Sexual Dimorphism and Discrimination of Barbel Steed (Hemibarbus labeo) in the Jinhe River, China: An Indicator of Habitat Status
Abstract
:1. Introduction
2. Materials and Methods
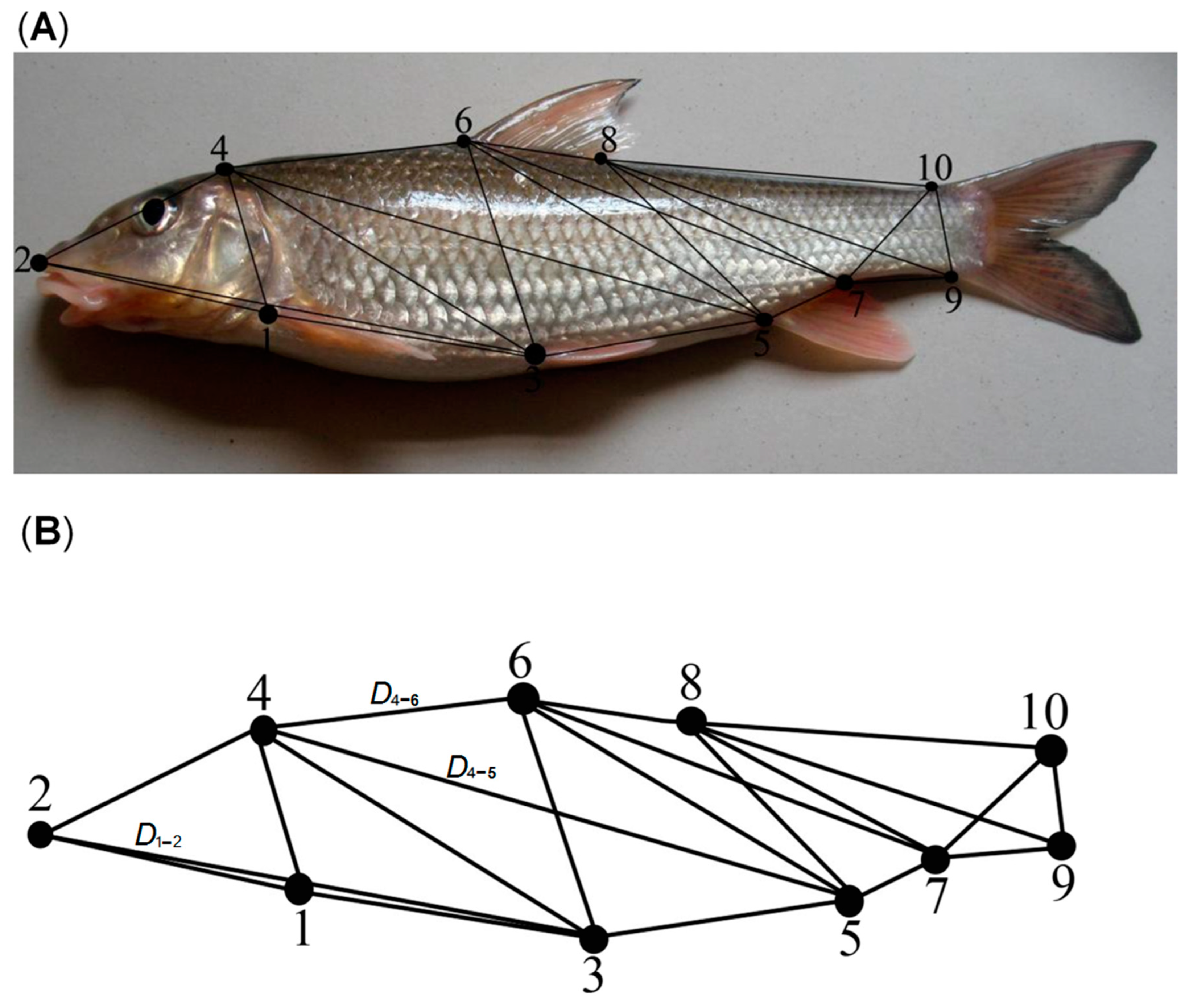
2.1. Sampling Collection and Preparation
2.2. Measurement
2.3. Data Analysis
3. Results
3.1. Sexual Dimorphism of H. labeo
3.2. Principal Component and R-Cluster Analyses of the Differences of the Mprphological Variables between Male and Female H. labeo
3.3. Sex Discriminant Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.; Clay, J.; Folke, C.; Lubchenco, J.; Monney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- James, H.T.; Geoff, L.A. Fish as food: Aquaculture’s contribution. EMBO Rep. 2001, 21, 958–963. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The state of world fisheries and aquaculture 2022. In Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Yin, M.C. Ecology of Fishes; Chinese Agriculture Press: Beijing, China, 1993; pp. 110–115. [Google Scholar]
- An, L.; Meng, Q.; Zhang, L.; Dong, X.; Li, X.; Li, W.; Zhu, S. Analysis of morphological indexes and discrimination of male and female Eryghroculter ilishaeformis. Freshw. Fish. 2017, 47, 36–41. [Google Scholar]
- Mustafa, A.A.; Mohammad, A.S.; Farrag MM, S.; Osmana, G. Sexual dimorphism of morphological characters in three carangid species from the Red Sea, Egypt. Iran. J. Ichthyol. 2021, 8, 223–235. [Google Scholar]
- McEvoy, F.J.; Tomkiewicz, J.; Støttrup, J.G.; Overton, J.L.; McEvoy, C.; Svalastoga, E. Determination of fish gender using fractal analysis of ultrasound images. Vet. Radiol. Ultrasound 2009, 50, 519–524. [Google Scholar] [CrossRef]
- Hedrick, A.V.; Temeles, E.J. The evolution of sexual dimorphism in animals: Hypotheses and tests. Trends Ecol. Evol. 1989, 4, 136–138. [Google Scholar] [CrossRef]
- Herler, J.; Kerschbaumer, M.; Mitteroecker, P.; Postl, L.; Sturmbauer, C. Sexual dimorphism and population divergence in the Lake Tanganyika cichlid fish genus. Tropheus. Front. Zool. 2010, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Parmentier, E.; Boistel, R.; Bahri, M.A.; Plenevaux, A.; Schwarzhans, W. Sexual dimorphism in the sonic system and otolith morphology of Neobythites gilli (Ophidiiformes). J. Zool. 2018, 305, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Meyer, A.; Kratochwil, C.F. Neural innervation as a potential trigger of morphological color change and sexual dimorphism in cichlid fish. Sci. Rep. 2020, 10, 12329. [Google Scholar] [CrossRef]
