Enhancing Sugarcane Growth and Improving Soil Quality by Using a Network-Structured Fertilizer Synergist
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of FS
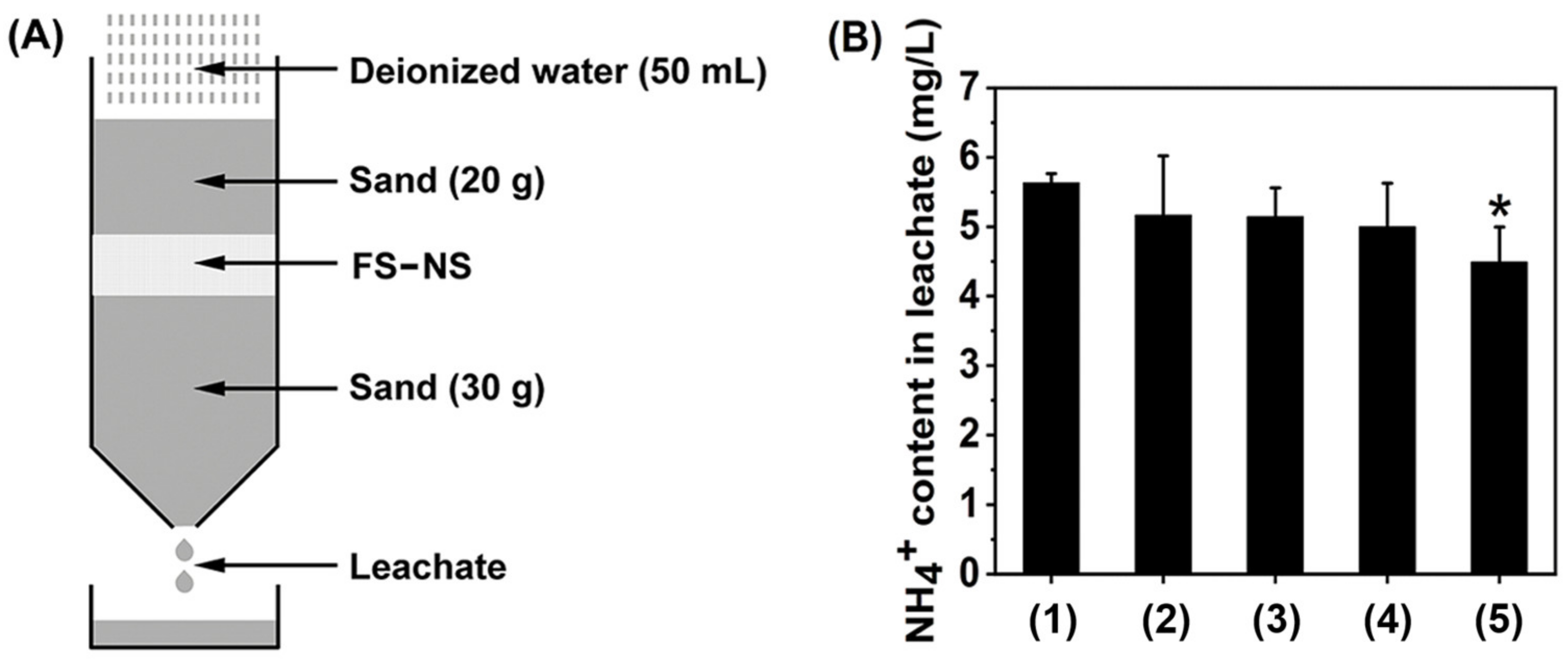
2.2. Leaching Test of FS
2.3. Characterization of FS and FS-NS
2.4. Field Tests
2.5. Data Statistical Analysis
3. Results
3.1. FS Ability to Control Leaching Loss
3.2. Network-Structured of FS and Interaction in the FS-NS System
3.3. Effects of FS on Agronomic of Sugarcane in Field Test
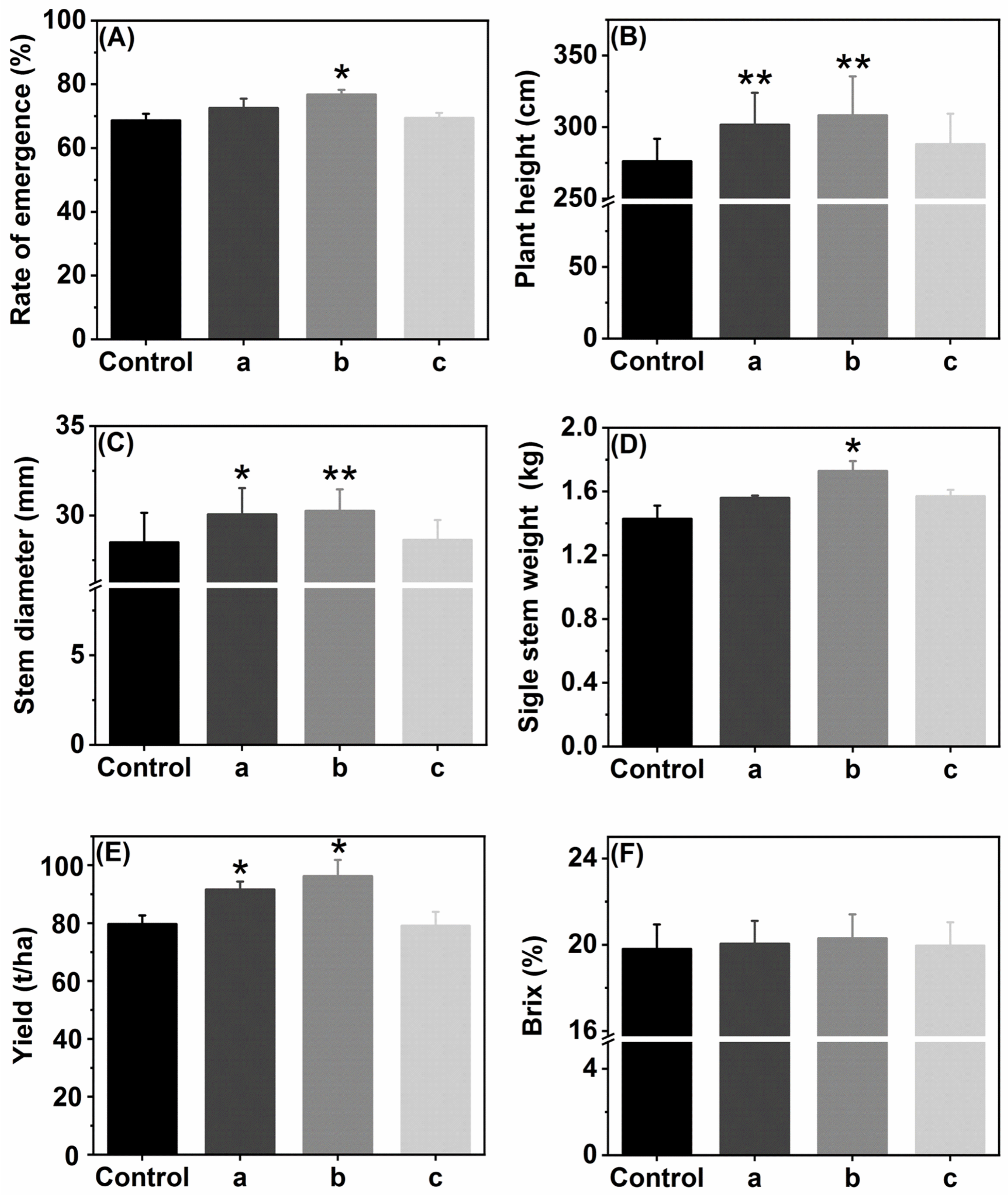
3.3.1. Effects of FS in Different Regions in Field
3.3.2. Agronomic Traits in Plot Test
3.3.3. Effects of FS on Physicochemical Properties of Sugarcane
3.3.4. FS Affects the Physicochemical Properties of Soil
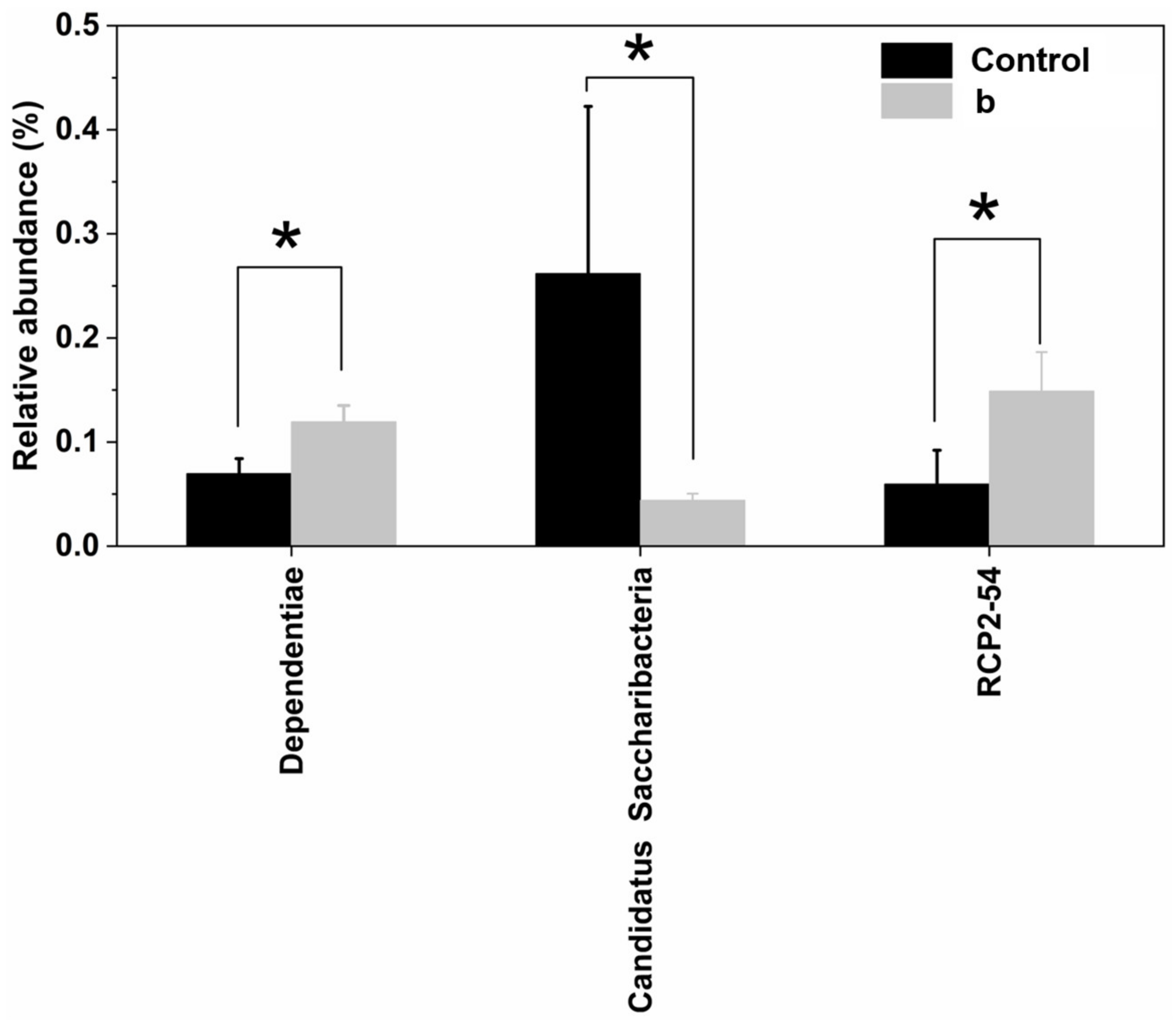
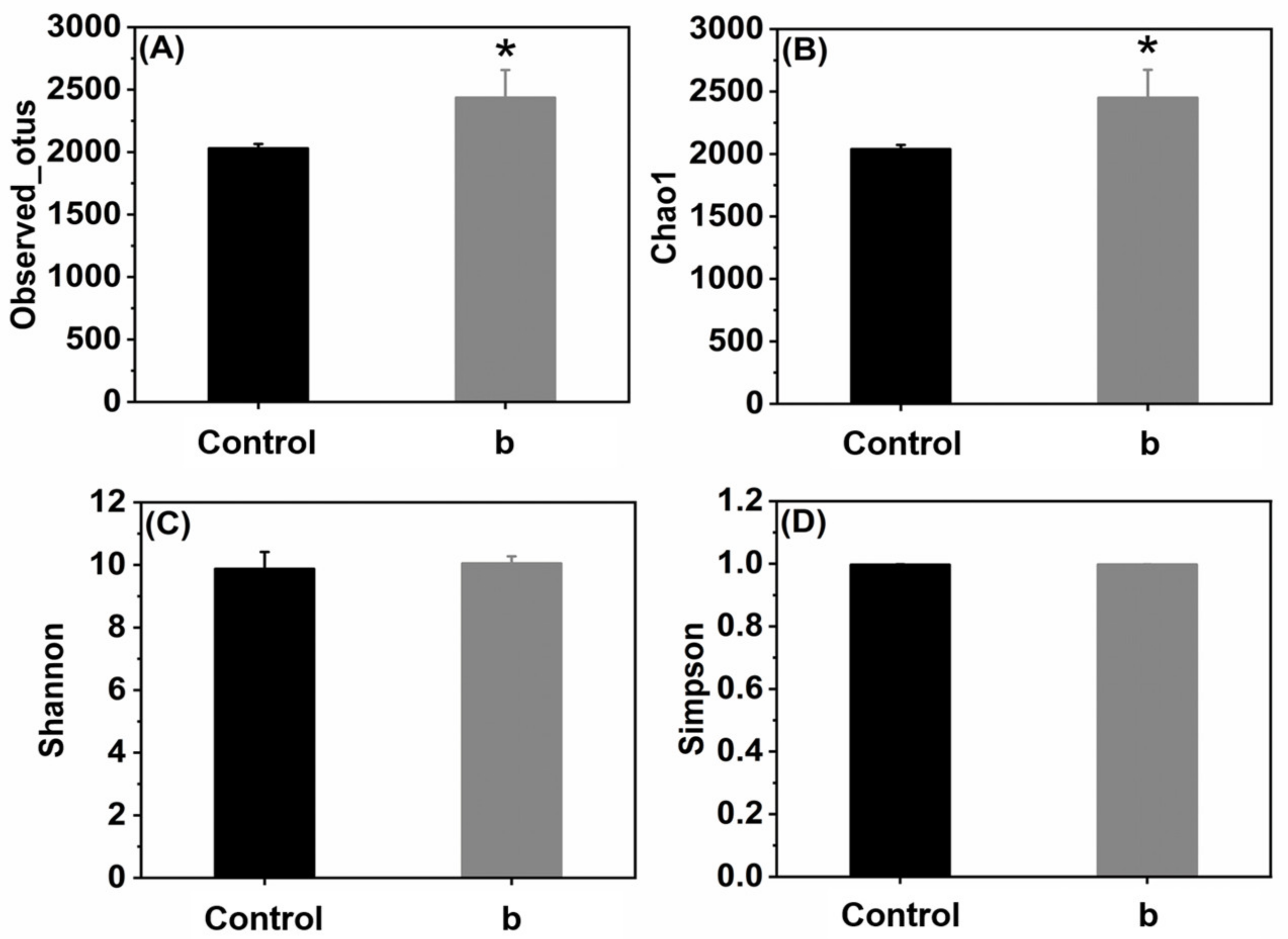
3.3.5. The Effect of FS on Bacterial Diversity in Rhizosphere Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced efficiency fertilisers: A review of formulation and nutrient release patterns. J. Sci. Food Agric. 2015, 95, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of fertilizers for enhanced nitrogen use efficiency—Trends and perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, H.; Ocheuze Trivelin, P.C.; Michelucci Contin, T.L.; Ferreira Dias, F.L.; Rossetto, R.; Marcelino, R.; Coimbra, R.B.; Quaggio, J.A. Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci. Agric. 2008, 65, 397–401. [Google Scholar] [CrossRef]
- Scivittaro, W.B.; Nunes Goncalves, D.R.; Campos do Vale, M.L.; Ricordi, V.G. Nitrogen losses by ammonia volatilization and lowland rice response to NBPT urease inhibitor-treated urea. Cienc. Rural. 2010, 40, 1283–1289. [Google Scholar] [CrossRef]
