The Biosynthesis of Nickel Oxide Nanoparticles: An Eco-Friendly Approach for Azo Dye Decolorization and Industrial Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and the Isolation of Bacterial Strain
2.2. The Biosynthesis of NiO-NPs by the Shewanella Species
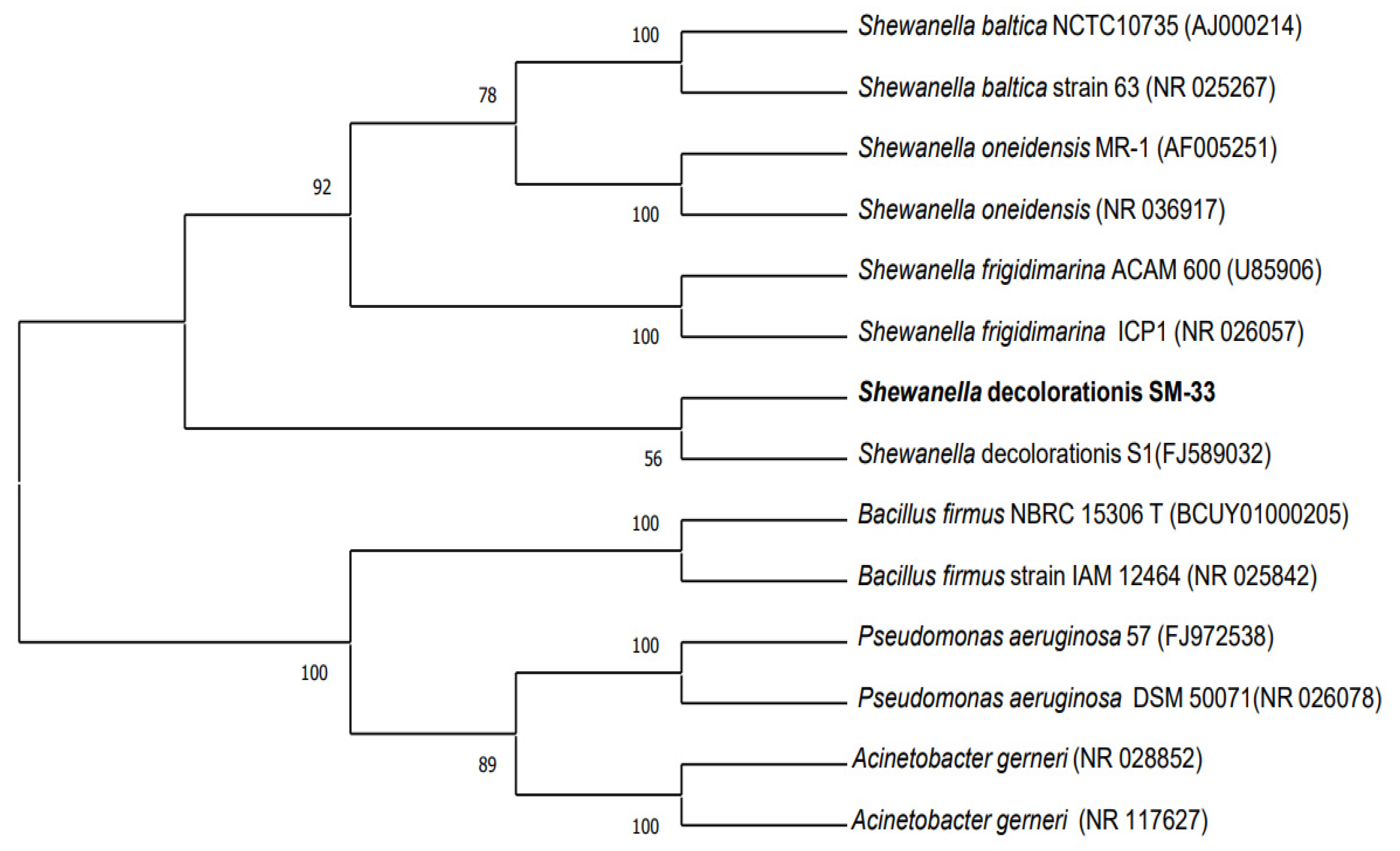
2.3. Molecular Characterization and Phylogenetic Analysis
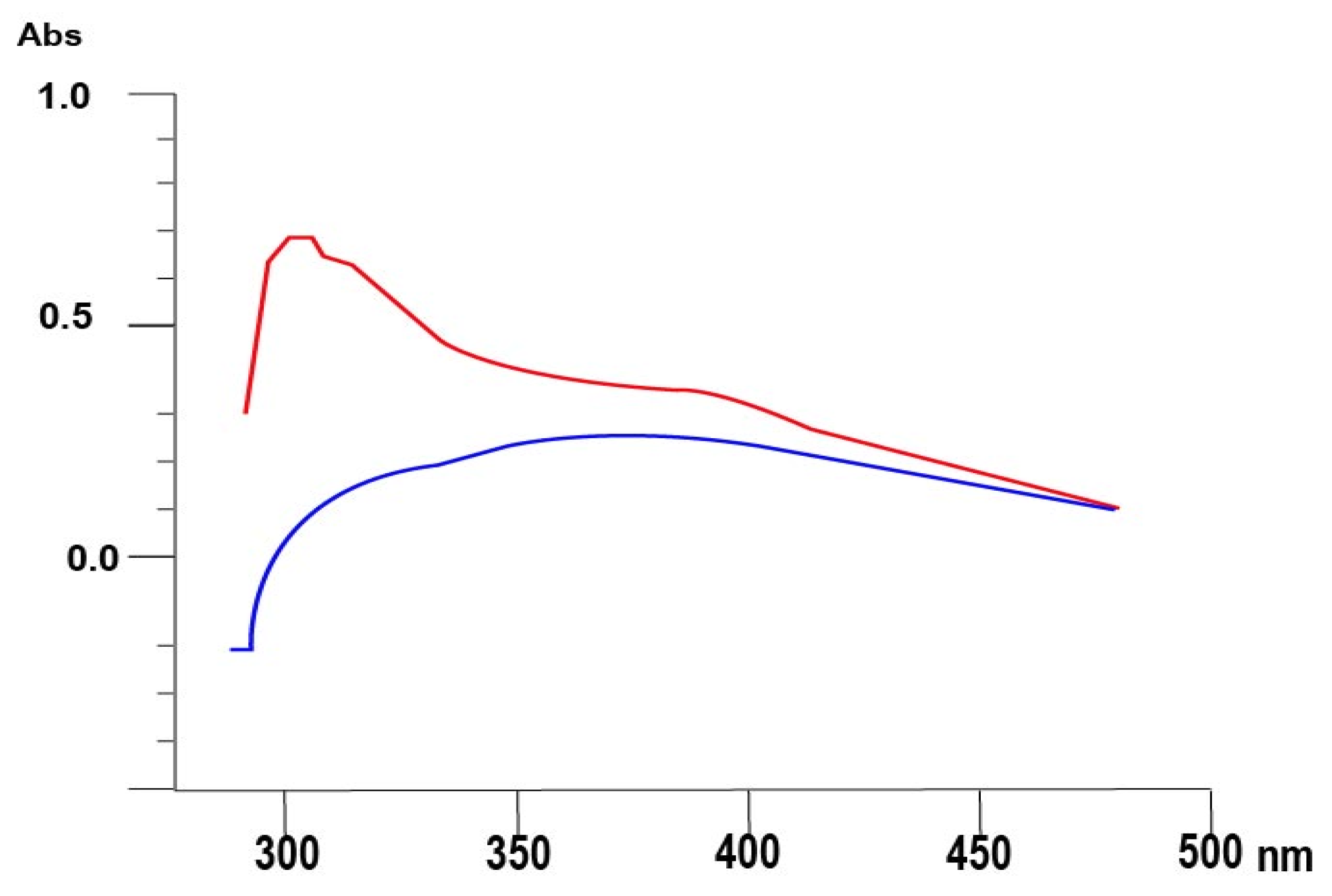
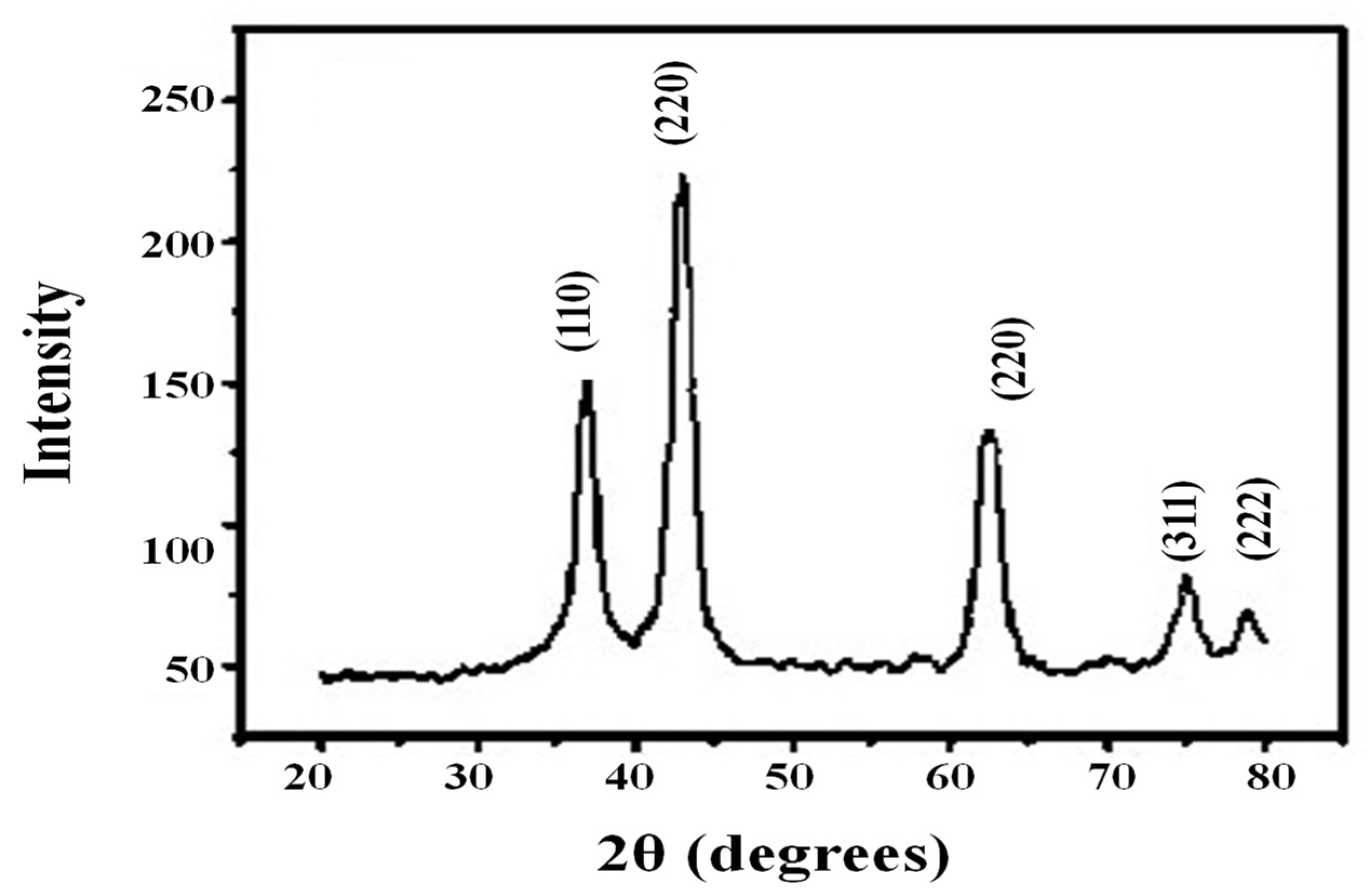
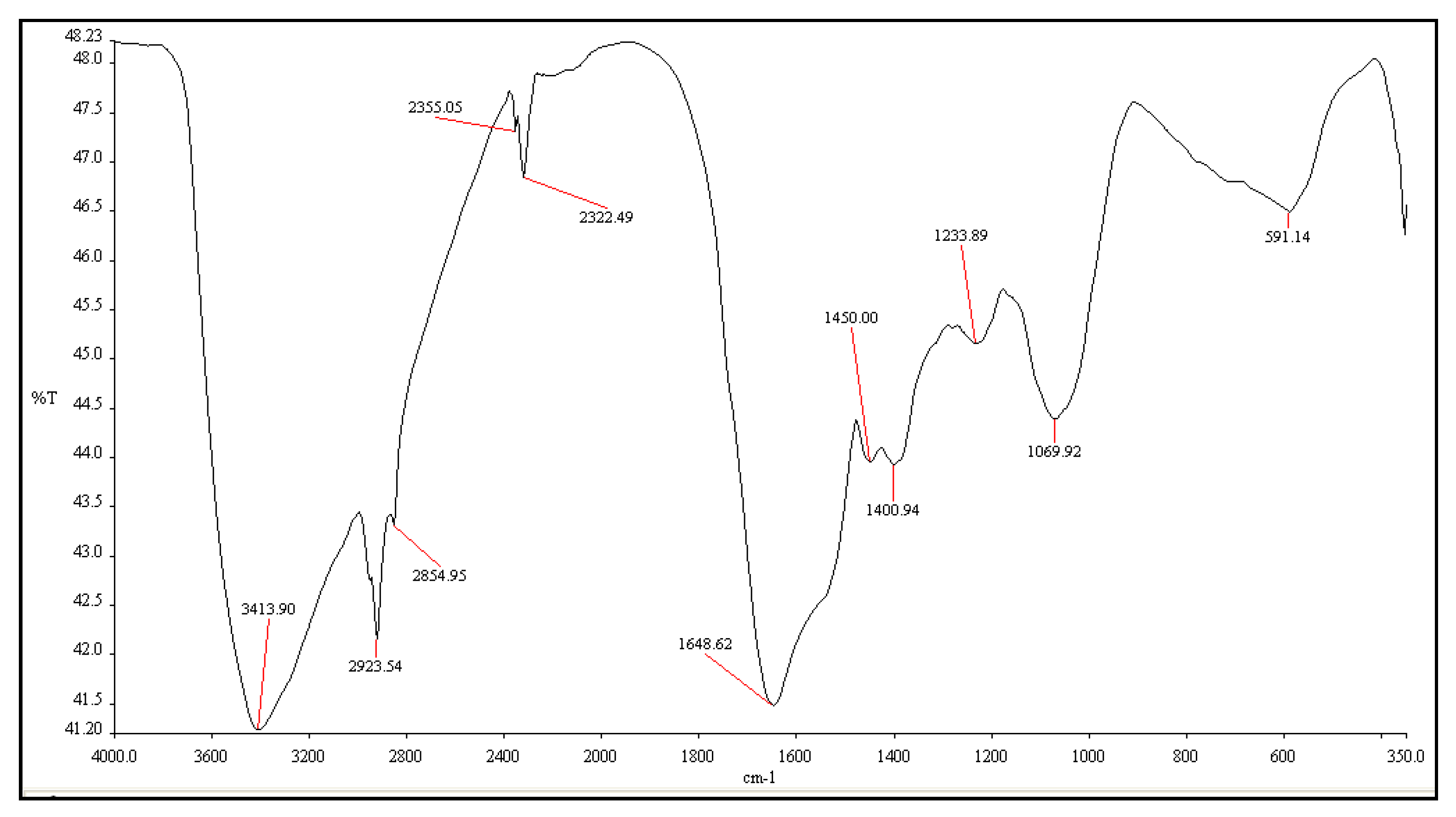
2.4. The Characterization of NiO-NPs
2.5. The Photocatalytic Degradation of Azo Dyes
2.6. The Application of NiO-NPs for Industrial Wastewater Treatment
2.6.1. pH Analysis Method
2.6.2. Electrical Conductivity Analysis Method
2.6.3. Chemical Oxygen Demand (COD) Analysis Method
2.6.4. Phosphorus
2.6.5. Sulfates
3. Results
3.1. Molecular Characterization and Phylogenetic Analysis
3.2. Characterization of NiO Nanoparticles
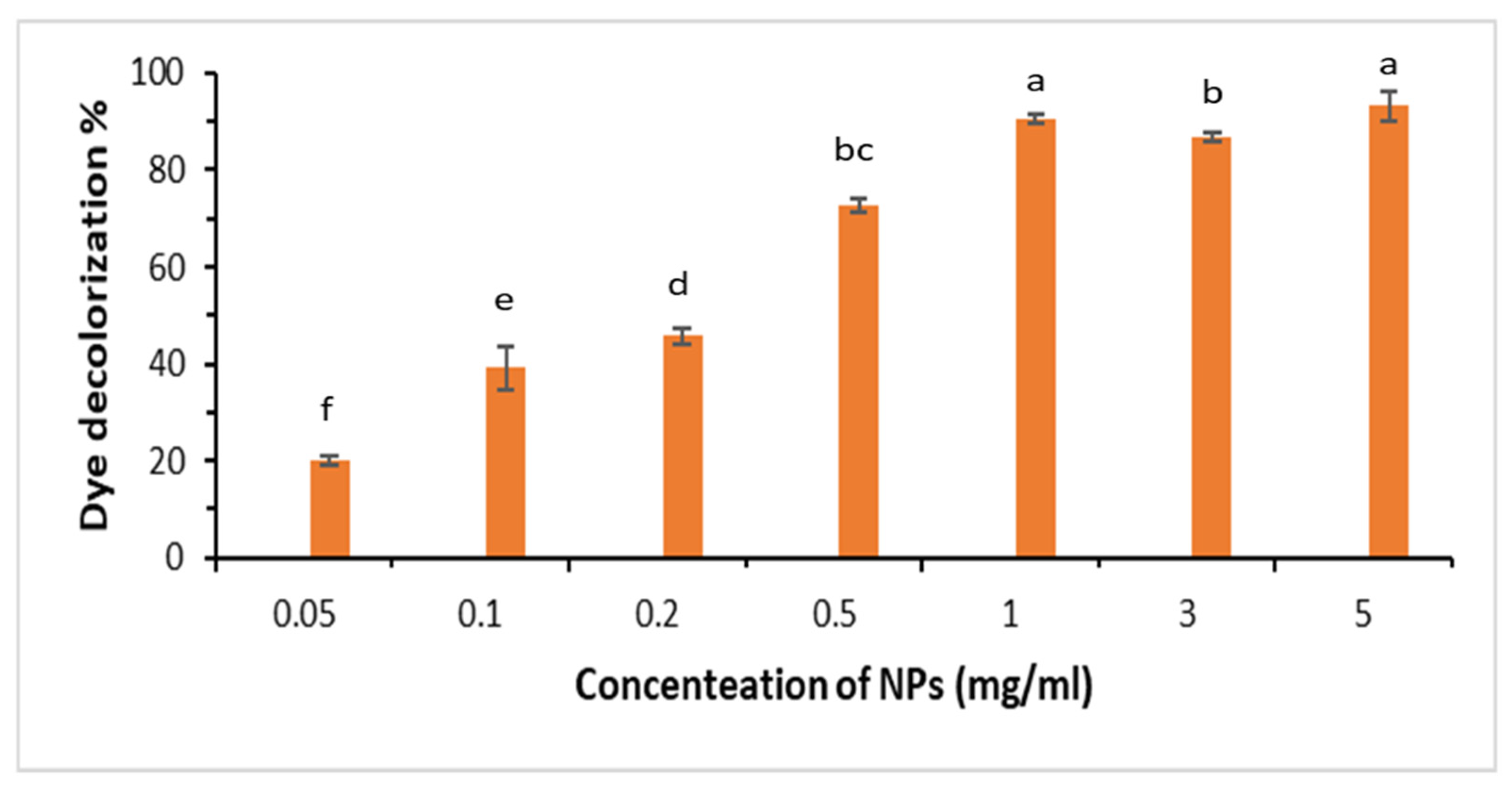
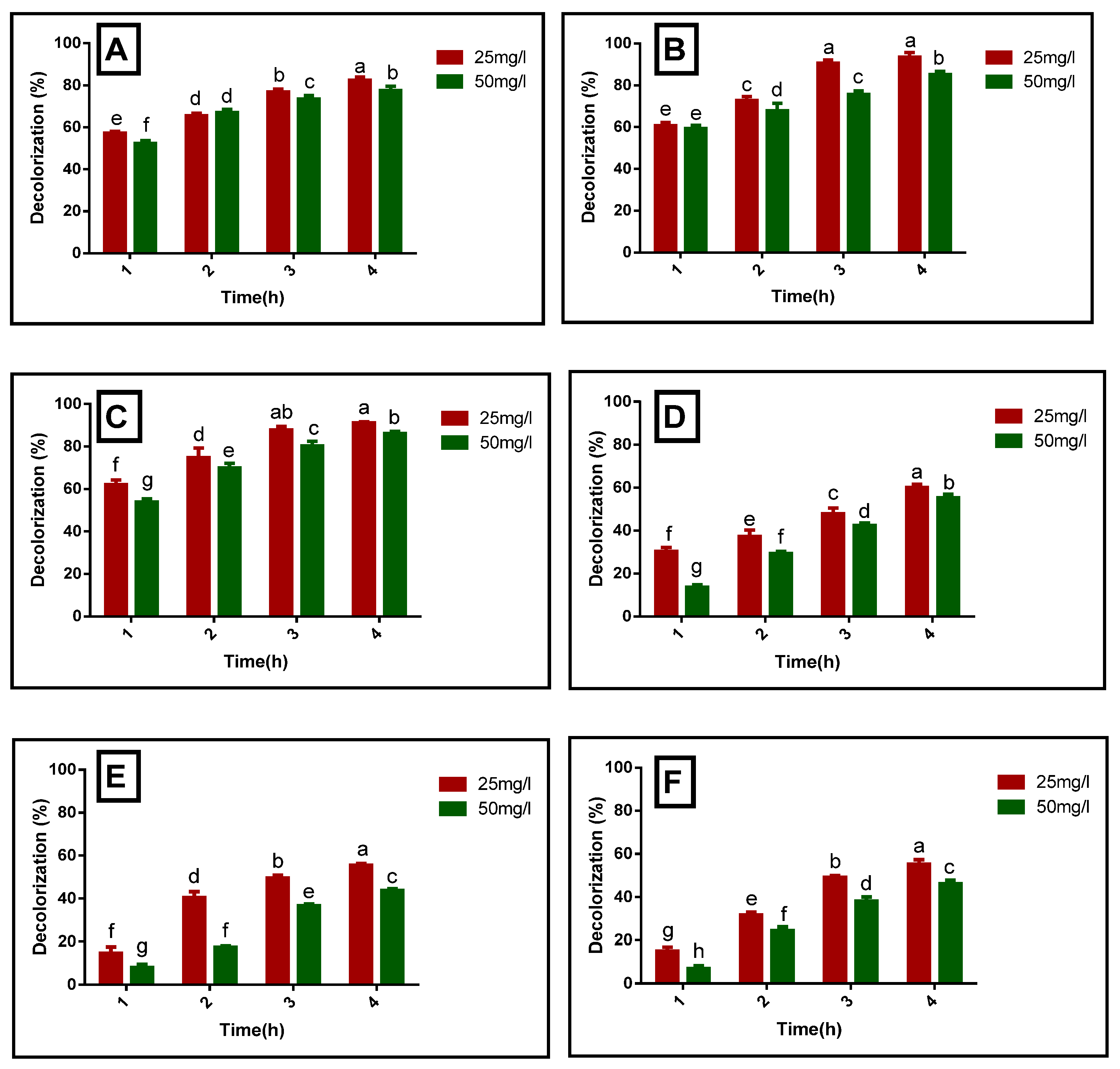
3.3. Photocatalytic Degradation of Organic Azo Dyes by Using Biogenic NiO-NPs
3.4. Treatment of Industrial Wastewater by Using Biosynthesized Nickel Oxide Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastopoulos, I.; Hosseini-Bandegharaei, A.; Fu, J.; Mitropoulos, A.C.; Kyzas, G.Z. Use of Nanoparticles for Dye Adsorption: Review. J. Dispers. Sci. Technol. 2017, 39, 836–847. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive Removal of Dyes from Aqueous Solution onto Carbon Nanotubes: A Review. Adv. Colloid Interface Sci. 2013, 193–194, 24–34. [Google Scholar] [CrossRef]
- Routoula, E.; Patwardhan, S.V. Degradation of Anthraquinone Dyes from Effluents: A Review Focusing on Enzymatic Dye Degradation with Industrial Potential. Environ. Sci. Technol. 2020, 54, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Deniz, F. Adsorption Properties of Low-Cost Biomaterial Derived from Prunus amygdalus L. for Dye Removal from Water. Sci. World J. 2013, 2013, 961671. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar]
