Abstract
Sex identification is linked to sexual dimorphism and is an important study issue in fish biology and aquaculture. However, owing to the unmarked sexual heteromorphism between adult Hemibarbus labeo, it is often difficult to distinguish their sex by visual observation. This study aimed to find a simple and reliable morphometric criterion for the sex identification of H. labeo using discriminant models. Forty-two morphometric traits of sixty-eight H. labeo individuals collected from the Jinhe River were measured, and 41 standardized features were calculated and analyzed. Eight trait variables from 41 standardized attributes were screened using stepwise discriminant analysis. The total classification accuracy of the model was 95.59%. Twelve standardized features significantly differed between male and female H. labeo individuals (p < 0.05). The condition factor, body height, dorsal fin coxal length, the distance between the pelvic and anal fins, and body length were significantly greater in females than in males (p < 0.05), suggesting that females of H. labeo in the Jinhe River were plumper than males, with a larger body size, but a smaller caudal peduncle. These results implied that the sex identification of H. labeo can be performed using the discriminant equation established in this study. This study provides a theoretical basis for endangered fish species protection and their artificial propagation.
1. Introduction
The role of aquaculture in the supply of aquatic products worldwide has reached a consensus [1,2]. Fish and other aquaculture products have become primary means of human access to animal proteins [3]. Artificial reproduction technology is a breakthrough in aquaculture and has solved the problem of seedling species in marine and freshwater economic fish cultures. The identification of male and female fish is the first requisite of artificial reproduction and the key to ensuring the success of artificial reproduction, because accurate sex determination of fish is the basis of the proper sex ratio of male and female pro-fish.
Sexual dimorphism is a phenomenon in which fish exhibit distinct external characteristics between males and females [4,5,6]. Sex identification is a fundamental study in fish biology and aquaculture, and its results provide a theoretical basis for precious fish conservation and artificial reproduction [7]. Studies have found that many species of female and male fish show more pronounced differences in body size, minority body structure, and body surface color [8,9,10,11]. However, many fish do not have obvious sexual dimorphisms, such as Acrossocheilus wenchowensis [12], Acanthorhodeus chankaensi [13], and Eryghroculter ilishaeformis [14]. Sex identification methods, such as anatomy and the application of molecular biology techniques, have many limitations and are not suitable for the protection of fish or their practical application. It is a simple and practical method to discriminate between females and males based on external characteristics and has been successfully applied in many fish, such as Anguilla japonica [15], Ilisha elongata [16], Scatophagus argus [17], and Hemibarbus maculatus [18]. Establishing discriminant models based on morphological traits, the comprehensively classified accuracy can reach more than 85%.
Hemibarbus labeo (Pallas, 1776) belongs to the Cyprinidae family and is commonly known as barbel steed. It is distributed widely in plain rivers of East Asia. It is an ecologically critical and valuable indigenous fish species and is one of the 50 main freshwater fishes economically important in China [19,20]. Owing to environmental pollution, overfishing, and other factors, there has been a sharp decline in the wild resources of H. labeo in many rivers. The artificial breeding of H. labeo has been performed in different regions, such as the Fujiang, Wusuli, Yalu, Liaoning, Oujiang, and Qiantang Rivers, to save this precious resource [21,22,23,24,25]. Moreover, H. labeo has the wide distribution, medium life span, the fast growth rate, high fertility, early sexual maturity, and benthic nature. Therefore, it is considered a river indicator species and is used to evaluate the health status of rivers [26].
The population ecology of H. labeo in many water bodies has been studied, including the Heilongjiang Basin [20,23], the Yangtze River Basin [21,22,27,28], the Pearl River Basin [26] in China, Chikugo River in Japan [29], and Goe-san Lake in Korea [30]. In a study of the reproductive biology of H. labeo in the Ganjiang River of China [27], we observed that the morphological differences between wild female and male individuals are not apparent. For example, the characteristics of the female cloaca protruding outwards [23] are not evident, and the annual sexual maturity of H. labeo populations in the Ganjiang River is not synchronized [27]. Generally, adult male H. labeo fish have a similar abdominal bulge as adult female fish because of the large amount of food consumed during the breeding season [27]. This study systematically analyzed the morphological indicators of H. labeo in the Ganjiang River, and a sex identification model was established. We attempted to use a combination of different transformation methods of standardized data and multivariate statistical techniques to create a discriminant model with high sex identification accuracy, laying a necessary foundation for the artificial reproduction of H. labeo and providing a reference method for the sex identification of other fishes.
