Winter Bloom of Marine Cyanobacterium, Trichodesmium erythraeum and Its Relation to Environmental Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection, Enumeration and Identification
2.3. Environmental Factors
2.4. Statistical Analysis
3. Results
3.1. Blooms of Trichodesmium erythraeum
3.2. Morphological Variations among T. erythraeum Colonies
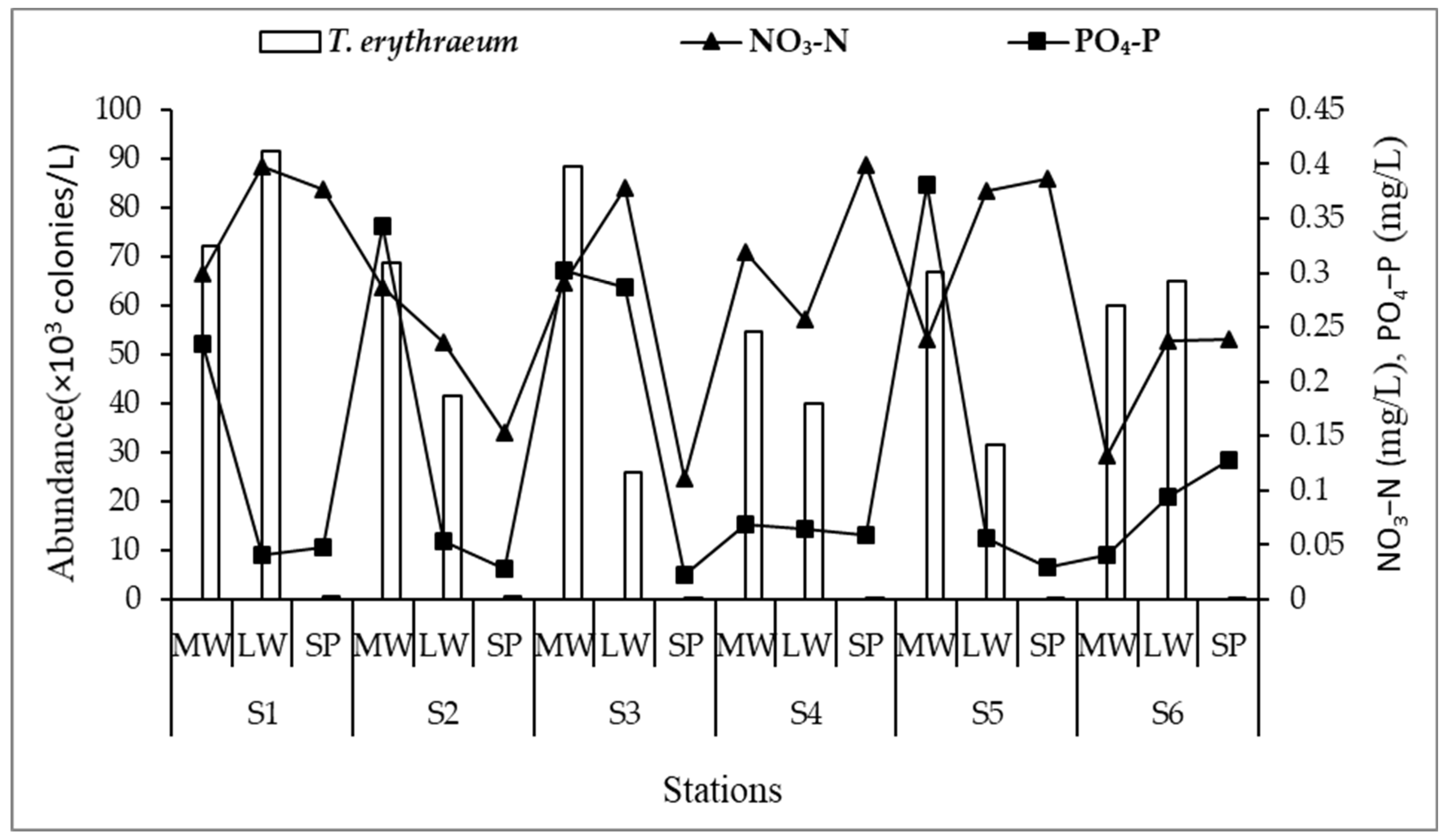
3.3. Relationship between T. erythraeum Colonies and Environmental Factors
3.4. T. erythraeum Assemblage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gobler, C.J. Climate Change and Harmful Algal Blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef] [PubMed]
- Malmqvist, B.; Rundle, S.D.; Covich, A.P.; Hildrew, A.G.; Robinson, C.T.; Townsend, C.R. Prospects for streams and rivers: An ecological perspective. In Aquatic Systems: Trends and Global Perspectives; Polunin, N., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 19–29. [Google Scholar]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.-Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Lundholm, N.; Churro, C.; Fraga, S.; Hoppenrath, M.; Iwataki, M.; Larsen, J.; Mertens, K.; Moestrup, Ø.; Zingone, A. (Eds.) IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. 2009. Available online: https://www.marinespecies.org/hab (accessed on 18 February 2022).
