Abstract
Chitosan, the deacetylated derivative of chitin, is a biopolymer with many applications in different sectors, such as pharmaceutical, food, and wastewater treatment, amongst others. It can be used as a source for synthesizing bioadsorbents modified with chelators and nanoparticles for the removal of pollutants. In this report, we conducted an exergy analysis to evaluate the large-scale production of chitosan-based bioadsorbents modified with iron nanoparticles and chelators. The objective was to identify energy inefficiencies and propose technological enhancements to improve energy utilization. The process was simulated using Aspen Plus V.10® software, enabling the quantification of chemical and physical exergies for the species and streams involved. We calculated process irreversibilities, exergy losses, waste exergy, and utility exergy flows for each stage and the overall process. These findings provide valuable insights into optimizing energy utilization in the production of chitosan-based bioadsorbents. The overall exergy efficiency was 4.98%, with the washing and drying stages of nanoparticles and adsorbent synthesis accounting for the largest contribution to process irreversibilities and exergy destruction. To increase the global exergy efficiency of the process, it is proposed to implement process improvement strategies, such as mass or energy integration, to obtain better energy performance.
1. Introduction
Seafood is a significant food source in numerous developing countries. According to reports from the Food and Agriculture Organization (FAO) [1], global shrimp catches reach approximately 3.4 million tons per year, with an estimated 50% of the shellfish’s net weight comprising shells [2]. Consequently, the substantial waste generated during manual and industrial seafood processing has become a pressing environmental concern [3]. Considering the above, various technological alternatives have been developed to valorize these residues by synthesizing value-added products. Chitin, one of the most common compounds found in the exoskeletons of shellfish, is widely used to manufacture chitosan [4].
Chitosan is a polysaccharide primarily composed of repeated units of D-glucosamine. It is obtained through the partial de-N-acetylation of chitin, another polysaccharide. In recent years, chitosan has gained considerable interest as a functional biopolymer due to its diverse and intriguing physicochemical and biological properties. It finds applications in various fields such as food, agriculture, medicine, pharmaceuticals, and cosmetics [5]. Chitosan is a biocompatible, water-soluble polymer with various beneficial properties. It is non-toxic and demonstrates antibacterial, antifungal, and antitumor activities [6]. Moreover, it exhibits immuno-enhancing effects in animals and displays antimicrobial properties against a wide range of phytopathogens. The commercial production of chitosan involves several sequential steps, including grinding crustacean shells into a fine powder and deacetylating the powder using a strong alkali [7].
Currently, there is growing interest in sustainable and green-chemistry approaches for synthesizing new materials for emerging industries. Industrial wastewater treatment poses a significant global challenge, with limited water reuse and costly purification being major issues. Stricter laws and regulations have sparked great interest in effective wastewater treatment technologies in recent years [8]. A variety of processes and technologies have been reported in the literature for water treatment, such as chemical coagulation and flocculation [9,10], filtration [11], ultraviolet (UV) disinfection and ozonation [12,13], and membrane separation techniques [14]. Amongst these methods, adsorption has emerged as an effective, efficient, and cost-effective technology [15]. Adsorbents, which can be of mineral, organic, or biological origin, are selected based on their specific applications. While polymeric adsorbents have been extensively used for removing organic pollutants from industrial wastewater, there is growing interest in adsorbents derived from biomasses, organic residues, and biopolymers due to their abundant availability and environmental concerns associated with the disposal of residual biomasses and wastes [16].
In this way, and due to its capacity to adsorb pigments, chitosan is being investigated for use in wastewater treatment as an adsorbent [17]. As a result, it has been functionalized with different compounds to enhance the selectivity and overall efficiency of the adsorption process [18]. Amongst the doping agents used for increasing the adsorption capacity of chitosan are TiO2 [19], magnetite [20], ZnO [19], pandan leaves [21], and metal–organic frameworks [22]. Therefore, synthesizing a novel material with enhanced adsorption and degradation capacity for water pollutants is attractive. Specifically, if applied to industrial development, there is a great opportunity to utilize shellfish waste materials for the production of modified chitosan.
In this matter, it has been found that modifying biopolymers, such as chitosan, with chelating agents like thiourea (CH4N2S) can enhance their selectivity towards certain metals and dyes. Chelating agents contain amine and thiol groups in their structure, which greatly contribute to the selective adsorption of ions. This is because thiol groups can only form stable complexes with the elements, thereby increasing the number of active sites on the surface of the chitosan [23]. Although chitosan modified with thiourea exhibits higher adsorption capacity and selectivity, its recovery efficiency in aqueous solutions remains challenging in industrial applications. To address this issue and avoid the high operating costs associated with specialized separation devices, modification of the bioadsorbents with magnetic particles is proposed. Magnetic separation technology has gained significant attention because of its easy and efficient separation process through the application of an external magnetic field [24].
