Study on the MOF Frame Pt-TiO2 Hybrid Photocatalyst and Its Photocatalytic Performance
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Reagents
2.2. Experimental Instruments
2.3. Experimental Methods and Conditions
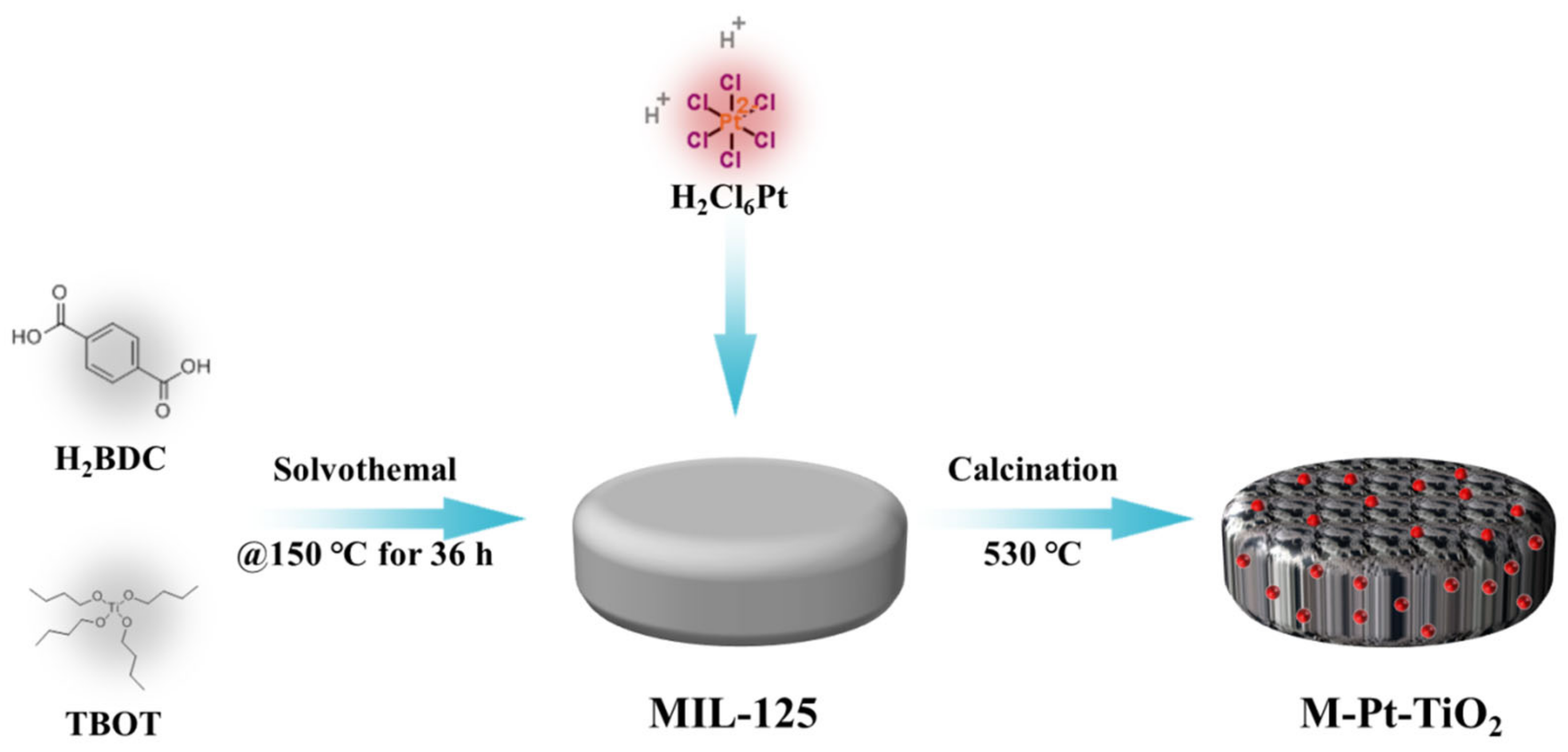
2.3.1. Preparation of Catalysts
2.3.2. Characterization of Catalysts
2.3.3. Photocatalyst Performance Testing
3. Results and Discussion
3.1. Characterisation of M-Pt-TiO2 Photocatalysts
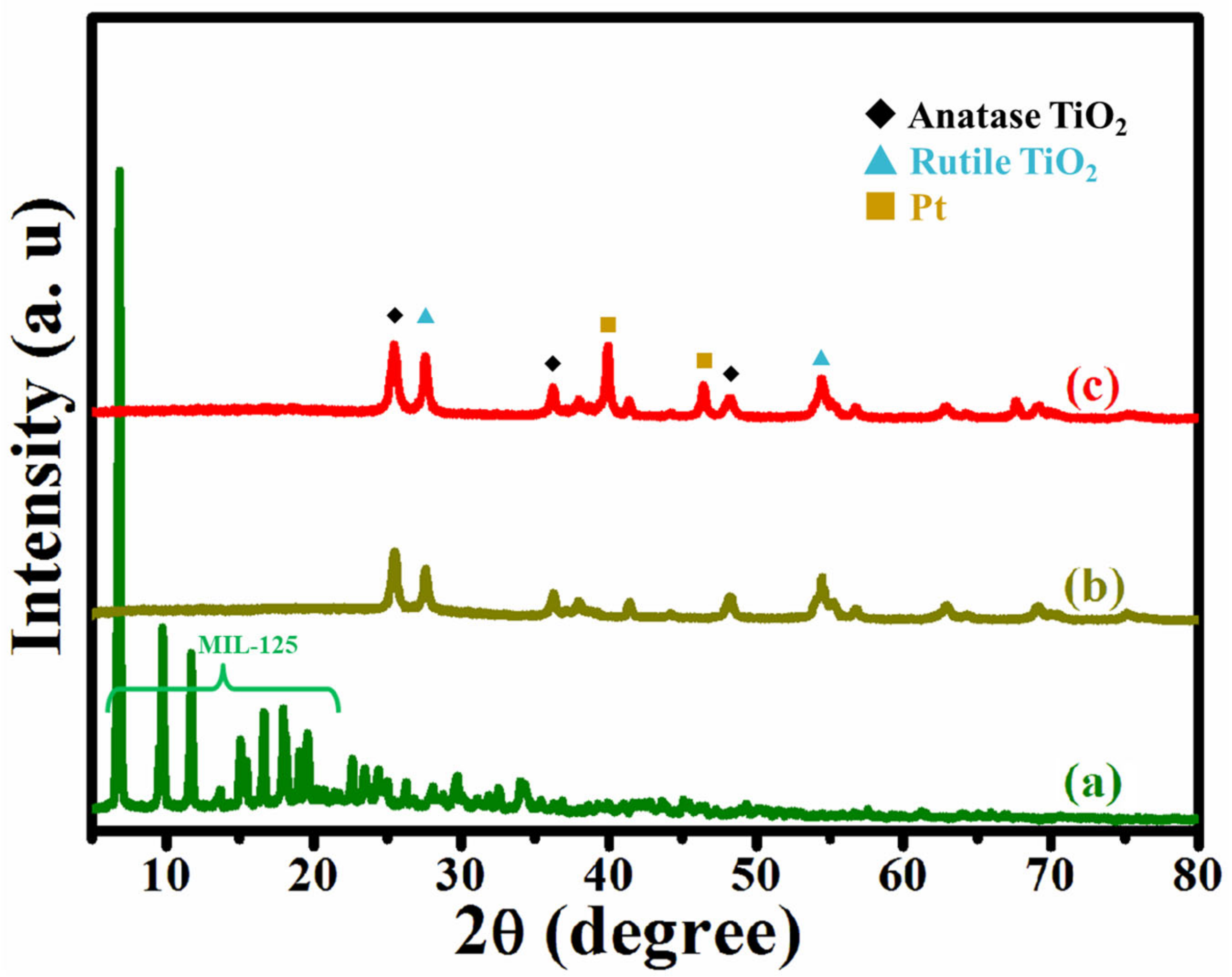
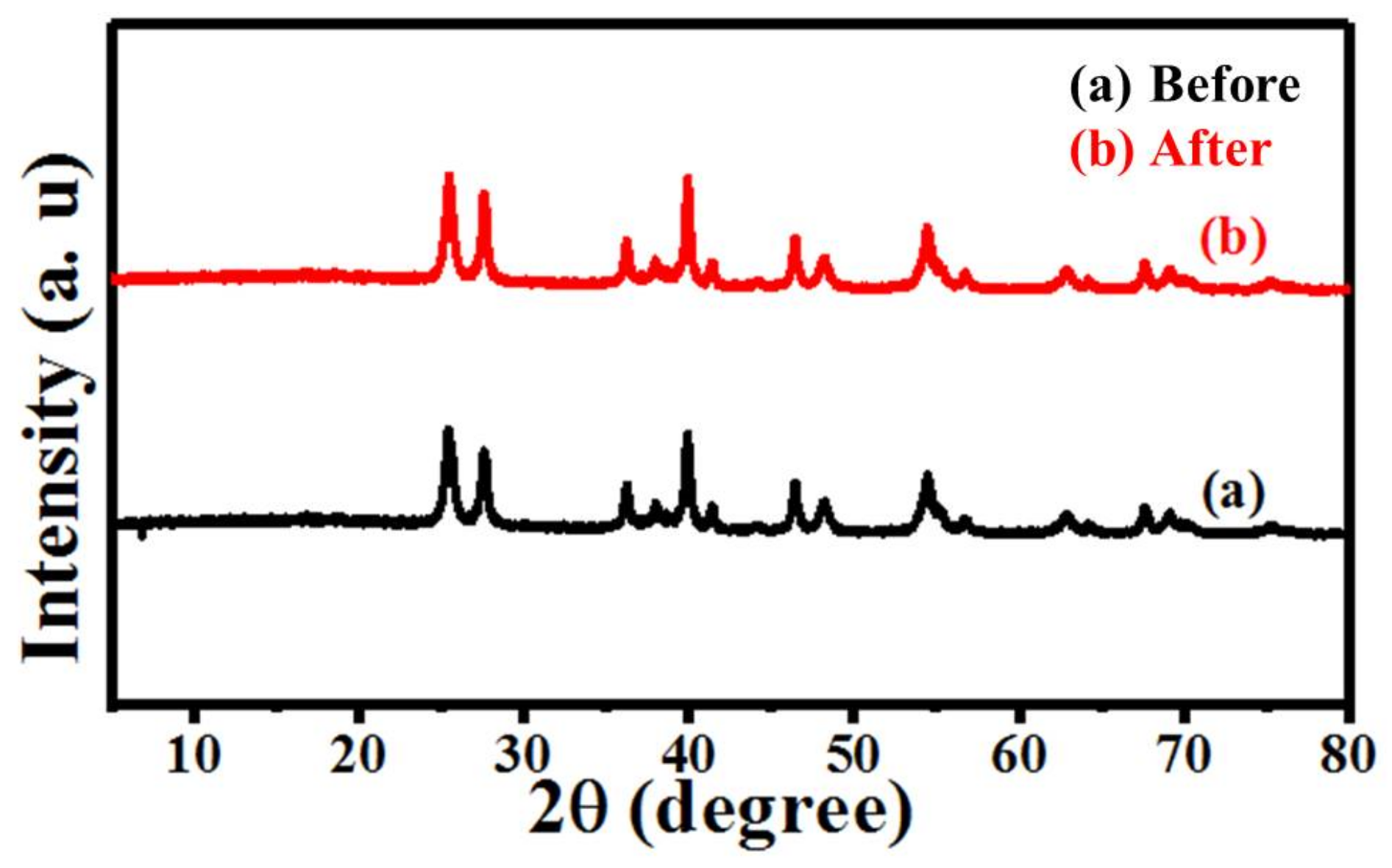
3.1.1. X-ray Diffraction Pattern
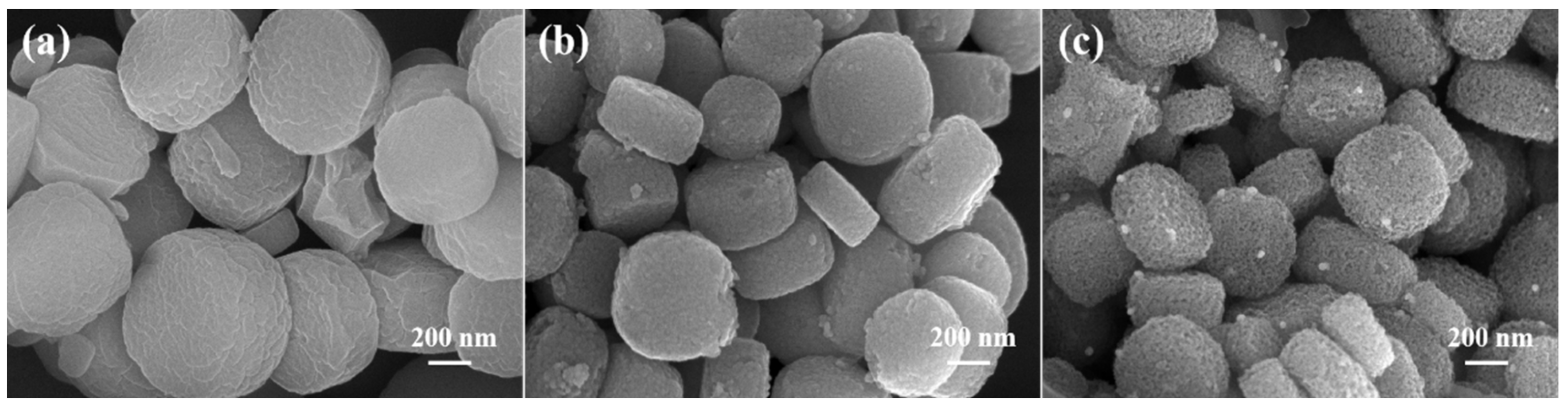
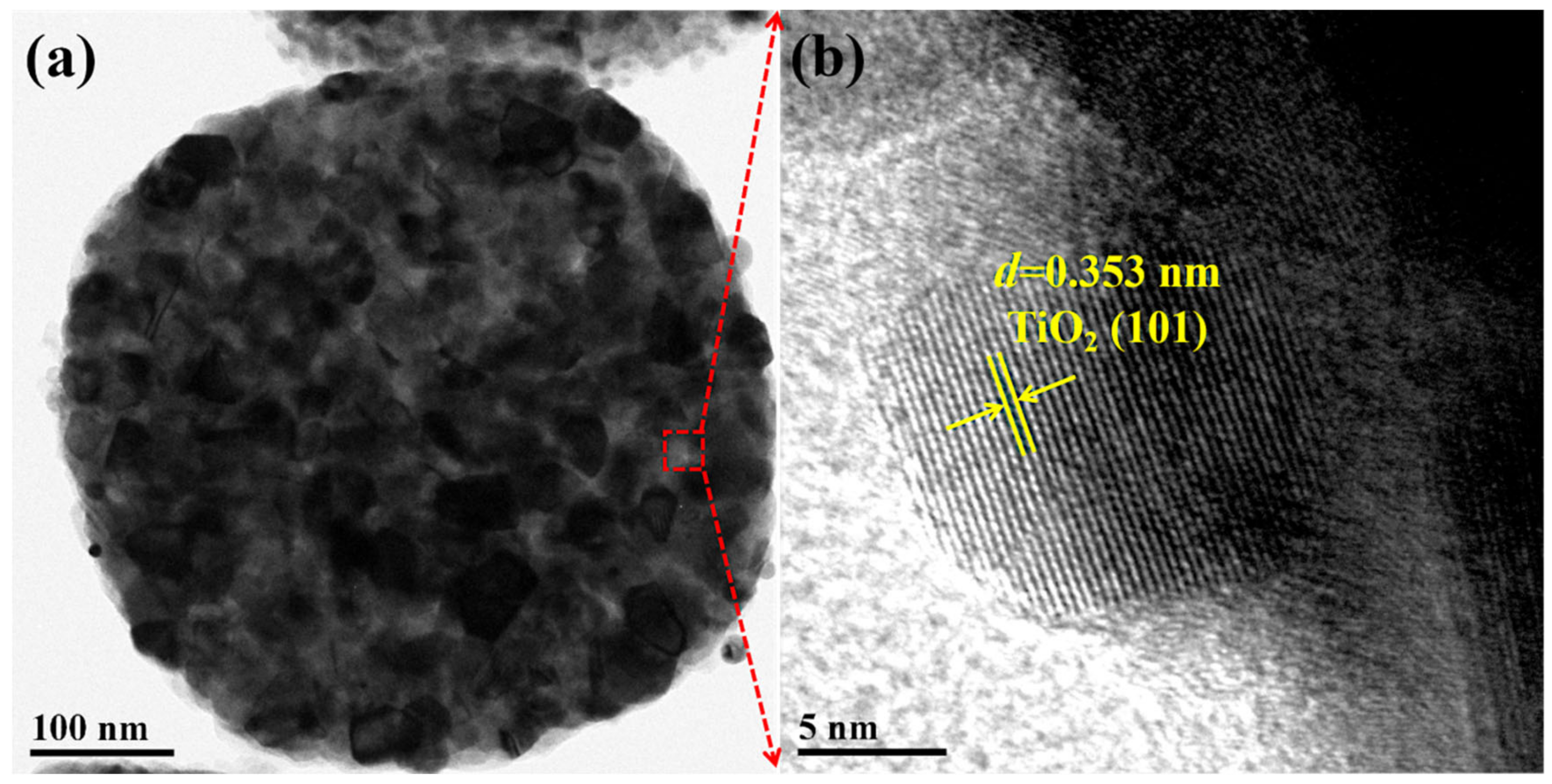
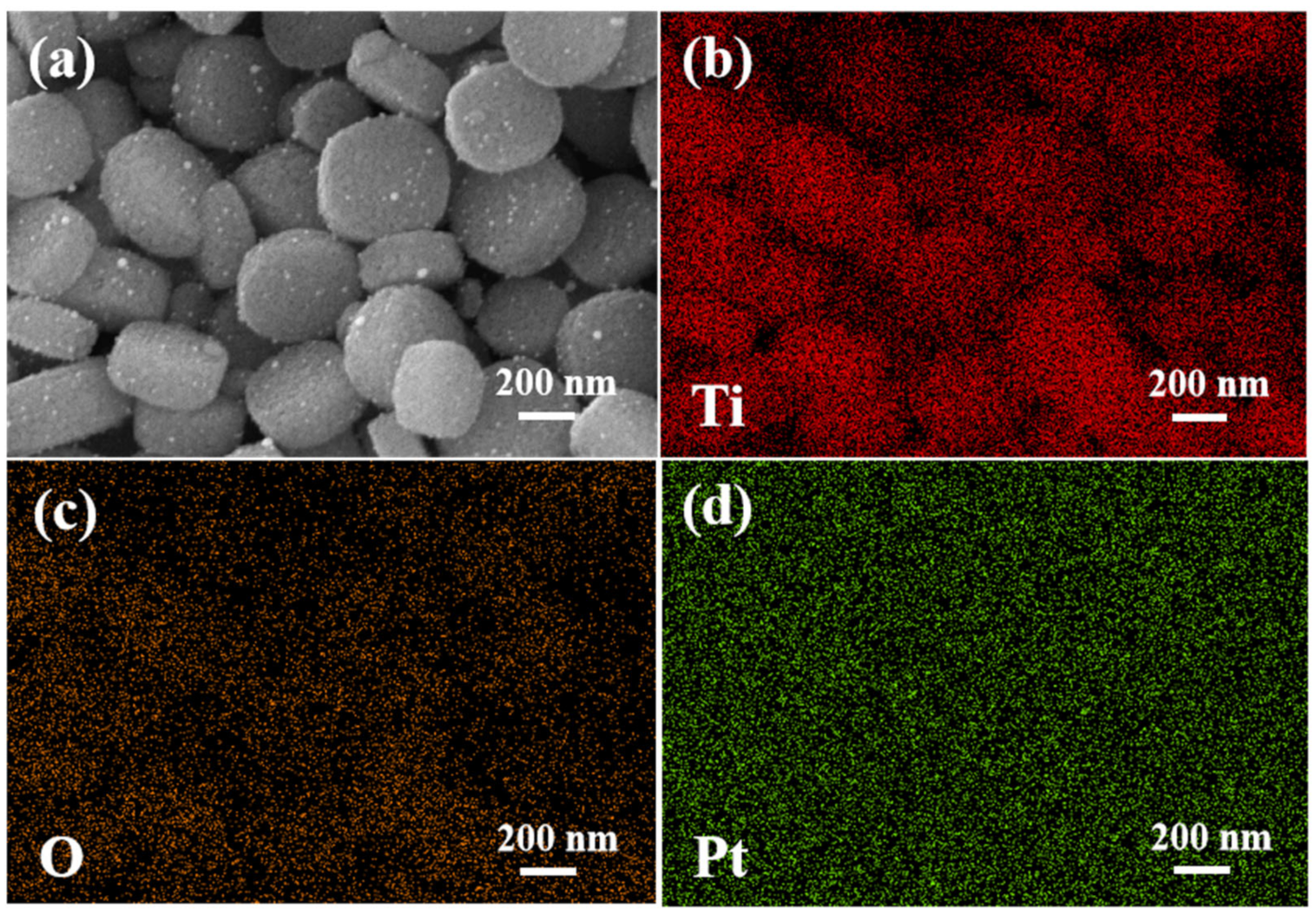
3.1.2. SEM and TEM Analysis
3.1.3. FTIR Infrared Spectroscopy
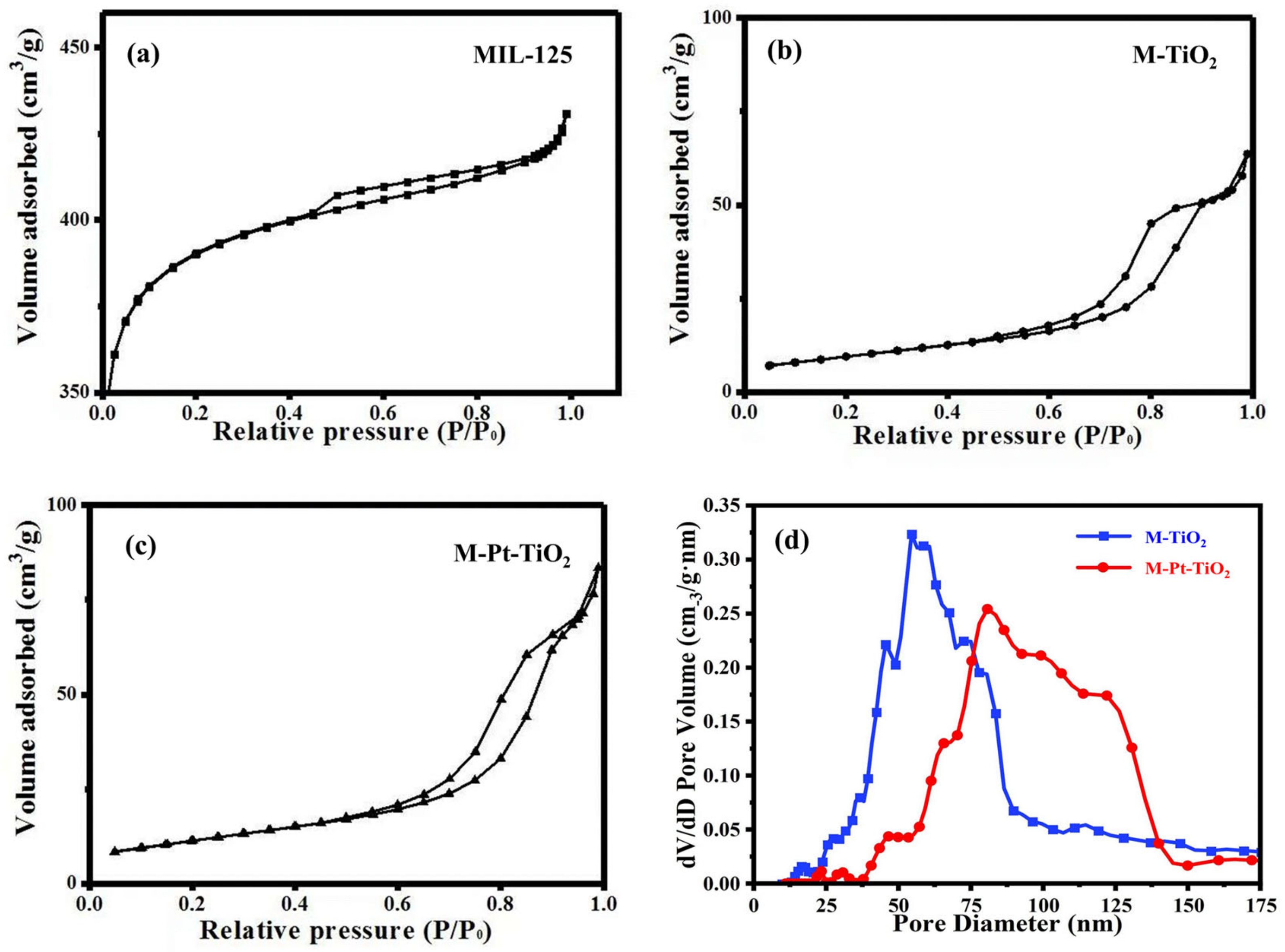
3.1.4. BET Analysis
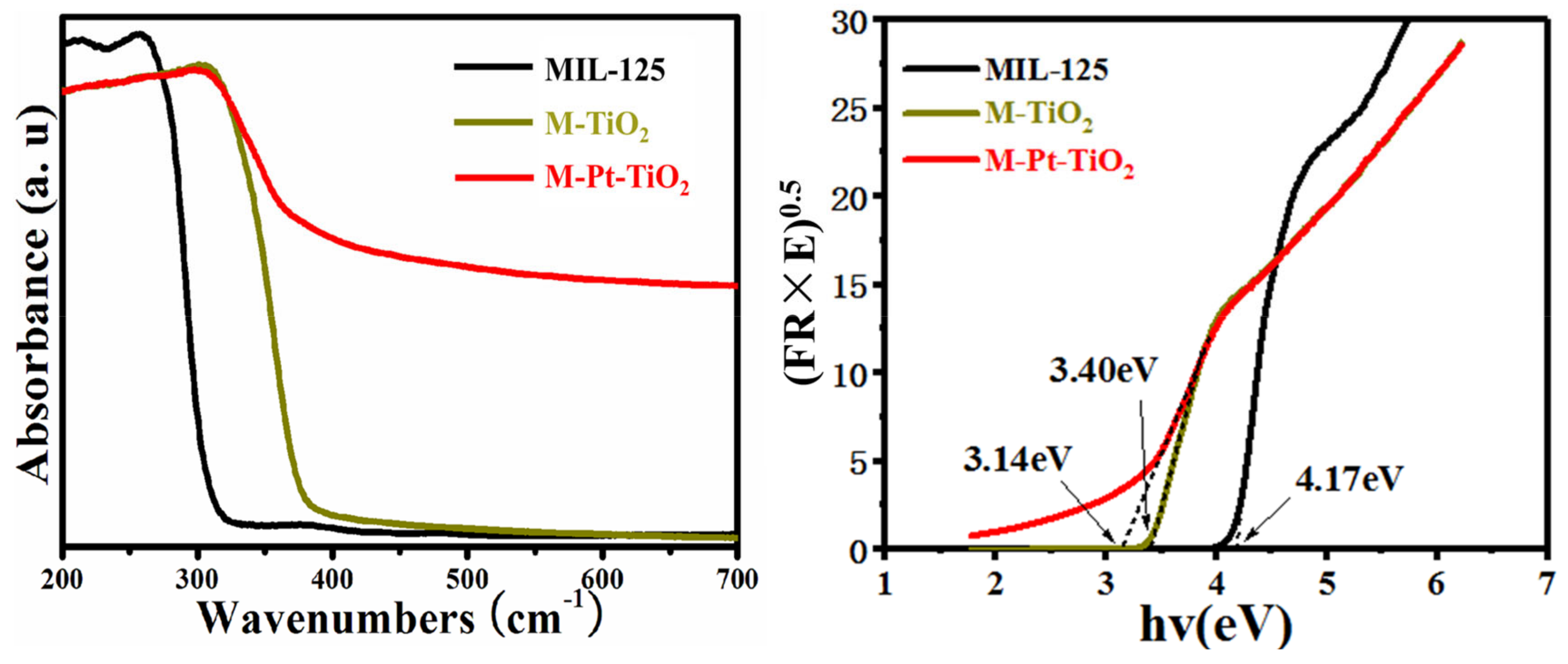
3.1.5. Solid UV–Vis
3.1.6. XPS Analysis
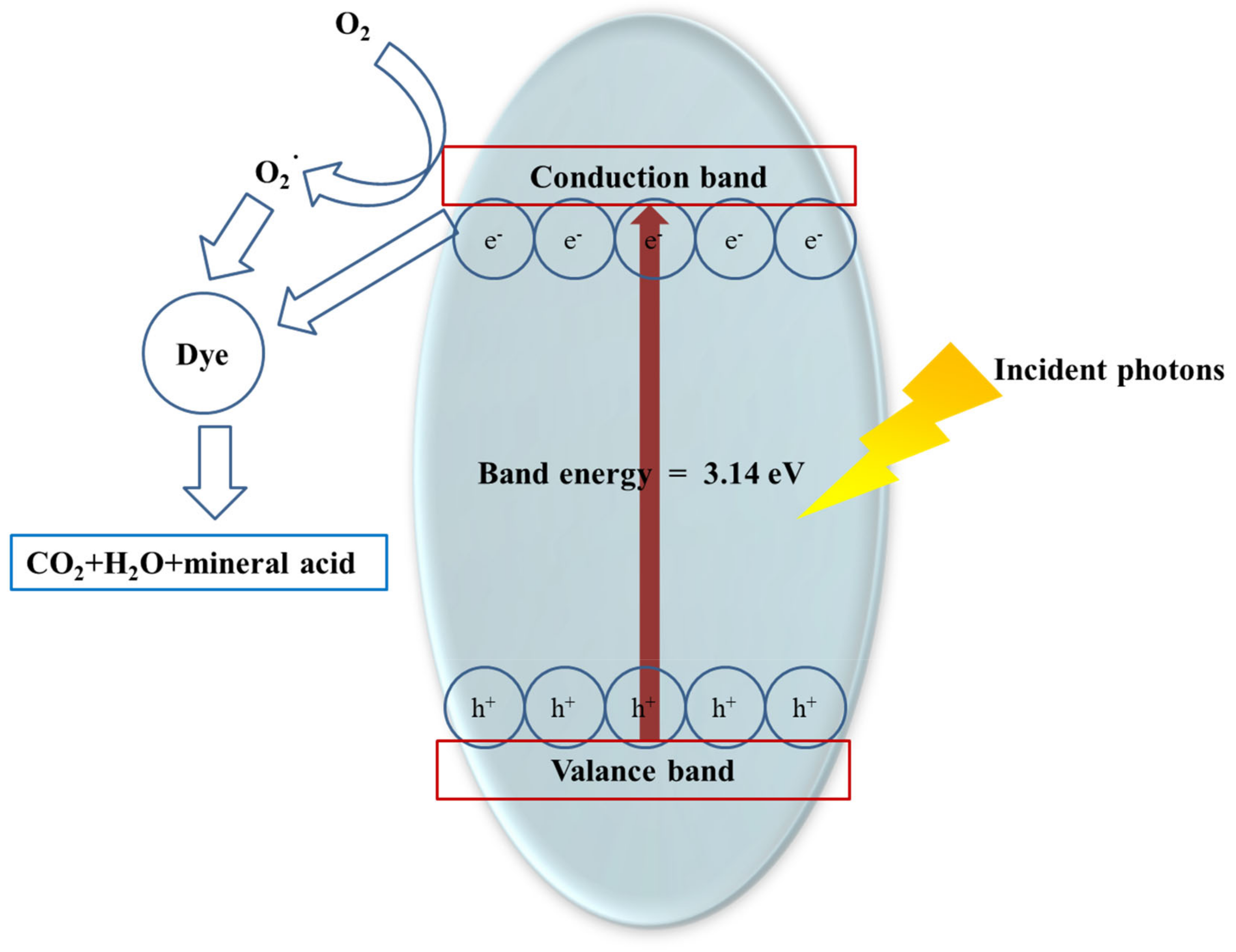
3.2. Analysis of the Photocatalytic Degradation Effect of M-Pt-TiO2
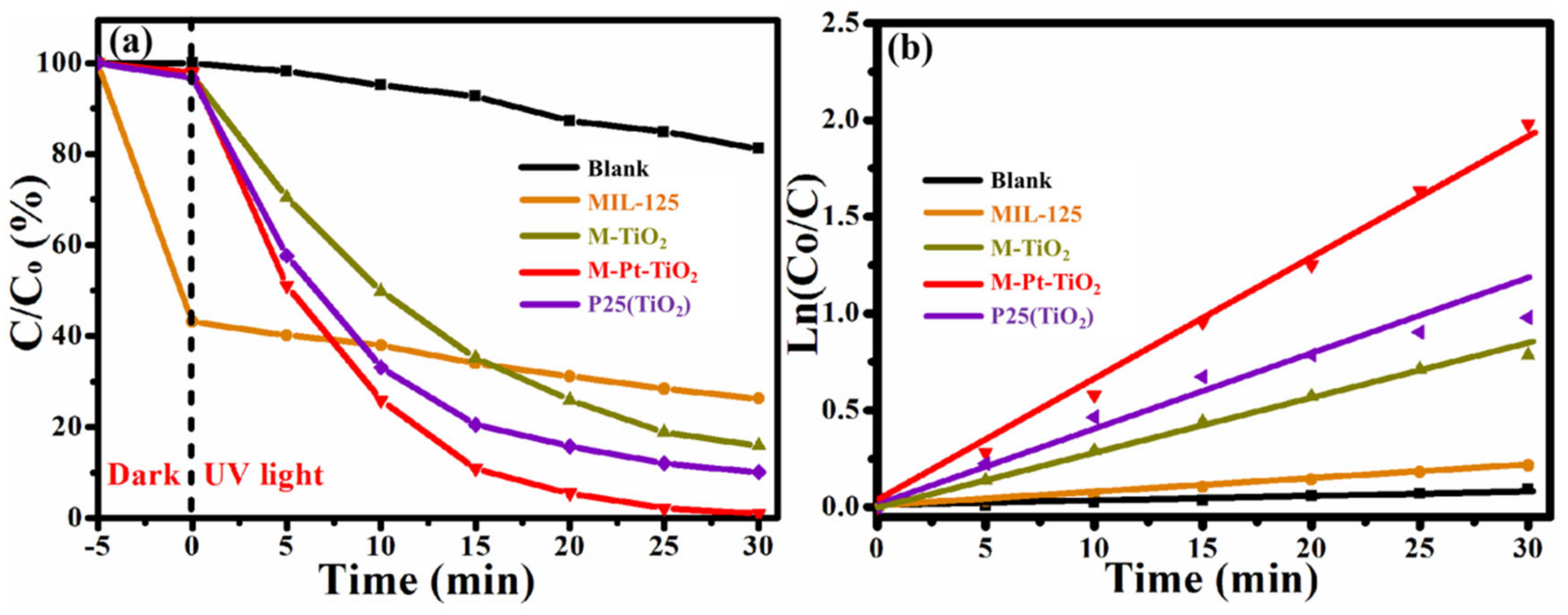
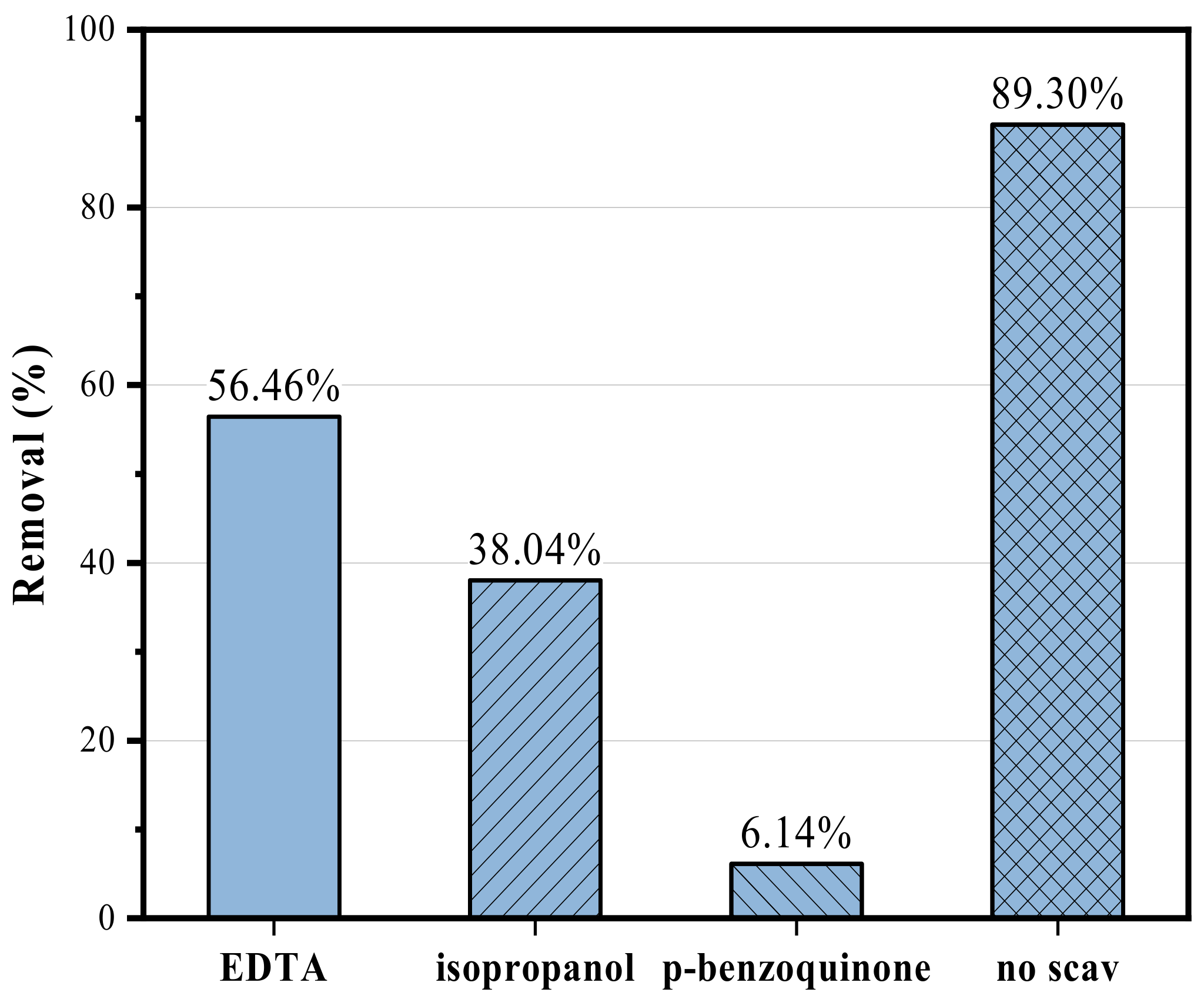
3.2.1. Experimental Results and Analysis of M-Pt-TiO2 Photocatalytic Degradation of Rhodamine B