- Keramati, M.; Ayati, B. Petroleum Wastewater Treatment Using a Combination of Electrocoagulation and Photocatalytic Process with Immobilized ZnO Nanoparticles on Concrete Surface. Process Saf. Environ. Prot. 2019, 126, 356–365. [Google Scholar] [CrossRef]
- Danouche, M.; El Arroussi, H.; El Ghachtouli, N. Bioremoval of Acid Red 14 Dye by Wickerhamomyces Anomalus Biomass: Kinetic and Thermodynamic Study, Characterization of Physicochemical Interactions, and Statistical Optimization of the Biosorption Process. Biomass Convers. Biorefinery 2022, 1–20. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.; Negm, F.; Khalid, A.; Shaharoona, B.; Hussain, S.; Nadeem, S.M.; Crowley, D.E. Yeast Extract Promotes Decolorization of Azo Dyes by Stimulating Azoreductase Activity in Shewanella Sp. Strain IFN4. Ecotoxicol. Environ. Saf. 2016, 124, 42–49. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Methylene Blue on Low-Cost Adsorbents: A Review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Subedi, S.K. An Introduction to Nanotechnology and Its Implications. Himal. Phys. 2015, 5, 78–81. [Google Scholar] [CrossRef]
- Rangasamy, M. Nano Technology: A Review. J. Appl. Pharm. Sci. 2011, 1, 8–16. [Google Scholar]
- Haidri, I.; Shahid, M.; Hussain, S.; Shahzad, T.; Mahmood, F.; Hassan, M.U.; Al-Khayri, J.M.; Aldaej, M.I.; Sattar, M.N.; Rezk, A.A.-S. Efficacy of Biogenic Zinc Oxide Nanoparticles in Treating Wastewater for Sustainable Wheat Cultivation. Plants 2023, 12, 3058. [Google Scholar] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C. Nanoparticles and Nanotechnology Research. J. Nanoparticle Res. 1999, 1, 1–6. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. Applications of Nanotechnology in Daily Life. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–143. [Google Scholar]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from Aqueous Solutions by Adsorption on Kaolin: Kinetic and Equilibrium Studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Adsorption of Anionic Surfactant on Alumina and Reuse of the Surfactant-Modified Alumina for the Removal of Crystal Violet from Aquatic Environment. J. Environ. Sci. Health Part A 2005, 40, 167–182. [Google Scholar] [CrossRef]