However, there is a lack of literature on the industrial-scale production of these bioadsorbents. Therefore, it is necessary to assess the scalability of the process. Exergy analysis is a valuable tool that goes beyond the limitations of the first law of thermodynamics. It helps identify the main sources of energy losses and provides insights into the thermal and energy efficiencies of the process. Exergy analysis allows for the quantification of parameters such as irreversibilities, exergy flows through residues, and exergy of utilities, amongst others. This approach is used to screen and evaluate sustainable production alternatives. Several studies have applied exergy analysis to chemical processes. For instance, Pelvan and Ozilgen [25] conducted an energy and exergy assessment of packaged tea production processes and waste valorization. Ma et al. [26] analyzed the calcium carbide production process using energy and exergy assessment. The exergetic efficiency of bio-oil production through fast pyrolysis from different feedstocks was also calculated, showing potential for energy utilization when pyrolysis product streams are recycled [27]. Since exergy analyses require knowledge of thermodynamic properties and process flows, computer-aided process engineering (CAPE) tools, such as process simulation software, are employed. Kartal and Özveren [28] used Aspen HYSYS for energy and exergy analyses of an entrained bed gasifier/GT/Kalina cycle model for CO2 co-gasification of waste tires and biochar.
The potential of a macroalgae-based biorefinery for the production of lactic acid was investigated from an exergetic point of view, finding overall exergy efficiencies of 66.65%, 90.3%, and 90.93% when using steam explosion, organosolov, and microbial, respectively, as pretreatment methods [29]. In another study, the energy efficiency of different biorefinery schemes utilizing sugarcane bagasse as the feedstock was investigated through process simulation and exergy analysis, they assessed the performance of these schemes. The findings demonstrated the utility of exergy analysis as an effective tool for evaluating novel technologies with sustainability objectives [30]. By employing exergy analysis, it was possible to gain valuable insights into the energy utilization and efficiency of the biorefinery processes. This approach proved to be instrumental in diagnosing areas that required improvement and optimizing the overall sustainability performance of the biorefinery schemes. The study highlights the significance of considering exergy analysis as a diagnostic instrument when pursuing sustainability goals in the development and assessment of new technologies in the biorefinery sector.
This research aims to evaluate the industrial-scale performance of chitosan-based bioadsorbent modified with iron nanoparticles and chelators using exergy assessment. Thiourea was chosen as the chelant agent, considering experimental data and previous information published by the research group [31]. The process scaling was carried out using computer-aided process engineering (CAPE), alongside with data obtained from laboratory-scale synthesis of the bioadsorbents [31]. This approach offers valuable insights into exergy flows, irreversibilities, unit efficiency, and other important factors, providing a comprehensive understanding of the process performance and identifying potential areas for improvement from an exergy/energy standpoint. Conserving energy quality contributes to improving economic, environmental, and process safety aspects, such as the extension of social impacts. Therefore, this exergy analysis contributes to generating insights that enable process improvement from a sustainability perspective. The novelty of this work is that for the first time, the exergetic analysis methodology is used to evaluate, from an energy quality perspective, the emerging process of producing chitosan-based bioadsorbents modified with magnetite nanoparticles and thiourea. The aim is to make an initial approximation to predict its behavior at an industrial scale from an energy standpoint.
2. Materials and Methods
The production process of the chitosan-based adsorbent functionalized with Fe3O4 nanomaterial was subjected to exergy analysis using Aspen Plus® software. The simulation was conducted considering a processing capacity of 90.36 kg/h for Fe3O4 and 180.71 kg/h for chitosan, taking into account the specific conditions in the northern region of Colombia as room temperature, availability of freshwater, and availability of process utilities, among others. To perform the simulation, necessary information such as operating conditions, mass balances, and energy balances was gathered from experimental data and relevant literature sources. The simulation aimed to replicate the actual production process and analyze the exergy performance of each step. By inputting the specific parameters and constraints of the system, the Aspen Plus® software provided valuable insights into the energy efficiency and exergy losses throughout the process. The results obtained from the simulation were crucial for identifying areas of improvement and optimizing the overall exergetic performance of the chitosan-based adsorbent production process. By calculating irreversibilities and exergy losses at each process step, critical stages were identified, providing insights for process optimization [32].
2.1. Process Description
To determine the critical areas from an energy perspective, the process was divided into nine steps (Figure 1).
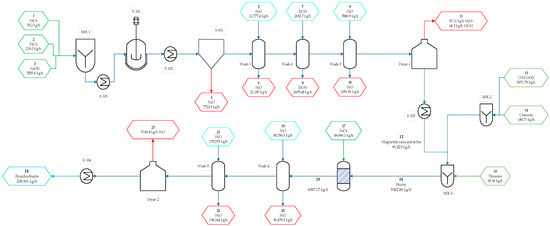
Figure 1.
Stages of the production process of chitosan microbeads modified with thiourea-magnetite nanoparticles for exergy analysis.
The stages of the process are described in Table 1:

Table 1.
Stages of the production process of chitosan microbeads modified with thiourea-magnetite nanoparticles.
2.2. Thermodynamic Modelling
The exergy analysis conducted in this study was made considering the process configuration, operating conditions, compositions, and mass flow of the process streams. Some basic assumptions made for performing the complete exergy analysis of the process were:
- The process operates in steady state and constant flow;
- Kinetic and potential exergies were not taken into account, considering that their magnitudes are not significant in comparison with the physical and chemical exergies [38,39];
- Temperature of reference is 298.15 K.
2.3. Exergy Analysis
The governing equations for the exergetic analysis are outlined below. It is important to note that the exergy balance deviates from the energy conservation principle in this analysis, as it takes into account exergy losses associated with system irreversibilities [40]. Exergy analysis enables the identification and quantification of unused energy within a system or process, making it a valuable tool for identifying potential optimization conditions to improve the process. It also facilitates the evaluation and selection of different alternatives, thereby contributing to the optimal design of the process [41].