- Koelln, O.T.; Junqueira Franco, H.C.; Ferreira, D.A.; Vargas, V.P.; de Quassi Castro, S.A.; Cantarella, H.; Caldana, C.; Ocheuze Trivelin, P.C. Root extracts of Bracchiaria humidicola and Saccharum spontaneum to increase N use by sugarcane. Sci. Agric. 2016, 73, 34–42. [Google Scholar] [CrossRef]
- Yan, X.; Jin, J.-Y.; Ping, H.E.; Liang, M.-Z. Recent Advances on the Technologies to Increase Fertilizer Use Efficiency. Agric. Sci. China 2008, 7, 469–479. [Google Scholar] [CrossRef]
- Yang, X.; Geng, J.; Li, C.; Zhang, M.; Tian, X. Cumulative release characteristics of controlled-release nitrogen and potassium fertilizers and their effects on soil fertility, and cotton growth. Sci. Rep. 2016, 6, 39030. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Yin, J.; Chadwick, D.; Norse, D.; Lu, Y.; Liu, X.; Chen, X.; Zhang, F.; Powlson, D.; et al. Enhanced-efficiency fertilizers are not a panacea for resolving the nitrogen problem. Glob. Chang. Biol. 2018, 24, e511–e521. [Google Scholar] [CrossRef]
- Di Bella, L.P.; Stacey, S.P.; Benson, A.; Royle, A.; Holzberger, G. An assessment of controlled release fertiliser in the Herbert cane-growing region [of Australia]. Int. Sugar J. 2013, 115, 868–872. [Google Scholar]
- Mustafa, A.; Athar, F.; Khan, I.; Chattha, M.U.; Nawaz, M.; Shah, A.N.; Mahmood, A.; Batool, M.; Aslam, M.T.; Jaremko, M.; et al. Improving crop productivity and nitrogen use efficiency using sulfur and zinc-coated urea: A review. Front. Plant Sci. 2022, 13, 942384. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.Y.; Sulaiman, S.A. Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Wegener, M.; Ou, Y.G.; Yang, D.T.; Liu, Q.T.; Zheng, D.K. Mechanising sugarcane harvesting in China: A review. Int. Sugar J. 2014, 116, 272–277. [Google Scholar]
