Effects of Acidic/Alkaline Contamination on the Physical and Mechanical Properties of Silty Clay
Abstract
1. Introduction
2. Materials and Methods
3. Physical Experiment
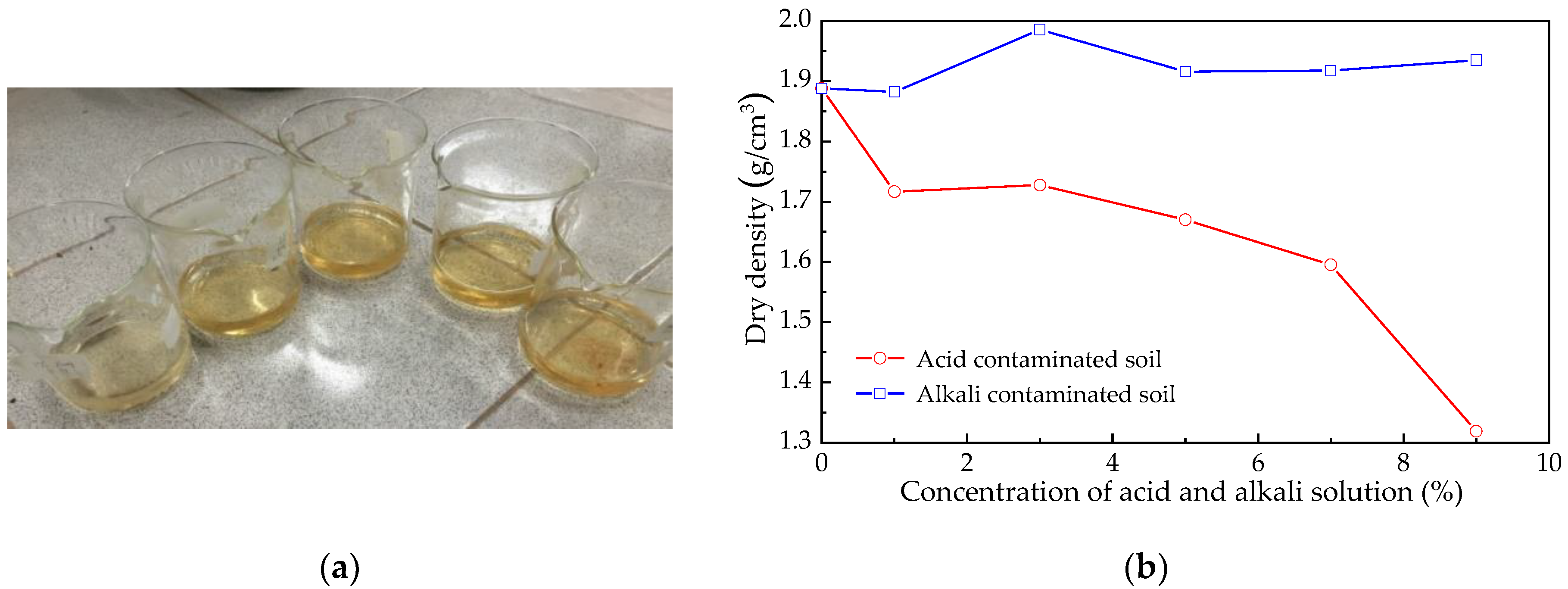
3.1. Apparent Feature Changes
3.2. Weight and Density Change
3.3. Specific Gravity Changes
4. Mechanical Experiment
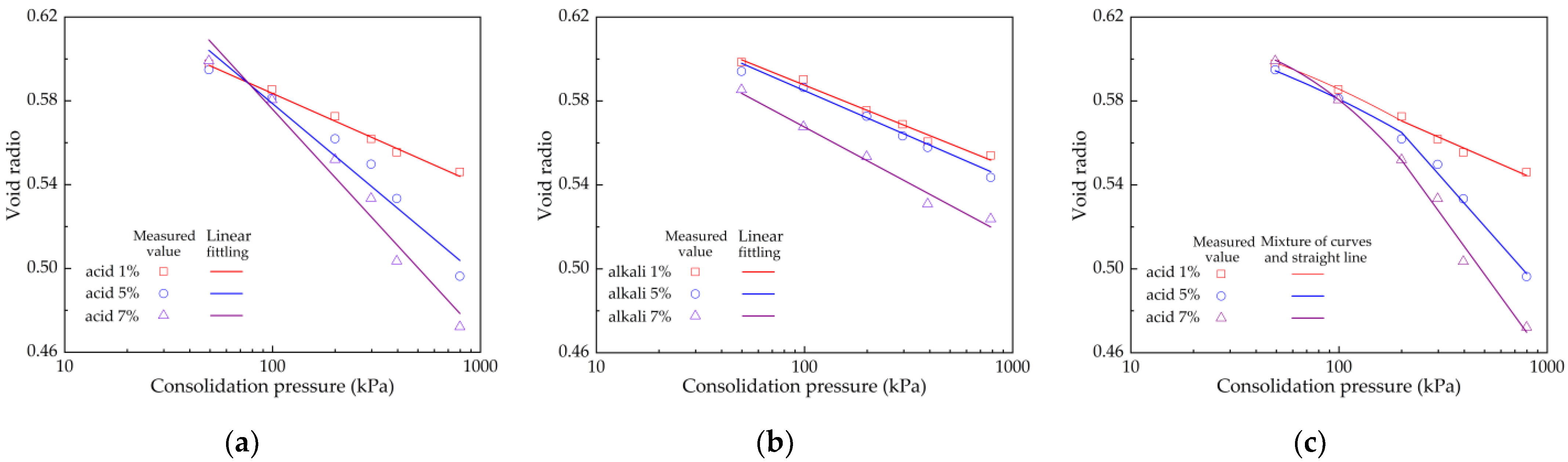
4.1. Compressive Property Research
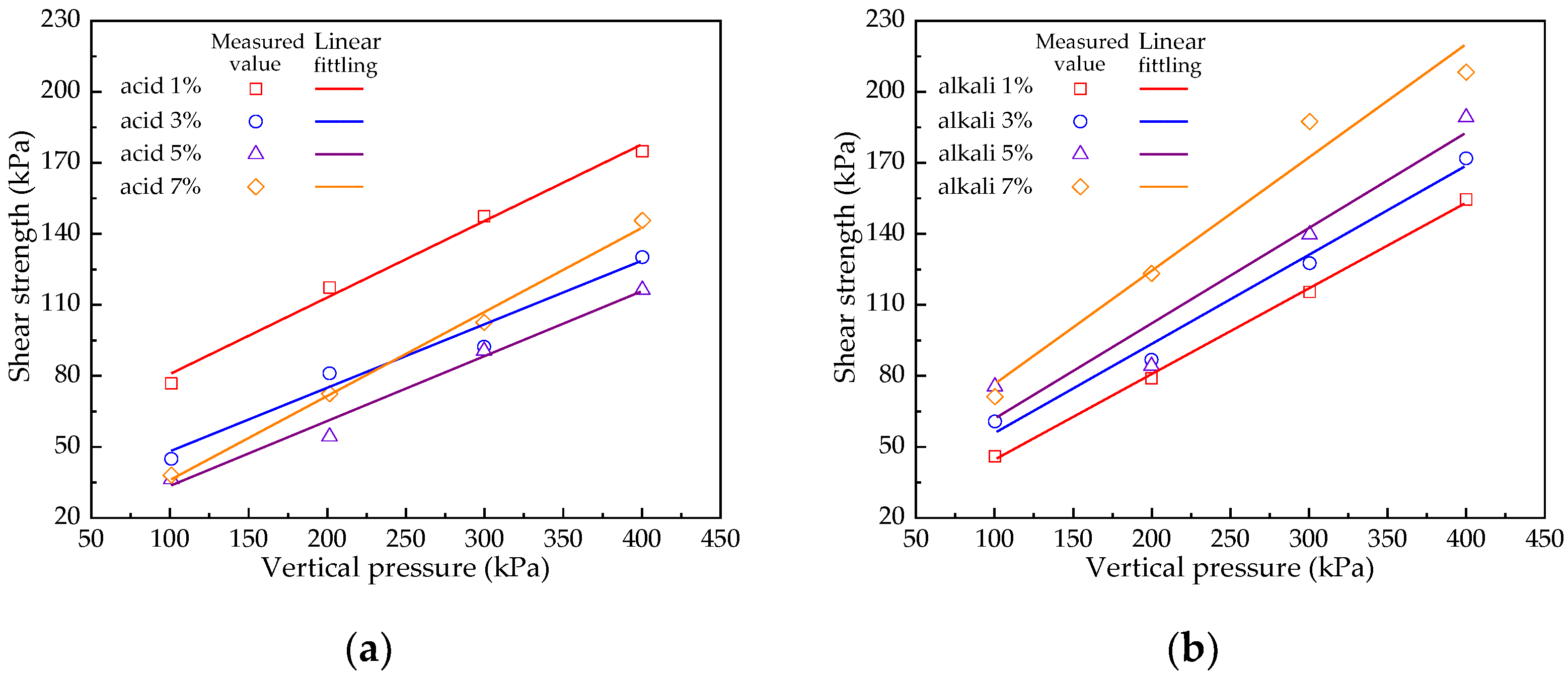
4.2. Shear Strength Research
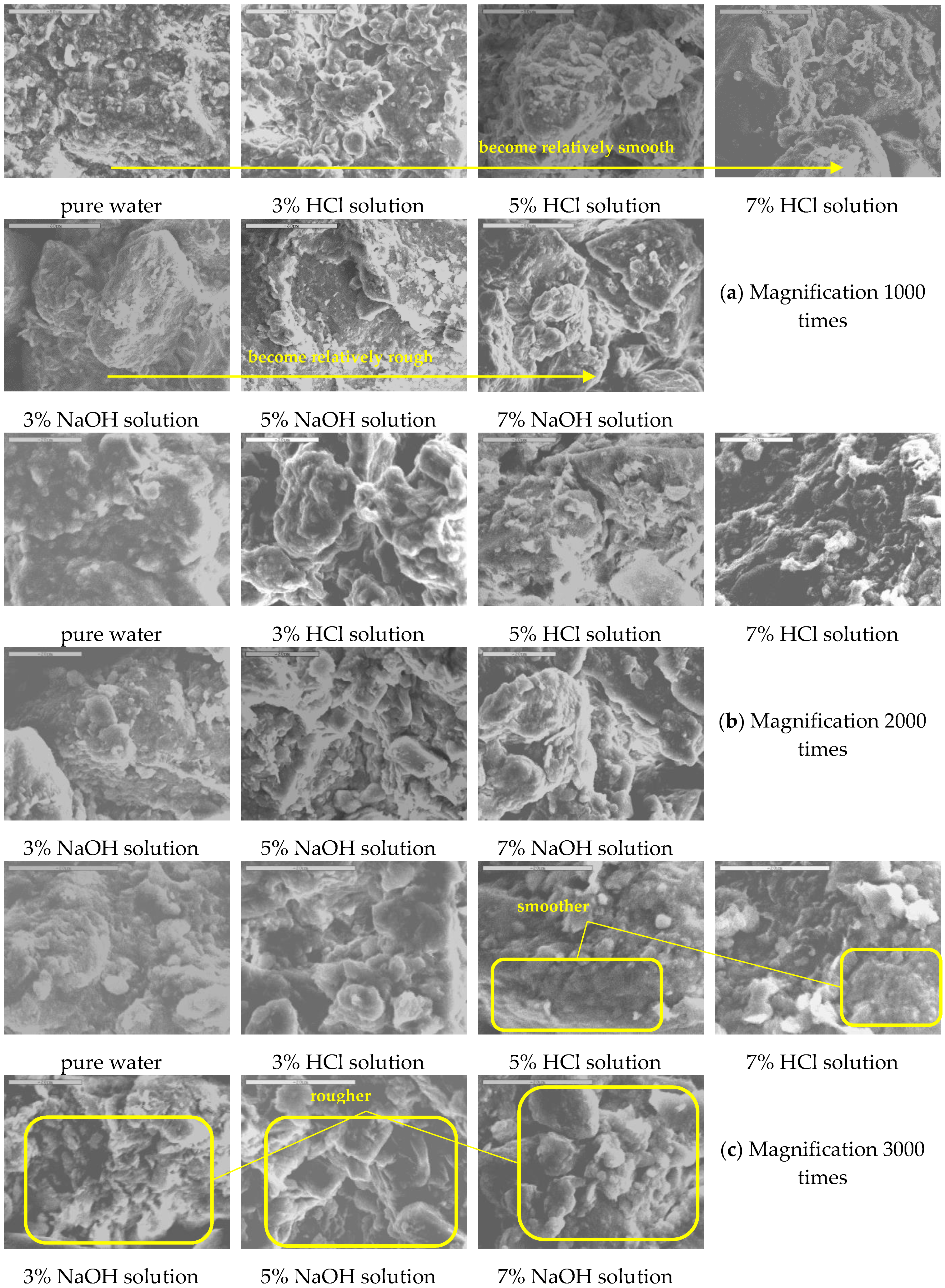
4.3. Microstructure Observation
5. Discussion
5.1. Application and Limitation
5.2. Contamination Mechanism
5.3. Comparison and Promotion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, G.J.; Shi, J.Y.; Yang, Y.; Jiang, Z.Q. Wedge method for landfill stability analysis considering temperature increase. Waste Manag. Res. 2022, 40, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Li, J.C.; Yang, C.B.; Zhu, B.; Zhan, L.T. Centrifuge modeling of municipal solid waste landfill failures induced by rising water levels. Can. Geotech. J. 2017, 54, 1739–1751. [Google Scholar] [CrossRef]
- Jayaweera, M.; Gunawardana, B.; Gunawardana, M.; Karunawardena, A.; Dias, V.; Premasiri, S.; Dissanayake, J.; Manatunge, J.; Wijeratne, N.; Karunarathne, D.; et al. Management of municipal solid waste open dumps immediately after the collapse: An integrated approach from Meethotamulla open dump, Sri Lanka. Waste Manag. 2019, 95, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Bonaparte, R.; Bachus, R.C.; Gross, B.A. Geotechnical stability of waste fills: Lessons learned and continuing challenges. J. Geotech. Geoenviron. 2020, 146, 05020010. [Google Scholar] [CrossRef]