To carry out an exergy analysis, the principles of the first and second laws of thermodynamics are used to quantify inefficiencies and understand their origin and cause. For this purpose, a mass and energy balance must be performed, which are fundamental conditions for conducting the exergy balance and evaluating the system’s performance. The exergy balance of a facility collects the inflows of exergy entering the system and the outflows of exergy obtained from it. These outflows of exergy leaving the system can be divided into two categories: useful exergy flows and residual exergy flows [42]. Through the exergy analysis, the amount of exergy lost in each component of the process is calculated. In the case of a steady-state exergy balance, the destruction of exergy is related to the net exergies of mass transfer, work, and heat transfer through Equation (2).
The exergy associated with work in a system where there is no change in volume is equal to the work performed by the system itself, and is calculated by Equation (3):
For the calculation of exergy by heat, Equation (4) is used based on Carnot efficiency, which represents the fraction of energy transferred from a heat source at temperature T that can be converted into work in an environment at the reference temperature To:
The exergy related to mass flow, in the absence of electrical, magnetic, nuclear, and surface tension effects, is defined as shown in Equation (5), where kinetic () and potential () exergies can be considered negligible for being significantly low compared to chemical and physical exergies:
The physical exergy by each component is defined by the Equation (6):
Regarding physical exergy, if the stream behaves as an ideal gas with constant heat capacity, Equation (7) can be used. Alternatively, if the stream is in a solid or liquid state with a constant specific heat, Equation (8) can be applied.
Chemical exergy by each component is determined by the standard formation energy of the compound plus the stoichiometric sum of the standard chemical exergies of its constituent elements, which can be calculated using Equation (9):
The chemical exergy of a mixture is defined by its components and their respective compositions, as shown in Equation (10):
The measurement of total irreversibilities in the system, denoted as , involved calculating the difference between the exergy input and output of the product streams or stage outputs using Equation (11). Additionally, Equation (12) was used to determine the non-avoidable irreversibilities, referred to as by considering the exergy values of the products and residues relative to the total exergy output. These equations allowed for the quantification of the system’s irreversibilities and provided insights into the energy losses and inefficiencies associated with the process.
The calculation of the exergies of the output products, is performed by the sum of the exergies associated with the mass transfer of the streams leaving the stage and containing the desired product; in our case this is the chitosan-based bioadsorbent modified with iron nanoparticles and chelators. Similarly, the calculation of the exergy lost with the residual streams of the process is performed by identifying in each stage the exergies associated with the mass transfer of the residual streams leaving the process. After that, they are summed up to obtain the total exergy lost due to waste throughout the process.
Once the exergy of the products and the exergy of the waste has been calculated for each stage of the process, the calculation of the unavoidable exergy losses is performed using Equation (12). To achieve this, the exergy of the total input to each stage is subtracted from the exergy that exits with the mass streams, i.e., both the exergy of the products and the exergy of the waste. With the obtained information, the exergy destroyed in each stage is calculated, which corresponds to both avoidable and unavoidable losses, using Equation (11). The total exergy destroyed in the process can also be calculated by summing the irreversibilities of each stage.
The exergy efficiency ( can be calculated as Equation (13) and, highlights that losses and internal irreversibilities are to be assessed to improve the performance of the process. The contribution of the process by stage i to global exergy () is determined as Equation (14).
Using Equation (14), the percentage of the total exergy destroyed attributed to each stage is calculated. This information helps determine which stages are critical in the process. The percentage of exergetic efficiency for each stage is calculated using Equation (14), taking into account that the required information (exergy destroyed and total input exergy) is only for the specific stage being studied. With the overall input exergy associated with mass transfer and the total input exergy from industrial services, we proceed to calculate the total input exergy. Using this value and the total irreversibilities of the process, we can calculate the overall exergy of the process by once again applying Equation (14).
For the exergy assessment, initially the physical and chemical exergies for each stream in the process were calculated based on information from simulation and the literature. Subsequently, the total exergy for each stream, the exergy of the heat input, and the work-related exergy of each piece of equipment were calculated. The process was divided into the following 9 stages: (1) Raw material mixing for iron nanoparticle synthesis, (2) Reaction, (3) Magnetic separation, (4) Iron nanoparticle washing, (5) Iron nanoparticle drying, (6) Raw material mixing for the production chitosan-based bioadsorbent modified with iron nanoparticles and thiourea, (7) Formation of chitosan-based bioadsorbent modified with iron nanoparticles and thiourea, (8) Modified bioadsorbent washing, and (9) Bioadsorbent modification drying. These stages were individually assessed to determine their performance. The total input exergy of each stage was then calculated, taking into account the input exergy by mass and the utilities, in order to determine the total irreversibilities, exergy attributable to residues, and losses at both the stage and global levels. Finally, the exergy efficiency of each stage and their contribution to exergy loss were measured.
