Buckwheat Production and Value-Added Processing: A Review of Potential Western Washington Cropping and Food System Applications
Abstract
1. Introduction
1.1. Food System Approach
1.2. Origin and Global Production
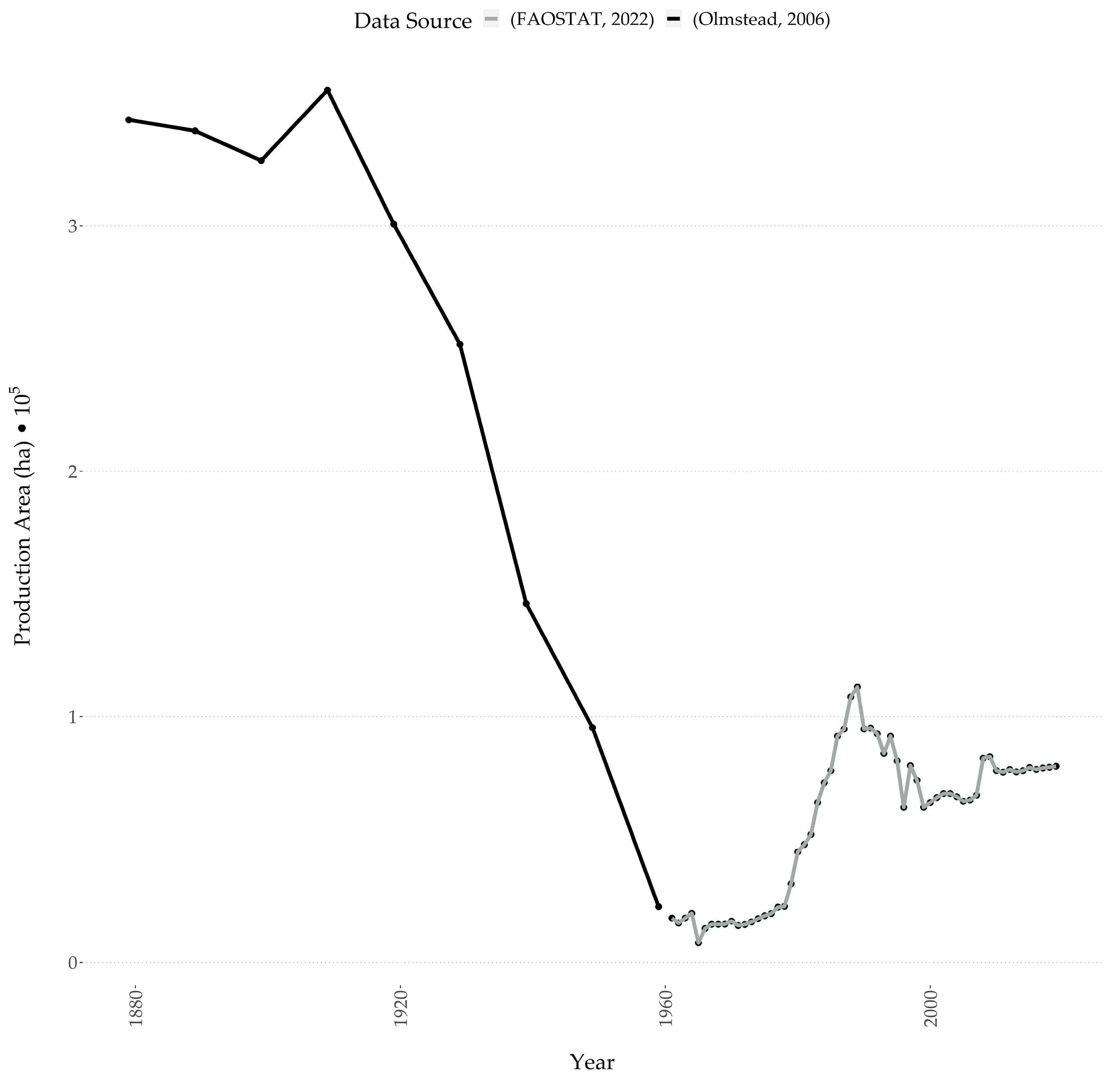
1.3. Buckwheat Production in Washington State
1.4. Market Potential
2. Crop Production
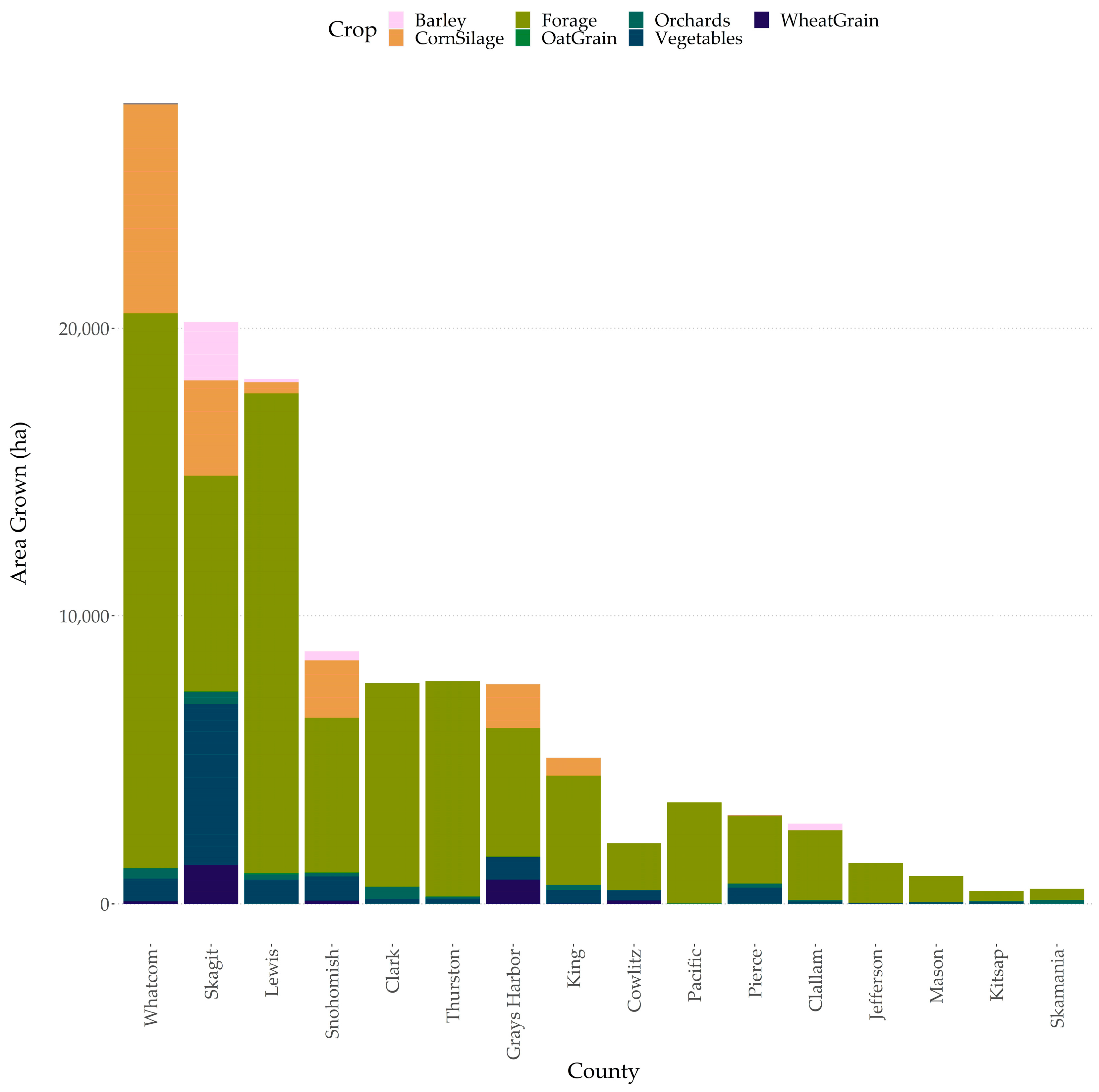
2.1. Washington State Cropping Systems
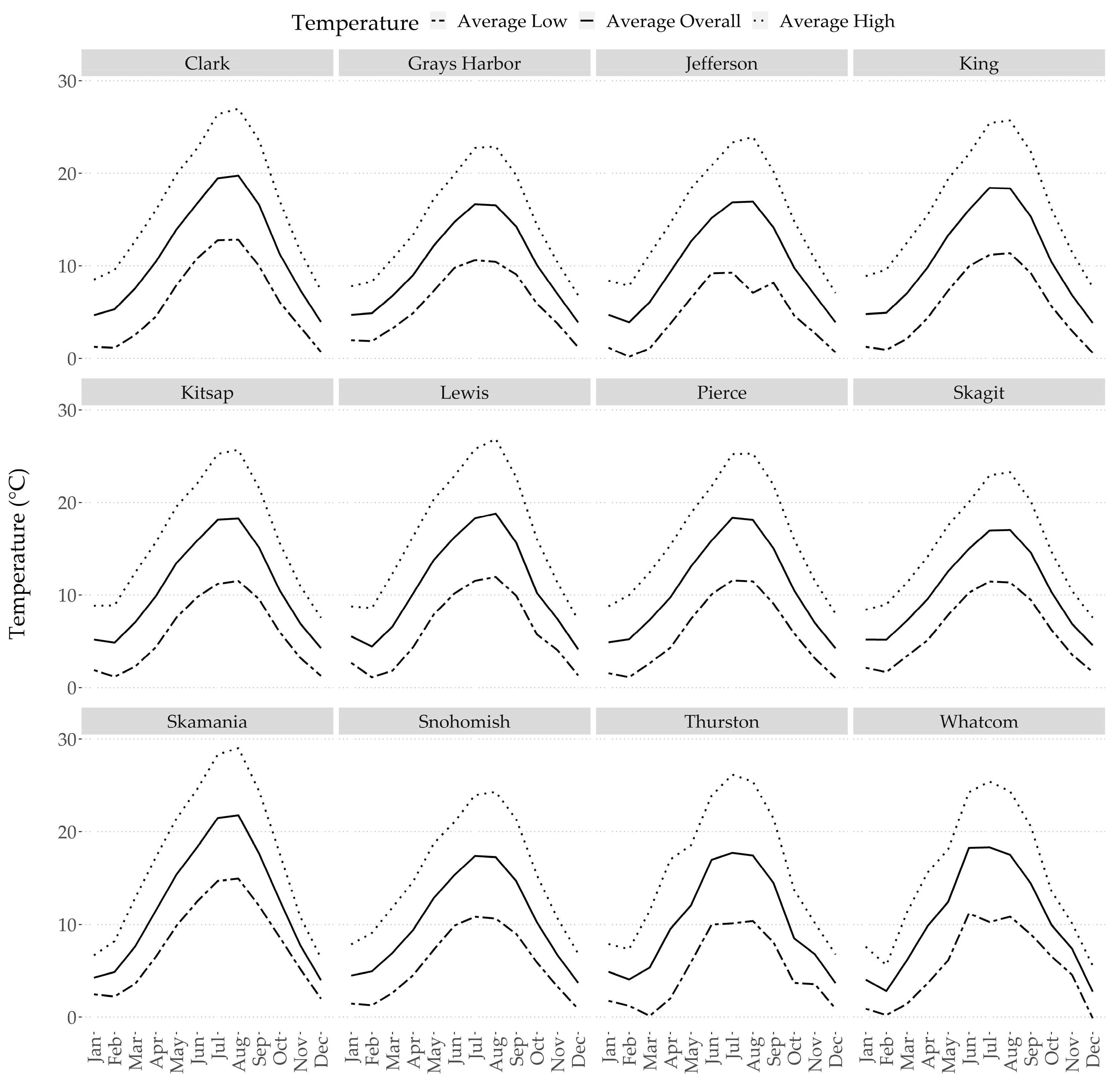
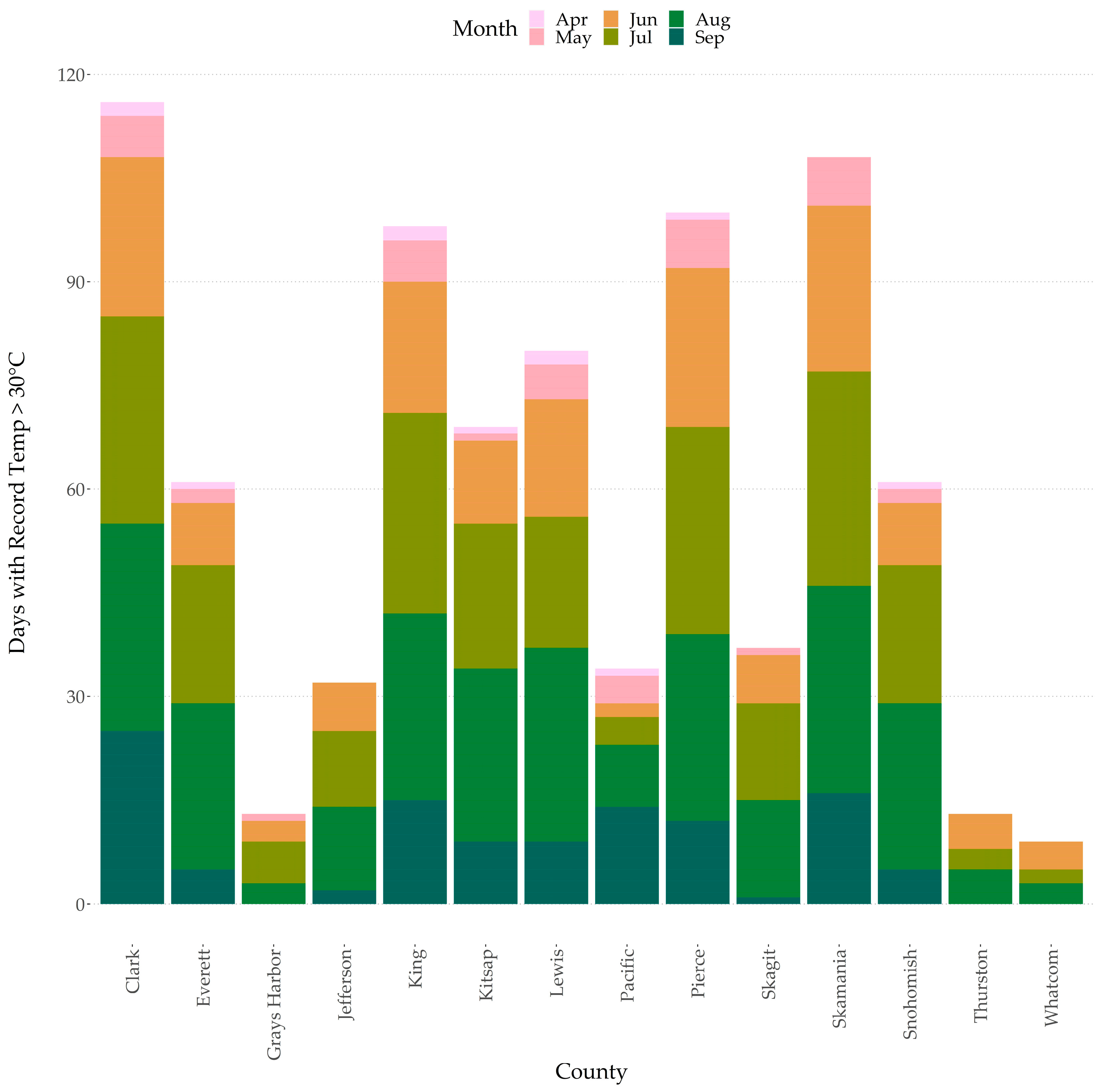
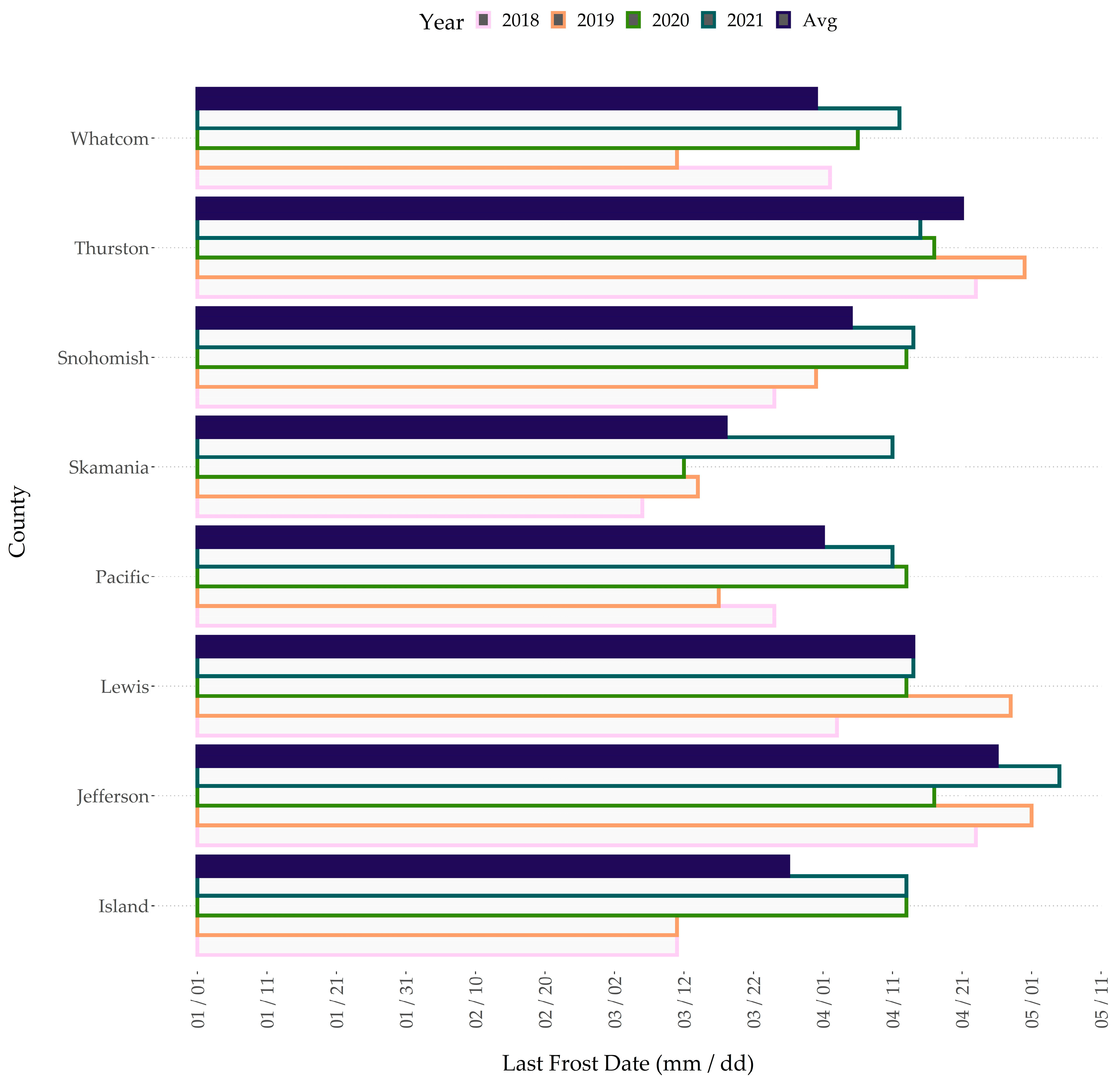
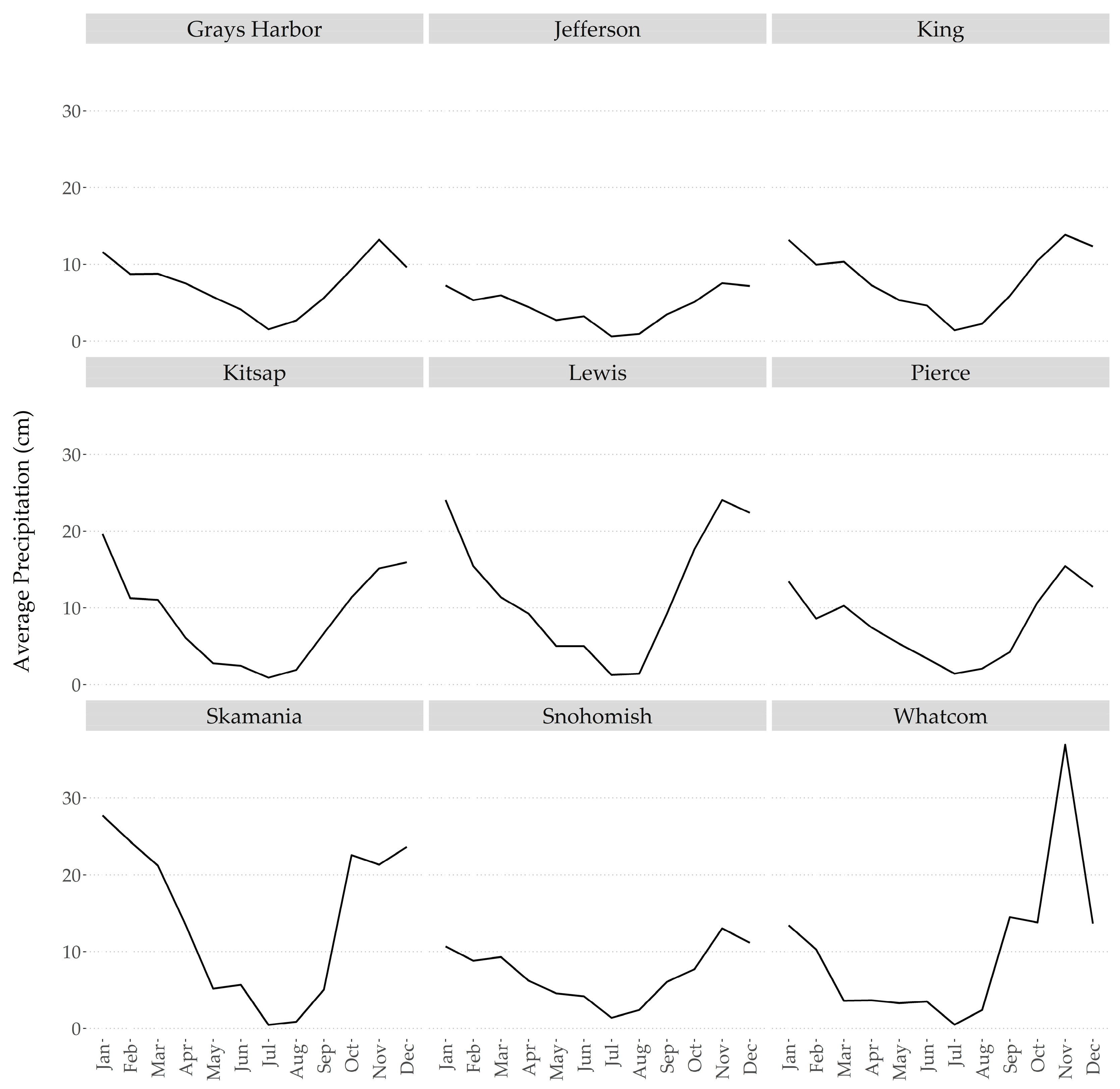
2.2. Abiotic Stresses within Western Washington
2.3. Buckwheat Breeding for Western Washington
3. Food Application
3.1. Physicochemical Characteristics of Buckwheat
3.1.1. Proximate Composition
3.1.2. Functional Properties
3.2. Buckwheat Fractions
3.2.1. Protein
3.2.2. Starch
3.3. Product Development
3.3.1. Bread
3.3.2. Cookies
3.3.3. Cakes
3.3.4. Pasta
3.3.5. Noodles
3.3.6. Beer and Malting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilar, J.; Gramig, G.G.; Hendrickson, J.R.; Archer, D.W.; Forcella, F.; Liebig, M.A. Crop Species Diversity Changes in the United States: 1978–2012. PLoS ONE 2015, 10, e0136580. [Google Scholar] [CrossRef]
- Wen, W.; Li, Z.; Shao, J.; Tang, Y.; Zhao, Z.; Yang, J.; Ding, M.; Zhu, X.; Zhou, M. The Distribution and Sustainable Utilization of Buckwheat Resources under Climate Change in China. Plants 2021, 10, 2081. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Yasui, Y.; Ohnishi, O. Original Birthplace of Cultivated Common Buckwheat Inferred from Genetic Relationships among Cultivated Populations and Natural Populations of Wild Common Buckwheat Revealed by AFLP Analysis. Genes Genet. Syst. 2005, 80, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Onishi, O. Population Genetics of Cultivated Common Buckwheat, Fagopyrum esculentum Moench. X. Diffusion Routes Revealed by RAPD Markers. Genes Genet. Syst. 1996, 71, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, O. Population Genetics of Cultivated Common Buckwheat, Fagopyrum esculentum Moench. VIII. Local Differentiation of Land Races in Europe and the Silk Road. Jpn. J. Genet. 1993, 68, 303–316. [Google Scholar] [CrossRef][Green Version]
- Food and Agriculture Organization of the United Nations FAOSTAT Database. Available online: http://www.fao.org/faostat/en/#home (accessed on 1 January 2022).
- Zhou, M.; Tang, Y.; Deng, X.; Ruan, C.; Kreft, I.; Tang, Y.; Wu, Y. Overview of Buckwheat Resources in the World. In Buckwheat Germplasm in the World; Zhou, M., Kreft, I., Suvorova, G., Tang, Y., Woo, S.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–7. ISBN 9780128110065. [Google Scholar]
- Zhang, K.; He, M.; Fan, Y.; Zhao, H.; Gao, B.; Yang, K.; Li, F.; Tang, Y.; Gao, Q.; Lin, T.; et al. Resequencing of Global Tartary Buckwheat Accessions Reveals Multiple Domestication Events and Key Loci Associated with Agronomic Traits. Genome Biol. 2021, 22, 23. [Google Scholar] [CrossRef]
- Podolska, G.; Gujska, E.; Klepacka, J.; Aleksandrowicz, E. Bioactive Compounds in Different Buckwheat Species. Plants 2021, 10, 961. [Google Scholar] [CrossRef]
