRef]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in agricultural soils: Impact, actors and mitigation. Curr. Opin. Biotechnol. 2018, 50, 166–173. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Ma, S.; Zheng, X.; Wang, Z.; Lu, C. Effects of commonly used nitrification inhibitors dicyandiamide (DCD), 3,4-dimethylpyrazole phosphate (DMPP), and nitrapyrin—On soil nitrogen dynamics and nitrifiers in three typical paddy soils. Geoderma 2020, 380, 114637. [Google Scholar] [CrossRef]
- Danmarks-Statistik. Landbrugsstatistik. Available online: https://www.dst.dk/ (accessed on 1 December 2022).
- Pedersen, I.F.; Nyord, T.; Sørensen, P. Tine tip width and placement depth by row-injection of cattle slurry influence initial leaf N and P concentrations and final yield of silage maize. Eur. J. Agron. 2022, 133, 126418. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Zhao, X.Q.; Chen, Y.L.; Zhang, L.Y.; Shen, R.F. Case of a stronger capability of maize seedlings to use ammonium being responsible for the higher 15N recovery efficiency of ammonium compared with nitrate. Plant Soil 2019, 440, 293–309. [Google Scholar] [CrossRef]
- Miller, M.H.; Robinson, J.B.; Gillham, R.W. Self-Sealing of earthen liquid manure storage ponds: I. A Case study1. J. Environ. Qual. 1985, 14, 533–538. [Google Scholar] [CrossRef]
- Petersen, S.O.; Nissen, H.H.; Lund, I.; Ambus, P. Redistribution of slurry in soil as influenced by organic matter content and injection method. J. Environ. Qual. 2003, 32, 2399–2409. [Google Scholar] [CrossRef]
- Barrington, S.F.; Madramootoo, C.A. Investigating seal formation from manure infiltration into soils. Trans. ASAE 1989, 32, 851–856. [Google Scholar] [CrossRef]
- Krogh, L.; Greve, M.H. Evaluation of world reference base for soil resources and FAO soil map of the world using nationwide grid soil data from Denmark. Soil Use Manag. 1999, 15, 157–166. [Google Scholar] [CrossRef]
- Petersen, S.O.; Hoffmann, C.C.; Schäfer, C.-M.; Blicher-Mathiesen, G.; Elsgaard, L.; Kristensen, K.; Larsen, S.E.; Torp, S.B.; Greve, M.H. Annual emissions of CH4 and N2O, and ecosystem respiration, from eight organic soils in Western Denmark managed by agriculture. Biogeosciences 2012, 9, 403–422. [Google Scholar] [CrossRef]
- Pedersen, A.R.; Petersen, S.O.; Schelde, K. A comprehensive approach to soil atmosphere trace-gas flux estimation with static chambers. Eur. J. Soil Sci. 2010, 61, 888–902. [Google Scholar] [CrossRef]
- Hou, Y.; Velthof, G.L.; Oenema, O. Mitigation of ammonia, nitrous oxide and methane emissions from manure management chains: A meta-analysis and integrated assessment. Glob. Chang. Biol. 2015, 21, 1293–1312. [Google Scholar] [CrossRef]
- Guenzi, W.D.; Beard, W.E. Volatile fatty acids in a redox controlled cattle manure slurry. J. Environ. Qual. 1981, 10, 479–482. [Google Scholar] [CrossRef]
- van Groenigen, J.W.; Kasper, G.J.; Velthof, G.L.; Van den Pol van Dasselaar, A.; Kuikman, P.J. Nitrous oxide emissions from silage maize fields under different mineral nitrogen fertilizer and slurry applications. Plant Soil 2004, 263, 101–111. [Google Scholar] [CrossRef]
- Rochette, P.; van Bochove, E.; Prévost, D.; Angers, D.; Côté, D.; Bertrand, N. Soil carbon and nitrogen dynamics following application of pig slurry for the 19th consecutive year. II. Nitrous oxide fluxes and mineral nitrogen. Soil Sci. Soc. Am. J. 2000, 64, 1396–1403. [Google Scholar] [CrossRef]