3. Results and Discussion
The production of chitosan-based adsorbent functionalized with Fe3O4 nanomaterial was simulated using Aspen Plus® software. The process operated at a production rate of 2.03 tons per hour, and all the necessary chemical species were readily available in the software’s extensive database. This eliminated the need for manually adding or creating new molecules, streamlining the simulation process. The thermodynamic properties of the mixture were calculated based on the molecular structures of each species, and the non-random two liquids (NRTL) thermodynamic model was chosen due to the diverse nature of the substances present in the process, including polar, non-polar, and electrolyte components. Through the simulations performed in Aspen Plus®, the physical exergies of the streams within the production process were determined. Physical exergy provides valuable insights into the maximum useful work that can be extracted from a stream when it reaches thermodynamic equilibrium with the environment. Additionally, the chemical exergies of the compounds listed in Table 2 were obtained from the literature [43,44]. These chemical exergy values, obtained at standard conditions of 298.15 K and 1 bar, were utilized in the calculation of the chemical exergies of the streams, and were employed in the calculation of the chemical exergies of the streams.

Table 2.
Standard Chemical Exergies of the compounds present in the processes under study [43,44].
The result of the exergetic analysis for the main streams of the BMCMN synthesis process is presented in Table 3, obtained from the implementations of Equations (2)–(12).

Table 3.
Physical, chemical, and global exergies of the main streams in the BMCMN synthesis process.
The results of the overall exergetic performance of the evaluated alternatives are presented in Table 4. It was observed that the rejected exergy of the residues accounted for the largest proportion of the exergy destruction in the process. This highlights the significance of the generation of residues and the utilization of utilities in relation to the requirements of flows and equipment.

Table 4.
Global exergetic performance.
The exergy assessment of the bioadsorbent production process, specifically bio-chitosan-based adsorbents modified with chelators and TiO2 or TiO2-magnetite nanoparticles, was conducted by Meramo et al. [45], Their study reported total irreversibilities of 117 GJ h−1, 182 GJ h−1, and 217 GJ h−1 for the production of 155 kg h−1, 232 kg h−1, and 307 kg h−1 of each respective adsorbent. These values, when compared on a per kilogram basis, were higher than those obtained in the present study, indicating that the initial stage of the process significantly contributed to the irreversibilities observed. It is important to note that the aforementioned values solely accounted for the irreversibilities within the process itself and did not consider those associated with waste generation. Waste-related irreversibilities can contribute to energy losses during the process. Additionally, the exergy due to utilities, which represents the energy requirements of the process, was estimated to be approximately 5 GJ h−1. This suggests that industrial services have a relatively minor impact on the overall irreversibilities compared to the exergy contributed by the input mass. Hence, the development of novel treatment technologies holds promise for reducing energy consumption and improving the exergy input for industrial services. By implementing efficient and sustainable techniques, it is possible to reduce energy losses and enhance the overall exergetic performance of the bioadsorbent production process. This highlights the importance of continued research and innovation in the field of treatment technologies to optimize energy consumption and improve the sustainability of manufacture of bioadsorbent.
The bioadsorbent production alternative using chitosan, as presented in this study, exhibited a low global exergy efficiency of 4.98%. This finding aligns with reported data in the literature on bioadsorbent production processes, where exergetic efficiencies ranged from 0.04% [45] to 2.5% [46]. These results underscore the necessity for further enhancements in the energy efficiency of the process, as well as the potential for novel treatment technologies to bolster overall exergetic performance. The observed low efficiencies imply significant room for technological advancements to achieve improved exergetic and energy indicators, emphasizing the importance of ongoing research-and-development efforts in this field.
Furthermore, Figure 2 and Figure 3 help to provide a comprehensive analysis of exergy efficiency and exergy losses per stage, categorized by product type. The results shed light on the critical stages within the entire process. Specifically, stage 4, the washing of nanoparticles, emerged as a key stage in terms of exergetic considerations. It accounted for approximately 63% of the total irreversibilities, resulting in a substantial exergy destruction of 50 GJ/h [46]. The majority of these irreversibilities (approximately 99.6%) can be attributed to the exergy of residues, primarily stemming from the removal of a significant amount of water and residual ethanol. This highlights the need to address the inefficiencies in the washing stage to enhance the overall exergetic performance of the process. By implementing strategies aimed at minimizing exergy losses associated with water and residual ethanol removal, the exergy efficiency can be improved, resulting in a reduction in overall irreversibilities. These improvements contribute to a more sustainable and efficient production process for bioadsorbents. It is imperative to focus efforts on optimizing the exergetic performance of the washing stage to achieve the desired sustainability and efficiency goals. The chemical production process of dimethyl ether (DME) and methanol, fueled by 25% of the CO2 captured from a natural gas combined-cycle (NGCC) power plant and by green hydrogen generated through an electrolyzer, achieved an exergetic efficiency of 75.74%. The primary source of exergy destruction was attributed to the distillation column that separated the outlet stream of the reactor. Notably, the top-stage condenser emerged as the component with the most significant irreversibility (accounting for 45% of the total), closely followed by the methanol/DME synthesis reactor (24%) [47]. In the synthesis of Methyl chlorid an exergy efficiency of 87.3% was reached, in which the reactor had the highest contribution to exergy destruction, followed by the heat exchange network [48]. The recovery of low-emission alcohol from wastewater showed losses in the conventional extractive distillation process (CEDP), heat pump-assisted extractive distillation process (HPEDP), thermally coupled extractive distillation processes (TCEDP), and heat pump-assisted thermally coupled extractive distillation process (HPTCEDP) of 64.54%, 51.69%, 56.77%, and 46.04%, respectively. The exergy loss of the HPTCEDP was the lowest, indicating that mechanical heat pump technology can significantly enhance the thermodynamic efficiency of the process [49].
