Natural Biomass-Derived Porous Carbon from Water Hyacinth Used as Composite Cathode for Lithium Sulfur Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Porous Carbon
2.2. Composite Preparation of Water Hyacinth Porous Carbon_Sulfur
2.3. Characterization
2.4. Electrochemical Measurement
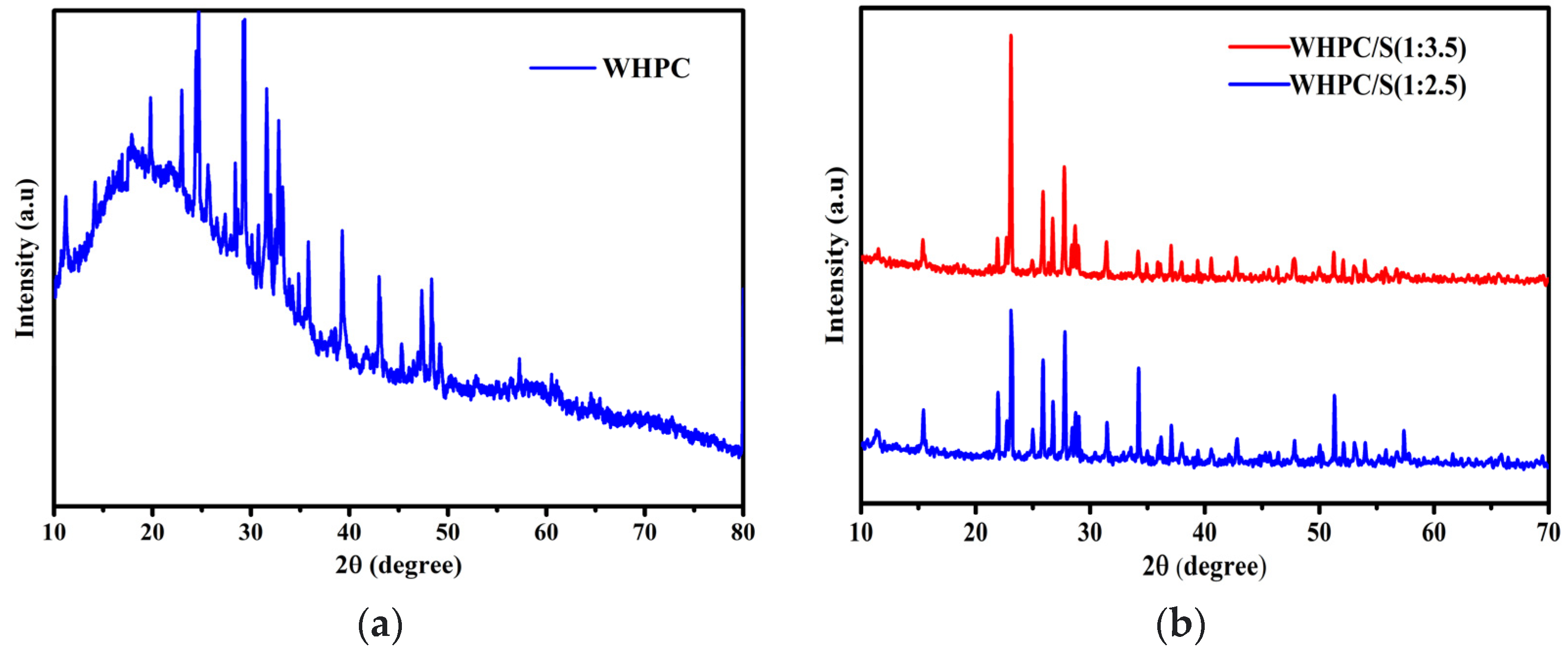
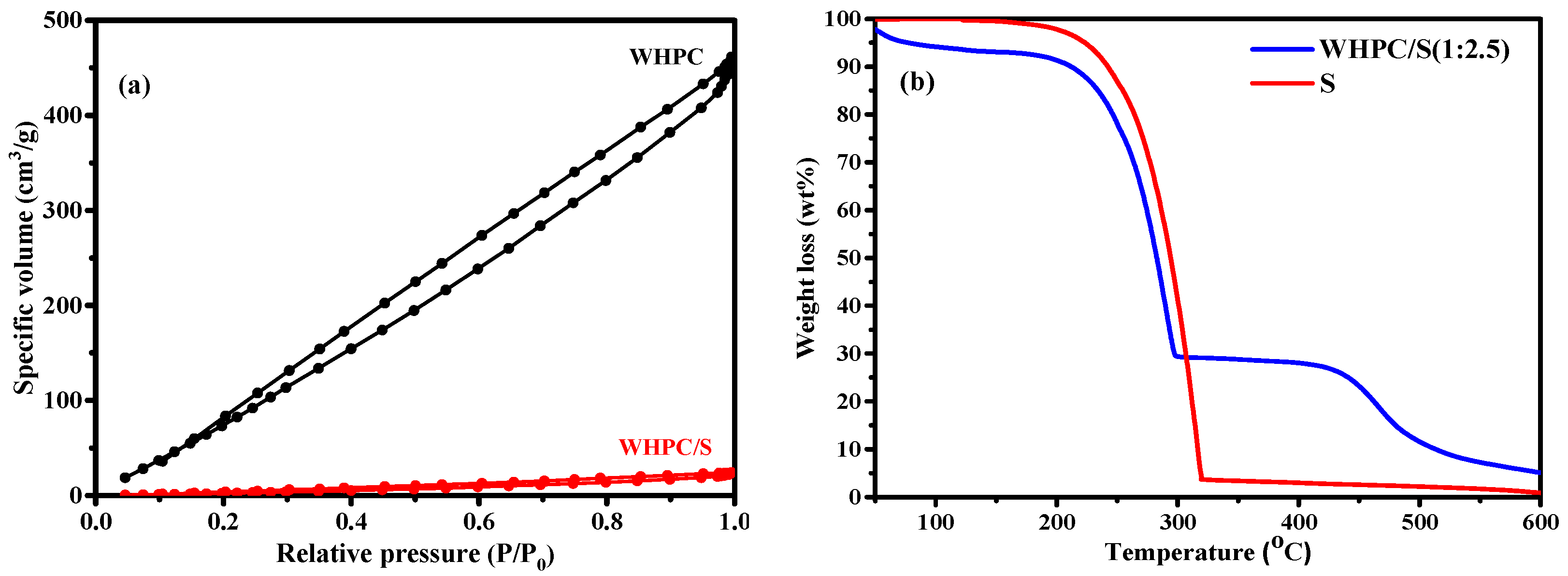
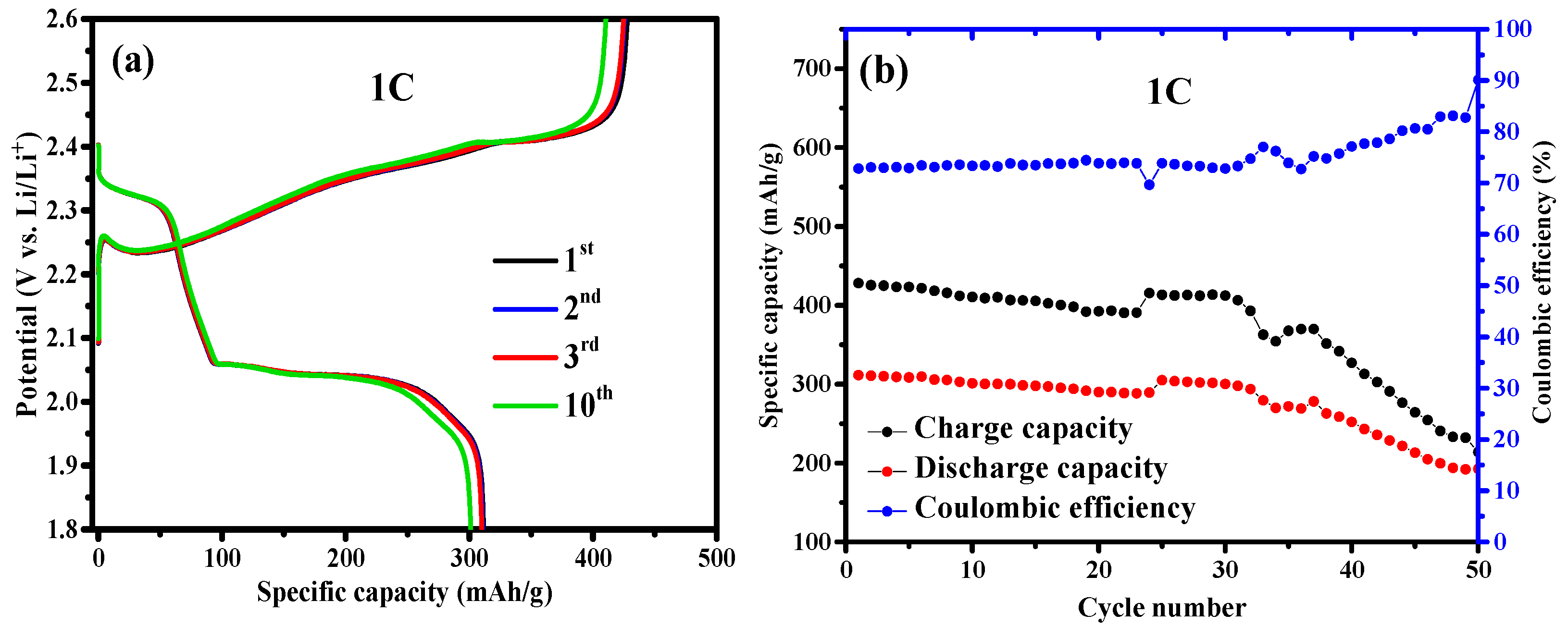
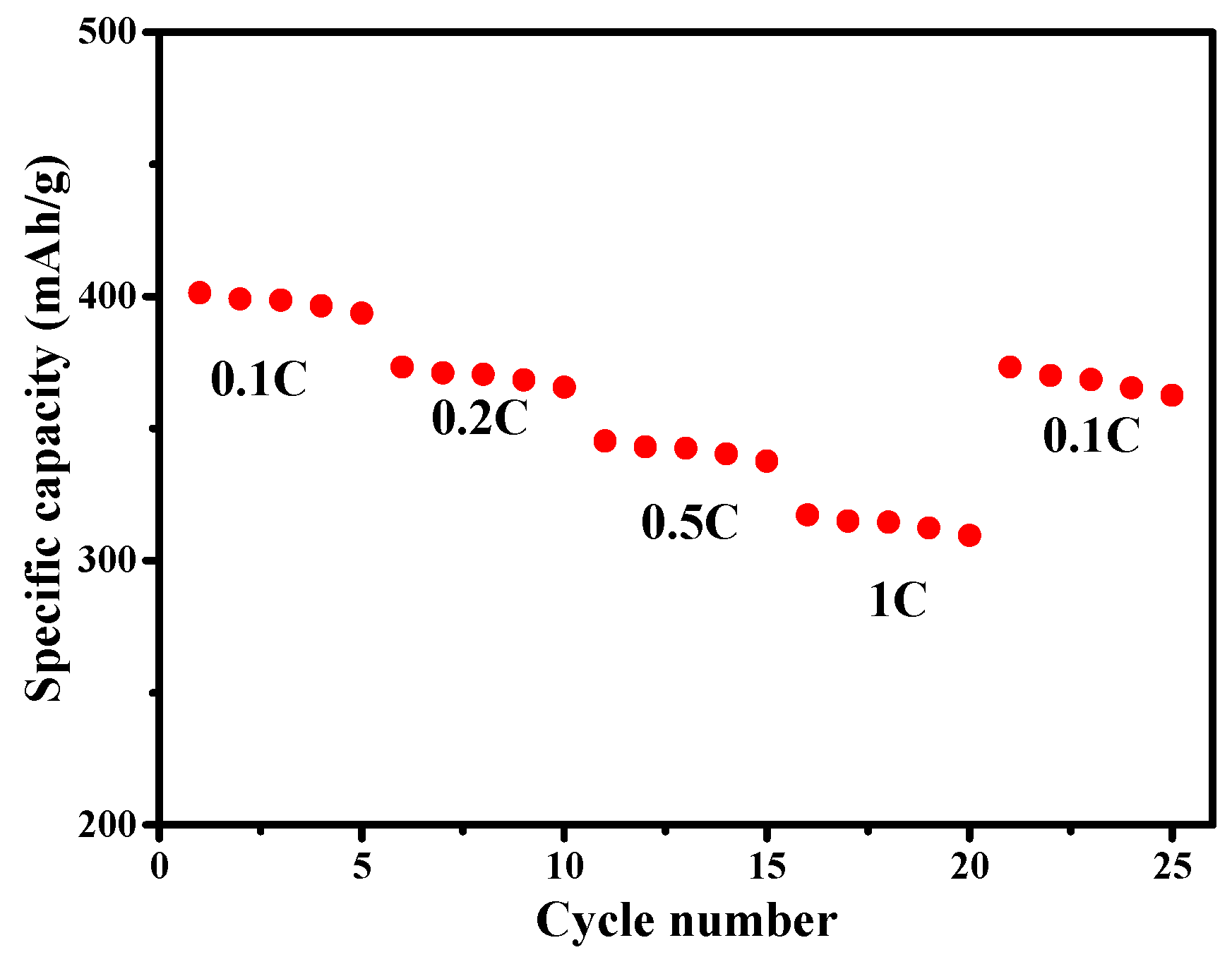
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ling, Z.; Wang, Z.; Wang, M.; Zhang, C.; Yu, G.; Wang, Y.; Dong, S.; Liu, Y.; Wang, J.; Qiu, J. Sustainable synthesis and assembly of biomass-derived B/N Co-Doped carbon nanosheets with ultrahigh aspect ratio for high-performance supercapacitors. Adv. Funct. Mater. 2016, 26, 111–119. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, Y.; Bi, X.; Zhou, T.; Zhou, J.; Zhao, J.; Miao, Z.; Yi, W.; Fu, P.; Zhuo, S. Biochar-based carbons with hierarchical micro-meso-macro porosity for high rate and long cycle life supercapacitors. J. Power Sources 2018, 376, 82–90. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Chen, K.; Zhang, N.; Zhao, X.; Zhao, F.; Dou, Z.; He, X.; Wang, L. Biomass-derived Activated Carbon for Rechargeable Lithium-Sulfur Batterais. Bioreources 2015, 1, 155–168. [Google Scholar]
- Chung, S.-H.; Manthiram, A. A Natural Carbonized Leaf as Polysulfide Diffusion Inhibitor for High-Performance Lithium-Sulfur Battery Cells. ChemSusChem 2014, 7, 1655–1661. [Google Scholar] [CrossRef]