- Sournia, A. Phytoplankton Manual; United Nations Educational, Scientific and Cultural Organization: Paris, France, 1978. [Google Scholar]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, J.; Zhou, F.; Zhai, H.; Zhang, D.; Yan, X. Summer distribution patterns of Trichodesmium spp. in the Changjiang (Yangtze River) Estuary and adjacent East China Sea shelf. Oceanologia 2017, 59, 248–261. [Google Scholar] [CrossRef]
- Capone, D.G.; Zehr, J.P.; Paerl, H.W.; Bergman, B.; Carpenter, E.J. Trichodesmium, a globally significant marine cyanobacterium. Science 1997, 276, 1221–1229. [Google Scholar] [CrossRef]
- Padmakumar, K.B.; Smitha, B.R.; Thomas, L.C.; Fanimol, C.L.; SreeRenjima, G.; Menon, N.R.; Sanjeevan, V.N. Blooms of Trichodesmium erythraeum in the South Eastern Arabian Sea during the onset of 2009 summer monsoon. Ocean Sci. J. 2010, 45, 151–157. [Google Scholar] [CrossRef]
- Tang, D.L.; Kester, D.R.; Ni, I.H.; Qi, Y.Z.; Kawamura, H. In situ and satellite observations of a harmful algal bloom and water condition at the Pearl River estuary in late autumn 1998. Harmful Algae 2003, 2, 89–99. [Google Scholar] [CrossRef]
- Karthik, R.; Padmavati, G. Temperature and salinity are the probable causative agent for the Trichodesmium erythraeum (Cyanophyceae) algal bloom on the Burmanallah coastal waters of South Andaman Island. World Appl. Sci. J. 2017, 35, 1271–1281. [Google Scholar] [CrossRef]
- Karl, D.; Michaels, A.; Bergman, B.; Capone, D.; Carpenter, E.; Letelier, R.; Lipschultz, L.; Paerl, H.; Sigman, D.; Stal, L. Dinitrogen fixation in the world’s oceans. In The Nitrogen Cycle at Regional to Global Scales; Boyer, E.W., Howarth, R.W., Eds.; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar] [CrossRef]
- Gradoville, M.R.; Crump, B.C.; Letelier, R.M.; Church, M.J.; White, A.E. Microbiome of Trichodesmium colonies from the North Pacific Subtropical Gyre. Front. Microbiol. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Devassy, V.P.; Bhattathiri, P.M.A.; Qasim, S.Z. Trichodesmium phenomenon. Indian J. Mar. Sci. 1978, 7, 168–186. [Google Scholar]
- Sathish, T.; Purushothaman, A.; Cicily, L.; Kuttippurath, J.; Padmakumar, K.B. Is Rising Trichodesmium Blooms an Indication of the Warming of Arabian Sea? In Proceedings of the International Symposium on Tropical Meteorology (INTROMET-2021)—Changing Climate: Consequences and Challenge, Online, 23–26 November 2021. [Google Scholar]
- Jyothibabu, R.; Madhu, N.V.; Murukesh, N.; Haridas, P.; Nair, K.K.C.; Venugopal, P. Intense blooms of Trichodesmium erythraeum (Cyanophyta) in the open waters along east coast of India. Indian J. Mar. Sci. 2003, 32, 165–167. [Google Scholar]
- Matondkar, S.G.P.; Parab, S.G.; Desa, E.; Dwivedi, R.M. Basin scale distribution of Trichodesmium spp. in the Arabian Sea using Ocean sat I/OCM. Remote sensing of the marine environment. In Proceedings of SPIE 6406. 64060V; Kawamura, Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–10. [Google Scholar]
- D’Silva, M.S.; Anil, A.C.; Naik, R.K.; D’costa, P.M. Algal blooms: A perspective from the coasts of India. Nat. Hazards 2012, 63, 1225–1253. [Google Scholar] [CrossRef]
- Narvekar, J.; Kumar, S.P. Mixed layer variability and chlorophyll a biomass in the Bay of Bengal. Biogeosciences 2014, 11, 3819–3843. [Google Scholar] [CrossRef]