- Luo, J.; Pan, Y.B.; Xu, L.; Grisham, M.P.; Zhang, H.; Que, Y. Rational regional distribution of sugarcane cultivars in China. Sci. Rep. 2015, 5, 15721. [Google Scholar] [CrossRef]
- Xie, J.; Yang, J.; Luo, Q.; Chunqiang, Y.; Pan, Q. Survey of sugarcane pests in Xianggui sugarcane area and countermeasures of pest control. J. Plant Dis. Pests 2020, 11, 13–16, 21. [Google Scholar]
- Jin, J. Changes in the efficiency of fertiliser use in China. J. Sci. Food Agric. 2012, 92, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, J. Spatiotemporal Changes of Chemical Fertilizer Application and Its Environmental Risks in China from 2000 to 2019. Int. J. Environ. Res. Public Health 2021, 18, 11911. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.; Yang, L.-T. Sugarcane Agriculture and Sugar Industry in China. Sugar Tech. 2014, 17, 1–8. [Google Scholar] [CrossRef]
- Xu, L.F.; Wang, K.L.; Zhu, H.H.; Hou, Y.; Zhang, W. Effects of different land use types on soil nutrients in karst region of Northwest Guangxi. Ying Yong Sheng Tai Xue Bao 2008, 19, 1013–1018. [Google Scholar] [PubMed]
- Zhang, L.; Yang, X.; Gao, D.; Wang, L.; Li, J.; Wei, Z.; Shi, Y. Effects of poly-gamma-glutamic acid (gamma-PGA) on plant growth and its distribution in a controlled plant-soil system. Sci. Rep. 2017, 7, 1–3. [Google Scholar]
- Yao, Y.; Dai, Q.; Gao, R.; Gan, Y.; Yi, X. Effects of rainfall intensity on runoff and nutrient loss of gently sloping farmland in a karst area of SW China. PLoS ONE 2021, 16, e0246505. [Google Scholar] [CrossRef]