- Dimitri, C.; Effland, A.; Conklin, N. The 20th Century Transformation of U.S. Agriculture and Farm Policy; U.S. Department of Agriculture: Washington, DC, USA, 2005; Volume 3. [Google Scholar]
- U.S. Department of Agriculture. USDA NASS 2017 Census of Agriculture-United States Summary and State Data; U.S. Department of Agriculture: Washington, DC, USA, 2019; Volume 1. [Google Scholar]
- U.S. Department of Agriculture. USDA NASS 2017 Census of Agriculture—County Data—Field Crops; U.S. Department of Agriculture: Washington, DC, USA, 2019. [Google Scholar]
- Simoes, A.; Hidalgo, C. The Economic Complexity Observatory: An Analytical Tool for Understanding the Dynamics of Economic Development. In Proceedings of the Workshops at the Twenty-Fifth AAAI Conference on Artificial Intelligence, San Francisco, CA, USA, 7–11 August 2011. [Google Scholar]
- Olmstead, A.L.; Rhode, P.W. TABLE Da746–754 Hay, Rye, and Buckwheat—Acreage, Production, and Price: 1839–1997. In Historical Statistics of the United States; Carter, S.B., Gartner, S.S., Haines, M.R., Olmstead, A.L., Sutch, R., Wright, G., Eds.; Cambridge University Press: Cambridge, UK, 2006; Volume 2, pp. 4–109. [Google Scholar]
- Hills, K.; Goldberger, J.; Jones, S. Commercial Bakers’ View on the Meaning of “Local” Wheat and Flour in Western Washington State. J. Agric. Food Syst. Community Dev. 2013, 3, 13–32. [Google Scholar] [CrossRef]
- NOAA National Center for Environmental Information U.S. Climate Atlas: 1991–2020 Normals. Available online: https://www.ncei.noaa.gov/access/climateatlas/ (accessed on 4 April 2023).