- Kumar, P.S.; Joshiba, G.J.; Femina, C.C.; Varshini, P.; Priyadharshini, S.; Karthick, M.S.A.; Jothirani, R. A Critical Review on Recent Developments in the Low-Cost Adsorption of Dyes from Wastewater. Desalination Water Treat. 2019, 172, 395–416. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.-H.; Bhatti, H.N.; Nouren, S. Dyes Adsorption Using Clay and Modified Clay: A Review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q.M. Magnetic Nanocomposites with Mesoporous Structures: Synthesis and Applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of Nanoparticles: Technological Concepts and Future Applications. J. Nanoparticle Res. 2007, 10, 507–517. [Google Scholar] [CrossRef]
- Shafqat, U.; Hussain, S.; Shahzad, T.; Shahid, M.; Mahmood, F. Elucidating the Phytotoxicity Thresholds of Various Biosynthesized Nanoparticles on Physical and Biochemical Attributes of Cotton. Chem. Biol. Technol. Agric. 2023, 10, 30. [Google Scholar]
- Maurer-Jones, M.A.; Bantz, K.C.; Love, S.A.; Marquis, B.J.; Haynes, C.L. Toxicity of Therapeutic Nanoparticles. Nanomedicine 2009, 4, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of Nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.I.; Coker, V.S.; Charnock, J.M.; Pattrick, R.A.D.; Mosselmans, J.F.W.; Law, N.; Beveridge, T.J.; Lloyd, J.R. Microbial Manufacture of Chalcogenide-Based Nanoparticles via the Reduction of Selenite Using Veillonella atypica: An in Situ EXAFS Study. Nanotechnology 2008, 19, 155603. [Google Scholar] [CrossRef]
- Noman, M.; Shahid, M.; Ahmed, T.; Niazi, M.B.K.; Hussain, S.; Song, F.; Manzoor, I. Use of Biogenic Copper Nanoparticles Synthesized from a Native Escherichia Sp. as Photocatalysts for Azo Dye Degradation and Treatment of Textile Effluents. Environ. Pollut. 2020, 257, 113514. [Google Scholar]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Rouch, D.A.; Lee, B.T.O.; Morby, A.P. Understanding Cellular Responses to Toxic Agents: A Model for Mechanism-Choice in Bacterial Metal Resistance. J. Ind. Microbiol. 1995, 14, 132–141. [Google Scholar] [CrossRef]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Nikolova, M.P.; Sadasivuni, K.K.; Mahdizadeh, H.; Verma, U. Structural Studies of Bio-Mediated NiO Nanoparticles for Photocatalytic and Antibacterial Activities. Inorg. Chem. Commun. 2020, 113, 107755. [Google Scholar] [CrossRef]