- Song, X.; Gao, Y.; Green, S.M.; Dungait, J.A.J.; Peng, T.; Quine, T.A.; Xiong, B.; Wen, X.; He, N. Nitrogen loss from karst area in China in recent 50 years: An in-situ simulated rainfall experiment’s assessment. Ecol. Evol. 2017, 7, 10131–10142. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Wu, Z.; Jiang, J.; Wu, Y.; Feng, H.; Brown, I.G.; Chu, P.K.; Yu, Z. Controlling nitrogen migration through micro-nano networks. Sci. Rep. 2014, 4, 3665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, P.; Chi, Y.; Wang, D.; Wang, P.; Liu, N.; Cai, D.; Wu, Z.; Zhong, N. Controlling the Hydrolysis and Loss of Nitrogen Fertilizer (Urea) by using a Nanocomposite Favors Plant Growth. ChemSusChem 2017, 10, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Bauw, D.H.; Wilde, P.; Rood, G.A.; Aalbers, T.G.J.C. A standard leaching test, including solid phase extraction, for the determination of PAH leachability from waste materials. Chemosphere 1991, 22, 713–722. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, L.; Wang, D.; Bi, Z.; Fu, Z. Sampling and analysis of airborne ammonia in workplaces of China. J. Occup. Health 2020, 62, e12100. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.-Q.; Guo, L.-P.; Wang, Z.-H.; Li, S.-N.; Peng, T.; Li, Q.; Yao, Y.-Y.; Qi, Z.-M.; Nie, J.-T. Soil ecological effect of attapulgite and its application prospect in ecological planting of Chinese materia medica. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2020, 45, 2031–2035. [Google Scholar]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-gamma-glutamic acid: Production, properties and applications. Microbiol.-Sgm. 2015, 161, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gui, W.; Wei, J.; Cui, Y.; Li, P.; Jia, Z.; Kong, P. Functionalized attapulgite for the adsorption of methylene blue: Synthesis, characterization, and adsorption mechanism. ACS Omega 2021, 6, 19586–19595. [Google Scholar] [CrossRef]