- Xu, D.; Lin, Z.; Lei, H. Sexual dimorphism in morphological traits and female individual fecundity of Acrossocheilus wenchowensis. J. Shanghai Jiaotong Univ. 2006, 24, 335–340. [Google Scholar]
- Chen, S.; Zhao, R.; Qi, D.; Fan, X.; Lei, H.; Lin, Z. Sexual dimorphism in morphological traits and female individual fecundity of Acanthorhodeus chankaensis Dybowsky. J. Shanghai Jiaotong Univ. 2013, 31, 61–66+78. [Google Scholar]
- Chen, S.; Zhao, R.; Zhang, Y.; Fan, X.; Ding, G.; Lin, Z. Sexual dimorphism in morphology and female fecundity of Erythroculter ilishaeformis from rivers in Yuyao. J. Lishui Univ. 2013, 35, 11–15. [Google Scholar]
- Guo, H.; Wei, K.; Xie, Z.; Tang, W.; Shen, L.; Gu, S.; Wu, J.; Chen, W. Analysis of morphological index system and discrimination of male and female silver eels (Anguilla japonica) collected at the Yangtze River Estuary. J. Fish. China 2011, 35, 1–9. [Google Scholar]
- Ni, H.; Chen, X. Analysis of shape index system and discriminant of male and female of Ilisha elongata. J. Biomath. 2003, 18, 224–228. [Google Scholar]
- Wu, B.; Zhang, M.; Deng, S.; Shi, S.; Li, G.; Zhu, C. Analysis of morphological index and discrimination of male and female Scatophagus argus. J. Shanghai Ocean. Univ. 2014, 23, 64–69. [Google Scholar]
- Tuo, Y.; Xiao, T.; Wang, H. Discrimination of Sexual Dimorphism through External Morphology of spotted steed (Hemibarbus maculatus) in the Yuanhe River, China. Appl. Ecol. Environ. Res. 2020, 18, 1539–1550. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Q.; Yue, P.; Huang, H. Economic Animals in China—Freshwater Fish; Science Press: Beijing, China, 1979; pp. 86–87. [Google Scholar]
- Никoльскии Г, В. Fishes in Amur River Basin; Science Press: Beijing, China, 1956; pp. 190–195. [Google Scholar]
- He, J.S.; He, X.F.; Yan, T.M. Observation on the embryonic development of Hemibarbus labeo (Pallas) in lower reaches of Fujiang River. J. Southwest China Norm. Univ. 1999, 24, 225–231. [Google Scholar]
- Xu, W.; Li, C.T.; Geng, L.W.; Sun, H.W.; Liu, X.Y. Growth and reproduction of reared Hemibarbus labeo in Wusuli River. J. Fish. Sci. China 2009, 16, 550–555. [Google Scholar]