- Brouwer, B.O.; Murphy, K.M.; Jones, S.S. Plant Breeding for Local Food Systems: A Contextual Review of End-Use Selection for Small Grains and Dry Beans in Western Washington. Renew. Agric. Food Syst. 2016, 31, 172–184. [Google Scholar] [CrossRef]
- Ostrom, M.; Donovan, C. Organizational Dimensions of Farmers Markets in Washington State; Washington State University: Pullman, WA, USA, 2018; pp. 1–26. [Google Scholar]
- Sedej, I.; Sakač, M.; Mandić, A.; Mišan, A.; Pestorić, M.; Šimurina, O.; Čanadanović-Brunet, J. Quality Assessment of Gluten-Free Crackers Based on Buckwheat Flour. LWT 2011, 44, 694–699. [Google Scholar] [CrossRef]
- Jan, U.; Gani, A.; Ahmad, M.; Shah, U.; Baba, W.N.; Masoodi, F.A.; Maqsood, S.; Gani, A.; Wani, I.A.; Wani, S.M. Characterization of Cookies Made from Wheat Flour Blended with Buckwheat Flour and Effect on Antioxidant Properties. J. Food Sci. Technol. 2015, 52, 6334–6344. [Google Scholar] [CrossRef] [PubMed]
- Torbica, A.; Hadnadev, M.; Dapčević Hadnadev, T. Rice and Buckwheat Flour Characterisation and Its Relation to Cookie Quality. Food Res. Int. 2012, 48, 277–283. [Google Scholar] [CrossRef]
- Hussain, A.; Kaul, R. Formulation and Characterization of Buckwheat-Barley Supplemented Multigrain Biscuits. Curr. Res. Nutr. Food Sci. 2018, 6, 873–881. [Google Scholar] [CrossRef]
- Wronkowska, M.; Haros, M.; Soral-Śmietana, M. Effect of Starch Substitution by Buckwheat Flour on Gluten-Free Bread Quality. Food Bioprocess Technol. 2013, 6, 1820–1827. [Google Scholar] [CrossRef]
- De Arcangelis, E.; Cuomo, F.; Trivisonno, M.C.; Marconi, E.; Messia, M.C. Gelatinization and Pasta Making Conditions for Buckwheat Gluten-Free Pasta. J. Cereal Sci. 2020, 95, 103073. [Google Scholar] [CrossRef]
- Bouasla, A.; Wójtowicz, A. Rice-Buckwheat Gluten-Free Pasta: Effect of Processing Parameters on Quality Characteristics and Optimization of Extrusion-Cooking Process. Foods 2019, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Schoenlechner, R.; Drausinger, J.; Ottenschlaeger, V.; Jurackova, K.; Berghofer, E. Functional Properties of Gluten-Free Pasta Produced from Amaranth, Quinoa and Buckwheat. Plant Foods Hum. Nutr. 2010, 65, 339–349. [Google Scholar] [CrossRef]
- Fu, M.; Sun, X.; Wu, D.; Meng, L.; Feng, X.; Cheng, W.; Gao, C.; Yang, Y.; Shen, X.; Tang, X. Effect of Partial Substitution of Buckwheat on Cooking Characteristics, Nutritional Composition, and in Vitro Starch Digestibility of Extruded Gluten-Free Rice Noodles. LWT Food Sci. Technol. 2020, 126, 109332. [Google Scholar] [CrossRef]
- Guo, X.N.; Wei, X.M.; Zhu, K.X. The Impact of Protein Cross-Linking Induced by Alkali on the Quality of Buckwheat Noodles. Food Chem. 2017, 221, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.A.; Menard, C.; Roudaut, G.; Brennan, C.S. Amaranth, Millet and Buckwheat Flours Affect the Physical Properties of Extruded Breakfast Cereals and Modulates Their Potential Glycaemic Impact. Starch Stärke 2012, 64, 392–398. [Google Scholar] [CrossRef]
- Cadenas, R.; Caballero, I.; Nimubona, D.; Blanco, C.A. Brewing with Starchy Adjuncts: Its Influence on the Sensory and Nutritional Properties of Beer. Foods 2021, 10, 1726. [Google Scholar] [CrossRef]
- Deng, Y.; Lim, J.; Lee, G.H.; Hanh Nguyen, T.T.; Xiao, Y.; Piao, M.; Kim, D. Brewing Rutin-Enriched Lager Beer with Buckwheat Malt as Adjuncts. J. Microbiol. Biotechnol. 2019, 29, 877–886. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. US Bureau of Labor Statistics Occupational Employment and Wage Statistics: Bakers; U.S. Department of Agriculture: Washington, DC, USA, 2021. [Google Scholar]
- Mefford, C.; Cohen, S.; Calen, M.; Haring, D.; McLennon, M.; Lobel, B.; Tarhouni, Z. Washington Beer: Economic Impacts in Washington State; Community Attributes Inc: Seattle, WA, USA, 2019. [Google Scholar]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac Disease: Understanding the Gluten-Free Diet. Eur. J. Nutr. 2016, 56, 449–459. [Google Scholar] [CrossRef]
- Thompson, T.; Dennis, M.; Higgins, L.A.; Lee, A.R.; Sharrett, M.K. Gluten-Free Diet Survey: Are Americans with Coeliac Disease Consuming Recommended Amounts of Fibre, Iron, Calcium and Grain Foods? J. Hum. Nutr. Diet. 2005, 18, 163–169. [Google Scholar] [CrossRef]
- Woomer, J.S.; Adedeji, A.A. Current Applications of Gluten-Free Grains—A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 14–24. [Google Scholar] [CrossRef]
- Christoph, M.J.; Larson, N.; Hootman, K.C.; Miller, J.M.; Neumark-Sztainer, D. Who Values Gluten-Free? Dietary Intake, Behaviors, and Sociodemographic Characteristics of Young Adults Who Value Gluten-Free Food. J. Acad. Nutr. Diet. 2018, 118, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Matlock, T. Organic: A Thriving Agricultural Segment. Available online: https://www.usda.gov/media/blog/2020/10/28/organic-thriving-agriculture-segment (accessed on 27 September 2023).
- Saturni, L.; Ferretti, G.; Bacchetti, T. The Gluten-Free Diet: Safety and Nutritional Quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef]
- Qin, P.; Wang, Q.; Shan, F.; Hou, Z.; Ren, G. Nutritional Composition and Flavonoids Content of Flour from Different Buckwheat Cultivars. Int. J. Food Sci. Technol. 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Granato, D.; Nunes, D.S.; Barba, F.J. An Integrated Strategy between Food Chemistry, Biology, Nutrition, Pharmacology, and Statistics in the Development of Functional Foods: A Proposal. Trends Food Sci. Technol. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Krkošková, B.; Mrázová, Z. Prophylactic Components of Buckwheat. Food Res. Int. 2005, 38, 561–568. [Google Scholar] [CrossRef]
- Scott, W.O. Crops for Emergency Plantings; University of Illinois Extension: Urbana, IL, USA, 1980; Circular72. [Google Scholar]
- Myers, R.L. How to Grow Buckwheat; Thomas Jefferson Agricultural Institute: Columbia, MO, USA, 2002; pp. 1–4. [Google Scholar]
- Bavec, M.; Bavec, F.; Plazovnik, C.; Grobelnik Mlakar, S. Buckwheat Leaf Area Index and Yield Performance Depending on Plant Population under Full-Season and Stubble-Crop Growing Periods. Bodenkultur 2006, 57, 5–12. [Google Scholar]
- Pavek, P.L.S. Plant Guide for Buckwheat; USDA-Natural Resources Conservation Service Plant Materials Center: Pullman, WA, USA, 2016; pp. 1–6. [Google Scholar]
- Creamer, N.G.; Baldwin, K.R. An Evaluation of Summer Cover Crops for Use in Vegetable Production Systems in North Carolina. HortScience 2000, 35, 600–603. [Google Scholar] [CrossRef]
- Smith, R.G.; Atwood, L.W.; Warren, N.D. Increased Productivity of a Cover Crop Mixture Is Not Associated with Enhanced Agroecosystem Services. PLoS ONE 2014, 9, e97351. [Google Scholar] [CrossRef]
- Holmes, A.A.; Thompson, A.A.; Wortman, S.E. Species-Specific Contributions to Productivity and Weed Suppression in Cover Crop Mixtures. Agron. J. 2017, 109, 2808–2819. [Google Scholar] [CrossRef]
- Wauters, V.M.; Grossman, J.M.; Pfeiffer, A.; Cala, R. Ecosystem Services and Cash Crop Tradeoffs of Summer Cover Crops in Northern Region Organic Vegetable Rotations. Front. Sustain. Food Syst. 2021, 5, 635955. [Google Scholar] [CrossRef]
- Ghahremani, S.; Ebadi, A.; Tobeh, A.; Hashemi, M.; Sedghi, M.; Gholipoouri, A.; Barker, A.V. Short-Term Impact of Monocultured and Mixed Cover Crops on Soil Properties, Weed Suppression, and Lettuce Yield. Commun. Soil Sci. Plant Anal. 2021, 52, 406–415. [Google Scholar] [CrossRef]
- Shabbir, A.; Hickman, L.; Walsh, M. The Weed-Suppressive Ability of Summer Cover Crops in the Northern Grains Region of Australia. Agronomy 2022, 12, 1831. [Google Scholar] [CrossRef]
- Björkman, T.; Shail, J.W. Using a Buckwheat Cover Crop for Maximum Weed Suppression after Early Vegetables. Horttechnology 2013, 23, 575–580. [Google Scholar] [CrossRef]
- Kumar, V.; Brainard, D.C.; Bellinder, R.R. Suppression of Powell Amaranth (Amaranthus powellii) by Buckwheat Residues: Role of Allelopathy. Weed Sci. 2009, 57, 66–73. [Google Scholar] [CrossRef]
- Osipitan, O.A.; Dille, J.A.; Assefa, Y.; Radicetti, E.; Ayeni, A.; Knezevic, S.Z. Impact of Cover Crop Management on Level of Weed Suppression: A Meta-Analysis. Crop Sci. 2019, 59, 833–842. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Sugimoto, H.; Yamada, M. Buckwheat Seedlings May Inhibit Other Plant Growth by Allelopathic Substances. Environ. Control Biol. 2007, 45, 27–32. [Google Scholar] [CrossRef][Green Version]