- Guan, Y.; Song, C.; Gan, Y.; Li, F.-M. Increased maize yield using slow-release attapulgite-coated fertilizers. Agron. Sustain. Dev. 2014, 34, 657–665. [Google Scholar] [CrossRef]
- Shih, I.L.; Van, Y.T. The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 2001, 79, 207–225. [Google Scholar] [CrossRef]
- Guo, J.; Shi, W.; Li, J.; Zhai, Z. Effects of poly-gamma-glutamic acid and poly-gamma-glutamic acid super absorbent polymer on the sandy loam soil hydro-physical properties. PLoS ONE 2021, 16, e0245365. [Google Scholar]
- Cheng, W.D.; Wang, Y.Y.; Lin, H.M.; Liu, X.Z.; Yang, J.J. Response of applying palygorskite and fertilization on growth rules of Radix Hedysari. Zhong Yao Cai 2010, 33, 657–661. [Google Scholar]
- Ji, G.; Xu, L.; Lyu, Q.; Liu, Y.; Gong, X.; Li, X.; Yan, Z. Poly-gamma-glutamic acid production by simultaneous saccharification and fermentation using corn straw and its fertilizer synergistic effect evaluation. Bioprocess Biosyst. Eng. 2021, 44, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, K.L.; Fu, Z.Y.; Chen, H.S.; Zhang, W.; Shi, Z.H. Hydrological characteristics of calcareous soil with contrasting architecture on dolomite slope of Northwest Guangxi. Ying Yong Sheng Tai Xue Bao 2017, 28, 2186–2196. [Google Scholar]
- Bajaj, I.; Singhal, R. Poly (glutamic acid)-An emerging biopolymer of commercial interest. Bioresour. Technol. 2011, 102, 5551–5561. [Google Scholar] [CrossRef]
- Ma, H.; Li, P.; Liu, X.; Li, C.; Zhang, S.; Wang, X.; Tao, X. Poly-gamma-glutamic acid enhanced the drought resistance of maize by improving photosynthesis and affecting the rhizosphere microbial community. BMC Plant Biol. 2022, 22, 1–5. [Google Scholar]
