Potential Changes in Soil Microbial Composition under 1,2-Dichlorobenzene Contamination
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Microcosm Preparation
2.2. Anaerobic Microcosm Experiments
2.3. Soil Chemical and Microbial Experiments
2.4. Data Analysis and Statistical Analysis
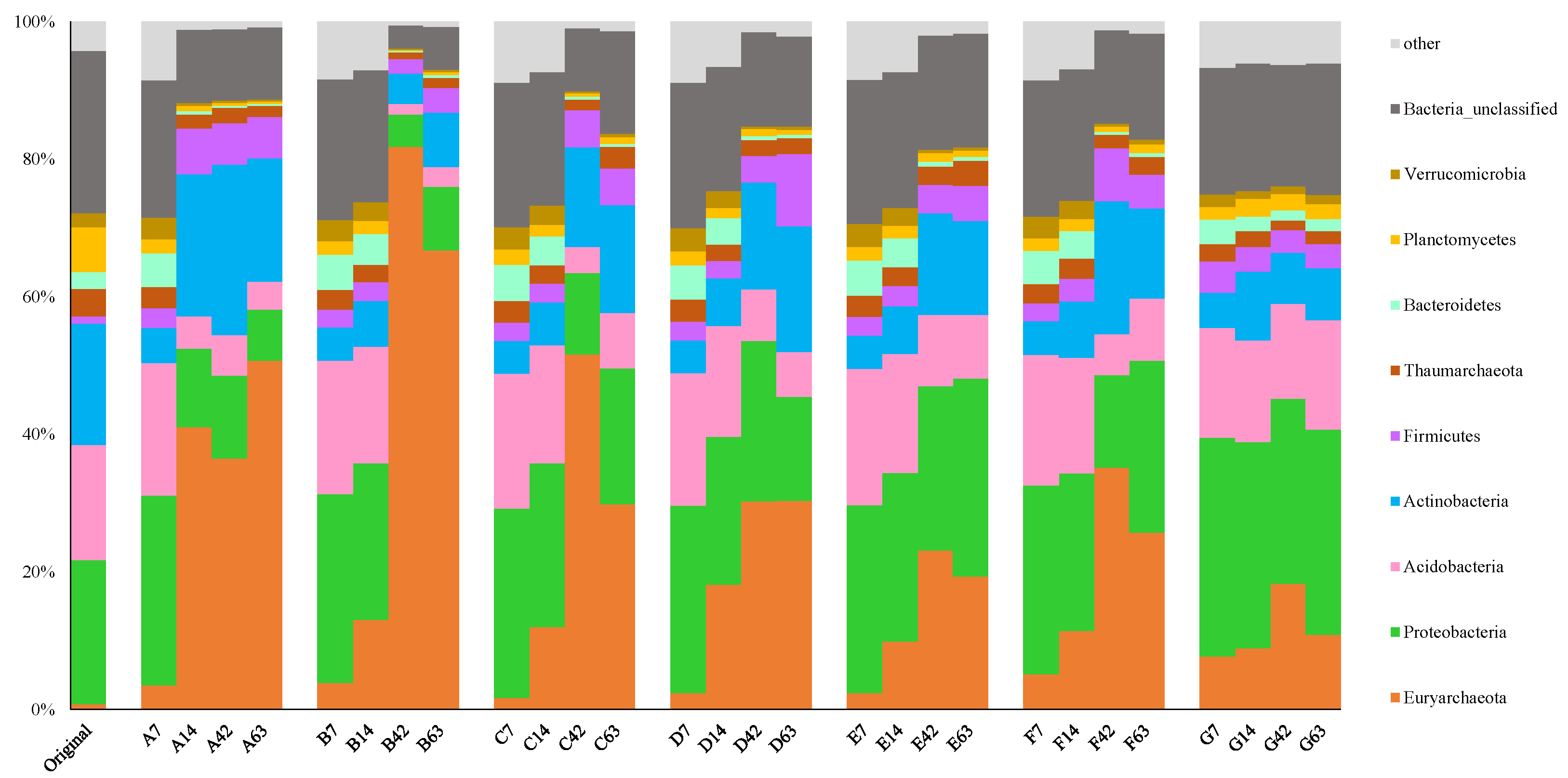
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,2-DCB | 1,2-dichlorobenzene |
| 1,4-DCB | 1,4-dichlorobenzene |
| γ-HCH | gamma-hexachlorocyclohexane |
| DNAPL | dense non-aqueous phase liquids |
| NGS | next-generation sequencing |
| CO2 | carbon dioxide |
| CH4 | methane |
| rRNA | ribosomal ribonucleic acid |
| PCR | polymerase chain reaction |
| OTU | operational taxonomic units |
| bphA | biphenyl dioxygenase subunit alpha |
| RDA | redundant analysis |
| DDT | dichlorodiphenyltrichloroethane |
References
- Lin, H.F.; Ravikrishna, R.; Valsaraj, K.T. Reusable adsorbents for dilute solution separation. 6. Batch and continuous reactors for the adsorption and degradation of 1,2-dichlorobenzene from dilute wastewater streams using titania as a photocatalyst. Sep. Purif. Technol. 2002, 28, 87–102. [Google Scholar] [CrossRef]
- Robinson, M.; Bercz, J.P.; Ringhand, H.P.; Condie, L.W.; Parnell, M.J. Ten- and Ninety-Day Toxicity Studies of 1,2–Dichlorobenzene Administered by Oral Gavage to Sprague-Dawley Rats. Drug Chem. Toxicol. 1991, 14, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Akram, R.; Turan, V.; Hammad, H.M.; Ahmad, S.; Hussain, S.; Hasnain, A.; Maqbool, M.M.; Rehmani, M.I.A.; Rasool, A.; Masood, N. Fate of organic and inorganic pollutants in paddy soils. In Environmental Pollution of Paddy Soils; Springer: Cham, Switzerland, 2018; pp. 197–214. [Google Scholar]
- Valentin, L.; Nousiainen, A.; Mikkonen, A. Introduction to organic contaminants in soil: Concepts and risks. Emerg. Org. Contam. Sludges 2013, 24, 1–29. [Google Scholar]
- Kumar, G.; Lal, S.; Soni, S.K.; Maurya, S.K.; Shukla, P.K.; Chaudhary, P.; Bhattacherjee, A.K.; Garg, N. Mechanism and kinetics of chlorpyrifos co-metabolism by using environment restoring microbes isolated from rhizosphere of horticultural crops under subtropics. Front. Microbiol. 2022, 13, 891870. [Google Scholar]