- Kumar, V.; Brainard, D.C.; Bellinder, R.R. Suppression of Powell Amaranth (Amaranthus powellii), Shepherd’s-Purse (Capsella bursa-pastoris), and Corn Chamomile (Anthemis arvensis) by Buckwheat Residues: Role of Nitrogen and Fungal Pathogens. Weed Sci. 2008, 56, 271–280. [Google Scholar] [CrossRef]
- Kumar, V.; Brainard, D.C.; Bellinder, R.R.; Hahn, R.R. Buckwheat Residue Effects on Emergence and Growth of Weeds in Winter-Wheat (Triticum aestivum) Cropping Systems. Weed Sci. 2011, 59, 567–573. [Google Scholar] [CrossRef]
- Szwed, M.; Wiczkowski, W.; Szawara, D.; Ralph, N.; Marcin, L.O. Allelopathic Influence of Common Buckwheat Root Residues on Selected Weed Species. Acta Physiol. Plant 2019, 41, 92. [Google Scholar] [CrossRef]
- Dahnke, W.C.; Fanning, C.; Cattanach, A. Fertilizing Buckwheat; North Dakota State University Circular Series; North Dakota State University: Fargo, ND, USA, 1992. [Google Scholar]
- Björkman, T. Buckwheat Production: Planting; Agronomy Fact Sheet Series; Cornell University Cooperative Extension: Ithaca, NY, USA, 2010; p. 2. [Google Scholar]
- Kaiser, D.E.; Lamb, J.A.; Eliason, R. Fertilizer Guidelines for Agronomic Crops in Minnesota; University of Minnesota Extension: Minneapolis, MN, USA, 2011; pp. 1–44. [Google Scholar]
- Wang, C.; Ruan, R.W.; Yuan, X.H.; Hu, D.; Yang, H.; Li, Y.; Yi, Z.L. Effects of Nitrogen Fertilizer and Planting Density on the Lignin Synthesis in the Culm in Relation to Lodging Resistance of Buckwheat. Plant Prod. Sci. 2015, 18, 218–227. [Google Scholar] [CrossRef]
- Schulte Auf’m Erley, G.; Kaul, H.P.; Kruse, M.; Aufhammer, W. Yield and Nitrogen Utilization Efficiency of the Pseudocereals Amaranth, Quinoa, and Buckwheat under Differing Nitrogen Fertilization. Eur. J. Agron. 2005, 22, 95–100. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Nie, J.; Wang, C.; Huang, K.; Zhang, Y.; Zhang, Y.; She, H.; Liu, X.; Ruan, R.; et al. Effects of Nitrogen Fertilizer and Planting Density on the Leaf Photosynthetic Characteristics, Agronomic Traits and Grain Yield in Common Buckwheat (Fagopyrum esculentum M.). Field Crop Res. 2018, 219, 160–168. [Google Scholar] [CrossRef]
- Gao, L.; Bai, W.; Xia, M.; Wan, C.; Wang, M.; Wang, P.; Gao, X.; Gao, J. Diverse Effects of Nitrogen Fertilizer on the Structural, Pasting, and Thermal Properties of Common Buckwheat Starch. Int. J. Biol. Macromol. 2021, 179, 542–549. [Google Scholar] [CrossRef]
- Wan, C.; Gao, L.; Wang, J.; Lei, X.; Wu, Y.; Gao, J. Proteomics Characterization Nitrogen Fertilizer Promotes the Starch Synthesis and Metabolism and Amino Acid Biosynthesis in Common Buckwheat. Int. J. Biol. Macromol. 2021, 192, 342–349. [Google Scholar] [CrossRef]
- Norbäck, D.; Wieslander, G. A Review on Epidemiological and Clinical Studies on Buckwheat Allergy. Plants 2021, 10, 607. [Google Scholar] [CrossRef]
- Berglund, D.R. Buckwheat Production; NDSU Extension: Fargo, ND, USA, 2019; p. A687. [Google Scholar]
- Lyon, D.J.; Thorne, M.E.; Jha, P.; Kumar, V.; Waters, T. Volunteer Buckwheat Control in Wheat. Crop Forage Turfgrass Manag. 2019, 5, 190033. [Google Scholar] [CrossRef]
- Michiyama, H.; Tsuchimoto, K.; Tani, K.I.; Hirano, T.; Hayashi, H.; Campbell, C. Influence of Day Length on Stem Growth, Flowering, Morphology of Flower Clusters, and Seed-Set in Buckwheat (Fagopyrum esculentum Moench). Plant Prod. Sci. 2005, 8, 44–50. [Google Scholar] [CrossRef]
- Michiyama, H.; Arikuni, M.; Hirano, T.; Hayashi, H. Influence of Day Length before and after the Start of Anthesis on the Growth, Flowering and Seed-Setting in Common Buckwheat (Fagopyrum esculentum Moench). Plant Prod. Sci. 2003, 6, 235–242. [Google Scholar] [CrossRef]
- Quinet, M.; Cawoy, V.; Lefèvre, I.; Van Miegroet, F.; Jacquemart, A.L.; Kinet, J.M. Inflorescence Structure and Control of Flowering Time and Duration by Light in Buckwheat (Fagopyrum esculentum Moench). J. Exp. Bot. 2004, 55, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Arduini, I.; Masoni, A.; Mariotti, M. A Growth Scale for the Phasic Development of Common Buckwheat. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 215–228. [Google Scholar] [CrossRef]
- Płażek, A.; Hura, K.; Hura, T.; Słomka, A.; Hornyák, M.; Sychta, K. Synthesis of Heat-Shock Proteins HSP-70 and HSP-90 in Flowers of Common Buckwheat (Fagopyrum esculentum) under Thermal Stress. Crop Pasture Sci. 2020, 71, 760–767. [Google Scholar] [CrossRef]
- Michiyama, H.; Yoshimura, K.; Hirano, T.; Hayashi, H. Influence of Air Temperature on Varietal Differences between Summer and Autumn Ecotype in Buckwheat. In Proceedings of the 10th International Symposium on Buckwheat, Yangling, China, 1 January 2007; pp. 242–246. [Google Scholar]
- Kumar, G.; Srivastava, A. Cytomorphological and Biochemical Impact of Temperature Stress in Buckwheat (Fagopyrum esculentum Moench). Int. J. Environ. Sci. Toxicol. Res. 2015, 3, 134–143. [Google Scholar]
- Płażek, A.; Słomka, A.; Kopeć, P.; Dziurka, M.; Hornyák, M.; Sychta, K.; Pastuszak, J.; Dubert, F. Effects of High Temperature on Embryological Development and Hormone Profile in Flowers and Leaves of Common Buckwheat (Fagopyrum esculentum Moench). Int. J. Mol. Sci. 2019, 20, 1705. [Google Scholar] [CrossRef]
- Hornyák, M.; Płażek, A.; Kopeć, P.; Dziurka, M.; Pastuszak, J.; Szczerba, A.; Hura, T. Photosynthetic Activity of Common Buckwheat (Fagopyrum esculentum Moench) Exposed to Thermal Stress. Photosynthetica 2020, 58, 45–53. [Google Scholar] [CrossRef]
- WSU AgWeathernet Weather Data. Available online: https://weather.wsu.edu/ (accessed on 30 May 2023).
- Kalinová, J.; Moudrý, J. Evaluation of Frost Resistance in Varieties of Common Buckwheat (Fagopyrum esculentum Moench). Plant Soil Environ. 2003, 49, 410–413. [Google Scholar] [CrossRef]
- Bjorkman, T. Causes of Poor Stand Establishment after Heavy Rains. In Proceedings of the 8th International Symposium on Buckwheat, Chunchon, Republic of Korea, 30 August–2 September 2001; pp. 134–137. [Google Scholar]
- Choi, J.Y.; Cho, S.W.; Chun, J.B.; Kwon, S.J.; Roy, S.K.; Sung, J.K.; Woo, S.H.; Sakagami, J.I. Morpho-Physiological Response of Common Buckwheat (Fagopyrum esculentum) to Flooding Stress at Different Growth Stages. J. Crop Sci. Biotechnol. 2021, 24, 41–49. [Google Scholar] [CrossRef]
- Murayama, S.; Suyama, Y.; Yanokuchi, Y. Varietal Difference of Pre-Germination Flooding Tolerance in Buckwheat. In Proceedings of the 9th International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2004; pp. 143–145. [Google Scholar]
- Sakata, K.; Ohsawa, R. Varietal Differences of Flood Tolerance during Germination and Selection of the Tolerant Lines in Common Buckwheat. Plant Prod. Sci. 2006, 9, 395–400. [Google Scholar] [CrossRef]
- Suzuki, T.; Noda, T.; Morishita, T.; Ishiguro, K.; Otsuka, S.; Brunori, A. Present Status and Future Perspectives of Breeding for Buckwheat Quality. Breed. Sci. 2020, 70, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Luthar, Z.; Zhou, M.; Golob, A.; Germ, M. Breeding Buckwheat for Increased Levels and Improved Quality of Protein. Plants 2021, 10, 14. [Google Scholar] [CrossRef]
- Ohsawa, R. Current Status and Prospects of Common Buckwheat Breeding in Japan. Breed. Sci. 2020, 70, 3–12. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Noda, T.; Ishiguro, K.; Otsuka, S.; Katsu, K. Breeding of Buckwheat to Reduce Bitterness and Rutin Hydrolysis. Plants 2021, 10, 791. [Google Scholar] [CrossRef]
- Joshi, D.C.; Chaudhari, G.V.; Sood, S.; Kant, L.; Pattanayak, A.; Zhang, K.; Fan, Y.; Janovská, D.; Meglič, V.; Zhou, M. Revisiting the Versatile Buckwheat: Reinvigorating Genetic Gains through Integrated Breeding and Genomics Approach. Planta 2019, 250, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Yasui, Y. Buckwheat Heteromorphic Self-Incompatibility: Genetics, Genomics and Application to Breeding. Breed. Sci. 2020, 70, 32–38. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency Variety Registration. Available online: https://inspection.canada.ca/plant-varieties/variety-registration/eng/1299175847046/1299175906353 (accessed on 8 May 2023).