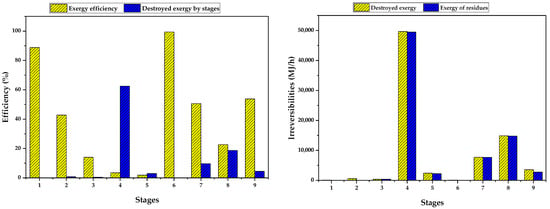
Figure 2.
Exergy efficiency and contribution to exergy loss per stage.
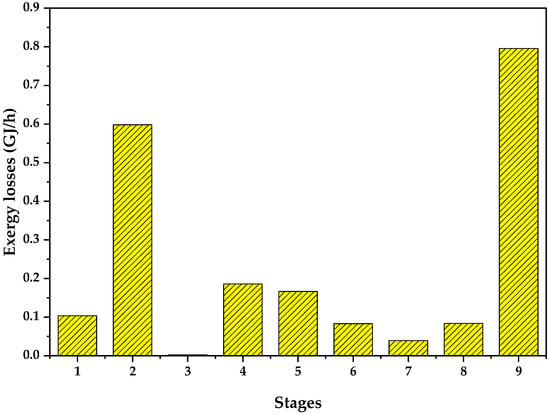
Figure 3.
Exergy loss per stage.
The analysis revealed that the washing of microbeads in Step 8 emerged as the second largest contributor to exergy destruction, underscoring the importance of the purification steps. Interestingly, stages 3 and 8 demonstrated improved exergetic efficiency, thanks to the utilization of residual streams from these stages as inputs for the corresponding washings in stages 4 and 8. Conversely, stage 5 (nanoparticles drying step) exhibited low exergetic efficiency, presenting significant opportunities for enhancement through optimization techniques. Optimization strategies, such as mass and energy integration, could be implemented to reduce reliance on industrial services and fresh materials, like ethanol, in the purification stages. By employing these strategies, the exergetic efficiency of the process can be enhanced, resulting in minimized exergy losses and a more sustainable and resource-efficient production of bioadsorbents. The implementation of such optimization measures would contribute to the overall improvement of the processes performance and its environmental footprint [45].
Stages 1 and 3, shown to be highly efficient. The mixing stages are usually more exergetically efficient because they primarily involve mass transfer and do not have significant energy losses. Compared to other stages of the process, such as chemical reactions or separations, mixing has lower exergy destruction and therefore higher efficiency in transferring and distributing thermodynamic properties of the streams. Additionally, mixing tends to be a simpler and less complex operation from a thermodynamic perspective, which contributes to its higher exergy efficiency, as reported by Gholami et al. [50] and Peralta-Ruiz et al. [41].
The purpose of analyzing of the contributions of each stage to the non-avoidable exergy losses of the system was to gain a deeper understanding of the critical stages, while also excluding the exergy destruction due to residues. The outcomes of this analysis are depicted in Figure 3, providing a visual representation of the significant contributions of each stage to the overall inefficiencies of the system. By focusing solely on the non-avoidable exergy losses, it becomes easier to identify specific stages that require attention and improvement, thus optimizing the overall exergy performance of the process. This information is invaluable for strategic targeting and enhancements of the system’s efficiency.
Based on the analysis of non-avoidable exergy losses presented in Figure 3, it can be observed that the stages with the highest irreversibilities are associated to the drying of the chitosan-based bioadsorbent and the reaction for magnetite nanoparticle production [32]. These particular stages are greatly influenced by the demands of utilities, such as the extraction of energy from the streams that is not effectively utilized, as seen in the cooling cycles. This contributes significantly to the unavoidable exergy losses experienced throughout the process. Conversely, stage 3, which involves magnetic separation, exhibits a minimal exergy loss compared to the other evaluated stages. Moreover, it is important to emphasize that the overall exergy losses are relatively small when compared to the exergy of the residues generated in the process. These findings emphasize the need for further optimization and improvement in the utilization of energy and resources within the system, aiming to reduce the overall exergy losses and enhance the overall sustainability of the process.
In the exergy assessment performed for an integrated plant that recovers NGL and produces electricity via waste gas for the energy conversion process [51], it was found that the high exergy destruction in the steam power cycle is due to the high calorific value of fuel in it. Otherwise, the steam cycle does not display substantial exergy destructions since the working fluid is steam produced through the steam cycle process. The 78% exergy destruction in the NGL recovery plant was caused by the heat exchangers. Also, it was found that an infinitesimal amount of exergy was destroyed in the condenser.
In Figure 4, the contribution of each stage to the total destroyed exergy is shown. The washing stage of the iron nanoparticles represents the highest losses in the process, accounting for 66.2%. This is mainly due to the use of ethanol as a solvent, where a significant portion of the exergy is destroyed due to concentration and pressure differences between the stream being washed and the solvent used. The subsequent stages that contribute to the exergy destruction are the washing of the bioadsorbents and the formation of the bioadsorbents, with values ranging from 10–13%. The drying stages of the nanoparticles and bioadsorbents contribute 3.8% and 4.9%, respectively. The remaining stages have contributions below 1%, indicating that there is minimal exergy loss in the stages associated with mixing and the formation reactions of the nanoparticles and bioadsorbents. Additionally, there is the possibility of utilizing the solvents used during the washing stages, as well as reusing the processed water.