- Li, L.; Nawaz, T.; Ferryman, J. PETS 2015: Datasets and Challenge. In Proceedings of the 2015 12th IEEE International Conference on Advanced Video and Signal Based Surveillance (AVSS), Karlsruhe, Germany, 25–28 August 2015. [Google Scholar]
- Fei, J.; Cui, Y.; Wang, A.; Zhu, P.; Li, J. Noble Metal Nanochains through Helical Self-Assembly. Chem. Commun. 2010, 46, 2310–2312. [Google Scholar] [CrossRef]
- Sathyavathi, S.; Manjula, A.; Rajendhran, J.; Gunasekaran, P. Extracellular Synthesis and Characterization of Nickel Oxide Nanoparticles from Microbacterium Sp. MRS-1 towards Bioremediation of Nickel Electroplating Industrial Effluent. Bioresour. Technol. 2014, 165, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Constanti, M. Room Temperature Biogenic Synthesis of Multiple Nanoparticles (Ag, Pd, Fe, Rh, Ni, Ru, Pt, Co, and Li) by Pseudomonas Aeruginosa SM1. J. Nanoparticle Res. 2012, 14, 831. [Google Scholar] [CrossRef]
- Khoshhesab, Z.M.; Sarfaraz, M.; Asadabad, M.A. Preparation of ZnO Nanostructures by Chemical Precipitation Method. Synth. React. Inorg. Met. Nano-Metal Chem. 2011, 41, 814–819. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [PubMed]
- Tiwari, M.; Jain, P.; Hariharapura, R.C.; Narayanan, K.; Bhat, U.; Udupa, N.; Rao, J.V. Biosynthesis of Copper Nanoparticles Using Copper-Resistant Bacillus Cereus, a Soil Isolate. Process Biochem. 2016, 51, 1348–1356. [Google Scholar]
- Griffiths, P.R.; de Haseth, J.A. Fourier Transform Infrared Spectrometry, 2nd ed; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Varshney, R.; Bhadauria, S.; Gaur, M.S.; Pasricha, R. Characterization of Copper Nanoparticles Synthesized by a Novel Microbiological Method. JOM 2010, 62, 102–104. [Google Scholar]
- Roy, K.; Ghosh, C.K.; Sarkar, C.K. Degradation of Toxic Textile Dyes and Detection of Hazardous Hg2+ by Low-Cost Bioengineered Copper Nanoparticles Synthesized Using Impatiens Balsamina Leaf Extract. Mater. Res. Bull. 2017, 94, 257–262. [Google Scholar]