- Shenoi, S.S.C.; Shankar, D.; Shetye, S.R. Differences in heat budgets of the near-surface Arabian Sea and Bay of Bengal: Implications for the summer monsoon. J. Geophys. Res. Oceans 2002, 107, 5-1. [Google Scholar] [CrossRef]
- Jyothibabu, R.; Maheswaran, P.A.; Madhu, N.V.; Mohammed Ashraf, T.T.; Gerson, V.J.; Haridas, P.C.; Venugopal, P.; Revichandran, C.; Nair, K.K.C.; Gopalakrishnan, T.C. Differential response of winter cooling on biological production in the northeastern Arabian Sea and northwestern Bay of Bengal. Curr. Sci. 2004, 87, 783–791. [Google Scholar]
- Madhu, N.V.; Jyothibabu, R.; Maheswaran, P.A.; John Gerson, V.; Gopalakrishnan, T.C.; Nair, K.K.C. Lack of seasonality in phytoplankton standing stock (chlorophyll a) and production in the western Bay of Bengal. Cont. Shelf Res. 2006, 26, 1868–1883. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef]
- Islam, M.S. Perspectives of the coastal and marine fisheries of the Bay of Bengal, Bangladesh. Ocean Coast. Manag. 2003, 46, 763–796. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.K.; Biswas, H.; De, T.K.; Jana, T.K. Fluxes of nutrients from the tropical River Hooghly at the land-ocean boundary of Sundarbans, NE Coast of Bay of Bengal, India. J. Mar. Syst. 2006, 62, 9–21. [Google Scholar] [CrossRef]
- Patra, P.K.; Kumar, M.D.; Mahowald, N.; Sarma, V.V.S.S. Atmospheric deposition and surface stratification as controls of contrasting chlorophyll abundance in the North Indian Ocean. J. Geophys. Res. Oceans 2007, 112, C05029. [Google Scholar] [CrossRef]
- Rashid, T.; Hoque, S.; Akter, S. Pollution in the Bay of Bengal: Impact on Marine Ecosystem. Open J. Mar. Sci. 2015, 5, 55–63. [Google Scholar] [CrossRef]
- Rahman, M.R. Blue Economy and Maritime Cooperation in the Bay of Bengal: Role of Bangladesh. Procedia Eng. 2017, 194, 356–361. [Google Scholar] [CrossRef]
- Khan, M.G. Bangladesh coastal and marine fisheries, and environment. In Sustainable Management of Fisheries Resources of the Bay of Bengal; Hussain, M.G., Hoq, M.E., Eds.; Support to BOBLME Project; Bangladesh Fisheries Research Institute: Mymensingh, Bangladesh, 2010; pp. 1–35. [Google Scholar]
- Alam, M.W.; Xiangmin, X.; Ahamed, R. Protecting the marine and coastal water from land-based sources of pollution in the northern Bay of Bengal: A legal analysis for implementing a national comprehensive act. Environ. Chall. 2021, 4, 100154. [Google Scholar] [CrossRef]
- Sarker, S.; Hussain, F.A.; Assaduzzaman, M.; Failler, P. Blue economy and climate change: Bangladesh perspective. J. Ocean Coast. Econ. 2019, 6, 6. [Google Scholar] [CrossRef]
- Capone, D.G.; Subramaniam, A.; Montoya, J.P.; Voss, M.; Hamborg, C.; Johansen, A.M.; Siefert, R.L.; Carpenter, E.J. An extensive bloom of the N₂-fixing cyanobacterium Trichodesmium erythraeum in the central Arabian Sea. Mar. Ecol. Prog. Ser. 1998, 172, 281–292. [Google Scholar] [CrossRef]
- Bergman, B.; Sandh, G.; Lin, S.; Larsson, J.; Carpenter, E.J. Trichodesmium—A widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiol. Rev. 2013, 37, 286–302. [Google Scholar] [CrossRef]
- Negri, A.P.; Bunter, O.; Jones, B.; Llewellyn, L. Effects of the bloom-forming alga Trichodesmium erythraeum on the pearl oyster Pinctada maxima. Aquaculture 2004, 232, 91–102. [Google Scholar] [CrossRef]