- Huang, Y.; Zhang, W.; Pang, S.; Chen, J.; Bhatt, P.; Mishra, S.; Chen, S. Insights into the microbial degradation and catalytic mechanisms of chlorpyrifos. Environ. Res. 2021, 194, 110660. [Google Scholar] [CrossRef]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Kumar, G.; Lal, S.; Maurya, S.K.; Bhattacherjee, A.K.; Chaudhary, P.; Gangola, S.; Rajan, S. Exploration of Klebsiella pneumoniae M6 for paclobutrazol degradation, plant growth attributes, and biocontrol action under subtropical ecosystem. PLoS ONE 2021, 16, e0261338. [Google Scholar] [CrossRef]
- Rein, W.D.; Krishna, G. Centrolobular hepatic necrosis related to covalent binding of metabolites of halogenated aromatic hydrocarbons. Exp. Mol. Pathol. 1973, 18, 80–99. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; MosbÆK, H.; Siegrist, R.L.; Bjerg, P.L. In Situ Chemical Oxidation of Contaminated Soil and Groundwater Using Persulfate: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Qu, J.; Tian, X.; Zhang, X.; Yao, J.; Xue, J.; Li, K.; Zhang, B.; Wang, L.; Zhang, Y. Free radicals-triggered reductive and oxidative degradation of highly chlorinated compounds via regulation of heat-activated persulfate by low-molecular-weight organic acids. Appl. Catal. B Environ. 2022, 310, 121359. [Google Scholar] [CrossRef]
- Qu, J.; Xu, Y.; Zhang, X.; Sun, M.; Tao, Y.; Zhang, X.; Zhang, G.; Ge, C.; Zhang, Y. Ball milling-assisted preparation of N-doped biochar loaded with ferrous sulfide as persulfate activator for phenol degradation: Multiple active sites-triggered radical/non-radical mechanism. Appl. Catal. B Environ. 2022, 316, 121639. [Google Scholar] [CrossRef]
- Sun, M.; Qu, J.; Han, T.; Xue, J.; Li, K.; Jiang, Z.; Zhang, G.; Yu, H.; Zhang, Y. Resource utilization of bovine bone to prepare biochar as persulfate activator for phenol degradation. J. Clean. Prod. 2023, 383, 135415. [Google Scholar] [CrossRef]
- Hamdi, H.; Benzarti, S.; Manusadžianas, L.; Aoyama, I.; Jedidi, N. Bioaugmentation and biostimulation effects on PAH dissipation and soil ecotoxicity under controlled conditions. Soil Biol. Biochem. 2007, 39, 1926–1935. [Google Scholar] [CrossRef]