- USDA Agricultural Marketing Service Plant Variety Protection Office—Scanned and Redacted Issued Certificates. Available online: https://apps.ams.usda.gov/CMS/ (accessed on 8 May 2023).
- Bhinder, S.; Kaur, A.; Singh, B.; Yadav, M.P.; Singh, N. Proximate Composition, Amino Acid Profile, Pasting and Process Characteristics of Flour from Different Tartary Buckwheat Varieties. Food Res. Int. 2020, 130, 108946. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Arif, M.; Khan, T.U.; Khan, M.I.; Bangash, J.A. Nutritional Evaluation of Common Buckwheat of Four Different Villages of Gilgit-Baltistan. J. Agric. Biol. Sci. 2013, 8, 264–266. [Google Scholar]
- Lu, L.; Murphy, K.; Baik, B.K. Genotypic Variation in Nutritional Composition of Buckwheat Groats and Husks. Cereal Chem. 2013, 90, 132–137. [Google Scholar] [CrossRef]
- Tien, N.N.T.; Trinh, L.N.D.; Inoue, N.; Morita, N.; Van Hung, P. Nutritional Composition, Bioactive Compounds, and Diabetic Enzyme Inhibition Capacity of Three Varieties of Buckwheat in Japan. Cereal Chem. 2018, 95, 615–624. [Google Scholar] [CrossRef]
- USDA Agricultural Research Service FoodData Central: Foundation Foods. Available online: https://fdc.nal.usda.gov (accessed on 27 September 2023).
- Shahzadi, N.A.; Butt, M.S.; Rehman, S.U.; Sharif, K.A. Chemical Characteristics of Various Composite Flours. Int. J. Agric. Biol. 2005, 07, 105–108. [Google Scholar]
- Nasir, M.; Anjum, F.M.; Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Minhas, R. Effect of Moisture on the Shelf Life of Wheat Flour. Int. J. Agric. Biol. 2003, 5, 458–459. [Google Scholar]
- Mahapatra, A.K.; Lan, Y.; Harris, D.L. Influence of Moisture Content and Temperature on Thermal Conductivity and Thermal Diffusivity of Rice Flours. Int. J. Food Prop. 2011, 14, 675–683. [Google Scholar] [CrossRef]
- Subramanian, S.; Viswanathan, R. Thermal Properties of Minor Millet Grains and Flours. Biosyst. Eng. 2003, 84, 289–296. [Google Scholar] [CrossRef]
- Jongsutjarittam, O.; Charoenrein, S. The Effect of Moisture Content on Physicochemical Properties of Extruded Waxy and Non-Waxy Rice Flour. Carbohydr. Polym. 2014, 114, 133–140. [Google Scholar] [CrossRef]
- Bilge, G.; Sezer, B.; Eseller, K.E.; Berberoglu, H.; Koksel, H.; Boyaci, I.H. Ash Analysis of Flour Sample by Using Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2016, 124, 74–78. [Google Scholar] [CrossRef]
- Kweon, M.; Martin, R.; Souza, E. Effect of Tempering Conditions on Milling Performance and Flour Functionality. Cereal Chem. 2009, 86, 12–17. [Google Scholar] [CrossRef]
- Kinsella, J.E. Functional Properties of Soy Proteins. J. Am. Oil Chem. Soc. 1979, 56, 242–258. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, R.; Hasan, S.; Zzaman, W.; Rana, M.R.; Ahmed, S.; Roy, M.; Sayem, A.; Matin, A.; Raposo, A.; et al. A Comprehensive Review on Bio-Preservation of Bread: An Approach to Adopt Wholesome Strategies. Foods 2022, 11, 319. [Google Scholar] [CrossRef]
- Ortiz de Erive, M.; Wang, T.; He, F.; Chen, G. Development of High-Fiber Wheat Bread Using Microfluidized Corn Bran. Food Chem. 2020, 310, 125921. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Jiang, H.; Yu, X.; Jane, J.L. Physicochemical and Functional Properties of Whole Legume Flour. LWT 2014, 55, 308–313. [Google Scholar] [CrossRef]
- Wolf, W.J. Soybean Proteins: Their Functional, Chemical, and Physical Properties. J. Agric. Food Chem. 1970, 18, 969–976. [Google Scholar] [CrossRef]
- Aluwi, N.A.; Murphy, K.M.; Ganjyal, G.M. Physicochemical Characterization of Different Varieties of Quinoa. Cereal Chem. 2017, 94, 847–856. [Google Scholar] [CrossRef]
- Kaushal, P.; Kumar, V.; Sharma, H.K. Comparative Study of Physicochemical, Functional, Antinutritional and Pasting Properties of Taro (Colocasia esculenta), Rice (Oryza sativa) Flour, Pigeonpea (Cajanus cajan) Flour and Their Blends. LWT Food Sci. Technol. 2012, 48, 59–68. [Google Scholar] [CrossRef]
- Raikos, V.; Neacsu, M.; Russell, W.; Duthie, G. Comparative Study of the Functional Properties of Lupin, Green Pea, Fava Bean, Hemp, and Buckwheat Flours as Affected by PH. Food Sci. Nutr. 2014, 2, 802–810. [Google Scholar] [CrossRef]
- Felli, R.; Aris Yang, T.; Nadiah Wan Abdullah, W.; Zzaman, W. Effects of Incorporation of Jackfruit Rind Powder on Chemical and Functional Properties of Bread. Trop. Life Sci. Res. 2018, 29, 113–126. [Google Scholar] [CrossRef]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.; et al. Biofortification of Wheat with Zinc through Zinc Fertilization in Seven Countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Chandra, S.; Singh, S.; Kumari, D. Evaluation of Functional Properties of Composite Flours and Sensorial Attributes of Composite Flour Biscuits. J. Food Sci. Technol. 2015, 52, 3681–3688. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and Functional Characterization of Kidney Bean and Field Pea Protein Isolates: A Comparative Study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M.; Singh, V. Nutrients and Antinutrients in Cowpea and Horse Gram Flours in Comparison to Chickpea Flour: Evaluation of Their Flour Functionality. Food Chem. 2012, 131, 462–468. [Google Scholar] [CrossRef]
- Román, L.; Reguilón, M.P.; Gómez, M. Physicochemical Characteristics of Sauce Model Systems: Influence of Particle Size and Extruded Flour Source. J. Food Eng. 2018, 219, 93–100. [Google Scholar] [CrossRef]
- Acevedo, B.A.; Thompson, C.M.B.; Foutel, N.S.G.; Chaves, M.G.; Avanza, M. V Effect of Different Treatments on the Microstructure and Functional and Pasting Properties of Pigeon Pea (Cajanus cajan L.), Dolichos Bean (Dolichos lablab L.) and Jack Bean (Canavalia ensiformis) Flours from the North-East Argentina. Int. J. Food Sci. Technol. 2017, 52, 222–230. [Google Scholar] [CrossRef]
- Fu, B.X. Asian Noodles: History, Classification, Raw Materials, and Processing. Food Res. Int. 2008, 41, 888–902. [Google Scholar] [CrossRef]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S.; Barrow, C.J. Interfacial and Emulsifying Properties of Lentil Protein Isolate. Food Chem. 2012, 134, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Srichuwong, S.; Sunarti, T.C.; Mishima, T.; Isono, N.; Hisamatsu, M. Starches from Different Botanical Sources II: Contribution of Starch Structure to Swelling and Pasting Properties. Carbohydr. Polym. 2005, 62, 25–34. [Google Scholar] [CrossRef]
- Mariotti, M.; Zardi, M.; Lucisano, M.; Pagani, M.A. Influence of the Heating Rate on the Pasting Properties of Various Flours. Starch/Staerke 2005, 57, 564–572. [Google Scholar] [CrossRef]
- De Bock, P.; Daelemans, L.; Selis, L.; Raes, K.; Vermeir, P.; Eeckhout, M.; Van Bockstaele, F. Comparison of the Chemical and Technological Characteristics of Wholemeal Flours Obtained from Amaranth (Amaranthus sp.), Quinoa (Chenopodium quinoa) and Buckwheat (Fagopyrum sp.) Seeds. Foods 2021, 10, 651. [Google Scholar] [CrossRef]
- Wei, Y.M.; Hu, X.Z.; Zhang, G.Q.; Ouyang, S.H. Studies on the Amino Acid and Mineral Content of Buckwheat Protein Fractions. Nahr. Food 2003, 47, 114–116. [Google Scholar] [CrossRef]
- Javornik, B.; Kreft, I. Characterization of Buckwheat Proteins. Fagopyrum 1984, 4, 30–38. [Google Scholar]
- Guo, X.; Yao, H. Fractionation and Characterization of Tartary Buckwheat Flour Proteins. Food Chem. 2006, 98, 90–94. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; O’Mahoney, J.A. Composition, Protein Profile and Rheological Properties of Pseudocereal-Based Protein-Rich Ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef]