- Shariatmadari, N.; Machado, S.L.; Noorzad, A.; Karimpour–Fard, M. Municipal solid waste effective stress analysis. Waste Manag. Res. 2009, 29, 2918–2930. [Google Scholar] [CrossRef]
- Torretta, V.; Ferronato, N.; Katsoyiannis, I.A.; Tolkou, A.K.; Airoldi, M. Novel and conventional technologies for landfill leachates treatment: A review. Sustainability 2017, 9, 9. [Google Scholar] [CrossRef]
- Townsend, T.G.; Powell, J.; Jain, P.; Xu, Q.Y.; Tolaymat, T.; Reinhart, D. Sustainable Practices for Landfill Design and Operation; Springer: New York, NY, USA, 2015; pp. 267–280. [Google Scholar]
- Todorov, M.; Kostov, V. Prediction of the development of long term deformations in deposit bodies. In Proceedings of the 17th International Multidisciplinary Scientific Geo Conference SGEM 2017, Vienna, Austria, 27–29 November 2017. [Google Scholar]
- Anandarajah, A.; Zhao, D. Triaxial behavior of kaolinite in different pore fluids. J. Geotech. Geoenviron. 2000, 126, 148–156. [Google Scholar] [CrossRef]
- Ratnaweera, P.; Meegoda, J. Shear strength and stress-strain behavior of contaminated soils. Geotech. Test J. 2006, 29, 133–140. [Google Scholar]
- Gratchev, I.; Towhata, I. Stress-strain characteristics of two natural soils subjected to long-term acidic contamination. Soils Found. 2013, 53, 469–476. [Google Scholar] [CrossRef]
- Koupai, A.J.; Fatahizadeh, M.; Mosaddeghi, M.R. Effect of pore water pH on mechanical properties of clay soil. Bull. Eng. Geol. Environ. 2020, 79, 1461–1469. [Google Scholar] [CrossRef]