- Bhat, S.R.; Verlencar, X.N. Some enigmatic aspects of marine cyanobacterial genus, Trichodesmium. Curr. Sci. 2006, 91, 18–19. [Google Scholar]
- Detoni, A.M.S.; Costa, L.D.F.; Pacheco, L.A.; Yunes, J.S. Toxic Trichodesmium bloom occurrence in the southwestern South Atlantic Ocean. Toxicon 2016, 110, 51–55. [Google Scholar] [CrossRef]
- Gianesella-Galvao, S.M.F.; Costa, M.P.F.; Kutner, M.B.B. Bloom of Oscillatoria (Trichodesmium) erythraea (Ehr.) Kutz. in coastal waters of the southern Atlantic. Publ. Espec. Inst. Español De Oceanogr. 1995, 11, 133–140. [Google Scholar]
- Rorig, L.R.; Yunes, J.S.; Kuroshima, K.N.; Schetinni, C.A.F.; Pezzuto, P.R.; Proença, L.O.A. Studies in the ecology and toxicity of Trichodesmium spp. blooms in southern Brazilian coastal waters. In Harmful Algae; Reguera, B., Blanco, J., Fernandez, M.L., Wyatt, T., Eds.; Xunta de Galicia and IOC- UNESCO: Paris, France, 1998; Volume 1, pp. 22–25. [Google Scholar]
- Rorig, L.R.; Guimaraes, S.C.P.; Luigli, D.O.; Proença, L.A.O.; Manzoni, G.C.; Marenzi, A.C. Monitoraçao de microalgas planct onicas potencialmente toxicas na area de maricultura da enseada de Armaçao de Itapocoroy—Penha—SC. Notas. Tec. Facimar. 1998, 2, 71–79. [Google Scholar]
- Naithirithi, T.C.; Lima, A.K.A.; Chellappa, T. Occurrence and dominance of an invasive toxin producing marine cyanobacteria into mangrove environment of the Potengi river estuary, in Natal, Rio Grande do Norte State, Brazil. Arq. De Ciências Do Mar 2005, 38, 19–27. [Google Scholar] [CrossRef]
- Silva, L.M. Ocorrencia de cianobact ^ erias no estu ario e costa adjacente a Lagoa dos Patos, Rio Grande, RS: Avaliaçao preliminar dos riscos a balneabilidade.~Master dissertation. In Programa de Pos-Graduaç ao em Oceanogra~fia Biologica; FURG: Rio Grande, Brazil, 2005; p. 144. [Google Scholar]
- Siqueira, A.; Kolm, H.E.; Brandini, F.P. Offshore distribution patterns of the cyanobacterium Trichodesmium erythraeum Ehrenberg and associated phyto- and bacterioplankton in the southern Atlantic Coast (Parana, Brazil). Braz. Arch. Biol. Technol. 2006, 49, 323–337. [Google Scholar] [CrossRef]
- Carvalho, M.; Giamessela, S.M.F.; Saldanha-Correa, F.M.P. Trichodesmium erythraeum bloom on the continental shelf off Santos, southeast Brazil. Braz. J. Oceanogr. 2008, 56, 307–311. [Google Scholar] [CrossRef]
- Proença, L.A.O.; Tamanaha, M.S.; Fonseca, R.S. Screening the toxicity and toxin content of blooms of the cyanobacterium Trichodesmium erythraeum (ehrenberg) in Northeast Brazil. J. Venom. Anim. Toxins 2009, 15, 204–215. [Google Scholar] [CrossRef]
- Sato, S.; Paranagua, M.N.; Eskinaza, E. On the mechanism of red tide of Trichodesmium in Recife northeastern Brazil, with some considerations of the relation to the human disease Tamandare fever. Trop. Oceanogr. 2016, 5, 7–49. [Google Scholar] [CrossRef][Green Version]
- Zhang, D.S.; Lu, D.D.; Li, H.L.; Ni, X.B.; Wang, C.S.; Jiang, Z.B. Seasonal dynamics of Trichodesmium in the northern East China Sea. Cont. Shelf Res. 2014, 88, 161–170. [Google Scholar] [CrossRef]