2. Materials and Methods
2.1. Sampling Collection and Preparation
A total of 68 adult H. labeo fish, with 35 females and 33 males, were captured from the Shanggao area (114°28′–115°10′ E, 28°02′–28°25′ N) of the Jinhe River using gill nets and floor cages with a mesh size of 20–30 mm and a mesh size of 5 mm, respectively, from March 2014 to June 2016. The Jinhe River, located in Southeastern China, is a tributary of the Ganjiang River. The main river is 307 km2 in length and has a natural gap of 391 m. The average ratio of the river to falls was 0.129%, and the river covers a drainage area of 7853 km2, approximately 10% of the Ganjiang River Basin area. The Jinjiang Basin is crisscrossed by rivers and a developed water system that includes 19 streams. The geological structure is valley plains and hilly land with loose soil containing red clay. The average width of the river is 126.46 m, the average flow is 20.45 m3/s, the mean velocity is 0.21 m/s, and the average depth is 0.77 m.
The samples were transported to the laboratory and anesthetized using MS-222. Photographs of each individual were obtained from the left side using a digital camera (Nikon D5200, Tokyo, Japan). Subsequently, the samples were sacrificed for sex identification following a protocol approved by Hunan Agricultural University ACUC (permit number: 2017NK1032).
2.2. Measurement
Body length (BL), body height (BH), head length (HL), head height (HH), head width (HW), mouth width (MB), mouth length (ML), eye diameter (ED), interorbital width (IW), snout length (SL), post-orbital length (PL), caudal peduncle length (CH), caudal peduncle height (CL), pectoral fin length (PFL), the distance between the snout and the dorsal fin (DSL), the distance between the snout and the pelvic fin (PSL), dorsal fin long-throned length (DFL), dorsal fin coxal length (DFCL), caudal fin length (CFL), and distance between the pelvic and anal fins (PAFD) were measured using a Vernier caliper, as described in [31,32]. Fish body weight (W) was measured using an electronic balance with a precision of 10 mg (Sartorius, Göttingen, Germany). The samples were then dissected to determine the sex based on the appearance of the gonads. The condition factor (K) was calculated using Equation (1):
where BL is the body length, and W is the body weight.
A total of 10 landmarks were marked on the left surface of each sample based on the truss network hypothesis (Figure 1A), as described in [33,34,35]. Twenty-one truss network measurements were constructed, including D1–4, D2–4, …, and D9–10. The truss parameter measurement was the distance between 2 of the 10 landmarks (Figure 1B).
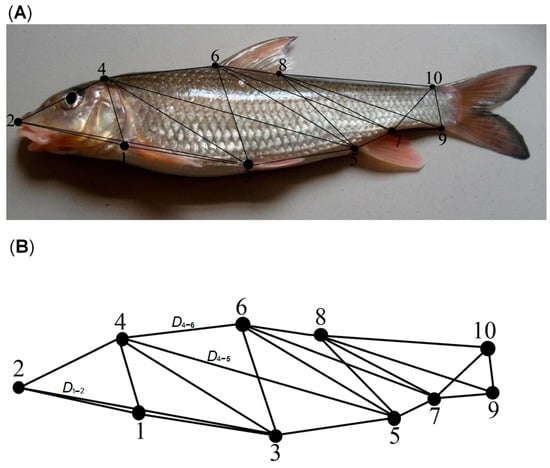
Figure 1.
Schematic measurement for the truss network (A) and the processed truss network (B). 1, origin of the pectoral fin; 2, tip of the snout; 3, origin of the pelvic fin; 4, most anterior scales on the skull; 5, origin of the anal fin; 6, origin of the dorsal fin; 7, terminus of the anal fin; 8, terminus of the dorsal fin; 9, ventral origin of the caudal fin; and 10, dorsal origin of the caudal fin. Truss parameter measurements were the distances between 2 of the 10 landmarks. For instance, D1–2 denotes the distance between landmarks 1 and 2.
2.3. Data Analysis
All data were tabulated using Microsoft Excel 2007, and statistical analyses were performed using SPSS 19 and Statistica 10. Three types of data from traditional morphological traits, the truss network traits, and a combination of the truss network traits and traditional morphological traits were analyzed in this study as previously described [14]. Moreover, to remove the effect of the fish body size, the data obtained by combining traditional morphological traits and the truss network traits were transformed to the ratio (Mr), the logarithmic ratio (Mlog), and the logarithmic heterocorrelation index (MALLOM), as shown in Equations (2)–(4), respectively, as previously described [36,37,38]:
where M is the observed character measurement of each sample, b is the slope of the linear regression of log10M on log10SL for all samples, SL is the body length of each sample, and SLmean is the mean standard length for all samples. The sexual heteromorphism index (SHI) was calculated using Equation (5), as described in [39,40]:
where BLmean.min is the mean body length of the sex with a smaller body size and BLmean.max is the mean body length of the sex with a larger body size. The Student’s t-test was used to compare the BL and 41 morphological parameters of H. labeo. Differences with p of < 0.05 were considered statistically significant.
Principal component analysis (PCA) with non-parametric permutational multivariate analysis of variance (PERMANOVA) [41] was performed using the R vegan package [42] to test for significant differences between male and female H. labeo. Cluster analysis was performed using R-clustering with the Pearson’s correlation coefficient. Discriminant analysis was used to analyze and screen out the traits with significant differences between male and female samples using a stepwise regression method, following by the construction of sex discriminant models for H. labeo.