- Gan, G.M.; Zhang, Y.G.; Zhang, X.F.; Wang, Z.J. Cytological studies on fertilization in Hemibarbus labeo. Acta Hydro Biol. Sin. 2006, 30, 284–291. [Google Scholar]
- Luo, X.N.; Li, J.; Jin, G.H.; Liu, Y.X.; Li, J.W.; Yang, P.M.; Xia, D.M.; Liu, B.Y. Fry and Juvenile Rearing of Hemibarbus labeo in an Earthen Pond. Fish. Sci. 2013, 32, 102–105. [Google Scholar]
- Lian, Q.P.; Mi, G.Q.; Yao, Z.L.; Hu, T.J.; Li, Q.; Liu, S.L.; Wang, Y.C. A Study on Artificial Propagation and Embryonic Development of Hemibarbus labeo in Oujiang River. Acta Agric. Univ. Jiangxiensis 2014, 36, 181–186. [Google Scholar]
- Jia, Y.T.; Chen, Y.F. River health assessment in a large river: Bioindicators of fish population. Ecol. Indic. 2013, 26, 24–32. [Google Scholar] [CrossRef]
- Tuo, Y.; Deng, Z.Q.; Hong, L.; Liao, L.Y. Morphological Biology of Hemibarbus maculatus Bleeker in Ganjiang River. J. Yichun Univ. 2016, 38, 89–92+122. [Google Scholar]
- Tuo, Y.; Li, J.; Xiao, T.; Wang, H. Sexual discrimination and fecundity of barbel steed (Hemibarbus labeo) in the Jinjiang river, china. Appl. Ecol. Environ. Res. 2021, 19, 1783–1797. [Google Scholar] [CrossRef]
- Takeshita, N.; Kimura, S. Age and Growth of the Cyprinid Fish Hemibarbus barbus in the Chikugo River. Nippon. Suisan Gakkaishi 1991, 57, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.O.; Zhang, M.M.; Oh, C.W.; Baek, J.M.; Song, K.J. Age and Growth of Barbel Steed Hemibarbus labeo in Goe-san Lake in Korea. Fish. Aquat. Sci. 2012, 15, 353–359. [Google Scholar] [CrossRef]
- Laboratory of Institute of Hydrobiology of Hubei Province. Fishes in the Yangtze River; Science Press: Beijing, China, 1976; pp. 79–80. [Google Scholar]
- Lin, Z.; Lei, H.; Lin, Z.; Hua, H. Sexual dimorphism and female reproductive output of Hemibarbus maculates. J. Shanghai Jiaotong Univ. 2005, 23, 284–288. [Google Scholar]
- Strauss, R.M.; Bookstein, F.L. The truss: Body form reconstruction in morphometrics. Syst. Zool. 1982, 31, 113–135. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology Cambridge; Cambridge University Press: New York, NY, USA, 1997. [Google Scholar]
- Corti, M.; Thorpe, R.S.; Sola, L.; Sbordoni, V.; Cataudella, S. Multivariate morphometrics in aquaculture:a case study of six stocks of the common carp (Cyprinnus carpio) from Italy. Can. J Fish. Aquat. Sci. 1988, 45, 1548–1554. [Google Scholar] [CrossRef]