- Allen, S.J.; Koumanova, B. Decolourisation of water/wastewater using adsorption. J. Univ. Chem. Technol. Metal. 2005, 40, 175–192. [Google Scholar]
- Soltani, R.D.C.; Safari, M. Periodate-Assisted Pulsed Sonocatalysis of Real Textile Wastewater in the Presence of MgO Nanoparticles: Response Surface Methodological Optimization. Ultrason. Sonochem. 2016, 32, 181–190. [Google Scholar]
- Lingaraju, K.; Raja Naika, H.; Nagabhushana, H.; Jayanna, K.; Devaraja, S.; Nagaraju, G. Biosynthesis of Nickel Oxide Nanoparticles from Euphorbia heterophylla (L.) and Their Biological Application. Arab. J. Chem. 2020, 13, 4712–4719. [Google Scholar] [CrossRef]
- Zaki, S.; El Kady, M.F.; Abd-El-Haleem, D. Biosynthesis and Structural Characterization of Silver Nanoparticles from Bacterial Isolates. Mater. Res. Bull. 2011, 46, 1571–1576. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; Hussain, S.; Niazi, M.B.K.; Shahid, M.; Song, F. Biogenic Copper Nanoparticles Synthesized by Using a Copper-Resistant Strain Shigella Flexneri SNT22 Reduced the Translocation of Cadmium from Soil to Wheat Plants. J. Hazard. Mater. 2020, 398, 123175. [Google Scholar] [PubMed]
- Bashir, A.K.H.; Razanamahandry, L.C.; Nwanya, A.C.; Kaviyarasu, K.; Saban, W.; Mohamed, H.E.A.; Ntwampe, S.K.O.; Ezema, F.I.; Maaza, M. Biosynthesis of NiO Nanoparticles for Photodegradation of Free Cyanide Solutions under Ultraviolet Light. J. Phys. Chem. Solids 2019, 134, 133–140. [Google Scholar] [CrossRef]
- Zorkipli, N.N.M.; Kaus, N.H.M.; Mohamad, A.A. Synthesis of NiO Nanoparticles through Sol-Gel Method. Procedia Chem. 2016, 19, 626–631. [Google Scholar] [CrossRef]
- Sabouri, Z.; Akbari, A.; Hosseini, H.A.; Hashemzadeh, A.; Darroudi, M. Bio-Based Synthesized NiO Nanoparticles and Evaluation of Their Cellular Toxicity and Wastewater Treatment Effects. J. Mol. Struct. 2019, 1191, 101–109. [Google Scholar]
- Rong, X.; Qiu, F.; Zhang, C.; Fu, L.; Wang, Y.; Yang, D. Adsorption–Photodegradation Synergetic Removal of Methylene Blue from Aqueous Solution by NiO/Graphene Oxide Nanocomposite. Powder Technol. 2015, 275, 322–328. [Google Scholar]