- Tiwari, B.; Tuladhar, G.; Marui, H. Variation in residual shear strength of the soil with the salinity of pore fluid. J. Geotech. Geoenviron. 2005, 131, 1445–1456. [Google Scholar] [CrossRef]
- Spagnoli, G.; Fernandez-Steeger, T.; Feinendegen, M.; Stanjek, H.; Azzam, R. The influence of dielectric constant and electrolyte concentration of the pore fluids on the undrained shear strength of smectite. Soils Found. 2010, 50, 757–763. [Google Scholar] [CrossRef]
- Nguyen, X.P.; Cui, Y.J.; Tang, A.M.; Deng, Y.F.; Li, X.L.; Wouters, L. Effects of pore water chemical composition on the hydro-mechanical behavior of natural stiff clay. Eng. Geol. 2013, 166, 52–64. [Google Scholar] [CrossRef]
- Alibeiki, Y.; Hassanlourad, M.; Ghasemipanah, A. Effect of acidic and alkaline pore fluid on the mechanical properties of fine-grained soil. Bull. Eng. Geol. Environ. 2022, 81, 512. [Google Scholar] [CrossRef]
- Gratchev, I.; Sassa, K. Cyclic behavior of fine-grained soils at different pH values. J. Geotech. Geoenviron. 2009, 135, 271–279. [Google Scholar] [CrossRef]
- Kamon, M.; Ying, C.; Katsumi, T. Effect of acid rain on physicochemical and engineering properties of soils. Soils Found. 1997, 37, 23–32. [Google Scholar] [CrossRef]
- Hu, W.L.; Cheng, W.C.; Wang, L.; Xue, Z.F. Micro-structural characteristics deterioration of intact loess under acid and saline solutions and resultant macro-mechanical properties. Soil. Till. Res. 2022, 220, 105382. [Google Scholar] [CrossRef]
- Hu, W.L.; Cheng, W.C.; Wen, S.J.; Rahman, M.M. Effects of chemical contamination on microscale structural characteristics of intact loess and resultant macroscale mechanical properties. Catena 2021, 203, 105361. [Google Scholar] [CrossRef]