- Turan, C. A Note on The Examination of Morphometric Differentiation Among Fish Populations: The Truss System. Turk. J. Zool. 1999, 23, 259–263. [Google Scholar]
- Turan, C.; Ergüden, D.; Gürlek, M.; Başusta, N.; Turan, F. Morphometric structuring of the anchovy(Engraulis encrasicolus L.) in the Black, Aegean and northeastern Mediterranean seas. Turk. J. Vet. Anim. Sci. 2004, 28, 865–871. [Google Scholar]
- Pinheiro, A.; Teixeira, C.M.; Rego, A.L.; Marques, J.F.; Cabral, H.N. Genetic and morphological variation of Solea lascaris (Risso, 1810) along the Portuguese coast. Fish. Res. 2005, 73, 67–78. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Lovich, J.E. Sexual dimorphism in turtles with emphasis on the slider turtle (Trachemys scripta). Herpetol. Monogr. 1990, 4, 1–29. [Google Scholar] [CrossRef]
- Shou, L.; Du, W.G.; Shu, L. Sexual dimorphism and fecundity in the gold-stripe pond frog (Pelophylax plancyi) and the terrestrial frog (Fejervarya limnocharis). Acta Ecol. Sin. 2005, 25, 664–668. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Emlen, S.T.; Oring, L.W. Ecology, sexual selection, and the evolution of mating system. Science 1977, 197, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Lande, R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 1980, 34, 292–305. [Google Scholar] [CrossRef]
- Abecia, J.E.; Luiz, O.J.; Crook, D.A.; Banks, S.C.; Wedd, D.; King, A.J. Sex and male breeding state predict intraspecific trait variation in mouthbrooding fishes. J. Fish Biol. 2022, 101, 550–559. [Google Scholar] [CrossRef]
- Parker, G.A. The evolution of sexual size dimorphism in fish. J. Fish. Biol. 1992, 41, 1–20. [Google Scholar] [CrossRef]
- Pyron, M. Sexual size dimorphism and phylogeny in North American minnows. Biol. J. Linn. Soc. 1996, 57, 327–341. [Google Scholar] [CrossRef]
- Erlandsson, A.; Ribbink, A.J. Patterns of sexual size dimorphism in African cichlid fishes. S. Afr. J. Sci. 1997, 93, 498–508. [Google Scholar]
- Hu, Y. Sexual Dimorphism and Female Individual Fecundity of Six Species of Gobioninae in the Jialing River, Southwest China; China West Normal University: Nanchong, China, 2017. [Google Scholar]
- Fan, X.; Lin, Z.; Lu, J.; Qiu, Y.; Chen, C.; Cao, Y.; Qi, D. Sexual dimorphism in morphological traits and female individual fecundity of Odontobutis obscurus. J. Shanghai Jiaotong Univ. 2009, 28, 587–591+623. [Google Scholar]