- Khairnar, S.D.; Shrivastava, V.S. Facile Synthesis of Nickel Oxide Nanoparticles for the Degradation of Methylene Blue and Rhodamine B Dye: A Comparative Study. J. Taibah Univ. Sci. 2019, 13, 1108–1118. [Google Scholar]
- Saifuddin, N.; Nian, C.Y.; Zhan, L.W.; Ning, K.X. Chitosan-Silver Nanoparticles Composite as Point-of-Use Drinking Water Filtration System for Household to Remove Pesticides in Water. Asian J. Biochem. 2011, 6, 142–159. [Google Scholar]
- Prabhu, S.; Thangadurai, T.D.; Bharathy, P.V.; Kalugasalam, P. Synthesis and Characterization of Nickel Oxide Nanoparticles Using Clitoria Ternatea Flower Extract: Photocatalytic Dye Degradation under Sunlight and Antibacterial Activity Applications. Results Chem. 2022, 4, 100285. [Google Scholar]
- Ahsan, H.; Shahid, M.; Imran, M.; Mahmood, F.; Siddique, M.H.; Ali, H.M.; Niazi, M.B.K.; Hussain, S.; Shahbaz, M.; Ayyub, M. Photocatalysis and Adsorption Kinetics of Azo Dyes by Nanoparticles of Nickel Oxide and Copper Oxide and Their Nanocomposite in an Aqueous Medium. PeerJ 2022, 10, e14358. [Google Scholar]
- Rafique, M.A.; Kiran, S.; Javed, S.; Ahmad, I.; Yousaf, S.; Iqbal, N.; Afzal, G.; Rani, F. Green Synthesis of Nickel Oxide Nanoparticles Using Allium Cepa Peels for Degradation of Congo Red Direct Dye: An Environmental Remedial Approach. Water Sci. Technol. 2021, 84, 2793–2804. [Google Scholar]
- Ghazal, S.; Khandannasab, N.; Hosseini, H.A.; Sabouri, Z.; Rangrazi, A.; Darroudi, M. Green Synthesis of Copper-Doped Nickel Oxide Nanoparticles Using Okra Plant Extract for the Evaluation of Their Cytotoxicity and Photocatalytic Properties. Ceram. Int. 2021, 47, 27165–27176. [Google Scholar]
- Naseem, S.; Rawal, R.S.; Pandey, D.; Suman, S.K. Immobilized Laccase: An Effective Biocatalyst for Industrial Dye Degradation from Wastewater. Environ. Sci. Pollut. Res. 2023, 30, 84898–84917. [Google Scholar]