- Goswami, M.; Chakraborty, P.; Mukherjee, K.; Dey, S.; Mitra, G.; Tribedi, P. Bioaugmentation and biostimulation: A potential strategy for environmental remediation. J. Microbiol. Exp. 2018, 6, 223–231. [Google Scholar] [CrossRef]
- Odokuma, L.; Dickson, A.A. Bioremediation of a crude oil polluted tropical rain forest soil. Glob. J. Environ. Sci. 2003, 2, 29–40. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Chaudhary, P.; Gangola, S. Chlorpyrifos degradation using binary fungal strains isolated from industrial waste soil. Biologia 2021, 76, 3071–3080. [Google Scholar] [CrossRef]
- Dutta, N.; Thomsen, K.; Ahring, B.K. Degrading chlorinated aliphatics by reductive dechlorination of groundwater samples from the Santa Susana Field Laboratory. Chemosphere 2022, 298, 134115. [Google Scholar] [CrossRef]
- Xu, Z.-J.; Spain Jim, C.; Zhou, N.-Y.; Li, T. Biodegradation of 3-Chloronitrobenzene and 3-Bromonitrobenzene by Diaphorobacter sp. Strain JS3051. Appl. Environ. Microbiol. 2022, 88, e02437-21. [Google Scholar] [CrossRef]
- Li, T.; Gao, Y.-Z.; Xu, J.; Zhang, S.-T.; Guo, Y.; Spain Jim, C.; Zhou, N.-Y. A Recently Assembled Degradation Pathway for 2,3-Dichloronitrobenzene in Diaphorobacter sp. Strain JS3051. mBio 2021, 12, e02231-21. [Google Scholar] [CrossRef]
- Zhao, X.; Bai, S.; Li, C.; Yang, J.; Ma, F. Bioaugmentation of atrazine removal in constructed wetland: Performance, microbial dynamics, and environmental impacts. Bioresour. Technol. 2019, 289, 121618. [Google Scholar] [CrossRef]
- Rhykerd, R.L.; Crews, B.; McInnes, K.J.; Weaver, R.W. Impact of bulking agents, forced aeration, and tillage on remediation of oil-contaminated soil. Bioresour. Technol. 1999, 67, 279–285. [Google Scholar] [CrossRef]
- Elektorowicz, M. Bioeemediation of petroleum-contaminated clayey soil with pretreatment. Environ. Technol. 1994, 15, 373–380. [Google Scholar] [CrossRef]
- Lorah, M.M.; Walker, C.W.; Baker, A.C.; Teunis, J.A.; Majcher, E.H.; Brayton, M.J.; Raffensperger, J.P.; Cozzarelli, I.M. Hydrogeologic Characterization and Assessment of Bioremediation of Chlorinated Benzenes and Benzene in Wetland Areas, Standard Chlorine of Delaware, Inc. Superfund Site, New Castle County, Delaware, 2009–2012; US Department of the Interior/US Geological Survey: Reston, VA, USA, 2014.