- Fan, X.; Lin, Z.; Ding, X.; Zhu, J. Sexual size dimorphism and female individual fecundity of Silurus asotus and Clarias fuscus. Acta Ecol. Sin. 2014, 34, 555–563. [Google Scholar]
- Li, J.; Lian, Z.; Wu, Z.; Zeng, L.; Mu, L.; Yuan, Y.; Bai, H.; Guo, Z.; Mai, K.; Tu, X.; et al. Artificial intelligence-based method for the rapid detection of fish parasites (Ichthyophthirius multifiliis, Gyrodactylus kobayashii, and Argulus japonicus). Aquaculture 2023, 563, 738790. [Google Scholar] [CrossRef]
- Lee, P.G. Process control and artificial intelligence software for aquaculture. Aquac. Eng. 2000, 23, 13–36. [Google Scholar] [CrossRef]
- Memarzadeh, M.; Britten, G.L.; Worm, B.; Boettiger, C. Rebuilding global fisheries under uncertainty. Proc. Natl. Acad. Sci. 2019, 116, 15985–15990. [Google Scholar] [CrossRef]
- Chen, W.; Wang, P.P.; Xiao, S.J.; Liu, Y.; Ye, K.; Chen, Q.K.; Wang, Z.Y. Analysis of morphological index system and sexual differences of large yellow croaker (Larimichthys crocea). J. Jimei Univ. 2014, 19, 401–408. [Google Scholar]
- Zhang, H.Y.; Chen, S.Q.; Wang, T.; Zhou, G.Q.; Yin, S.W. Analysis of morphological index system and sexual differences of Odontobutis potamophila. Jiangsu Agric. Sci. 2018, 46, 138–142. [Google Scholar]
- Li, S.; Cai, W.; Zhou, B. Morphological and biochemical genetic variations among populations of blunt snout bream (Megalobrama amblycephala). J. Fish. China 1991, 15, 204–211. [Google Scholar]


| Characters | Female | Male | t-Value | p-Value | ||
|---|---|---|---|---|---|---|
| No. of Samples | Mean ± S.D. | No. of Samples | Mean ± S.D. | |||
| BL | 35 | 21.25 ± 3.25 | 33 | 18.48 ± 4.09 | 3.10 | 0.003 |
| D1–2 | 35 | 0.66 ± 0.03 | 33 | 0.67 ± 0.03 | −1.65058 | 0.104 |
| D1–3 | 35 | 0.78 ± 0.03 | 33 | 0.77 ± 0.03 | 0.12613 | 0.900 |
| D2–3 | 35 | 1.02 ± 0.02 | 33 | 1.03 ± 0.02 | −1.13756 | 0.259 |
| D2–4 | 35 | 0.66 ± 0.05 | 33 | 0.65 ± 0.06 | 0.78714 | 0.434 |
| D1–4 | 35 | 0.42 ± 0.12 | 33 | 0.50 ± 0.06 | −3.74263 | <0.001 |
| D3–4 | 35 | 0.86 ± 0.04 | 33 | 0.88 ± 0.02 | −2.34524 | 0.022 |
| D3–5 | 35 | 0.72 ± 0.03 | 33 | 0.71 ± 0.03 | 1.53544 | 0.129 |
| D4–6 | 35 | 0.71 ± 0.04 | 33 | 0.73 ± 0.03 | −2.11645 | 0.038 |
| D3–6 | 35 | 0.66 ± 0.07 | 33 | 0.69 ± 0.05 | −2.00059 | 0.0496 |
| D4–5 | 35 | 1.07 ± 0.03 | 33 | 1.08 ± 0.02 | −1.37553 | 0.174 |