3. Results
3.1. Sexual Dimorphism of H. labeo
Of the 68 H. labeo samples, 35 were females with an average BL of 21.25 ± 3.25 cm (15.35–30.28 cm), and 33 were males with an average BL of 18.48 ± 4.09 cm (12.50–27.50 cm). The BL of females was significantly higher than that of males (independent t-test, t = 3.10, p < 0.01; Table 1). The SHI was 0.13. The BL correlated significantly with other morphological traits, with the Pearson’s correlation coefficients ranging from 0.480 to 0.959 (Pearson’s correlation analysis, p < 0.05; Appendix A). Males D1–4, D3–4, D3–6, D4–6, D7–9, D8–9, and D9–10 were significantly larger than those for females (independent t-test, p < 0.05; Table 1), whereas the BH, the DFCL, the PAFD, and the K were significantly smaller than those for females (independent t-test, p < 0.05; Table 1).

Table 1.
Comparison of external characteristics of male and female Hemibarbus labeo. Except for the body length (BL, cm), character parameters were transformed by dividing by the BL. Data are presented as mean ± standard deviation. Male and female individual transition variables were compared using t-tests. D1–2, distance from the snout tip to the origin of the pectoral fin; D2–3, distance from the snout tip to the pelvic fin origin; D1–3, distance from the origin of the pectoral fin to the origin of the pelvic fin; D2–4, distance from the snout tip to the most anterior of scales on skull; D3–4, distance from the origin of the pelvic fin to the most anterior of scales on skull; D4–5, distance from the most anterior of scales on skull to the origin of the anal fin; D5–6, distance from the origin of the anal fin to the origin of the dorsal fin; D4–6, distance from the most anterior of scales on skull to the origin of the dorsal fin; D7–9, distance from the back end of the anal fin base to the ventral origin of the caudal fin; D7–10, distance from the back end of the anal fin base to the dorsal origin of the caudal fin; D8–9, distance from the back end of the dorsal fin base to the ventral origin of the caudal fin; D9–10, distance from the back end of the anal fin base to the dorsal origin of the caudal fin peduncle; CH, caudal peduncle height; CL, caudal peduncle length; DSL, distance between the snout and the dorsal fin; PSL, distance between the snout and the pelvic fin; PFL, pectoral fin length; CFL, caudal fin length; DFCL, dorsal fin coxal length; DFL, dorsal fin long-throned length; K, condition factor; and PAFD, distance between the pelvic and anal fins.
3.2. Principal Component and R-Cluster Analyses of the Differences of the Mprphological Variables between Male and Female H. labeo
The first two principal components (eigenvalue: ≥1) were extracted from the principal component analyses of the 42 morphological characteristic variables of male and female individuals, which explained 83.63% of the variance (Table 2). The BL, the D1–3, the D2–3, the D4–5, the D5–6, the D7–10, the HL, the PSL, the DSL, and the DFCL had high positive loading coefficients in PC1 (explaining 76.90% of the variance), representing the body shape and growth factors. The condition factor K had the highest positive loading coefficient in PC2, explaining 6.73% of the variance in Table 2, describing the body fatness factor. The morphological features of male and female H. labeo overlapped considerably (Figure 2A), even though male and female H. labeo were significantly different in terms of morphological characteristics (PERMANOVA, F = 8.316, p = 0.010).

Table 2.
Loading of the first two axes of a principal component analysis on 42 morphological variables in Hemibarbus labeo. The major contributing variables of the principal components are shown in bold. BL, body length (cm); D1–2, distance from the snout tip to the origin of the pectoral fin; D2–3, distance from the snout tip to the pelvic fin origin; D1–3, distance from the origin of the pectoral fin to the origin of the pelvic fin; D2–4, distance from the snout tip to the most anterior of scales on skull; D3–4, distance from the origin of the pelvic fin to the most anterior of scales on skull; D4–5, distance from the most anterior of scales on skull to the origin of the anal fin; D5–6, distance from the origin of the anal fin to the origin of the dorsal fin; D4–6, distance from the most anterior of scales on skull to the origin of the dorsal fin; D7–9, distance from the back end of the anal fin base to the ventral origin of the caudal fin; D7–10, distance from the back end of the anal fin base to the dorsal origin of the caudal fin; D8–9, distance from the back end of the dorsal fin base to the ventral origin of the caudal fin; D9–10, distance from the back end of the anal fin base to the dorsal origin of the caudal fin peduncle; CH, caudal peduncle height; CL, caudal peduncle length; DSL, distance between the snout and the dorsal fin; PSL, distance between the snout and the pelvic fin; PFL, pectoral fin length; CFL, caudal fin length; DFCL, dorsal fin coxal length; PAFD, distance between the pelvic and anal fins; DFL, dorsal fin long-throned length; and K, condition factor.