- Nestler, H.; Kiesel, B.; Kaschabek, S.R.; Mau, M.; Schlömann, M.; Balcke, G.U. Biodegradation of chlorobenzene under hypoxic and mixed hypoxic-denitrifying conditions. Biodegradation 2007, 18, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, Y.; Zhang, Q.; Xu, J.; Crowley, D. Coupling between pentachlorophenol dechlorination and soil redox as revealed by stable carbon isotope, microbial community structure, and biogeochemical data. Environ. Sci. Technol. 2015, 49, 5425–5433. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, S.; Cheng, J.; Guo, C.; Shen, C.; He, J.; Yang, Y.; Hu, P.; Xu, J.; He, Y. Potential role of methanogens in microbial reductive dechlorination of organic chlorinated pollutants in situ. Environ. Sci. Technol. 2021, 55, 5917–5928. [Google Scholar] [CrossRef]
- Haigler, B.E.; Nishino, S.F.; Spain, J. Degradation of 1, 2-dichlorobenzene by a Pseudomonas sp. Appl. Environ. Microbiol. 1988, 54, 294–301. [Google Scholar] [CrossRef]
- Ziagova, M.; Liakopoulou-Kyriakides, M. Comparison of cometabolic degradation of 1, 2-dichlorobenzene by Pseudomonas sp. and Staphylococcus xylosus. Enzym. Microb. Technol. 2007, 40, 1244–1250. [Google Scholar] [CrossRef]
- Liu, H.; Yang, C.; Ding, C.; Li, Z.; Liang, H. Isolation, Identification and Degradation Characterization of 1, 2-Dichlorobenzene Degrading Strain. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2020; pp. 1–5. [Google Scholar]
- Cui, G.; Chien, M.-F.; Suto, K.; Inoue, C. Analysis of sTable 1, 2-dichlorobenzene-degrading enrichments and two newly isolated degrading strains, Acidovorax sp. sk40 and Ralstonia sp. sk41. Appl. Microbiol. Biotechnol. 2017, 101, 6821–6828. [Google Scholar] [CrossRef]
- Liang, H.; Chen, A.; Li, Z.; Ashraf, M.A.; Ding, C. Influences of 1, 2-dichlorobenzene on bacterial community structure in wetland soil. Sains Malays. 2016, 45, 129–134. [Google Scholar]
- Fan, L.; Ni, J.; Wu, Y.; Zhang, Y. Treatment of bromoamine acid wastewater using combined process of micro-electrolysis and biological aerobic filter. J. Hazard. Mater. 2009, 162, 1204–1210. [Google Scholar] [CrossRef]
- Trueba-Santiso, A.; Palau, J.; Soder-Walz, J.M.; Vicent, T.; Marco-Urrea, E. Assessment of aerobic biodegradation of lower-chlorinated benzenes in contaminated groundwater using field-derived microcosms and compound-specific carbon isotope fractionation. J. Environ. Sci. 2022, 118, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Shokralla, S.; Spall, J.L.; Gibson, J.F.; Hajibabaei, M. Next-generation sequencing technologies for environmental DNA research. Mol. Ecol. 2012, 21, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Sogin, M.L.; Morrison, H.G.; Huber, J.A.; Welch, D.M.; Huse, S.M.; Neal, P.R.; Arrieta, J.M.; Herndl, G.J. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl. Acad. Sci. USA 2006, 103, 12115–12120. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonák, J.; Lind, K.; Sindelka, R.; Sjöback, R.; Sjögreen, B.; Strömbom, L.; et al. The real-time polymerase chain reaction. Mol. Asp. Med. 2006, 27, 95–125. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Zhang, M.; Zhang, N.; Guo, C.; Hao, C.; Zhang, S.; Shi, C.; Sheng, Y.; Chen, Z. Metagenomic characterization of a novel enrichment culture responsible for dehalogenation of 1,2,3-trichloropropane to allyl chloride. J. Environ. Chem. Eng. 2022, 10, 108907. [Google Scholar] [CrossRef]
- Classen, A.T.; Boyle, S.I.; Haskins, K.E.; Overby, S.T.; Hart, S.C. Community-level physiological profiles of bacteria and fungi: Plate type and incubation temperature influences on contrasting soils. FEMS Microbiol. Ecol. 2003, 44, 319–328. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, H.; Zhu, H.; Fu, S.; Yao, Q. Effects of longterm soil storage on the metabolic activity and functional groups of soil microbial community. Microbiol. China 2015, 42, 1017–1024. [Google Scholar]