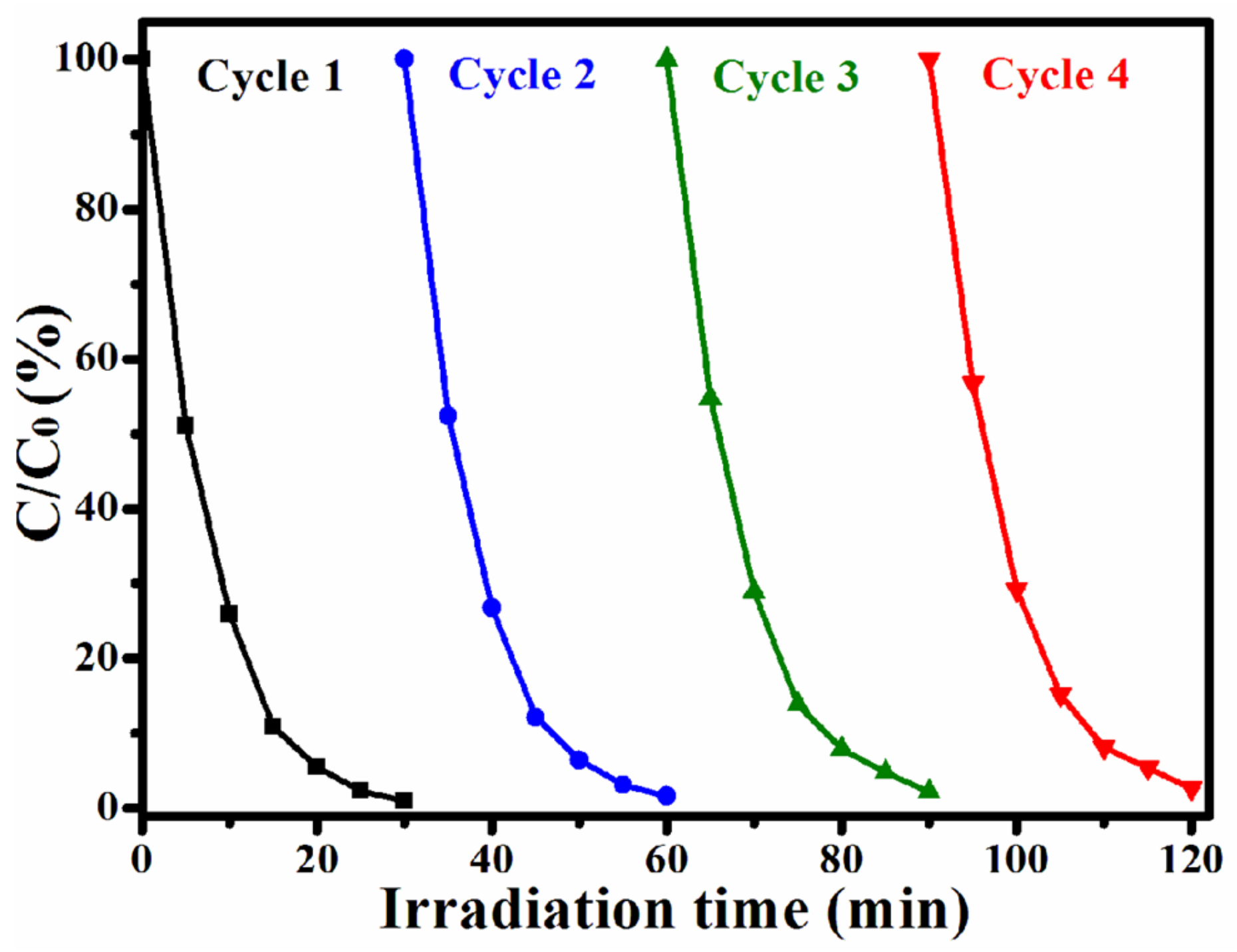
3.2.2. Experimental Analysis of the Recyclability of M-Pt-TiO2 Photocatalytic Degradation of Rhodamine B
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, S.-Y.; Guo, J.-H.; Zhao, C.; Peng, Z.; Gong, C.-H.; Yu, L.-G.; Liu, X.-H.; Zhang, J.-W. Preparation of Cellulose/Graphene Oxide Composite Membranes and Their Application in Removing Organic Contaminants in Wastewater. J. Nanosci. Nanotechnol. 2019, 19, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Leena, V.B.; Rajubhai, K.M. Visible/solar light active photocatalysts for organic effluent treatment: Fundamentals, mechanisms and parametric review. Renew. Sustain. Energy Rev. 2017, 76, 1393–1421. [Google Scholar]
- Zheng, Q.; Durkin, D.P.; Elenewski, J.E.; Sun, Y.; Banek, N.A.; Hua, L.; Chen, H.; Wagner, M.J.; Zhang, W.; Shuai, D. Visible-Light-Responsive Graphitic Carbon Nitride: Rational Design and Photocatalytic Applications for Water Treatment. Environ. Sci. Technol. 2016, 50, 12938–12948. [Google Scholar] [CrossRef]
- Kakarla, R.R.; Mahbub, H.; Vincent, G.G. Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal. A Gen. 2015, 489, 1–16. [Google Scholar]