- Fernando, S. Production of Protein-Rich Pulse Ingredients through Dry Fractionation: A Review. LWT 2021, 141, 110961. [Google Scholar] [CrossRef]
- Yoshimoto, Y.; Egashira, T.; Hanashiro, I.; Ohinata, H.; Takase, Y.; Takeda, Y. Molecular Structure and Some Physicochemical Properties of Buckwheat Starches. Cereal Chem. 2004, 81, 515–520. [Google Scholar] [CrossRef]
- Rayner, M.; Timgren, A.; Sjöö, M.; Dejmek, P. Quinoa Starch Granules: A Candidate for Stabilising Food-Grade Pickering Emulsions. J. Sci. Food Agric. 2012, 92, 1841–1847. [Google Scholar] [CrossRef]
- Qian, J.; Rayas-Duarte, P.; Grant, L. Partial Characterization of Buckwheat (Fagopyrum esculentum) Starch. Cereal Chem. 1998, 75, 365–373. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Chen, L.; Li, X. Study on Supramolecular Structural Changes of Ultrasonic Treated Potato Starch Granules. Food Hydrocoll. 2012, 29, 116–122. [Google Scholar] [CrossRef]
- Gao, J.; Kreft, I.; Chao, G.; Wang, Y.; Liu, X.; Wang, L.; Wang, P.; Gao, X.; Feng, B. Tartary Buckwheat (Fagopyrum Tataricum Gaertn.) Starch, a Side Product in Functional Food Production, as a Potential Source of Retrograded Starch. Food Chem. 2016, 190, 552–558. [Google Scholar] [CrossRef]
- Zhu, F. Buckwheat Starch: Structures, Properties, and Applications. Trends Food Sci. Technol. 2016, 49, 121–135. [Google Scholar] [CrossRef]
- Horstmann, S.W.; Lynch, K.M.; Arendt, E.K. Starch Characteristics Linked to Gluten-Free Products. Foods 2017, 6, 29. [Google Scholar] [CrossRef]
- Nikolić, V.; Simić, M.; Kandić, V.; Dodevska, M.; Titan, P.; Dodig, D.; Žilić, S. Pasting Properties and the Baking Functionality of Whole-Grain Wheat Flour with Different Amylose and Dietary Fibers Content. J. Food Process. Preserv. 2021, 46, e15805. [Google Scholar] [CrossRef]
- Gregori, M.; Kreft, I. Breakable Starch Granules in a Low-Amylose Buckwheat (Fagopyrum esculentum Moench) Mutant. J. Food, Agric. Environ. 2012, 10, 258–262. [Google Scholar]
- Li, W.; Lin, R.; Corke, H. Physicochemical Properties of Common and Tartary Buckwheat Starch. Cereal Chem. 1997, 74, 79–82. [Google Scholar] [CrossRef]
- Lin, L.Y.; Hsieh, Y.J.; Liu, H.M.; Lee, C.C.; Mau, J.L. Flavor Components in Buckwheat Bread. J. Food Process. Preserv. 2009, 33, 814–826. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Shen, M.; Guo, X.; Lv, M.; Wang, M. Changes in Physicochemical Properties and in Vitro Digestibility of Tartary Buckwheat and Sorghum Starches Induced by Annealing. Starch/Staerke 2016, 68, 709–718. [Google Scholar] [CrossRef]
- Lu, L.; Baik, B.K. Starch Characteristics Influencing Resistant Starch Content of Cooked Buckwheat Groats. Cereal Chem. 2015, 92, 65–72. [Google Scholar] [CrossRef]
- Van Hung, P.; Maeda, T.; Morita, N. Waxy and High-Amylose Wheat Starches and Flours-Characteristics, Functionality and Application. Trends Food Sci. Technol. 2006, 17, 448–456. [Google Scholar] [CrossRef]
- Maningat, C.C.; Seib, P.A. Understanding the Physicochemical and Functional Properties of Wheat Starch in Various Foods. Cereal Chem. 2010, 87, 305–314. [Google Scholar] [CrossRef]
- Ibrahim, U.K.; Salleh, R.M.; Maqsood-ul-Haque, S.N.S. Bread towards Functional Food: An Overview. ETP Int. J. Food Eng. 2015, 1, 39–43. [Google Scholar] [CrossRef][Green Version]
- Hager, A.S.; Arendt, E.K. Influence of Hydroxypropylmethylcellulose (HPMC), Xanthan Gum and Their Combination on Loaf Specific Volume, Crumb Hardness and Crumb Grain Characteristics of Gluten-Free Breads Based on Rice, Maize, Teff and Buckwheat. Food Hydrocoll. 2013, 32, 195–203. [Google Scholar] [CrossRef]
- Lin, L.Y.; Wang, H.E.; Lin, S.D.; Liu, H.M.; Mau, J.L. Changes in Buckwheat Bread during Storage. J. Food Process. Preserv. 2013, 37, 285–290. [Google Scholar] [CrossRef]
- Mariotti, M.; Pagani, M.A.; Lucisano, M. The Role of Buckwheat and HPMC on the Breadmaking Properties of Some Commercial Gluten-Free Bread Mixtures. Food Hydrocoll. 2013, 30, 393–400. [Google Scholar] [CrossRef]
- Torbica, A.; Hadnadev, M.; Dapčević, T. Rheological, Textural and Sensory Properties of Gluten-Free Bread Formulations Based on Rice and Buckwheat Flour. Food Hydrocoll. 2010, 24, 626–632. [Google Scholar] [CrossRef]
- Christa, K.; Soral-Mietana, M.; Lewandowicz, G. Buckwheat Starch: Structure, Functionality and Enzyme in Vitro Susceptibility upon the Roasting Process. Int. J. Food Sci. Nutr. 2009, 60, 140–154. [Google Scholar] [CrossRef]
- Baljeet, S.Y.; Ritika, B.Y.; Yadav, R. Studies on Functional Properties and Incorporation of Buckwheat Flour for Biscuit Making. Int. Food Res. J. 2010, 17, 1067–1076. [Google Scholar]
- Chopra, N.; Dhillon, B.; Puri, S. Formulation of Buckwheat Cookies and Their Nutritional, Physical, Sensory and Microbiological Analysis. Int. J. Adv. Biotechnol. Res. 2014, 5, 381–387. [Google Scholar]
- Giami, S.Y.; Achinewhu, S.C.; Ibaakee, C. The Quality and Sensory Attributes of Cookies Supplemented with Fluted Pumpkin (Telfairia occidentalis Hook) Seed Flour. Int. J. Food Sci. Technol. 2005, 40, 613–620. [Google Scholar] [CrossRef]
- Singh, B.; Bajaj, M.; Kaur, A.; Sharma, S.; Sidhu, J.S. Studies on the Development of High-Protein Biscuits from Composite Flours. Plant Foods Hum. Nutr. 1993, 43, 181–189. [Google Scholar] [CrossRef]
- McWatters, K.H. Cookies Baking Porperties of Defatted Peanut, Soybean, and Field Pea Flours. Cereal Chem. 1978, 55, 853–863. [Google Scholar]
- Yamsaengsung, R.; Berghofer, E.; Schoenlechner, R. Physical Properties and Sensory Acceptability of Cookies Made from Chickpea Addition to White Wheat or Whole Wheat Flour Compared to Gluten-Free Amaranth or Buckwheat Flour. Int. J. Food Sci. Technol. 2012, 47, 2221–2227. [Google Scholar] [CrossRef]
- Altindag, G.; Certel, M.; Erem, F.; Ilknur Konak, Ü. Quality Characteristics of Gluten-Free Cookies Made of Buckwheat, Corn, and Rice Flour with/without Transglutaminase. Food Sci. Technol. Int. 2015, 21, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Farzana, T.; Fatema, J.; Hossain, F.B.; Afrin, S.; Rahman, S.S. Quality Improvement of Cakes with Buckwheat Flour, and Its Comparison with Local Branded Cakes. Curr. Res. Nutr. Food Sci. 2021, 9, 570–577. [Google Scholar] [CrossRef]
- Srivastava, T.; Kumari, A.; Wasnik, P.; Kumar, P.; Singh, A. Development of Gluten Free Buckwheat Cake and Optimization of Its Baking Process. Int. J. Chem. Stud. 2018, 6, 268–273. [Google Scholar]
- Cecchini, C.; Menesatti, P.; Antonucci, F.; Costa, C. Trends in Research on Durum Wheat and Pasta, a Bibliometric Mapping Approach. Cereal Chem. 2020, 97, 581–588. [Google Scholar] [CrossRef]
- Güngörmüşler, M.; Başınhan, İ.; Üçtuğ, F.G. Optimum Formulation Determination and Carbon Footprint Analysis of a Novel Gluten-Free Pasta Recipe Using Buckwheat, Teff, and Chickpea Flours. J. Food Process. Preserv. 2020, 44, e14701. [Google Scholar] [CrossRef]
- Triboï, E.; Martre, P.; Triboï-Blondel, A.M. Environmentally-Induced Changes in Protein Composition in Developing Grains of Wheat Are Related to Changes in Total Protein Content. J. Exp. Bot. 2003, 54, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, K.X.; Guo, X.N.; Brijs, K.; Zhou, H.M. Natural Additives in Wheat-Based Pasta and Noodle Products: Opportunities for Enhanced Nutritional and Functional Properties. Compr. Rev. Food Sci. Food Saf. 2014, 13, 347–357. [Google Scholar] [CrossRef]
- Sultan, T.; Ahmed, A.; Qayyum, A.; Mumtaz, A.; Khalid, N. Effect of Buckwheat Supplementation on the Quality Parameters of Pasta. Pak. J. Agric. Res. 2020, 33, 422–691. [Google Scholar] [CrossRef]