- Shiau, Y.-J.; Chiu, C.-Y. Changes in soil biochemical properties in a cedar plantation invaded by moso bamboo. Forests 2017, 8, 222. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Wang, H.C.; Chen, T.H.; Jien, S.H.; Tian, G.L.; Chiu, C.Y. Improvement in the biochemical and chemical properties of badland soils by thorny bamboo. Sci. Rep. 2017, 7, 40561. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.; Westcott, S.; Baxter, N.; Highlander, S.; Schloss, P. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.-H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef]
- Díaz, S.; Purvis, A.; Cornelissen, J.H.; Mace, G.M.; Donoghue, M.J.; Ewers, R.M.; Jordano, P.; Pearse, W.D. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol. Evol. 2013, 3, 2958–2975. [Google Scholar] [CrossRef]
- Ho, A.; Kerckhof, F.-M.; Luke, C.; Reim, A.; Krause, S.; Boon, N.; Bodelier, P.L.E. Conceptualizing functional traits and ecological characteristics of methane-oxidizing bacteria as life strategies. Environ. Microbiol. Rep. 2013, 5, 335–345. [Google Scholar] [CrossRef]
- Kearney, M.R.; Jusup, M.; McGeoch, M.A.; Kooijman, S.A.L.M.; Chown, S.L. Where do functional traits come from? The role of theory and models. Funct. Ecol. 2021, 35, 1385–1396. [Google Scholar] [CrossRef]
- Liu, Y.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol. Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef]
- Zhu, M.; Feng, X.; Qiu, G.; Feng, J.; Zhang, L.; Brookes, P.C.; Xu, J.; He, Y. Synchronous response in methanogenesis and anaerobic degradation of pentachlorophenol in flooded soil. J. Hazard. Mater. 2019, 374, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Remenár, M.; Karelová, E.; Harichová, J.; Zámocký, M.; Krčová, K.; Ferianc, P. Actinobacteria occurrence and their metabolic characteristics in the nickel-contaminated soil sample. Biologia 2014, 69, 1453–1463. [Google Scholar] [CrossRef]
- Cruz-Morales, P.; Ramos-Aboites, H.E.; Licona-Cassani, C.; Selem-Mójica, N.; Mejía-Ponce, P.M.; Souza-Saldívar, V.; Barona-Gómez, F. Actinobacteria phylogenomics, selective isolation from an iron oligotrophic environment and siderophore functional characterization, unveil new desferrioxamine traits. FEMS Microbiol. Ecol. 2017, 93, fix086. [Google Scholar] [CrossRef] [PubMed]
- Amin, D.H.; Abdallah, N.A.; Abolmaaty, A.; Tolba, S.; Wellington, E.M. Microbiological and molecular insights on rare Actinobacteria harboring bioactive prospective. Bull. Natl. Res. Cent. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
- Pankratov, T.A.; Kirsanova, L.A.; Kaparullina, E.N.; Kevbrin, V.V.; Dedysh, S.N. Telmatobacter bradus gen. nov., sp. nov., a cellulolytic facultative anaerobe from subdivision 1 of the Acidobacteria, and emended description of Acidobacterium capsulatum Kishimoto et al. 1991. Int. J. Syst. Evol. Microbiol. 2012, 62, 430–437. [Google Scholar] [CrossRef]
- Andrews, J.H.; Harris, R.F. r- and K-Selection and Microbial Ecology. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Springer: Boston, MA, USA, 1986; pp. 99–147. [Google Scholar] [CrossRef]
- Ward, N.L.; Challacombe, J.F.; Janssen, P.H.; Henrissat, B.; Coutinho, P.M.; Wu, M.; Xie, G.; Haft, D.H.; Sait, M.; Badger, J.; et al. Three Genomes from the Phylum Acidobacteria Provide Insight into the Lifestyles of These Microorganisms in Soils. Appl. Environ. Microbiol. 2009, 75, 2046–2056. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- McGrath, J.E.; Harfoot, C.G. Reductive dehalogenation of halocarboxylic acids by the phototrophic genera Rhodospirillum and Rhodopseudomonas. Appl. Environ. Microbiol. 1997, 63, 3333–3335. [Google Scholar] [CrossRef]
- Peng, P.; Zheng, Y.; Koehorst Jasper, J.; Schaap Peter, J.; Stams Alfons, J.M.; Smidt, H.; Atashgahi, S. Concurrent Haloalkanoate Degradation and Chlorate Reduction by Pseudomonas chloritidismutans AW-1T. Appl. Environ. Microbiol. 2017, 83, e00325-17. [Google Scholar] [CrossRef]