- Qin, X.; Liu, Y.; Wang, L.; Li, B.; Wang, H.; Xu, Y. Remediation of heavy metal-polluted alkaline vegetable soil using mercapto-grafted palygorskite: Effects of field-scale application and soil environmental quality. Environ. Sci. Pollut. Res. 2021, 28, 60526–60536. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.K.; Joseph, S.D.; Li, F.; Bai, Y.; Shang, Z.; Rawal, A.; Hook, J.M.; Munroe, P.R.; Donne, S.; Taherymoosavi, S.; et al. Pyrolysis of attapulgite clay blended with yak dung enhances pasture growth and soil health: Characterization and initial field trials. Sci. Total Environ. 2017, 607, 184–194. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Abrantes, I.; Barra Caracciolo, A.; Bevivino, A.; Ciancio, A.; Grenni, P.; Hrynkiewicz, K.; Kredics, L.; Proença, D.N. Belowground microbiota and the health of tree crops. Front. Microbiol. 2018, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Hu, J.; Jousset, A.J.A.R.O.E. Evolution, Systematics More than the sum of its parts: Microbiome biodiversity as a driver of plant growth and soil health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Panahpour, E.; Naseri, A. Assessing the effects of urea and nano-nitrogen chelate fertilizers on sugarcane yield and dynamic of nitrate in soil. Soil Sci. Plant Nutr. 2020, 66, 352–359. [Google Scholar] [CrossRef]
- Moreira, L.A.; Otto, R.; Cantarella, H.; Junior, J.L.; Azevedo, R.A.; de Mira, A.B. Urea-Versus ammonium nitrate-based fertilizers for green sugarcane cultivation. J. Soil Sci. Plant Nutr. 2021, 21, 1329–1338. [Google Scholar] [CrossRef]