- Yang, K.; Gao, Q.; Tan, Y.; Tian, W.; Zhu, L.; Yang, C. Microporous carbon derived from Apricot shell as cathode material for lithium–sulfur battery. Microporous Mesoporous Mater. 2014, 204, 235–241. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Li, Z.; Chen, P. Effect of mesoporous carbon microtube prepared by carbonizing the poplar catkin on sulfur cathode performance in Li/S batteries. J. Alloys Compd. 2015, 619, 298–302. [Google Scholar] [CrossRef]
- Wei, Y.; Yan, Y.; Zou, Y.; Shi, M.; Deng, Q.; Zhao, N.; Wang, J.; You, C.; Yang, R.; Xu, Y. Sulfonated polyaniline coated bamboo-derived biochar/sulfur cathode for Li-S batteries with excellent dual conductivity and polysulfides affinity. Electrochimica Acta 2019, 310, 45–57. [Google Scholar] [CrossRef]
- Noelia, M.; Caballero, A.; Morales, J.; Rodríguez-Castellon, E. Improved performance of electrodes based on carbonized olive stones/S composites by impregnating with mesoporous TiO2 for advanced LieS batteries. J. Power Sources 2016, 313, 21–29. [Google Scholar]
- Choi, C.; Seo, S.-D.; Kim, B.-K. Enhanced Lithium Storage in Hierarchically Porous Carbon Derived from Waste Tea Leaves. Sci. Rep. 2016, 6, 39099. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Lu, W.; Chen, C.; Tan, Y.; Li, B.; Zhang, C. Optimized synthesis of banana peel derived porous carbon and its application in lithium sulfur batteries. Mater. Res. Bull. 2019, 112, 269–280. [Google Scholar] [CrossRef]
- Xu, Z.; Geng, Z.; Yi, G.; Chen, C.; Xue, M.; Li, B.; Zhang, C. Corncob-derived Porous Carbon as an Interlayer Coating to Improve the Performance of Lithium Sulfur Battery. Int. J. Electrochem. Sci. 2017, 12, 4515–4527. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, Q.; Zhang, S.; Tang, H.; Li, L.; Pang, H. Macroporous Activated Carbon Derived from Rapeseed Shell for Lithium–Sulfur Batteries. Appl. Sci. 2017, 7, 1036. [Google Scholar] [CrossRef]