- Mehta, A.; Mishra, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Saleh, T.A.; Aminabhavi, T.M. Band Gap Tuning and Surface Modification of Carbon Dots for Sustainable Environmental Remediation and Photocatalytic Hydrogen Production—A Review. J. Environ. Manag. 2019, 250, 109486. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of Nanomaterials as Adsorbents in Heavy Metal Ion Removal from Waste Water: A Review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Mehwish, H.M.; Rajoka, M.S.R.; Xiong, Y.; Cai, H.; Aadil, R.M.; Mahmood, Q.; He, Z.; Zhu, Q. Green Synthesis of a Silver Nanoparticle Using Moringa Oleifera Seed and Its Applications for Antimicrobial and Sun-Light Mediated Photocatalytic Water Detoxification. J. Environ. Chem. Eng. 2021, 9, 105290. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdelkareem, A.; Said, H.A.; El-Belely, E.F.; Alkhalifah, D.H.M.; Alshallash, K.S.; Hassan, S.E.D. Phyco-Synthesized Zinc Oxide Nanoparticles Using Marine Macroalgae, Ulva Fasciata Delile, Characterization, Antibacterial Activity, Photocatalysis, and Tanning Wastewater Treatment. Catalysts 2022, 12, 756. [Google Scholar] [CrossRef]
- Puasa, N.A.; Hairom, N.H.H.; Dzinun, H.; Madon, R.H.; Ahmad, N.S.; Sidik, D.A.B.; Azmi, A.A.A.R. Photocatalytic Degradation of Palm Oil Mill Secondary Effluent in Presence of Zinc Oxide Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100413. [Google Scholar]
- Som, I.; Roy, M.; Saha, R. Advances in Nanomaterial-Based Water Treatment Approaches for Photocatalytic Degradation of Water Pollutants. ChemCatChem 2020, 12, 3409–3433. [Google Scholar] [CrossRef]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and Aluminium Based Adsorption Strategies for Removing Arsenic from Water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef]
- Dihom, H.R.; Al-Shaibani, M.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Sharma, A.; Khamidun, M.H. Bin Photocatalytic Degradation of Disperse Azo Dyes in Textile Wastewater Using Green Zinc Oxide Nanoparticles Synthesized in Plant Extract: A Critical Review. J. Water Process Eng. 2022, 47, 102705. [Google Scholar] [CrossRef]
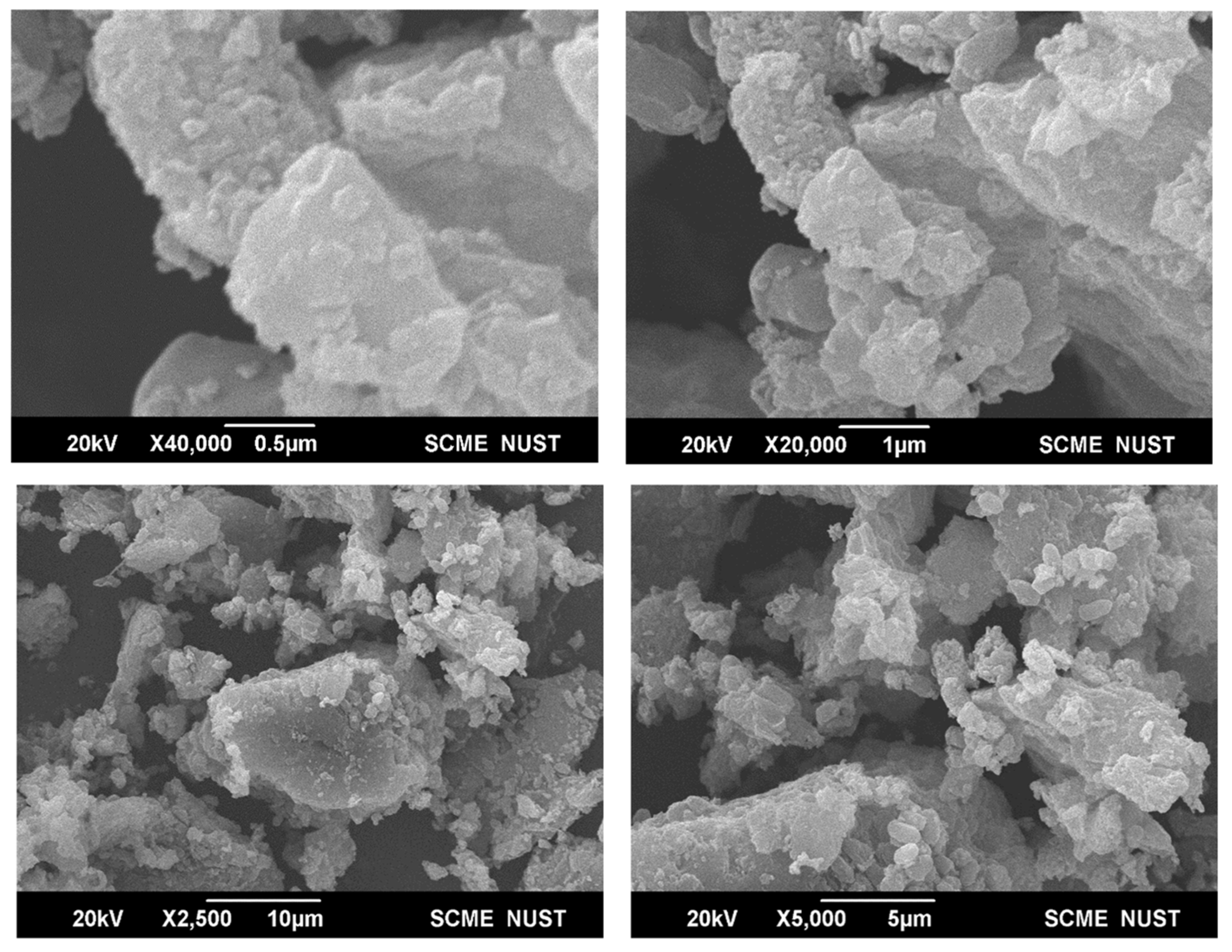

| Ingredients | Volume (µL) |
|---|---|
| PCR mix | 12.5 |
| Water | 9.00 |
| Forward primer | 0.75 |
| Reverse primer | 0.75 |
| Template | 2.00 |
| Total Volume | 25 |