- Olphen, H.V. An Introduction to Clay Colloid Chemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 226–235. [Google Scholar]
- Sridharan, A.; Rao, S.M.; Murthy, N.S. Liquid limit of kaolinitic soils. Geotechnique 1988, 38, 191–198. [Google Scholar] [CrossRef]
- Sridharan, A.; Prakash, K. Mechanisms controlling the undrained shear strength behaviour of clays. Can. Geotech. J. 1999, 36, 1030–1038. [Google Scholar] [CrossRef]
- Gratchev, I.; Sassa, K.; Osipov, V.; Fukuoka, H.; Wang, G. Undrained cyclic behavior of bentonite–sand mixtures and factors affecting it. Geotech. Geol. Eng. 2007, 25, 349–367. [Google Scholar] [CrossRef]
- Gratchev, I.; Towhata, I. Compressibility of natural soils subjected to long-term acidic contamination. Environ. Earth. Sci. 2011, 64, 193–200. [Google Scholar] [CrossRef]
- Gratchev, I.B.; Towhata, I. Effects of acidic contamination on the geotechnical properties of marine soils in Japan. In Proceedings of the Nineteenth (2009) International Offshore and Polar Engineering Conference, Osaka, Japan, 21–26 June 2009. [Google Scholar]
- Tang, H.; Liu, C.Y.; Wang, N.Q.; Li, H.H.; Wu, G.N.; Luo, J.Z.; Zeng, M.L. Influence of acidic substances on compression deformation characteristics of loess. Adv. Civ. Eng. 2021, 2021, 6614391. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S. Explanation of the influence of sodium chloride solution on volume deformation and permeability of normally consolidated clays. Materials 2019, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Han, P.J.; Yan, Y.B.; Bai, X.H. Study on mechanical properties of acidic and alkaline silty soil by electrochemical impedance spectroscopy. Int. J. Electrochem. Sci. 2018, 13, 10548–10563. [Google Scholar] [CrossRef]