- Nasalevich, M.A.; van der Veen, M.; Kapteijn, F.; Gascon, J. Metal–organic frameworks as heterogeneous photocatalysts: Advantages and challenges. CrystEngComm 2014, 16, 4919–4926. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Benyan, X.; Xu, B.; Liu, Y.; Wang, Z.; Wang, P.; Dai, Y.; Qin, X.; Zhang, X.; Huang, B. Photocatalytic Overall Water Splitting over MIL-125(Ti) upon CoPi and Pt Co-catalyst Deposition. ChemistryOpen 2017, 6, 701–705. [Google Scholar] [CrossRef]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef]
- An, Y.; Liu, Y.; An, P.; Dong, J.; Xu, B.; Dai, Y.; Qin, X.; Zhang, X.; Whangbo, M.; Huang, B. NiII Coordination to an Al-Based Metal-Organic Framework Made from 2-Aminoterephthalate for Photocatalytic Overall Water Splitting. Angew. Chem. Int. Ed. 2017, 56, 3036–3040. [Google Scholar] [CrossRef]
- An, Y.; Li, H.; Liu, Y.; Huang, B.; Sun, Q.; Dai, Y.; Qin, X.; Zhang, X. Photoelectrical, photophysical and photocatalytic properties of Al based MOFs: MIL-53(Al) and MIL-53-NH2(Al). J. Solid State Chem. 2016, 233, 194–198. [Google Scholar] [CrossRef]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. Multifunctional NH2-mediated zirconium metal–organic framework as an efficient visible-light-driven photocatalyst for selective oxidation of alcohols and reduction of aqueous Cr(vi). Dalton Trans. 