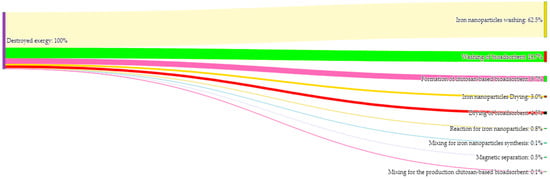
Figure 4.
Sankey irreversibilities flow diagram of the process by stages.
During the biorefinery process for cathecol production from lignin, finding that the biomass fractionation stage contributed to the 45% of exergy losses, mainly caused to the heating and condensers equipment associated with the higher heat transfer between input and output streams [52]. Under biorefinery approach of synthetic biofuel production via fast pyrolysis and hydro-upgrading of poplar wood, it was also obtained an overall 77.7% exergy efficiency, with the steam reforming reactor as the main source of inefficiencies, followed by the two hydrotreating reactors [53]. It was also shown for a lignocellulosic-based biorefinery annexed to a sugarcane mill for simultaneous lactic acid and electricity production, a total irreversibility rate of 205.4 M W for the biorefinery system, in which the steam generation unit was the main contributor with a 63% of the total [54].
4. Conclusions
This research employed computer-aided exergy analysis to evaluate the large-scale production of chitosan-based bioadsorbents modified with thiourea and magnetite. The stages of washing and drying for both nanoparticles and bioadsorbent were identified as the process sections with the highest irreversibilities, contributing to 63% of the total exergy losses. A significant portion (99.6%) of the irreversibilities were attributed to the exergy of residues, primarily associated with the residual water and ethanol. The overall exergy efficiency of the process was calculated at 4.96%, indicating suboptimal utilization of waste and water flows. To enhance the process, implementing strategies such as process integration is necessary. For future research, it is recommended to assess other sustainability aspects, including safety, health, and operational uncertainty. Additionally, incorporating process optimization strategies such as process intensification, energy integration, and process resilience would provide valuable insights to augment resource conservation, curtailing energy consumption, mitigating waste generation, and amplifying the overall exergy efficiency of the process. These enhancements would result in heightened cost-effectiveness, bolstered environmental sustainability, and heightened operational flexibility, thus culminating in the fortification and adaptability of the production framework.
Author Contributions
Conceptualization, Á.D.G.-D.; methodology, Á.D.G.-D.; software, F.B.-P. and G.C.-C.; validation, Á.D.G.-D.; formal analysis, F.B.-P. and G.C.-C.; investigation, F.B.-P., G.C.-C. and Á.D.G.-D.; resources, Á.D.G.-D.; data curation F.B.-P. and G.C.-C.; writing—original draft preparation, F.B.-P. and G.C.-C.; writing—review and editing, Á.D.G.-D.; visualization, F.B.-P. and G.C.-C.; supervision, Á.D.G.-D.; project administration, Á.D.G.-D.; and funding acquisition, Á.D.G.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Á.D.G.-D., upon reasonable request.
Acknowledgments
The authors thank the Universidad de Cartagena to provide the software and required equipment to successfully conclude this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ambigaipalan, P.; Shahidi, F. Bioactive peptides from shrimp shell processing discards: Antioxidant and biological activities. J. Funct. Foods 2017, 34, 7–17. [Google Scholar] [CrossRef]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Beevi, A.H.; Anand, M.; Ramakritinan, C.; Kumaraguru, A. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front. Lab. Med. 2018, 2, 30–35. [Google Scholar] [CrossRef]
- Muñoz, I.; Rodríguez, C.; Gillet, D.M.; Moerschbacher, B. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Optimization of chitin and chitosan production from shrimp wastes and characterization. J. Food Process. Preserv. 2018, 42, e13494. [Google Scholar] [CrossRef]
- Rebello, S.; Sali, S.; Jisha, M.; Reshmy, R.; Pugazhendhi, A.; Madhavan, A.; Binod, P.; Awasthi, M.K.; Pandey, A.; Sindhu, R. Chitosan a versatile adsorbent in environmental remediation in the era of circular economy-a mini review. Sustain. Chem. Pharm. 2023, 32, 101004. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; De Lima, M.A.B.; de Oliveira Franco, L.; De Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Bhojwani, S.; Topolski, K.; Mukherjee, R.; Sengupta, D.; El-Halwagi, M.M. Technology review and data analysis for cost assessment of water treatment systems. Sci. Total. Environ. 2019, 651, 2749–2761. [Google Scholar] [CrossRef]
- Rebah, F.; Mnif, W.; Siddeeg, S.M. Microbial Flocculants as an Alternative to Synthetic Polymers for Wastewater Treatment: A Review. Symmetry 2018, 10, 556. [Google Scholar] [CrossRef]