- Yang, K.; Yan, J.; He, R.; Li, D.; Li, Y.; Li, T.; Ren, B. Nitrogen-doped porous carbon was prepared from peony shell for the cathode material of lithium-sulfur battery. J. Electroanal. Chem. 2020, 861, 113922. [Google Scholar] [CrossRef]
- Qu, J.; Lv, S.; Peng, X.; Tian, S.; Wang, J.; Gao, F. Nitrogen-doped porous “green carbon” derived from shrimp shell: Combined effects of pore sizes and nitrogen doping on the performance of lithium sulfur battery. J. Alloys Compd. 2016, 671, 17–23. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Liu, X.; Zhang, Y.; Zhao, W.; Li, Y.; Qin, C.; Bakenov, Z. High specific surface area bimodal porous carbon derived from biomass reed flowers for high performance lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 569, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Barnscheidt, Y.; Peng, M.; Bettels, F.; Taoran, L.; Tao, H.; Fei, D.; Zhang, L. A Biomass-Based Integral Approach Enables Li-S Full Pouch Cells with Exceptional Power Density and Energy Density. Adv. Sci. 2021, 8, 2101182. [Google Scholar] [CrossRef]
- Rezania, S.; Ponraj, M.; Din, M.F.M.; Songip, A.R.; Sairan, F.M.; Chelliapan, S. The diverse applications of water hyacinth with main focus on sustainable energy and production for new era: An overview. Renew. Sustain. Energy Rev. 2015, 41, 943–954. [Google Scholar] [CrossRef]
- Rop, K.; Mbui, D.; Njomo, N.; Karuku, G.N.; Michira, I.; Ajayi, R.F. Biodegradable water hyacinth cellulose-graft-poly(ammonium acrylate-co-acrylic acid) polymer hydrogel for potential agricultural application. Heliyon 2019, 5, e01416. [Google Scholar] [CrossRef]