2013, 42, 13649–13657. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Jafari, A.J.; Gholami, M.; Sobhi, H.R.; Nourbakhsh, M.; Akbari-Adergani, B. Photocatalytic degradation of cefixime with MIL-125(Ti)-mixed linker decorated by g-C 3 N 4 under solar driven light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123874. [Google Scholar] [CrossRef]
- Kampouri, S.; Nguyen, T.N.; Spodaryk, M.; Palgrave, R.G.; Züttel, A.; Smit, B.; Stylianou, K.C. Concurrent Photocatalytic Hydrogen Generation and Dye Degradation Using MIL-125-NH2 under Visible Light Irradiation. Adv. Funct. Mater. 2018, 28, 1806368. [Google Scholar] [CrossRef]
- Chambers, M.B.; Wang, X.; Ellezam, L.; Ersen, O.; Fontecave, M.; Sanchez, C.; Rozes, L.; Mellot-Draznieks, C. Maximizing the Photocatalytic Activity of Metal-Organic Frameworks with Aminated-Functionalized Linkers: Substoichiometric Effects in MIL-125-NH2. J. Am. Chem. Soc. 2017, 139, 8222–8228. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Cheng, J.K.; Hu, Z.; Zhang, M.; Xu, Q.; Kang, Z.; Zhao, D. Metal-organic frameworks (MOFs) as precursors towards TiOx/C composites for photodegradation of organic dye. Rsc Adv. 2014, 4, 34221–34225. [Google Scholar] [CrossRef]
- Khaletskaya, K.; Pougin, A.; Medishetty, R.; Rösler, C.; Wiktor, C.; Strunk, J.; Fischer, R.A. Fabrication of Gold/Titania Photocatalyst for CO2 Reduction Based on Pyrolytic Conversion of the Metal–Organic Framework NH2-MIL-125(Ti) Loaded with Gold Nanoparticles. Chem. Mater. 2015, 27, 7248–7257. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, X.; Luo, Y.; Wang, L.; Cui, G.; Xie, F.; Wang, H.; Xie, Y.; Sun, X. An ultrafine platinum–cobalt alloy decorated cobalt nanowire array with superb activity toward alkaline hydrogen evolution. Nanoscale 2018, 10, 12302–12307. [Google Scholar] [CrossRef]
- Tai, C.; Liu, H.; Hu, Y. Fabrication of CdS/Pt/MIL-125 with Effective Spatial Separation for Improved Visible-Light Catalytic H-2 Evolution Using gamma-Ray Irradiation. ACS Sustain. Chem. Eng. 2020, 8, 18196–18205. [Google Scholar] [CrossRef]
- Jiang, F.; Zeng, L.; Li, S.; Liu, G.; Wang, S.; Gong, J. Propane Dehydrogenation over Pt/TiO2-Al2O3 Catalysts. ACS Catal. 2015, 5, 438–447. [Google Scholar] [CrossRef]