- Comfort, S.D.; Kelling, K.A.; Keeney, D.R.; Converse, J.C. The fate of nitrogen from injected liquid manure in a silt loam soil. J. Environ. Qual. 1988, 17, 317–322. [Google Scholar] [CrossRef]
- Petersen, S.O.; Henriksen, K.; Blackburn, T.H. Coupled nitrification-denitrification associated with liquid manure in a gel-stabilized model system. Biol. Fertil. Soils 1991, 12, 19–27. [Google Scholar] [CrossRef]
- Velthof, G.L.; Mosquera, J. The impact of slurry application technique on nitrous oxide emission from agricultural soils. Agric. Ecosyst. Environ. 2011, 140, 298–308. [Google Scholar] [CrossRef]
- Sørensen, P.; Jensen, E.S. Mineralization-immobilization and plant uptake of nitrogen as influenced by the spatial distribution of cattle slurry in soils of different texture. Plant Soil 1995, 173, 283–291. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Revsbech, N.P. Diffusion Chamber for Nitrogen-15 Determination of Coupled Nitrification-Denitrification around Soil-Manure Interfaces. Soil Sci. Soc. Am. J. 1994, 58, 795–800. [Google Scholar] [CrossRef]
- Molodovskaya, M.; Singurindy, O.; Richards, B.K.; Warland, J.; Johnson, M.S.; Steenhuis, T.S. Temporal variability of nitrous oxide from fertilized croplands: Hot moment analysis. Soil Sci. Soc. Am. J. 2012, 76, 1728–1740. [Google Scholar] [CrossRef]
- Di, W.; Cárdenas, L.M.; Calvet, S.; Brüggemann, N.; Loick, N.; Liu, S.; Bol, R. The effect of nitrification nhibitor on N2O, NO and N2 emissions under different soil moisture levels in a permanent grassland soil. Soil Biol. Biochem. 2017, 113, 153–160. [Google Scholar]
- Misselbrook, T.H.; Cardenas, L.M.; Camp, V.; Thorman, R.E.; Williams, J.R.; Rollett, R.J.; Chambers, B.J. An assessment of nitrification inhibitors to reduce nitrous oxide emissions from UK agriculture. Environ. Res. Lett. 2014, 9, 115006. [Google Scholar] [CrossRef]
- Dittert, K.; Bol, R.; King, R.; Chadwick, D.; Hatch, D. Use of a novel nitrification inhibitor to reduce nitrous oxide emission from N-15-labelled dairy slurry injected into soil. Rapid Commun. Mass Spectrom. 2001, 15, 1291–1296. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Sahrawat, K.L.; Berry, W.L.; Nakahara, K.; Ishikawa, T.; Watanabe, T.; Suenaga, K.; Rondon, M.; Rao, I. Scope and strategies for regulation of nitrification in agricultural systems—Challenges and opportunities. Crit. Rev. Plant Sci. 2006, 25, 303–335. [Google Scholar] [CrossRef]
- Chibuike, G.; Saggar, S.; Palmada, T.; Luo, J. The persistence and efficacy of nitrification inhibitors to mitigate nitrous oxide emissions from New Zealand pasture soils amended with urine. Geoderma Reg. 2022, 30, e00541. [Google Scholar] [CrossRef]
- Meng, X.; Ma, C.; Petersen, S.O. Sensitive control of N2O emissions and microbial community dynamics by organic fertilizer and soil interactions. Biol. Fertil. Soils 2022, 58, 771–788. [Google Scholar] [CrossRef]
- Bollmann, A.; Conrad, R. Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Glob. Chang. Biol. 1998, 4, 387–396. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Wu, D.; Well, R.; Cárdenas, L.M.; Fuβ, R.; Lewicka-Szczebak, D.; Köster, J.R.; Brüggemann, N.; Bol, R. Quantifying N2O reduction to N2 during denitrification in soils via isotopic mapping approach: Model evaluation and uncertainty analysis. Environ. Res. 2019, 179, 108806. [Google Scholar] [CrossRef] [PubMed]
- Friedl, J.; Scheer, C.; Rowlings, D.W.; Deltedsco, E.; Gorfer, M.; De Rosa, D.; Grace, P.R.; Müller, C.; Keiblinger, K.M. Effect of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on N-turnover, the N2O reductase-gene nosZ and N2O:N2 partitioning from agricultural soils. Sci. Rep. 2020, 10, 2399. [Google Scholar] [CrossRef]