- Yang, H.S.; Wei, H.; Yang, Y.L.; Li, J.Y.; Yin, X.L. Experimental study on laterite eroded by alkaline materials. Adv. Mater. Res. 2012, 368–373, 2781–2786. [Google Scholar] [CrossRef]
- Witteveen, P.; Ferrari, A.; Laloui, L. An experimental and constitutive investigation on the chemo-mechanical behaviour of a clay. Géotechnique 2013, 63, 244–255. [Google Scholar] [CrossRef]
- Abbaslou, H.; Hadifard, H.; Ghanizadeh, A.R. Effect of cations and anions on flocculation of dispersive clayey soils. Heliyon 2020, 6, e03462. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, X.A. Effects of carbonate on the structure and properties of loess and the corresponding mechanism: An experimental study of the Malan loess, Xi’an area, China. Bull. Eng. Geol. Environ. 2019, 78, 4965–4976. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.C.; Wei, J.H.; Song, J.L.; Yu, Y.X.; He, W.; Zhang, J.X. The physical-mechanical properties degradation mechanism and microstructure response of acid-alkali-contaminated Xiashu loess. Nat. Hazards 2021, 106, 2845–2861. [Google Scholar] [CrossRef]
- GB/T 50123 (2019); The China National Standards for Geotechnical Testing Method. Ministry of Housing and Urban-Rural Construction of the People’s Republic of China: Beijing, China, 2019. (In Chinese)
- Kang, Q.R.; Xia, Y.D.; Li, X.S.; Zhang, W.Z.; Feng, C.R. Study on the effect of moisture content and dry density on shear strength of silty clay based on direct shear test. Adv. Civ. Eng. 2022, 2022, 2213363. [Google Scholar] [CrossRef]