- Wójtowicz, A. Influence of Process Conditions on Selected Texture Properties of Precooked Buckwheat Pasta. Teka Kom. Motoryz. I Energetyki Rol. 2012, 12, 315–322. [Google Scholar]
- Wang, R.; Li, M.; Chen, S.; Hui, Y.; Tang, A.; Wei, Y. Effects of Flour Dynamic Viscosity on the Quality Properties of Buckwheat Noodles. Carbohydr. Polym. 2019, 207, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, Z.; Hao, X.; Cheng, Y.; Li, L. Impact of Calcium Hydroxide on the Textural Properties of Buckwheat Noodles. J. Texture Stud. 2012, 43, 227–234. [Google Scholar] [CrossRef]
- Han, X.M.; Xing, J.J.; Guo, X.N.; Zhu, K.X. Influence of the Addition of Extruded Endogenous Tartary Buckwheat Starch on Processing and Quality of Gluten-Free Noodles. Foods 2021, 10, 2693. [Google Scholar] [CrossRef]
- Han, X.M.; Xing, J.J.; Han, C.; Guo, X.N.; Zhu, K.X. The Effects of Extruded Endogenous Starch on the Processing Properties of Gluten-Free Tartary Buckwheat Noodles. Carbohydr. Polym. 2021, 267, 118170. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Piskuła, M.; Zieliński, H. Recent Advances in Development of Gluten-Free Buckwheat Products. Trends Food Sci. Technol. 2015, 44, 58–65. [Google Scholar] [CrossRef]
- Hatcher, D.W.; Bellido, G.G.; Anderson, M.J.; Dexter, J.E.; Head, D.; Izydorczyk, M. Investigation of Empirical and Fundamental Soba Noodle Texture Parameters Prepared with Tartary, Green Testa and Common Buckwheat. J. Texture Stud. 2011, 42, 490–502. [Google Scholar] [CrossRef]
- Lv, L.; Xia, Y.; Zou, D.; Han, H.; Wang, Y.; Fang, H.; Li, M. Fagopyrum tataricum (L.) Gaertn.: A Review on Its Traditional Uses, Phytochemical and Pharmacology. Food Sci. Technol. Res. 2017, 23, 1–7. [Google Scholar] [CrossRef]
- Sun, X.; Li, W.; Hu, Y.; Zhou, X.; Ji, M.; Yu, D.; Fujita, K.; Tatsumi, E.; Luan, G. Comparison of Pregelatinization Methods on Physicochemical, Functional and Structural Properties of Tartary Buckwheat Flour and Noodle Quality. J. Cereal Sci. 2018, 80, 63–71. [Google Scholar] [CrossRef]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Codină, G.G. Buckwheat and Amaranth as Raw Materials for Brewing, a Review. Plants 2022, 11, 756. [Google Scholar] [CrossRef]
- De Meo, B.; Freeman, G.; Marconi, O.; Booer, C.; Perretti, G.; Fantozzi, P. Behaviour of Malted Cereals and Pseudo-Cereals for Gluten-Free Beer Production. J. Inst. Brew. 2011, 117, 541–546. [Google Scholar] [CrossRef]
- Deželak, M.; Gebremariam, M.M.; Zarnkow, M.; Becker, T.; Košir, I.J. Part III: The Influence of Serial Repitching of Saccharomyces Pastorianus on the Production Dynamics of Some Important Aroma Compounds during the Fermentation of Barley and Gluten-Free Buckwheat and Quinoa Wort. J. Inst. Brew. 2015, 121, 387–399. [Google Scholar] [CrossRef]
- Yang, D.; Gao, X. Progress of the Use of Alternatives to Malt in the Production of Gluten-Free Beer. Crit. Rev. Food Sci. Nutr. 2022, 62, 2820–2835. [Google Scholar] [CrossRef] [PubMed]
| Variety | Year | Country | Submitted by | Issued Plant Breeder Rights or Plant Variety Protection Certificate |
|---|---|---|---|---|
| Mancan | 1974 | Canada | Agriculture and Agri-food Canada | |
| Manor | 1980 | Canada | Agriculture and Agri-food Canada | |
| Winsor Royal | 1983 | USA | Winsor Grain, Inc., Minnesota | Yes |
| AC Manisoba | 1996 | Canada | Agriculture and Agri-food Canada | |
| AC Springfield | 1997 | Canada | Springfield Mills | |
| Koban | 1999 | Canada | Agriculture and Agri-food Canada | |
| Koto | 2000 | Canada | Agriculture and Agri-food Canada | Yes |
| Koma | 2005 | Canada | Kade Research | Yes |
| Horizon | 2011 | Canada | Mancan Genetics | |
| Kenmar | 2016 | Canada | Springfield Mills | |
| Takane Ruby 2011 | 2016 | USA | Takano Co. Ltd., Japan | Yes |
| Aoi | 2017 | USA | McKay Seed Co. Inc., Washington | Yes |
| Agassiz | 2019 | Canada | Mancan Genetics |
| Bhinder et al. [94] | Tien et al. [97] | Khan et al. [95] | Lu et al. [96] | Qin et al. [40] | USDA Whole Wheat Flour [98] | USDA White All-Purpose, Enriched, Bleached Wheat Flour [98] | |
|---|---|---|---|---|---|---|---|
| G/W 1 | Unknown | G | G | G | Unknown | - | - |
| Number of varieties per species | T: 23 | T: 1 C: 1 Tetraploid T: 1 | C: 4 | C: 9 T: 1 | T: 21 C: 18 | - | - |
| Growing location/seed source | National Bureau of Plant Genetic Resources (Shimla, India) | T Miyake Seifun Co., Ltd. (Osaka, Japan) C Nikkoku Flour Milling Co., Ltd. (Nagano, Japan) Tetraploid T University of Japan | Gilgit/Baltistan, Pakistan | Seed from McKay Seed Company (Almira, WA, USA) grown in Eastern WA, USA | China (Guizhou, Shanxi, Ningxia, Inner Mongolia, Hebei, Liaoning) | - | - |
| Moisture (%) | 7.6–10.8 | - | 9.3–11.7 | - | - | 10.7 | 11.9 |
| Ash (%) | 1.8–2.8 | T: 2.4 C: 2.2 Tetraploid T: 2.1 | 1.1–1.6 | T: 1.8–2.1 C: 2.7 | T: 1.9–3.1 C: 1.3–3.1 | 1.6 | 0.5 |
| Crude fat (%) | 2.0–3.6 | T: 2.9 C: 2.4 Tetraploid T: 2.7 | 0.9–1.5 | - | T: 1.2–4.7 C: 1.5–5.4 | 2.5 | 1.0 |
| Crude fiber (%) | - | - | 0.8–1.0 | T: 3.6–7.6 C: 10.6 | T: 1.7–4.0 C: 1.3–3.1 | 10.7 | 2.7 |
| Protein (%) | 9.1–14.9 | T: 12.8 C: 13.3 Tetraploid T: 14.1 | 13.9–16.5 | T: 10.4–13.4 C: 17.9 | T: 6.8–15.0 C: 8.1–12.4 | 13.2 | 10.3 |
| Starch (%) | 66.3–72.5 | - | - | T: 72.8–76.8 C: 61.2 | T: 65.6–74.3 C: 65.9–78.1 | 72.0 | 76.3 |
| Bhinder et al. [94] | Raikos et al. [113] | Jan et al. [20] | |
|---|---|---|---|
| C/T 1 | T | Unknown | Unknown |
| WAC 2 (g/g) | 2.3–2.7 | 1.4 | 1.3 |
| OAC 3 (g/g) | 0.9–1.4 | - | 1.7 |
| ESI 4 (min) | 35.5–77.8 | 76.1 | - |
| EAI 5 (m2/g) | 33.5–59.8 | 40.0 | - |
| FC 6 (%) | 10.3–23.7 | 85.0 | 31.7 |
| FS 7 (%) | 6.2–14.3 | 65.0 | 74.4 |
| Functional Property | Ingredient Functionality | Food Application | References |
|---|---|---|---|
| WAC/WHC 1 | Increase viscosity/thickening Moisture retention | Custards Soups Baked goods Meat products | [106,110,113] |
| OAC/OHC 2 | Mouthfeel and texture flavor | Fried food (including battered/breaded food) | [106,109,114] |
| ESI and EAI 3 | Support emulsion formation and resistance to change overtime | Salad dressing Mayonnaise Sausage Sauces Soups Ice cream Milk Butter Frozen desserts | [109,112,115,116] |
| FC and FS 4 | Aeration Overrun | Baked goods Mousses Meringues Ice cream Whipped toppings | [94,113,117,118] |
| Pasting properties | Binding agent Thickening agent Gelling agent | Soup Sauces Asian noodles | [111,112,119,120,121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breslauer, R.; Nalbandian, E.; Reinman, T.; Rezaey, M.; Ganjyal, G.M.; Murphy, K.M. Buckwheat Production and Value-Added Processing: A Review of Potential Western Washington Cropping and Food System Applications. Sustainability 2023, 15, 14758. https://doi.org/10.3390/su152014758
Breslauer R, Nalbandian E, Reinman T, Rezaey M, Ganjyal GM, Murphy KM. Buckwheat Production and Value-Added Processing: A Review of Potential Western Washington Cropping and Food System Applications. Sustainability. 2023; 15(20):14758. https://doi.org/10.3390/su152014758
Chicago/Turabian StyleBreslauer, Rachel, Elizabeth Nalbandian, Tayler Reinman, Mahvash Rezaey, Girish M. Ganjyal, and Kevin M. Murphy. 2023. "Buckwheat Production and Value-Added Processing: A Review of Potential Western Washington Cropping and Food System Applications" Sustainability 15, no. 20: 14758. https://doi.org/10.3390/su152014758
APA StyleBreslauer, R., Nalbandian, E., Reinman, T., Rezaey, M., Ganjyal, G. M., & Murphy, K. M. (2023). Buckwheat Production and Value-Added Processing: A Review of Potential Western Washington Cropping and Food System Applications. Sustainability, 15(20), 14758. https://doi.org/10.3390/su152014758