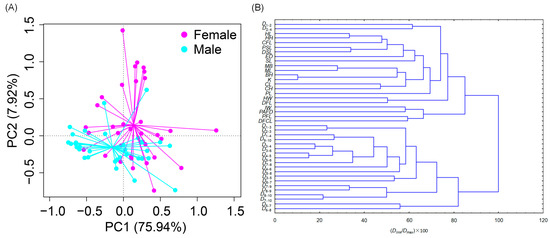
Figure 2.
The plots of the first and second components from PCA (A) and the R-cluster dendrogram of standardized morphological traits (B) of Hemibarbus labeo. D1–2, distance from the snout tip to the origin of the pectoral fin; D2–3, distance from the snout tip to the origin of the pelvic fin; D1–3, distance from the origin of the pectoral fin to the origin of the pelvic fin; D2–4, distance from the snout tip to the most anterior of scales on skull; D3–4, distance from the origin of the pelvic fin to the most anterior of scales on skull; D4–5, distance from the most anterior of scales on skull to the origin of the anal fin; D5–6, distance from the origin of the anal fin to the origin of the dorsal fin; D4–6, distance from the most anterior of scales on skull to the origin of the dorsal fin; D7–9, the distance from the back end of the anal fin base to the ventral origin of the caudal fin; D7–10, distance from the back end of the anal fin base to the dorsal origin of the caudal fin; D8–9, the distance from the back end of the dorsal fin base to the ventral origin of the caudal fin, D9–10, the distance from the back end of the anal fin base to the dorsal origin of the caudal fin peduncle; CH, caudal peduncle height; CL, caudal peduncle length; DSL, distance between the snout and the dorsal fin; PSL, distance between the snout and the pelvic fin; PFL, pectoral fin length; CFL, caudal fin length; DFCL, dorsal fin coxal length; DFL, dorsal fin long-throned length; K, condition factor; PAFD, distance between the pelvic and anal fins.
The R-cluster analysis of 41 standardized character parameters showed that the relative traits after normalization could be divided into two categories. Class I contained D1–2, D2–4, HL, HH, HW, SL, PL, IW, K, PAFD, and other characteristic parameters that mainly represented the fish head characteristics, body fatness and thinness, and abdominal characteristics. The second category contained D1–3, D2–3, D6–7, …, and D8–10, which mainly represented the body shape of H. labeo (Figure 2B).
3.3. Sex Discriminant Analysis
The discriminant analysis results showed that the comprehensive discrimination rate (CDR) of traditional features was 92.65% (male: 96.97%; female: 88.57%), the CDR of frame features was 94.12% (male: 96.97%; female: 91.43%), and the CDR of data from combined traditional morphological traits and frame features was 100%. The effects of the three transformation methods on the CDR of the data combining traditional morphological traits and frame features were compared, and the discriminant rates were 95.59% for Mr (male: 93.94%; female: 97.14%), 94.12% for Mlog (male: 90.91%; female: 97.14%), and 85.29% for MALLOM (male: 84.85%; female: 85.71%).
Stepwise regression screened out eight morphological parameters (i.e., PAFD, D8–9, PFL, BH, CFL, D4–6, CH, and DFCL), which successfully differentiated males and females through the standardized data of ratio transformation, and the sex discriminant equations of H. labeo were established using Equations (6) and (7):
The discriminant equations established through the test showed an extremely significant difference (Wilks’ lambda = 0.2734, F (8, 59) = 19.60, p < 0.001; Figure 3). Sixty-eight fish were identified using the sex-discriminant equation. Eight characteristic parameters of each fish in the equation were used to calculate Y1 and Y2. If Y1 > Y2, it is male; otherwise, it is female. After anatomical verification, only the sex of three fishes was incorrectly identified; the misidentification rate was 4.41%, and the accuracy rate was as high as 95.59% (Table 3).
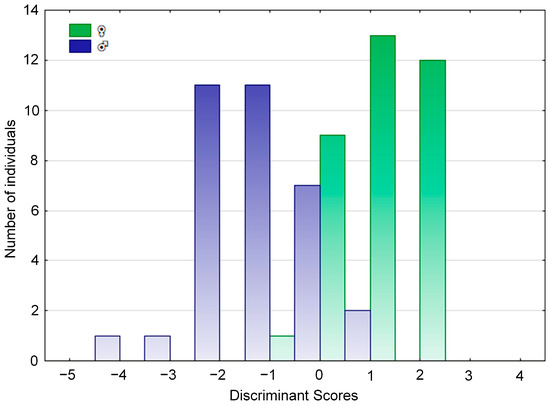
Figure 3.
Histogram of the frequency distributions of discriminant scores between male and female Hemibarbus labeo.

Table 3.
Classification results of male and female Hemibarbus labeo.