- Atashgahi, S.; Hornung, B.; van der Waals, M.J.; da Rocha, U.N.; Hugenholtz, F.; Nijsse, B.; Molenaar, D.; van Spanning, R.; Stams, A.J.M.; Gerritse, J.; et al. A benzene-degrading nitrate-reducing microbial consortium displays aerobic and anaerobic benzene degradation pathways. Sci. Rep. 2018, 8, 4490. [Google Scholar] [CrossRef] [PubMed]
- Oosterkamp, M.J.; Veuskens, T.; Talarico Saia, F.; Weelink, S.A.B.; Goodwin, L.A.; Daligault, H.E.; Bruce, D.C.; Detter, J.C.; Tapia, R.; Han, C.S.; et al. Genome Analysis and Physiological Comparison of Alicycliphilus denitrificans Strains BC and K601T. PLoS ONE 2013, 8, e66971. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.M.; Chapelle, F.H. Role for Acetotrophic Methanogens in Methanogenic Biodegradation of Vinyl Chloride. Environ. Sci. Technol. 1999, 33, 3473–3476. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.; Zhao, S.; He, J. Microbial synergistic interactions for reductive dechlorination of polychlorinated biphenyls. Sci. Total Environ. 2019, 666, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, B.; Dai, X.; Li, S.; Lu, G.; Zhou, Y. Structure and function of the bacterial communities during rhizoremediation of hexachlorobenzene in constructed wetlands. Environ. Sci. Pollut. Res. 2017, 24, 11483–11492. [Google Scholar] [CrossRef]
- Ito, K.; Takagi, K.; Kataoka, R.; Kiyota, H. Biochemical characterization of NADH: FMN oxidoreductase HcbA3 from Nocardioides sp. PD653 in catalyzing aerobic HCB dechlorination. J. Pestic. Sci. 2020, 45, 125–131. [Google Scholar] [CrossRef]
- Sanford, R.A.; Cole, J.R.; Tiedje, J.M. Characterization and Description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an Aryl-Halorespiring Facultative Anaerobic Myxobacterium. Appl. Environ. Microbiol. 2002, 68, 893–900. [Google Scholar] [CrossRef]
- Cui, Y.; Woo, S.G.; Lee, J.; Sinha, S.; Kang, M.S.; Jin, L.; Kim, K.K.; Park, J.; Lee, M.; Lee, S.T. Nocardioides daeguensis sp. nov., a nitrate-reducing bacterium isolated from activated sludge of an industrial wastewater treatment plant. Int. J. Syst. Evol. Microbiol. 2013, 63, 3727–3732. [Google Scholar] [CrossRef]
- Zumft, W.G.; Kroneck, P.M.H. Respiratory Transformation of Nitrous Oxide (N2O) to Dinitrogen by Bacteria and Archaea. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Saddler, G.S.; Bradbury, J.F. Xanthomonadaceae fam. nov. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–3. [Google Scholar] [CrossRef]
- Pérez-Castaño, R.; Bastida-Martínez, E.; Fernández-Zapata, J.; Polanco, M.d.C.; Galbis-Martínez, M.L.; Iniesta, A.A.; Fontes, M.; Padmanabhan, S.; Elías-Arnanz, M. Coenzyme B12-dependent and independent photoregulation of carotenogenesis across Myxococcales. Environ. Microbiol. 2022, 24, 1865–1886. [Google Scholar] [CrossRef]
- Marshall, T.; Pensini, E. Vitamin B12 and Magnesium: A Healthy Combo for the Degradation of Trichloroethylene. Water Air Soil Pollut. 2021, 232, 1–10. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-T.; Shiau, Y.-J. Potential Changes in Soil Microbial Composition under 1,2-Dichlorobenzene Contamination. Sustainability 2023, 15, 1432. https://doi.org/10.3390/su15021432
Huang W-T, Shiau Y-J. Potential Changes in Soil Microbial Composition under 1,2-Dichlorobenzene Contamination. Sustainability. 2023; 15(2):1432. https://doi.org/10.3390/su15021432
Chicago/Turabian StyleHuang, Wen-Ting, and Yo-Jin Shiau. 2023. "Potential Changes in Soil Microbial Composition under 1,2-Dichlorobenzene Contamination" Sustainability 15, no. 2: 1432. https://doi.org/10.3390/su15021432
APA StyleHuang, W.-T., & Shiau, Y.-J. (2023). Potential Changes in Soil Microbial Composition under 1,2-Dichlorobenzene Contamination. Sustainability, 15(2), 1432. https://doi.org/10.3390/su15021432