| Process | Condition | ||
|---|---|---|---|
| Pre-denaturation | 94 °C for 5 min | ||
| Denaturation | 94 °C for 1 min |  | |
| Annealing | 54 °C for 1 min | (30 cycles) | |
| Extension | 72 °C for 1 min | ||
| Final extension | 72 °C for 10 min |
| Parameters | Wastewater | Treated Wastewater | NEQS Limits | %Decrease |
|---|---|---|---|---|
| pH | 8.5 | 6.1 | 6–10 | 28.23 |
| EC (dS m−1) | 9.3 | 4.5 | Not given | 48.38 |
| Color Removal (%) | 0.53 | 0.07 | Not given | 86.79 |
| COD (mg L−1) | 1523 | 773 | 150 | 49.24 |
| TDS (mg L−1) | 1891 | 623 | 3500 | 67.05 |
| Sulfates (mg L−1) | 145.37 | 69.02 | 600 | 52.5 |
| Phosphates (mg L−1) | 27.64 | 13.96 | Not given | 49.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, S.; Mahmood, F.; Shafqat, U.; Hussain, S.; Shahid, M.; Batool, F.; Elnour, R.O.; Hashem, M.; Asseri, T.A.Y.; Shahzad, T. The Biosynthesis of Nickel Oxide Nanoparticles: An Eco-Friendly Approach for Azo Dye Decolorization and Industrial Wastewater Treatment. Sustainability 2023, 15, 14965. https://doi.org/10.3390/su152014965
Mustafa S, Mahmood F, Shafqat U, Hussain S, Shahid M, Batool F, Elnour RO, Hashem M, Asseri TAY, Shahzad T. The Biosynthesis of Nickel Oxide Nanoparticles: An Eco-Friendly Approach for Azo Dye Decolorization and Industrial Wastewater Treatment. Sustainability. 2023; 15(20):14965. https://doi.org/10.3390/su152014965
Chicago/Turabian StyleMustafa, Sadia, Faisal Mahmood, Usman Shafqat, Sabir Hussain, Muhammad Shahid, Fatima Batool, Rehab O. Elnour, Mohamed Hashem, Tahani A. Y. Asseri, and Tanvir Shahzad. 2023. "The Biosynthesis of Nickel Oxide Nanoparticles: An Eco-Friendly Approach for Azo Dye Decolorization and Industrial Wastewater Treatment" Sustainability 15, no. 20: 14965. https://doi.org/10.3390/su152014965
APA StyleMustafa, S., Mahmood, F., Shafqat, U., Hussain, S., Shahid, M., Batool, F., Elnour, R. O., Hashem, M., Asseri, T. A. Y., & Shahzad, T. (2023). The Biosynthesis of Nickel Oxide Nanoparticles: An Eco-Friendly Approach for Azo Dye Decolorization and Industrial Wastewater Treatment. Sustainability, 15(20), 14965. https://doi.org/10.3390/su152014965