- Li, Z.X.; Wang, J.D.; Yang, S.; Liu, S.H.; Li, Y.W. Characteristics of Microstructural Changes of Malan Loess in Yan’an Area during Creep Test. Water 2022, 14, 438. [Google Scholar] [CrossRef]
- Jha, A.K.; Sivapullaiah, P.V. Mechanism of improvement in the strength and volume change behavior of lime stabilized soil. Eng. Geol. 2015, 198, 53–64. [Google Scholar] [CrossRef]
- GB/T 16594 (2008); The China National Standards for General Rules for Measurement of Length in Micron Scale by SEM. State Administration for Market Regulation of the People’s Republic of China: Beijing, China, 2008. (In Chinese)
- Pesavento, F.; Schrefler, B.A.; Sciumè, G. Multiphase flow in deforming porous media: A review. Arch. Comput. Method Eng. 2017, 24, 423–448. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, R.; Cai, G.Q.; Hu, W.; Yang, G.C. Coupled thermo-hydro-mechanical mechanism in view of the soil particle rearrangement of granular thermodynamics. Comput. Geotech. 2021, 137, 104272. [Google Scholar] [CrossRef]
- Sivapullaiah, P.V.; Sankara, G.; Allam, M.M. Mineralogical changes and geotechnical properties of an expansive soil interacted with caustic solution. Environ. Earth. Sci. 2010, 60, 1189–1199. [Google Scholar] [CrossRef]
- Tong, F.; Ma, Q.; Hu, X. Triaxial shear test on hydrochloric acid-contaminated clay treated by lime, crushed concrete, and super absorbent polymer. Adv. Mater. Sci. Eng. 2019, 2019, 3865157. [Google Scholar] [CrossRef]
- Das, B.M. Advanced Soil Mechanics, 4th ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 3–8. [Google Scholar]