- Wei, H.; Ren, J.; Li, A.; Yang, H. Sludge dewaterability of a starch-based flocculant and its combined usage with ferric chloride. Chem. Eng. J. 2018, 349, 737–747. [Google Scholar] [CrossRef]
- Andreoli, F.; Sabogal-Paz, L. Household slow sand filter to treat groundwater with microbiological risks in rural communities. Water Res. 2020, 186, 116352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Srivastava, V.; Ambat, I.; Safaei, Z.; Sillanpää, M. Degradation of Ibuprofen by UV-LED/catalytic advanced oxidation process. J. Water Process. Eng. 2019, 31, 100808. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M. Membrane Separation Process in Wastewater and Water Purification. Membranes 2022, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent advances in heavy metal removal by chitosan based adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; El-Monaem, E.M.A.; Ziora, Z.M. Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Phasuphan, W.; Praphairaksit, N.; Imyim, A. Removal of ibuprofen, diclofenac, and naproxen from water using chitosan-modified waste tire crumb rubber. J. Mol. Liq. 2019, 294, 111554. [Google Scholar] [CrossRef]
- Farhadian, N.; Akbarzadeh, R.; Pirsaheb, M.; Jen, T.-C.; Fakhri, Y.; Asadi, A. Chitosan modified N, S-doped TiO2 and N, S-doped ZnO for visible light photocatalytic degradation of tetracycline. Int. J. Biol. Macromol. 2019, 132, 360–373. [Google Scholar] [CrossRef]
- Karimi, F.; Ayati, A.; Tanhaei, B.; Sanati, A.L.; Afshar, S.; Kardan, A.; Dabirifar, Z.; Karaman, C. Removal of metal ions using a new magnetic chitosan nano-bio-adsorbent; A powerful approach in water treatment. Environ. Res. 2022, 203, 111753. [Google Scholar] [CrossRef]
- Razmi, F.A.; Ngadi, N.; Wong, S.; Inuwa, I.M.; Opotu, L.A. Kinetics, thermodynamics, isotherm and regeneration analysis of chitosan modified pandan adsorbent. J. Clean. Prod. 2019, 231, 98–109. [Google Scholar] [CrossRef]
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, J.; Liu, Z. Adsorption of platinum(IV) and palladium(II) from aqueous solution by thiourea-modified chitosan microspheres. J. Hazard. Mater. 2009, 172, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.H.; Mahdavinia, G.R.; Massoumi, B.; Baghban, A.; Saraei, M. Ionically crosslinked magnetic chi-tosan/kappa-carrageenan bioadsorbents for removal of anionic eriochrome black-T. Int. J. Biol. Macromol. 2018, 113, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Pelvan, E.; Özilgen, M. Assessment of energy and exergy efficiencies and renewability of black tea, instant tea and ice tea production and waste valorization processes. Sustain. Prod. Consum. 2017, 12, 59–77. [Google Scholar] [CrossRef]
- Ma, S.; Lu, S.; Ma, H.; Li, R.; Xu, C.; Chen, M.; Zhang, H. Energy and exergy analysis of a new calcium carbide production process. Fuel Process. Technol. 2022, 226, 107070. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A.; Osgood-Jacobs, L.; Carlson, P.; Macken, N. Mass Balance, Energy, and Exergy Analysis of Bio-Oil Production by Fast Pyrolysis. J. Energy Resour. Technol. 2012, 134, 042001. [Google Scholar] [CrossRef]
- Kartal, F.; Özveren, U. Energy and exergy analysis of entrained bed gasifier/GT/Kalina cycle model for CO2 co-gasification of waste tyre and biochar. Fuel 2023, 331, 125943. [Google Scholar] [CrossRef]
- Chung, M.R.W.Y.; Tan, I.S.; Foo, H.C.Y.; Lam, M.K.; Lim, S. Potential of macroalgae-based biorefinery for lactic acid production from exergy aspect. Biomass-Convers. Biorefin. 2023, 13, 2623–2653. [Google Scholar] [CrossRef]
- Restrepo-Serna, D.L.; Martínez-Ruano, J.A.; Cardona-Alzate, C.A. Energy Efficiency of Biorefinery Schemes Using Sugarcane Bagasse as Raw Material. Energies 2018, 11, 3474. [Google Scholar] [CrossRef]
- Solano, R.A.; De León, L.D.; De Ávila, G.; Herrera, A.P. Polycyclic aromatic hydrocarbons (PAHs) adsorption from aqueous solution using chitosan beads modified with thiourea, TiO2 and Fe3O4 nanoparticles. Environ. Technol. Innov. 2021, 21, 101378. [Google Scholar] [CrossRef]
- González-Delgado, Á.D.; Moreno-Sader, K.A.; Martínez-Consuegra, J.D. Sustainable Biorefining of Shrimp: Developments from Computer Aided Process Engineering; Corporación Universitaria Minuto de Dios—UNIMINUTO: Bogotá, Colombia, 2022; (In Spanish). [Google Scholar] [CrossRef]
- Tao, K.; Dou, H.; Sun, K. Interfacial coprecipitation to prepare magnetite nanoparticles: Concentration and temperature dependence. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 115–122. [Google Scholar] [CrossRef]
- Alfaro, I.; Molina, L.; González, P.; Gaete, J.; Valenzuela, F.; Marco, J.F.; Sáez, C.; Basualto, C. Silica-coated magnetite nanoparticles functionalized with betaine and their use as an adsorbent for Mo(VI) and Re(VII) species from acidic aqueous solutions. J. Ind. Eng. Chem. 2019, 78, 271–283. [Google Scholar] [CrossRef]