- Yap-Dejeto, L.G.; Batula, H.S. Bloom of Trichodesmium (Oscillatoriales, Phormidiaceae) and seasonality of potentially harmful phytoplankton in San Pedro Bay, Leyte, Philippines. Rev. De Biol. Trop. 2016, 64, 897–911. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Brando, V.E.; Lønborg, C.; Leahy, S.M.; Dekker, A.G. Phenology of Trichodesmium spp. blooms in the Great Barrier Reef lagoon, Australia, from the ESA- MERIS 10-year mission. PLoS ONE 2018, 13, e0208010. [Google Scholar] [CrossRef]
- Kashem, M.A.; Siddiqui, A.A.M.; Islam, M.A. Coastal fisheries land use zoning and its potentials of Maheshkhali Upazila, Cox’s Bazar, Bangladesh. Int. J. Fish. Aquat. Stud. 2019, 7, 383–392. [Google Scholar]
- Khan, S.; Jahan, R.; Ahmed, M.U.; Rahman, M.A.; Haque, M.M.; Alom, M.Z. Dynamics of a Heterotrophic Dinoflagellate, Protoperidinium divergens, in the South-Eastern Coastal Waters of the Bay of Bengal. Annu. Res. Rev. Biol. 2019, 33, 1–9. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton Methodik. Verh. Des Int. Ver. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Janson, S.; Siddiqui, P.J.A.; Walsby, A.E.; Romans, K.M.; Carpenter, E.J.; Bergman, B. Cytomorohological characterization of the planktonic diazotrophic Cyanobacteria Trichodesmium erythraeum spp. from the Indian ocean and Caribbean and Sagassosea. J. Phycol. 1995, 31, 463–477. [Google Scholar] [CrossRef]
- Aziz, A.; Mohid, M. Structure, morphogenesis of calyptra and nomenclatural identity of Trichodesmium erythraeum Ehr. (Cyanobacteria) newly recorded off the Southwest coast of Bangladesh. Bangladesh J. Plant Taxon. 2020, 27, 273–282. [Google Scholar] [CrossRef]
- Carpenter, E.J. Physiology and ecology of marine planktonic Oscillatoria (Trichodesmium). Mar. Biol. Lett. 1983, 4, 69–85. [Google Scholar]
- Hegde, S.; Anil, A.C.; Patil, J.S.; Mitbavkar, S.; Krishnamurthy, V.; Gopalakrishna, V.V. Influence of environmental settings on the prevalence of Trichodesmium spp. in the Bay of Bengal. Mar. Ecol. Progress Ser. 2008, 356, 93–101. [Google Scholar] [CrossRef]
- Sahu, B.K.; Baliarsingh, S.K.; Lotliker, A.A.; Parida, C.; Srichandan, S.; Sahu, K.C. Winter thermal inversion and Trichodesmium dominance in north-western Bay of Bengal. Ocean Sci. J. 2017, 52, 301–306. [Google Scholar] [CrossRef]
- Bell, P.R.F.; Uwins, P.J.R.; Elmetri, I.; Phillips, J.A.; Fu, F.X.; Yago, A.J.E. Laboratory culture studies of Trichodesmium isolated from the Great Barrier Reef Lagoon, Australia. Hydrobiologia 2005, 532, 9–21. [Google Scholar] [CrossRef]
- Kumar, M.A.; Padmavati, G.; Pradeep, H.D. Occurrence of Trichodesmium erythraeum (Cyanophyte) bloom and its effects on the fish catch during April 2013, in the Andaman Sea. Appl. Environ. Res. 2015, 37, 49–57. [Google Scholar] [CrossRef]
- Boatman, T.G.; Oxborough, K.; Gledhill, M.; Lawson, T.; Geider, R.J. An integrated response of Trichodesmium erythraeum IMS101 growth and photo-physiology to iron, CO2, and light intensity. Front. Microbiol. 2018, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Dao, H.J.; Wiegand, C. Dynamics of cyanobacteria and cyanobacterial toxins and their correlation with environmental parameters in Tri An Reservoir, Vietnam. J. Water Health 2016, 14, 699–712. [Google Scholar] [CrossRef]
- Okogwu, O.I.; Ugwumba, A.I. Cyanobacteria abundance and its relationship to water quality in the Mid-Cross River floodplain, Nigeria. Rev. De Biol. Trop. 2009, 57, 33–43. [Google Scholar] [CrossRef]