| D5–6 | 35 | 0.86 ± 0.04 | 33 | 0.87 ± 0.02 | −1.24011 | 0.219 |
| D5–7 | 35 | 0.21 ± 0.07 | 33 | 0.22 ± 0.04 | −1.08063 | 0.284 |
| D6–8 | 35 | 0.46 ± 0.06 | 33 | 0.47 ± 0.05 | −1.30382 | 0.197 |
| D5–8 | 35 | 0.70 ± 0.07 | 33 | 0.71 ± 0.05 | −0.55710 | 0.579 |
| D6–7 | 35 | 0.90 ± 0.06 | 33 | 0.92 ± 0.03 | −1.81232 | 0.074 |
| D7–8 | 35 | 0.74 ± 0.05 | 33 | 0.75 ± 0.04 | −0.98835 | 0.327 |
| D7–9 | 35 | 0.38 ± 0.05 | 33 | 0.41 ± 0.05 | −2.63366 | 0.011 |
| D8–10 | 35 | 0.84 ± 0.04 | 33 | 0.85 ± 0.03 | −1.37056 | 0.175 |
| D8–9 | 35 | 0.45 ± 0.06 | 33 | 0.51 ± 0.04 | −4.43331 | <0.001 |
| D7–10 | 35 | 0.88 ± 0.03 | 33 | 0.89 ± 0.03 | −1.38254 | 0.171 |
| D9–10 | 35 | 0.26 ± 0.05 | 33 | 0.30 ± 0.03 | −3.50495 | <0.001 |
| HL | 35 | 0.73 ± 0.03 | 33 | 0.73 ± 0.03 | 0.32452 | 0.747 |
| HH | 35 | 0.50 ± 0.04 | 33 | 0.50 ± 0.03 | 0.74258 | 0.460 |
| HW | 35 | 0.40 ± 0.05 | 33 | 0.41 ± 0.05 | −0.85223 | 0.397 |
| SL | 35 | 0.33 ± 0.06 | 33 | 0.33 ± 0.06 | 0.39606 | 0.693 |
| PL | 35 | 0.31 ± 0.03 | 33 | 0.31 ± 0.05 | 0.08953 | 0.929 |
| IW | 35 | 0.23 ± 0.07 | 33 | 0.20 ± 0.08 | 1.84848 | 0.069 |
| ED | 35 | 0.09 ± 0.04 | 33 | 0.10 ± 0.03 | −0.81851 | 0.416 |
| MB | 35 | 0.15 ± 0.07 | 33 | 0.16 ± 0.09 | −0.41510 | 0.679 |
| ML | 35 | 0.18 ± 0.10 | 33 | 0.18 ± 0.08 | 0.00289 | 0.998 |
| BH | 35 | 0.72 ± 0.08 | 33 | 0.67 ± 0.05 | 2.90755 | 0.005 |
| CH | 35 | 0.50 ± 0.06 | 33 | 0.50 ± 0.07 | −0.19904 | 0.843 |
| CL | 35 | 0.27 ± 0.04 | 33 | 0.27 ± 0.03 | −0.34000 | 0.735 |
| PSL | 35 | 1.01 ± 0.02 | 33 | 1.00 ± 0.02 | 1.20229 | 0.234 |
| PFL | 35 | 0.57 ± 0.04 | 33 | 0.58 ± 0.04 | −1.08881 | 0.280 |
| CFL | 35 | 0.65 ± 0.05 | 33 | 0.66 ± 0.04 | −0.70806 | 0.481 |
| DSL | 35 | 0.96 ± 0.02 | 33 | 0.96 ± 0.02 | 0.06306 | 0.950 |
| DFCL | 35 | 0.42 ± 0.02 | 33 | 0.40 ± 0.03 | 2.62420 | 0.011 |
| DFL | 35 | 0.55 ± 0.06 | 33 | 0.55 ± 0.05 | 0.25689 | 0.798 |
| K | 35 | 2.12 ± 0.47 | 33 | 1.77 ± 0.32 | 3.53716 | <0.001 |
| PAFD | 35 | 0.72 ± 0.03 | 33 | 0.69 ± 0.04 | 4.55243 | <0.001 |
| Character | Component | |
|---|---|---|
| 1 | 2 | |
| D1–2 | 0.93 | 0.05 |
| D1–3 | 0.95 | −0.11 |
| D1–4 | 0.69 | −0.51 |
| D2–3 | 0.98 | −0.06 |
| D2–4 | 0.85 | 0.27 |
| D3–4 | 0.94 | −0.26 |
| D3–5 | 0.94 | −0.09 |
| D3–6 | 0.85 | −0.40 |
| D4–5 | 0.97 | −0.17 |
| D4–6 | 0.93 | −0.17 |
| D5–6 | 0.96 | −0.22 |