4. Discussion
Animal sexual size dimorphism (SSD) is usually explained as a result of sexual selection, ecological selection, and fertility selection [8,43,44,45]. Fish have three main SSD types: (1) female adults are larger than male adults; (2) female adults are smaller than male adults; and (3) males and females are similar in size, but there are differences in local characteristics [46,47,48]. Our results showed that the SSD of H. labeo in the Jinhe River belonged to the first type. The larger the female individual, the greater the fecundity, and the combined effect was biased towards the result of fertility selection, which was the same as that of H. maculatus in the Yuanhe River [18]. In the same habitat of the Ganjiang River Basin, the sexual heteromorphism indices (SHIs) of H. labeo (0.13) and H. maculatus (0.126) were highly similar, indicating that under the same current comprehensive selection effect, these two species in the same genus evolved with the heteromorphic body size of the two sexes. However, the two types of heteromorphism of H. labeo in the Ganjiang River and the Jialing River [49] are similar in size, with both being of the first type, indicating that the resources of H. labeo in the Yangtze River Basin are declining. To ensure racial reproduction, they tend to increase reproductive output and choose R countermeasures to resist environmental pressure.
Local characteristics D7–9, D8–9, and D9–10 of male H. labeo were larger than those of females, and the difference between the two sexes was that the male H. labeo in the Ganjiang River had a larger caudal peduncle, similar to that of H. maculatus [18], Odontobutis obscurus [50], and catfish (Clarias fuscus) [51]. It is speculated that males have faster locomotion and explosive swimming abilities compared to females, such as steering ability, which is important for chasing females when breeding, cooperating with their egg-laying behavior to achieve reproductive success, and is also conducive to nesting and fighting behavior. These are the results of long-term natural selection. Female local characteristics BH, K, and PAFD were significantly larger than males, which was related to the reproductive output of fish [32].
Our results showed that the type II standardized traits classified by R-clustering corresponded to principal component 1 obtained by PCA and both reflected the fish body shape factor, while the type I standardized traits corresponded to principal component 2, both reflecting the fish body fatness and thinness factor. Both analyses yielded similar results. Therefore, the differences between male and female H. labeo in the Jinhe River were mainly concentrated in two aspects: fish body size and fullness, which was similar to that of I. Elongata [16], S. argus [17], and H. maculatus [18].
Therefore, the practical applicability of these results should be considered. Although we measured 42 morphometric traits and calculated 41 standardized features of H. labeo in this study, our results showed that using eight morphological parameters, that is, PAFD, D8–9, PFL, BH, CFL, D4–6, CH, and DFCL, could successfully differentiate between males and females through the standardized data of ratio transformation. This result reduces the workload of measuring morphological parameters in practical applications. Although we dissected the fish samples analyzed in this study, we determined the sex of the fish to verify the effectiveness of our sex discrimination model. Our results showed that after anatomical verification, only three fish were wrong in sex judgment; the misjudgment rate of our model was 4.41%, and the accuracy rate was as high as 95.59%. Such high accuracy means that we can identify the sex of fish using our model rather than dissection. Moreover, considering artificial intelligence has been applied to fish management and parasite detection in aquaculture [52,53,54], to improve the efficiency of practical applications, it is also necessary to introduce computer programs to calculate the values of Y1 and Y2 and to try to automatically measure the morphological traits of fish photos and automatically convert data through artificial intelligence, which will greatly improve the efficiency of our model.
Owing to the incomplete or inaccurate selection of morphological features and the size of the fish body effects, the sex discrimination models established by many fish have low discrimination rates, such as 60.26–82.14% for Larimichthys crocea [55] and 71% for Odontobutis potamophila [56]. Our results showed that data obtained by combining traditional morphological traits and frame features could improve the discrimination accuracy of H. labeo in the Jinhe River, which was similar to those of H. maculatus [18] and Megalobrama amblycephala [57]. In addition, the first transformation method, body length ratio transformation, had the highest accuracy in discriminating between male and female H. labeo in the Jinhe River, with a comprehensive recognition rate of 95.59%. Whether this can improve the discrimination accuracy of H. labeo in other waters requires further study. Therefore, establishing a morphological discriminant equation is an effective tool to discriminate the males and females of H. labeo in the Jinhe River. The discriminant model was developed based on the sample ranged from 12.50 to 30.28 cm in body length and ranged from 22.40 to 418.00 g in weight. Whether this model can be used to discriminate H. labeo without the above ranges still needs to be further studied. Moreover, because wild resources of this fish in the Jinhe River are rare, the relatively small sample size also limited the accuracy of our model to some extent. Therefore, it is necessary to further analyze the accuracy of this model for identifying female and male H. labeo individuals in different rivers.