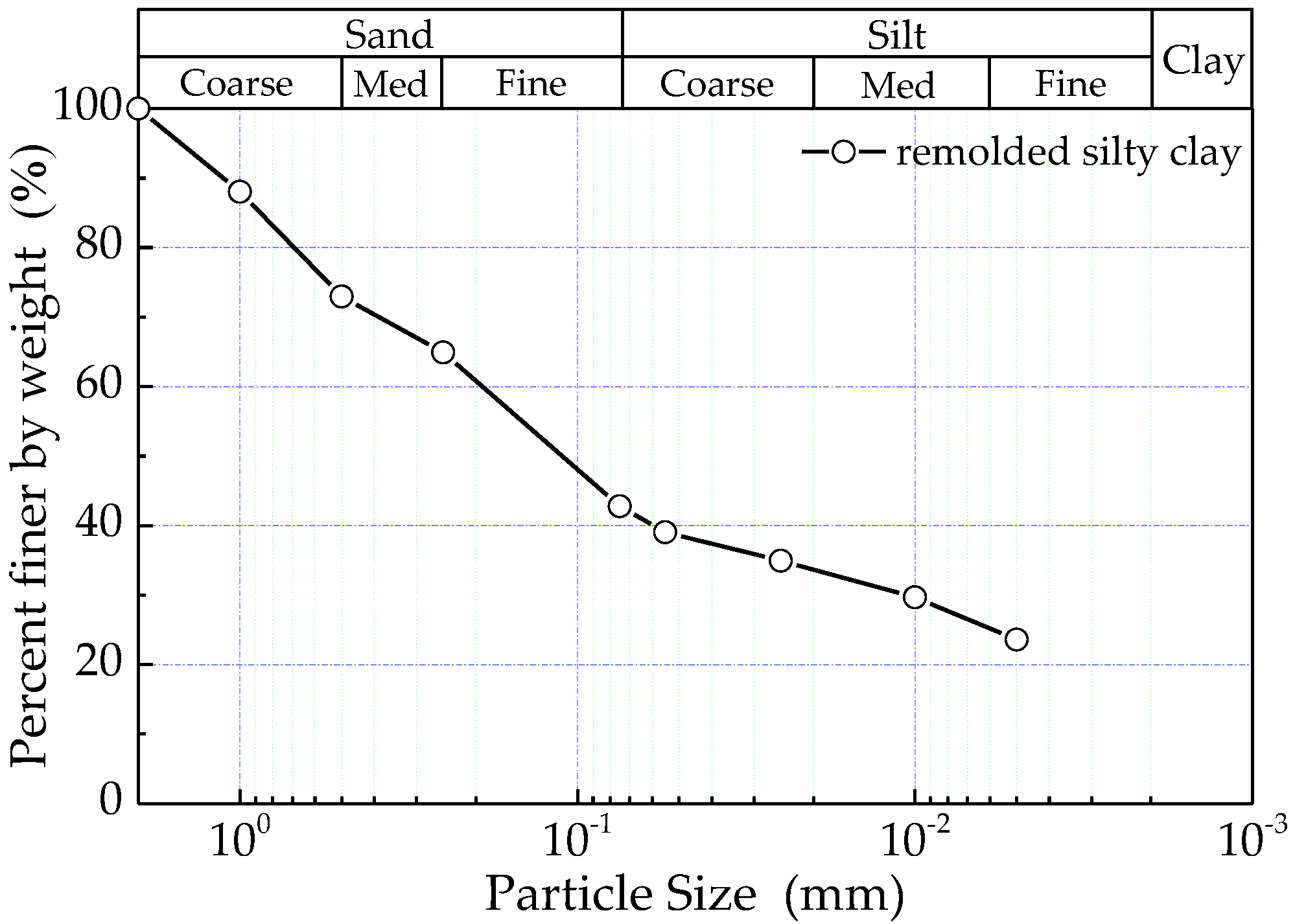

| Chemical Composition(%) | SiO2 | Al2O3 | MgO | CaO | K2O | ZnO | MnO | Fe2O3 | Others |
|---|---|---|---|---|---|---|---|---|---|
| -- | 58.86 | 17.33 | 12.63 | 4.17 | 2.06 | 1.96 | 1.12 | 0.98 | 0.89 |
| Mineral Composition(%) | Quartz | Plagioclase | Microcline | Calcite | Pyrite | Dolomite | Total Clay | ||
| -- | 39 | 11 | 4 | 23 | 2 | 5 | 56 | ||
| Concentration | Change in Weight (g) | Density (g/cm3) | Concentration | Change in Weight (g) | Density (g/cm3) |
|---|---|---|---|---|---|
| pure water | −0.820 | 1.88825 | — | — | — |
| acid 1% | −10.291 | 1.71673 | alkali 1% | −0.366 | 1.88215 |
| acid 3% | −9.635 | 1.72767 | alkali 3% | +0.450 | 1.98575 |
| acid 5% | −13.087 | 1.67013 | alkali 5% | +1.665 | 1.91600 |
| acid 7% | −17.574 | 1.59535 | alkali 7% | +1.762 | 1.91762 |
| acid 9% | −34.161 | 1.31890 | alkali 9% | +2.804 | 1.93498 |
| Concentration | Specific Gravity | Concentration | Specific Gravity |
|---|---|---|---|
| pure water | 2.6772 | — | — |
| acid 1% | 2.6541 | alkali 1% | 2.6764 |
| acid 3% | 2.5914 | alkali 3% | 2.7020 |
| acid 5% | 1.9268 1 | alkali 5% | 2.7028 |
| acid 7% | 2.5716 | alkali 7% | 2.7479 |
| acid 9% | 2.5283 | alkali 9% | 2.7500 |
| Soil Samples | λ | b | R2 | Soil Samples | λ | b | R2 |
|---|---|---|---|---|---|---|---|
| acid 1% | 0.019 | 0.6734 | 0.9923 | acid 1% curved segment | — | — | 0.9981 |
| acid 5% | 0.034 | 0.7384 | 0.9470 | acid 1% line segment | 0.019 | 0.6701 | 0.9652 |
| acid 7% | 0.047 | 0.7931 | 0.9677 | acid 5% curved segment | — | — | 0.9975 |