- Zhou, L.; Wei, Y.; Zhang, H.; Zhao, Q.; Zhao, Z.; Guo, Y.; Zhu, S.; Fu, H.; Cai, W. Pt-TiO2 Bilayered Hemispherical Nanoshells with Tunable Pt Distribution for Chemically Self-Propelled Colloidal Motors. ACS Appl. Nano Mater. 2022, 5, 18469–18478. [Google Scholar] [CrossRef]
- Fang, J.; Shi, F.; Bu, J.; Ding, J.; Xu, S.; Bao, J.; Ma, Y.; Jiang, Z.; Zhang, W.; Gao, C.; et al. One-Step Synthesis of Bifunctional TiO2 Catalysts and Their Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 7940–7948. [Google Scholar] [CrossRef]
- Peng, C.; Wang, H.; Yu, H.; Peng, F. (111) TiO2-x/Ti3C2: Synergy of active facets, interfacial charge transfer and Ti3+ doping for enhance photocatalytic activity. Mater. Res. Bull. 2017, 89, 16–25. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y. Synthesis of M@TiO2 (M = Au, Pd, Pt) Core–Shell Nanocomposites with Tunable Photoreactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]


| Reagents | Specifications | Manufacturer |
|---|---|---|
| Ammonium chloride | AR | Hangzhou Gopin Fine Chemicals Co.; Hangzhou, China |
| Methanol | AR | Hangzhou Gopin Fine Chemicals Co. |
| Anhydrous ethanol | AR | Hangzhou Gopin Fine Chemicals Co. |