- Yazdani, F.; Edrissi, M. Effect of pressure on the size of magnetite nanoparticles in the coprecipitation synthesis. Mater. Sci. Eng. B 2010, 171, 86–89. [Google Scholar] [CrossRef]
- Bui, T.Q.; Ton, S.N.-C.; Duong, A.T.; Tran, H.T. Size-dependent magnetic responsiveness of magnetite nanoparticles synthesised by co-precipitation and solvothermal methods. J. Sci. Adv. Mater. Devices 2018, 3, 107–112. [Google Scholar] [CrossRef]
- Hadadian, Y.; Sampaio, D.R.; Ramos, A.P.; Carneiro, A.A.; Mozaffari, M.; Cabrelli, L.C.; Pavan, T.Z. Synthesis and characterization of zinc substituted magnetite nanoparticles and their application to magneto-motive ultrasound imaging. J. Magn. Magn. Mater. 2018, 465, 33–43. [Google Scholar] [CrossRef]
- González-Delgado, Á.D.; García-Martínez, J.B.; Barajas-Solano, A.F. A Technoeconomic Resilience and Exergy Analysis Approach for the Evaluation of a Vaccine Production Plant in North-East Colombia. Sustainability 2023, 15, 287. [Google Scholar] [CrossRef]
- Arteaga-Diaz, S.; Gonzalez-Diaz, J.; Ojeda-Delgado, K.; Pajaro-Morales, M.; Gonzalez-Delgado, A. Computer-aided exergy analysis of a palm based-biorefinery for producing palm oil, kernel oil and hydrogen. Contemp. Eng. Sci. 2018, 11, 537–545. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; Alarcón-Suesca, C.; González-Delgado, Á.D. Exergetic sensibility analysis and environmental evaluation of chitosan production from shrimp exoskeleton in Colombia. J. Clean. Prod. 2020, 248, 119285. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; González-Delgado, A.-D.; Kafarov, V. Evaluation of alternatives for microalgae oil extraction based on exergy analysis. Appl. Energy 2013, 101, 226–236. [Google Scholar] [CrossRef]
- Rocco, M.; Colombo, E.; Sciubba, E. Advances in exergy analysis: A novel assessment of the Extended Exergy Accounting method. Appl. Energy 2014, 113, 1405–1420. [Google Scholar] [CrossRef]
- Hellström, D. An exergy analysis for a wastewater treatment plant-an estimation of the consumption of physical resources. Water Environ. Res. 1997, 69, 44–51. [Google Scholar] [CrossRef]
- Szargut, J. Appendix 1. Standard chemical exergy. In Exergy Method. Technical and Ecological Applications; WIT Press: Southampton, UK, 2007; pp. 489–494. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; Herrera-Barros, A.; González-Delgado, D. Evaluation of Large-Scale Production of Chitosan Microbeads Modified with Nanoparticles Based on Exergy Analysis. Energies 2019, 12, 1200. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; Urbina-Suaréz, N.; González-Delgado, D. Computer-aided environmental and exergy analyses of a large-scale production of chitosan microbeads modified with TiO2 nanoparticles. J. Clean. Prod. 2019, 237, 117804. [Google Scholar] [CrossRef]
- De Falco, M.; Natrella, G.; Capocelli, M.; Popielak, P.; Sołtysik, M.; Wawrzyńczak, D.; Majchrzak-Kucęba, I. Exergetic Analysis of DME Synthesis from CO2 and Renewable Hydrogen. Energies 2022, 15, 3516. [Google Scholar] [CrossRef]
- Gollangi, R.; Rao, K.N. Energetic, exergetic analysis and machine learning of methane chlorination process for methyl chloride production. Energy Environ. 2022, 0958305X221109604. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Liu, T.; Yin, K.; Dai, Y.; Zhu, Z.; Cui, P.; Wang, Y.; Zhong, L. Economic, environmental, and exergy analysis of an efficient separation process for recovering low-carbon alcohol from wastewater. J. Clean. Prod. 2022, 365, 132733. [Google Scholar] [CrossRef]
- Gholami, A.; Hajinezhad, A.; Pourfayaz, F.; Ahmadi, M.H. The effect of hydrodynamic and ultrasonic cavitation on biodiesel production: An exergy analysis approach. Energy 2018, 160, 478–489. [Google Scholar] [CrossRef]
- Aigba, P.A.; Emovon, I.; Samuel, O.D.; Enweremadu, C.C.; Abdeljawad, T.; Al-Mdallal, Q.M.; Afzal, A. Exergetic Assessment of Waste Gas to Energy in a Novel Integrated NGL Recovery and Power Generation Plant. Front. Energy Res. 2022, 9, 798896. [Google Scholar] [CrossRef]
- Mabrouk, A.; Erdocia, X.; Alriols, M.G.; Labidi, J. Economic analysis of a biorefinery process for catechol production from lignin. J. Clean. Prod. 2018, 198, 133–142. [Google Scholar] [CrossRef]
- Peters, J.F.; Petrakopoulou, F.; Dufour, J. Exergy analysis of synthetic biofuel production via fast pyrolysis and hydroupgrading. Energy 2015, 79, 325–336. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Mandegari, M.; Tabatabaei, M.; Farzad, S.; Soufiyan, M.M.; Görgens, J.F. Exergy analysis of a lignocellulosic-based biorefinery annexed to a sugarcane mill for simultaneous lactic acid and electricity production. Energy 2018, 149, 623–638. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).