- Khan, S.; Jahan, R.; Rahman, M.A.; Haque, M. Eutrophication enhances phytoplankton abundance in the Maheshkhali channel, Bay of Bengal, Bangladesh. Aust. J. Sci. Techno. 2019, 3, 141–147. [Google Scholar]
- Walsh, J.J.; Jolliff, J.K.; Darrow, B.P.; Lenes, J.M.; Milroy, S.P.; Remsen, A.; Dieterle, D.A.; Carder, K.L.; Chen, F.R.; Vargo, G.A.; et al. Red tides in the Gulf of Mexico: Where, when, and why? J. Geophys. Res. 2006, 111, 1. [Google Scholar] [CrossRef] [PubMed]
- Lenes, J.M.; Darrow, B.A.; Walsh, J.J.; Prospero, J.M.; He, R.; Weisberg, R.H.; Vargo, G.A.; Heil, C.A. Saharan dust and phosphatic fidelity: A three-dimensional biogeochemical model of Trichodesmium as a nutrient source for red tides on the West Florida Shelf. Cont. Shelf Res. 2018, 28, 1091–1115. [Google Scholar] [CrossRef]
- Gomes, H.R.; Goes, J.I.; Saino, T. Influence of physical processes and freshwater discharge on the seasonality of phytoplankton regime in the Bay of Bengal. Cont. Shelf Res. 2000, 20, s0278–s4343. [Google Scholar] [CrossRef]
- Hussain, M.G.; Hoq, M.E. Sustainable Management of Fisheries Resources of the Bay of Bengal; Support to Sustainable Management of the BOBLME Project; Bangladesh Fisheries Research Institute: Mymensingh, Bangladesh, 2010; SBOBLMEP Pub./Rep. 2. [Google Scholar]
- Qasim, S.Z. Some Observations on Trichodesmium blooms, 1st ed.; International Symposium on Taxonomy and Biology of Blue-Green Algae: Madras, India, 1970. [Google Scholar]
- Stal, L.J. Is the distribution of nitrogen-fixing cyanobacteria in the oceans related to temperature? Environ. Microbiol. 2009, 11, 1632–1645. [Google Scholar] [CrossRef]
- Sarker, M.M.; Hossain, M.B.; Islam, M.M.; Mustafa Kamal, A.H.; Idris, M.H. Unravelling the diversity and assemblage of phytoplankton in homestead ponds of central coastal belt, Bangladesh. Aqua. Res. 2021, 52, 167–184. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaika, N.A.; Alhomaidi, E.; Sarker, M.M.; An Nur, A.; Sadat, M.A.; Awal, S.; Mostafa, G.; Hasan, S.J.; Mahmud, Y.; Khan, S. Winter Bloom of Marine Cyanobacterium, Trichodesmium erythraeum and Its Relation to Environmental Factors. Sustainability 2023, 15, 1311. https://doi.org/10.3390/su15021311
Shaika NA, Alhomaidi E, Sarker MM, An Nur A, Sadat MA, Awal S, Mostafa G, Hasan SJ, Mahmud Y, Khan S. Winter Bloom of Marine Cyanobacterium, Trichodesmium erythraeum and Its Relation to Environmental Factors. Sustainability. 2023; 15(2):1311. https://doi.org/10.3390/su15021311
Chicago/Turabian StyleShaika, Nowrin Akter, Eman Alhomaidi, Md. Milon Sarker, Abdullah An Nur, Md. Ashfaq Sadat, Sadiqul Awal, Golam Mostafa, Shanur Jahedul Hasan, Yahia Mahmud, and Saleha Khan. 2023. "Winter Bloom of Marine Cyanobacterium, Trichodesmium erythraeum and Its Relation to Environmental Factors" Sustainability 15, no. 2: 1311. https://doi.org/10.3390/su15021311
APA StyleShaika, N. A., Alhomaidi, E., Sarker, M. M., An Nur, A., Sadat, M. A., Awal, S., Mostafa, G., Hasan, S. J., Mahmud, Y., & Khan, S. (2023). Winter Bloom of Marine Cyanobacterium, Trichodesmium erythraeum and Its Relation to Environmental Factors. Sustainability, 15(2), 1311. https://doi.org/10.3390/su15021311