| Isopropyl alcohol | AR | Shanghai Aladdin Reagent Co.; Shanghai, China |
| 2-Methylimidazole | AR | Shanghai Aladdin Reagent Co. |
| Tetra-butyl titanate | AR | Sigmar Aldridge, Miamisburg, OH, USA |
| Dimethylformamide | AR | Shanghai Aladdin Reagent Co. |
| Chloroplatinic acid | ACS, ≥99.0% | Shanghai Aladdin Reagent Co. |
| Titanium dioxide (P25) | AR | Hangzhou Gopin Fine Chemicals Co. |
| Terephthalic acid | AR | Hangzhou Gopin Fine Chemicals Co. |
| Rhodamine B | AR | Shanghai Aladdin Reagent Co. |
| EDTA | AR | Shanghai Aladdin Reagent Co. |
| p-Benzoquinone | Strong, AR | Hangzhou Gopin Fine Chemicals Co. |
| Deionised water | AR |
| Instruments | Models | Manufacturer |
|---|---|---|
| Ultrasonic cleaners | JP-040S | Shenzhen United Cleaning Equipment Co., Shenzhen, China |
| Constant temperature and pressure drying ovens | DHG-9035A | Shanghai Heng Yi Technology Co.; Shanghai, China |
| High-speed centrifuges | H-1850 | Hunan Xiang Yi Experimental Instrument Development Co.; Changsha, China |
| Digital magnetic stirrer | SZCL-24A | Hangzhou Youning Instrument Co.; Hangzhou, China |