| D5–7 | 0.86 | −0.14 |
| D5–8 | 0.76 | −0.33 |
| D6–7 | 0.90 | −0.14 |
| D6–8 | 0.86 | −0.04 |
| D7–8 | 0.90 | −0.30 |
| D7–9 | 0.82 | −0.02 |
| D7–10 | 0.95 | −0.18 |
| D8–9 | 0.80 | −0.34 |
| D8–10 | 0.89 | −0.16 |
| D9–10 | 0.90 | −0.31 |
| BL | 0.99 | −0.02 |
| HL | 0.96 | 0.18 |
| HH | 0.91 | 0.24 |
| HW | 0.87 | 0.20 |
| SL | 0.87 | 0.15 |
| PL | 0.92 | 0.11 |
| ED | 0.83 | 0.28 |
| IW | 0.82 | 0.00 |
| ML | 0.84 | 0.21 |
| MB | 0.77 | 0.43 |
| BH | 0.80 | 0.46 |
| CL | 0.80 | 0.27 |
| CH | 0.92 | 0.22 |
| PSL | 0.98 | 0.07 |
| PFL | 0.87 | 0.13 |
| CFL | 0.86 | 0.30 |
| DSL | 0.97 | 0.04 |
| DFCL | 0.95 | 0.09 |
| PAFD | 0.91 | 0.05 |
| DFL | 0.79 | 0.23 |
| K | 0.05 | 0.80 |
| Eigenvalues | 32.30 | 2.87 |
| Contribution rate (%) | 76.9 | 6.73 |
| Cumulative contribution rate (%) | 76.9 | 83.63 |
| Sex | Anatomical Identification | Predicted Result | Accuracy of Discrimination (%) | Total Discrimination Accuracy (%) | Transformations * | |
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Male | 33 | 31 | 2 | 93.94 | 95.59 | Equation (2) |
| Female | 35 | 1 | 34 | 97.14 | ||
| Male | 33 | 30 | 3 | 94.29 | 94.12 | Equation (3) |
| Female | 35 | 2 | 33 | 97.14 | ||
| Male | 33 | 27 | 6 | 81.82 | 83.82 | Equation (4) |
| Female | 35 | 5 | 30 | 85.71 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Tuo, Y.; Xiao, T.; Chen, C.; Fang, G. Sexual Dimorphism and Discrimination of Barbel Steed (Hemibarbus labeo) in the Jinhe River, China: An Indicator of Habitat Status. Sustainability 2023, 15, 993. https://doi.org/10.3390/su15020993
Li J, Tuo Y, Xiao T, Chen C, Fang G. Sexual Dimorphism and Discrimination of Barbel Steed (Hemibarbus labeo) in the Jinhe River, China: An Indicator of Habitat Status. Sustainability. 2023; 15(2):993. https://doi.org/10.3390/su15020993
Chicago/Turabian StyleLi, Jing, Yun Tuo, Tiaoyi Xiao, Cuihe Chen, and Guangwei Fang. 2023. "Sexual Dimorphism and Discrimination of Barbel Steed (Hemibarbus labeo) in the Jinhe River, China: An Indicator of Habitat Status" Sustainability 15, no. 2: 993. https://doi.org/10.3390/su15020993
APA StyleLi, J., Tuo, Y., Xiao, T., Chen, C., & Fang, G. (2023). Sexual Dimorphism and Discrimination of Barbel Steed (Hemibarbus labeo) in the Jinhe River, China: An Indicator of Habitat Status. Sustainability, 15(2), 993. https://doi.org/10.3390/su15020993