5. Conclusions
We measured and analyzed the morphological index system of male and female H. labeo in the Jinhe River and established a quantitatively morphological discriminant model for the sexual identification of H. labeo based on this, which solves the problem of sex identification of this species. This model can provide important support for the development of H. labeo aquaculture/breeding, which can serve as an indicator species to evaluate the health status of rivers. We validated the conclusion that combining traditional morphological feature data with the truss network data can increase fish sex discrimination accuracy. Simultaneously, we found that the different transformation methods used to normalize the data had a significant impact on the accuracy of H. labeo sex discrimination. Different fish species, the same species in different growth environments, and using different transformation methods can improve the accuracy of male and female identification models. The optimal male−female ratio for the artificial reproduction of H. labeo requires further study.
Author Contributions
Conceptualization, J.L., Y.T. and T.X.; methodology, Y.T.; software, J.L.; validation, Y.T., C.C. and G.F.; formal analysis, J.L. and Y.T.; investigation, Y.T.; resources, Y.T.; data curation, J.L.; writing—original draft preparation, J.L.; writing—review and editing, Y.T. and T.X.; visualization, J.L.; supervision, C.C. and G.F.; project administration, J.L.; funding acquisition, J.L., Y.T. and T.X. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Jiangxi Education Department in Science and Technology Project (grant number: No. GJJ190831) and the Innovation and Demonstration of Breeding Mode in Pond Healthy Colleges and Universities (grant number: No. 2017NK1032).
Institutional Review Board Statement
The animal study protocol was approved by Hunan Agricultural University ACUC (protocol code ACC2017007 and date of approval on 9 October 2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Figure A1.
Correlations between body length and other morphological parameters.
References
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.; Clay, J.; Folke, C.; Lubchenco, J.; Monney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef]
- James, H.T.; Geoff, L.A. Fish as food: Aquaculture’s contribution. EMBO Rep. 2001, 21, 958–963. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The state of world fisheries and aquaculture 2022. In Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Yin, M.C. Ecology of Fishes; Chinese Agriculture Press: Beijing, China, 1993; pp. 110–115. [Google Scholar]
- An, L.; Meng, Q.; Zhang, L.; Dong, X.; Li, X.; Li, W.; Zhu, S. Analysis of morphological indexes and discrimination of male and female Eryghroculter ilishaeformis. Freshw. Fish. 2017, 47, 36–41. [Google Scholar]
- Mustafa, A.A.; Mohammad, A.S.; Farrag MM, S.; Osmana, G. Sexual dimorphism of morphological characters in three carangid species from the Red Sea, Egypt. Iran. J. Ichthyol. 2021, 8, 223–235. [Google Scholar]
- McEvoy, F.J.; Tomkiewicz, J.; Støttrup, J.G.; Overton, J.L.; McEvoy, C.; Svalastoga, E. Determination of fish gender using fractal analysis of ultrasound images. Vet. Radiol. Ultrasound 2009, 50, 519–524. [Google Scholar] [CrossRef]
- Hedrick, A.V.; Temeles, E.J. The evolution of sexual dimorphism in animals: Hypotheses and tests. Trends Ecol. Evol. 1989, 4, 136–138. [Google Scholar] [CrossRef]
- Herler, J.; Kerschbaumer, M.; Mitteroecker, P.; Postl, L.; Sturmbauer, C. Sexual dimorphism and population divergence in the Lake Tanganyika cichlid fish genus. Tropheus. Front. Zool. 2010, 7, 4. [Google Scholar] [CrossRef]
- Parmentier, E.; Boistel, R.; Bahri, M.A.; Plenevaux, A.; Schwarzhans, W. Sexual dimorphism in the sonic system and otolith morphology of Neobythites gilli (Ophidiiformes). J. Zool. 2018, 305, 274–280. [Google Scholar] [CrossRef]
- Liang, Y.; Meyer, A.; Kratochwil, C.F. Neural innervation as a potential trigger of morphological color change and sexual dimorphism in cichlid fish. Sci. Rep. 2020, 10, 12329. [Google Scholar] [CrossRef]