- De Mendonca, H.V.; Martins, C.E.; Duarte da Rocha, W.S.; Vieira Borges, C.A.; Henry Balbaud Ometto, J.; Otenio, M.H. Biofertilizer replace urea as a source of nitrogen for sugarcane production. Water Air Soil Pollut. 2018, 229, 216. [Google Scholar] [CrossRef]
- Tarmizi, W.; Utami, S.N.H.; Hanudin, E. Influences of urea and Za fertilizers to soil chemical properties, N uptake and sugarcane growth in Ultisols Seputih Mataram, Lampung. Ilmu Pertan. Agric. Sci. 2018, 3, 29–35. [Google Scholar] [CrossRef]





| Site | F-control | F-a | F-b | F-c | F-d | F-Fertilizer |
|---|---|---|---|---|---|---|
| Heng County (2020) | 100% fertilizer (3000 kg/ha CF + 375 kg/ha U) | 100% fertilizer + 150 kg/ha FS | 100% fertilizer + 300 kg/ha FS | 80% fertilizer + 150 kg/ha FS | 80% fertilizer + 300 kg/ha FS | N-P2O5-K2O: 16-7-11 |
| Heng County (2021) | 100% fertilizer (2625 kg/ha CF + 375 kg/ha U) | 100% fertilizer + 300 kg/ha FS | 80% fertilizer + 300 kg/ha FS | N-P2O5-K2O: 12-7-10 | ||
| Huanjiang Maonan Autonomous County (2020) | 100% fertilizer (3750 kg/ha CF) | 100% fertilizer + 150 kg/ha FS | 100% fertilizer + 300 kg/ha FS | 100% fertilizer + 600 kg/ha FS | N-P2O5-K2O: 12-8-8 | |
| Huanjiang Maonan Autonomous County (2021) | 100% fertilizer (3000 kg/ha OF) | 92% fertilizer + 150 kg/ha FS | 84% fertilizer + 300 kg/ha FS | 68% fertilizer + 600 kg/ha FS | content of organic matter ≥ 34% | |
| Dahua Yao Autonomous County (2021) | 100% fertilizer (1125 kg/ha MF + 1125 kg/ha OF) | 100% fertilizer + 450 kg/ha FS | effective nutrient content of MF ≥ 32%, content of organic matter ≥ 45% | |||
| Wuming district (2021) | 100% fertilizer (2400 kg/ha CF) | 100% fertilizer + 300 kg/ha FS | N-P2O5-K2O: 16-7-11 | |||
| Da’an town(2021) | 100% fertilizer (1500 kg/ha CF) | 100% fertilizer + 300 kg/ha FS | N-P2O5-K2O: 12-7-10 |