| Muffle Furnaces | KSL-II00XX | Hefei Kepin Materials Technology Co.; Hefei, China |
| Name | Molecular Formula | Structural Formula | Molecular Weight |
|---|---|---|---|
| Rhodamine B (RhB) | C28H31C1N2O3 |  | 479.01 |
| Name | Specific Surface Area (m2.g−1) | Average Pore Size (nm) |
|---|---|---|
| MIL-125 | 1366.937 | / |
| M-TiO2 | 34.288 | 47.856 |
| M-Pt-TiO2 | 41.199 | 47.988 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, X.; Yuan, H.; Li, C. Study on the MOF Frame Pt-TiO2 Hybrid Photocatalyst and Its Photocatalytic Performance. Sustainability 2023, 15, 1403. https://doi.org/10.3390/su15021403
Mei X, Yuan H, Li C. Study on the MOF Frame Pt-TiO2 Hybrid Photocatalyst and Its Photocatalytic Performance. Sustainability. 2023; 15(2):1403. https://doi.org/10.3390/su15021403
Chicago/Turabian StyleMei, Xueqiao, Han Yuan, and Chunhu Li. 2023. "Study on the MOF Frame Pt-TiO2 Hybrid Photocatalyst and Its Photocatalytic Performance" Sustainability 15, no. 2: 1403. https://doi.org/10.3390/su15021403
APA StyleMei, X., Yuan, H., & Li, C. (2023). Study on the MOF Frame Pt-TiO2 Hybrid Photocatalyst and Its Photocatalytic Performance. Sustainability, 15(2), 1403. https://doi.org/10.3390/su15021403