- Zeng, G.; Zhou, B.; Yi, L.; Li, H.; Hu, X.; Li, Y. Green and facile fabrication of hierarchical N-doped porous carbon from water hyacinths for high performance lithium/sodium ion batteries. Sustain. Energy Fuels 2017, 2, 855–861. [Google Scholar] [CrossRef]
- Zheng, K.; Li, Y.; Zhu, M.; Yu, X.; Zhang, M.; Shi, L.; Cheng, J. The porous carbon derived from water hyacinth with well-designed hierarchical structure for supercapacitors. J. Power Sources 2017, 366, 270–277. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.S. Challenges and Prospects of Lithium Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From lithium to sodium: Cell chemistry of room suhue sodium–air and sodium–sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Swiderska-Mocek, A.; Rudnicka, R. Lithium sulfur battery with activated carbon cloth-sulfur cathode and ionic liquid as electrolyte. J. Power Sources 2015, 273, 162–167. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Liu, J. Biomass-derived porous carbon materials for advanced lithium sulfur batteries. J. Energy Chem. 2019, 34, 171–185. [Google Scholar] [CrossRef]
- Nurhilal, O.; Winarsih, S.; Hidayat, S.; Sumiarsa, D.; Risdiana, R. High Sulfur Content of Mesoporous Activated Carbon Composite Derived from Water Hyacinth. Sustainability 2021, 13, 12880. [Google Scholar] [CrossRef]
- Li, G.C.; Hu, J.J.; Li, G.R.; Ye, S.H.; Gao, X.P. Sulfur/activated-conductive carbon black composites as cathode materials for lithium/sulfur battery. J. Power Sources 2013, 240, 598–605. [Google Scholar] [CrossRef]