- Xu, D.; Lin, Z.; Lei, H. Sexual dimorphism in morphological traits and female individual fecundity of Acrossocheilus wenchowensis. J. Shanghai Jiaotong Univ. 2006, 24, 335–340. [Google Scholar]
- Chen, S.; Zhao, R.; Qi, D.; Fan, X.; Lei, H.; Lin, Z. Sexual dimorphism in morphological traits and female individual fecundity of Acanthorhodeus chankaensis Dybowsky. J. Shanghai Jiaotong Univ. 2013, 31, 61–66+78. [Google Scholar]
- Chen, S.; Zhao, R.; Zhang, Y.; Fan, X.; Ding, G.; Lin, Z. Sexual dimorphism in morphology and female fecundity of Erythroculter ilishaeformis from rivers in Yuyao. J. Lishui Univ. 2013, 35, 11–15. [Google Scholar]
- Guo, H.; Wei, K.; Xie, Z.; Tang, W.; Shen, L.; Gu, S.; Wu, J.; Chen, W. Analysis of morphological index system and discrimination of male and female silver eels (Anguilla japonica) collected at the Yangtze River Estuary. J. Fish. China 2011, 35, 1–9. [Google Scholar]
- Ni, H.; Chen, X. Analysis of shape index system and discriminant of male and female of Ilisha elongata. J. Biomath. 2003, 18, 224–228. [Google Scholar]
- Wu, B.; Zhang, M.; Deng, S.; Shi, S.; Li, G.; Zhu, C. Analysis of morphological index and discrimination of male and female Scatophagus argus. J. Shanghai Ocean. Univ. 2014, 23, 64–69. [Google Scholar]
- Tuo, Y.; Xiao, T.; Wang, H. Discrimination of Sexual Dimorphism through External Morphology of spotted steed (Hemibarbus maculatus) in the Yuanhe River, China. Appl. Ecol. Environ. Res. 2020, 18, 1539–1550. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Q.; Yue, P.; Huang, H. Economic Animals in China—Freshwater Fish; Science Press: Beijing, China, 1979; pp. 86–87. [Google Scholar]
- Никoльскии Г, В. Fishes in Amur River Basin; Science Press: Beijing, China, 1956; pp. 190–195. [Google Scholar]
- He, J.S.; He, X.F.; Yan, T.M. Observation on the embryonic development of Hemibarbus labeo (Pallas) in lower reaches of Fujiang River. J. Southwest China Norm. Univ. 1999, 24, 225–231. [Google Scholar]
- Xu, W.; Li, C.T.; Geng, L.W.; Sun, H.W.; Liu, X.Y. Growth and reproduction of reared Hemibarbus labeo in Wusuli River. J. Fish. Sci. China 2009, 16, 550–555. [Google Scholar]
- Gan, G.M.; Zhang, Y.G.; Zhang, X.F.; Wang, Z.J. Cytological studies on fertilization in Hemibarbus labeo. Acta Hydro Biol. Sin. 2006, 30, 284–291. [Google Scholar]
- Luo, X.N.; Li, J.; Jin, G.H.; Liu, Y.X.; Li, J.W.; Yang, P.M.; Xia, D.M.; Liu, B.Y. Fry and Juvenile Rearing of Hemibarbus labeo in an Earthen Pond. Fish. Sci. 2013, 32, 102–105. [Google Scholar]
- Lian, Q.P.; Mi, G.Q.; Yao, Z.L.; Hu, T.J.; Li, Q.; Liu, S.L.; Wang, Y.C. A Study on Artificial Propagation and Embryonic Development of Hemibarbus labeo in Oujiang River. Acta Agric. Univ. Jiangxiensis 2014, 36, 181–186. [Google Scholar]
- Jia, Y.T.; Chen, Y.F. River health assessment in a large river: Bioindicators of fish population. Ecol. Indic. 2013, 26, 24–32. [Google Scholar] [CrossRef]
- Tuo, Y.; Deng, Z.Q.; Hong, L.; Liao, L.Y. Morphological Biology of Hemibarbus maculatus Bleeker in Ganjiang River. J. Yichun Univ. 2016, 38, 89–92+122. [Google Scholar]
- Tuo, Y.; Li, J.; Xiao, T.; Wang, H. Sexual discrimination and fecundity of barbel steed (Hemibarbus labeo) in the Jinjiang river, china. Appl. Ecol. Environ. Res. 2021, 19, 1783–1797. [Google Scholar] [CrossRef]
- Takeshita, N.; Kimura, S. Age and Growth of the Cyprinid Fish Hemibarbus barbus in the Chikugo River. Nippon. Suisan Gakkaishi 1991, 57, 29–34. [Google Scholar] [CrossRef]
- Lee, W.O.; Zhang, M.M.; Oh, C.W.; Baek, J.M.; Song, K.J. Age and Growth of Barbel Steed Hemibarbus labeo in Goe-san Lake in Korea. Fish. Aquat. Sci. 2012, 15, 353–359. [Google Scholar] [CrossRef]
- Laboratory of Institute of Hydrobiology of Hubei Province. Fishes in the Yangtze River; Science Press: Beijing, China, 1976; pp. 79–80. [Google Scholar]
- Lin, Z.; Lei, H.; Lin, Z.; Hua, H. Sexual dimorphism and female reproductive output of Hemibarbus maculates. J. Shanghai Jiaotong Univ. 2005, 23, 284–288. [Google Scholar]
- Strauss, R.M.; Bookstein, F.L. The truss: Body form reconstruction in morphometrics. Syst. Zool. 1982, 31, 113–135. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology Cambridge; Cambridge University Press: New York, NY, USA, 1997. [Google Scholar]