| alkali 1% | 0.017 | 0.6673 | 0.9855 | acid 5% line segment | 0.049 | 0.8230 | 0.9871 |
| alkali 5% | 0.019 | 0.6712 | 0.9894 | acid 7% curved segment | — | — | 0.9999 |
| alkali 7% | 0.023 | 0.6750 | 0.9820 | acid 7% line segment | 0.059 | 0.8659 | 0.9748 |
| Soil Samples | c (kPa) | (°) | R2 | Soil Samples | c (kPa) | (°) | R2 |
|---|---|---|---|---|---|---|---|
| acid 1% | 48.04 | 18.00 | 0.9918 | alkali 1% | 9.49 | 19.75 | 0.9985 |
| acid 3% | 20.30 | 15.22 | 0.9620 | alkali 3% | 20.24 | 20.41 | 0.9880 |
| acid 5% | 5.15 | 15.54 | 0.9886 | alkali 5% | 22.79 | 21.70 | 0.9350 |
| acid 7% | 0.75 | 19.65 | 0.9939 | alkali 7% | 29.25 | 25.41 | 0.9630 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Y.; Cai, G.; Zhang, C.; Wang, X.; Shi, Y.; Li, J. Effects of Acidic/Alkaline Contamination on the Physical and Mechanical Properties of Silty Clay. Sustainability 2023, 15, 1317. https://doi.org/10.3390/su15021317
Shan Y, Cai G, Zhang C, Wang X, Shi Y, Li J. Effects of Acidic/Alkaline Contamination on the Physical and Mechanical Properties of Silty Clay. Sustainability. 2023; 15(2):1317. https://doi.org/10.3390/su15021317
Chicago/Turabian StyleShan, Yepeng, Guoqing Cai, Ce Zhang, Xiao Wang, Yehui Shi, and Jian Li. 2023. "Effects of Acidic/Alkaline Contamination on the Physical and Mechanical Properties of Silty Clay" Sustainability 15, no. 2: 1317. https://doi.org/10.3390/su15021317
APA StyleShan, Y., Cai, G., Zhang, C., Wang, X., Shi, Y., & Li, J. (2023). Effects of Acidic/Alkaline Contamination on the Physical and Mechanical Properties of Silty Clay. Sustainability, 15(2), 1317. https://doi.org/10.3390/su15021317