| Pore Diameter (nm) | Pore Volume (cm3/g) | Surface Area (m2/g) | |
|---|---|---|---|
| ATP | 3.819 | 0.292 | 73.80 |
| ATP-PGA | 3.823 | 0.303 | 74.62 |
| ATP-PGA-NS | 3.818 | 0.225 | 55.59 |
| Group | Scheme | Fertilizer Input | FS Input | Total Input | Total Output | Net Income |
|---|---|---|---|---|---|---|
| (RMB¥/ha) | (RMB¥/ha) | (RMB¥/ha) | (RMB¥/ha) | (RMB¥/ha) | ||
| Control | 100% CF | 12,600 | 0 | 12,600 | 35,844.29 | 23,244.29 |
| a | 100% CF + 10% FS | 12,600 | 900 | 13,500 | 41,229.56 | 27,729.56 |
| b | 100% CF + 20% FS | 12,600 | 1800 | 14,400 | 43,295.91 | 28,895.91 |
| c | 80% CF + 20% FS | 10,080 | 1440 | 11,520 | 35,619.28 | 24,099.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Cao, J.; Wang, Z.; Liu, L.; Yan, M.; Zhong, N.; Zhao, P. Enhancing Sugarcane Growth and Improving Soil Quality by Using a Network-Structured Fertilizer Synergist. Sustainability 2023, 15, 1428. https://doi.org/10.3390/su15021428
Zhao Y, Cao J, Wang Z, Liu L, Yan M, Zhong N, Zhao P. Enhancing Sugarcane Growth and Improving Soil Quality by Using a Network-Structured Fertilizer Synergist. Sustainability. 2023; 15(2):1428. https://doi.org/10.3390/su15021428
Chicago/Turabian StyleZhao, Yonglong, Jingjing Cao, Zhiqin Wang, Lu Liu, Meixin Yan, Naiqin Zhong, and Pan Zhao. 2023. "Enhancing Sugarcane Growth and Improving Soil Quality by Using a Network-Structured Fertilizer Synergist" Sustainability 15, no. 2: 1428. https://doi.org/10.3390/su15021428
APA StyleZhao, Y., Cao, J., Wang, Z., Liu, L., Yan, M., Zhong, N., & Zhao, P. (2023). Enhancing Sugarcane Growth and Improving Soil Quality by Using a Network-Structured Fertilizer Synergist. Sustainability, 15(2), 1428. https://doi.org/10.3390/su15021428