- Manoj, M.; Muhamed Ashraf, C.; Jasna, M.; Anilkumar, K.M.; Jinisha, B.; Pradeep, V.S.; Jayalekshmi, S. Biomass-derived, activated carbon-sulfur composite cathode with a bifunctional interlayer of functionalized carbon nanotubes for lithium-sulfur cells. J. Colloid Interface Sci. 2019, 535, 287–299. [Google Scholar] [CrossRef]

| Elements | Average | |
|---|---|---|
| Weight (%) | Atomic (%) | |
| C | 66.33 | 80.32 |
| O | 9.48 | 8.61 |
| S | 1.56 | 0.71 |
| Cl | 8.67 | 3.56 |
| K | 2.41 | 0.90 |
| Ca | 4.04 | 1.47 |
| Zn | 4.29 | 0.94 |
| Na | 3.22 | 2.09 |
| Total | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurhilal, O.; Hidayat, S.; Sumiarsa, D.; Risdiana, R. Natural Biomass-Derived Porous Carbon from Water Hyacinth Used as Composite Cathode for Lithium Sulfur Batteries. Sustainability 2023, 15, 1039. https://doi.org/10.3390/su15021039
Nurhilal O, Hidayat S, Sumiarsa D, Risdiana R. Natural Biomass-Derived Porous Carbon from Water Hyacinth Used as Composite Cathode for Lithium Sulfur Batteries. Sustainability. 2023; 15(2):1039. https://doi.org/10.3390/su15021039
Chicago/Turabian StyleNurhilal, Otong, Sahrul Hidayat, Dadan Sumiarsa, and Risdiana Risdiana. 2023. "Natural Biomass-Derived Porous Carbon from Water Hyacinth Used as Composite Cathode for Lithium Sulfur Batteries" Sustainability 15, no. 2: 1039. https://doi.org/10.3390/su15021039
APA StyleNurhilal, O., Hidayat, S., Sumiarsa, D., & Risdiana, R. (2023). Natural Biomass-Derived Porous Carbon from Water Hyacinth Used as Composite Cathode for Lithium Sulfur Batteries. Sustainability, 15(2), 1039. https://doi.org/10.3390/su15021039