- Corti, M.; Thorpe, R.S.; Sola, L.; Sbordoni, V.; Cataudella, S. Multivariate morphometrics in aquaculture:a case study of six stocks of the common carp (Cyprinnus carpio) from Italy. Can. J Fish. Aquat. Sci. 1988, 45, 1548–1554. [Google Scholar] [CrossRef]
- Turan, C. A Note on The Examination of Morphometric Differentiation Among Fish Populations: The Truss System. Turk. J. Zool. 1999, 23, 259–263. [Google Scholar]
- Turan, C.; Ergüden, D.; Gürlek, M.; Başusta, N.; Turan, F. Morphometric structuring of the anchovy(Engraulis encrasicolus L.) in the Black, Aegean and northeastern Mediterranean seas. Turk. J. Vet. Anim. Sci. 2004, 28, 865–871. [Google Scholar]
- Pinheiro, A.; Teixeira, C.M.; Rego, A.L.; Marques, J.F.; Cabral, H.N. Genetic and morphological variation of Solea lascaris (Risso, 1810) along the Portuguese coast. Fish. Res. 2005, 73, 67–78. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Lovich, J.E. Sexual dimorphism in turtles with emphasis on the slider turtle (Trachemys scripta). Herpetol. Monogr. 1990, 4, 1–29. [Google Scholar] [CrossRef]
- Shou, L.; Du, W.G.; Shu, L. Sexual dimorphism and fecundity in the gold-stripe pond frog (Pelophylax plancyi) and the terrestrial frog (Fejervarya limnocharis). Acta Ecol. Sin. 2005, 25, 664–668. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Emlen, S.T.; Oring, L.W. Ecology, sexual selection, and the evolution of mating system. Science 1977, 197, 215–223. [Google Scholar] [CrossRef]
- Lande, R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 1980, 34, 292–305. [Google Scholar] [CrossRef]
- Abecia, J.E.; Luiz, O.J.; Crook, D.A.; Banks, S.C.; Wedd, D.; King, A.J. Sex and male breeding state predict intraspecific trait variation in mouthbrooding fishes. J. Fish Biol. 2022, 101, 550–559. [Google Scholar] [CrossRef]
- Parker, G.A. The evolution of sexual size dimorphism in fish. J. Fish. Biol. 1992, 41, 1–20. [Google Scholar] [CrossRef]
- Pyron, M. Sexual size dimorphism and phylogeny in North American minnows. Biol. J. Linn. Soc. 1996, 57, 327–341. [Google Scholar] [CrossRef][Green Version]
- Erlandsson, A.; Ribbink, A.J. Patterns of sexual size dimorphism in African cichlid fishes. S. Afr. J. Sci. 1997, 93, 498–508. [Google Scholar]
- Hu, Y. Sexual Dimorphism and Female Individual Fecundity of Six Species of Gobioninae in the Jialing River, Southwest China; China West Normal University: Nanchong, China, 2017. [Google Scholar]
- Fan, X.; Lin, Z.; Lu, J.; Qiu, Y.; Chen, C.; Cao, Y.; Qi, D. Sexual dimorphism in morphological traits and female individual fecundity of Odontobutis obscurus. J. Shanghai Jiaotong Univ. 2009, 28, 587–591+623. [Google Scholar]
- Fan, X.; Lin, Z.; Ding, X.; Zhu, J. Sexual size dimorphism and female individual fecundity of Silurus asotus and Clarias fuscus. Acta Ecol. Sin. 2014, 34, 555–563. [Google Scholar]
- Li, J.; Lian, Z.; Wu, Z.; Zeng, L.; Mu, L.; Yuan, Y.; Bai, H.; Guo, Z.; Mai, K.; Tu, X.; et al. Artificial intelligence-based method for the rapid detection of fish parasites (Ichthyophthirius multifiliis, Gyrodactylus kobayashii, and Argulus japonicus). Aquaculture 2023, 563, 738790. [Google Scholar] [CrossRef]
- Lee, P.G. Process control and artificial intelligence software for aquaculture. Aquac. Eng. 2000, 23, 13–36. [Google Scholar] [CrossRef]
- Memarzadeh, M.; Britten, G.L.; Worm, B.; Boettiger, C. Rebuilding global fisheries under uncertainty. Proc. Natl. Acad. Sci. 2019, 116, 15985–15990. [Google Scholar] [CrossRef]
- Chen, W.; Wang, P.P.; Xiao, S.J.; Liu, Y.; Ye, K.; Chen, Q.K.; Wang, Z.Y. Analysis of morphological index system and sexual differences of large yellow croaker (Larimichthys crocea). J. Jimei Univ. 2014, 19, 401–408. [Google Scholar]
- Zhang, H.Y.; Chen, S.Q.; Wang, T.; Zhou, G.Q.; Yin, S.W. Analysis of morphological index system and sexual differences of Odontobutis potamophila. Jiangsu Agric. Sci. 2018, 46, 138–142. [Google Scholar]
- Li, S.; Cai, W.; Zhou, B. Morphological and biochemical genetic variations among populations of blunt snout bream (Megalobrama amblycephala). J. Fish. China 1991, 15, 204–211. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).