| Treatment | Application Method | Slurry Treatment | Sampling Position |
|---|---|---|---|
| Plac-Unt-Rw | Placed | Untreated | Row |
| Plac-Unt-IR | Placed | Untreated | Interrow |
| Plac-Viz-Rw | Placed | Vizura | Row |
| Plac-Viz-IR | Placed | Vizura | Interrow |
| Non-placed-Unt-Rw | Non-placed | Untreated | Row |
| Non-placed-Unt-IR | Non-placed | Untreated | Interrow |
| Non-placed-Viz-Rw | Non-placed | Vizura | Row |
| Non-placed-Viz-IR | Non-placed | Vizura | Interrow |
| Control | NA | NA | Row and interrow |
| Treatments | Num DF | Den DF | F | p |
|---|---|---|---|---|
| Ammonium-N | ||||
| Intercept | 1 | 93 | 643.74 | *** |
| Application method | 1 | 93 | 3.26 | ns |
| Slurry treatment | 1 | 93 | 16.61 | *** |
| Date | 10 | 93 | 25.88 | *** |
| Slurry application * slurry treatment | 1 | 93 | 0.18 | ns |
| Slurry treatment * date | 10 | 93 | 1.85 | ns |
| Nitrate-N | ||||
| Intercept | 1 | 94 | 7685.17 | *** |
| Application method | 1 | 94 | 2.40 | ns |
| Slurry treatment | 1 | 94 | 17.54 | *** |
| Date | 10 | 94 | 100.39 | *** |
| Application method * slurry treatment | 11 | 94 | 0.29 | ns |
| Slurry treatment * date | 10 | 94 | 3.16 | ** |
| Num DF | Den DF | F | p | |
|---|---|---|---|---|
| Within-row | ||||
| Intercept | 1 | 101 | 976.07 | *** |
| Application method | 1 | 101 | 1.66 | ns |
| Slurry treatment | 1 | 101 | 0.33 | ns |
| Date | 11 | 101 | 4.77 | *** |
| Application method * slurry treatment | 1 | 101 | 20.44 | ns |
| Slurry treatment * date | 11 | 101 | 2.18 | * |
| Inter-row | ||||
| Intercept | 1 | 225 | 2701.87 | *** |
| Application method | 1 | 225 | 5.75 | * |
| Slurry treatment | 1 | 225 | 0.07 | ns |
| Date | 11 | 225 | 10.83 | *** |
| Application method * slurry treatment | 1 | 225 | 25.98 | *** |
| Slurry treatment * date | 11 | 225 | 2.47 | ns |
| Num DF | Den DF | F | p | |
|---|---|---|---|---|
| Intercept | 1 | 123 | 705.57 | *** |
| Application method | 1 | 123 | 6.93 | ** |
| Slurry treatment | 1 | 123 | 0.08 | ns |
| Date | 1 | 123 | 0.11 | ns |
| Application method * slurry treatment | 1 | 123 | 3.64 | *** |
| Slurry treatment * date | 1 | 123 | 1.98 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghizadeh-Toosi, A.; Baral, K.R.; Sørensen, P.; Petersen, S.O. Short-Term Nitrous Oxide Emissions from Cattle Slurry for Silage Maize: Effects of Placement and the Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP). Sustainability 2023, 15, 15810. https://doi.org/10.3390/su152215810
Taghizadeh-Toosi A, Baral KR, Sørensen P, Petersen SO. Short-Term Nitrous Oxide Emissions from Cattle Slurry for Silage Maize: Effects of Placement and the Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP). Sustainability. 2023; 15(22):15810. https://doi.org/10.3390/su152215810
Chicago/Turabian StyleTaghizadeh-Toosi, Arezoo, Khagendra Raj Baral, Peter Sørensen, and Søren O. Petersen. 2023. "Short-Term Nitrous Oxide Emissions from Cattle Slurry for Silage Maize: Effects of Placement and the Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP)" Sustainability 15, no. 22: 15810. https://doi.org/10.3390/su152215810
APA StyleTaghizadeh-Toosi, A., Baral, K. R., Sørensen, P., & Petersen, S. O. (2023). Short-Term Nitrous Oxide Emissions from Cattle Slurry for Silage Maize: Effects of Placement and the Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP). Sustainability, 15(22), 15810. https://doi.org/10.3390/su152215810






