Abstract
The current study explored bioenergy, particularly biohythane (a combination of biohydrogen (bioH2) and biomethane (bioCH4)), production from cow dung and untreated domestic wastewater sludge to valorize the waste into a value-added product. The experimental study consisted of a two-step process: dark fermentation (DF) and anaerobic digestion (AD) with a range of processing conditions varying the temperature and pH (acidic, neutral, and basic). The study maintained thermophilic conditions (55 °C) for bioH2 production and mesophilic conditions (35 °C) for bioCH4 production. The highest yields of bioH2 and bioCH4 were obtained at a pH of 5.5 (108.04 mL H2/g VS) and a pH of 7.5 (768.54 mL CH4/g VS), respectively. Microorganisms, such as Lactobacillus brevis and Clostridium butyricum, in the wastewater sludge accelerated the conversion reaction resulting in the highest bioH2 yield for an acidic environment, while Clostridium and Bacilli enhanced bioCH4 yield in basic conditions. The maximum cumulative yield of biohythane was obtained under basic pH conditions (pH 7.5) through DF and AD, resulting in 811.12 mL/g VS and a higher volumetric energy density of 3.316 MJ/L as compared to other reaction conditions. The experimental data were modelled using a modified Gompertz’s model at a 95% confidence interval and showed the best-fitting data from experimental and simulation results for biohythane production. The regression coefficient R2 value was highly significant at 0.995 and 0.992 for bioH2 and bioCH4 with the change in pH during biohythane production. Thus, this study presented an effective pathway to utilize untreated domestic wastewater sludge as an inoculum, showcasing the potential of biohythane production and the generation of valuable metabolic end-products across a broad range of pH conditions.
1. Introduction
Energy is a basic requirement for human civilization and around 85.4% of the energy demand comes from conventional energy sources, compared to 14.6% from renewable sources [1]. The widespread use of conventional fossil fuels has created environmental concerns and has necessitated a shift to renewable energy sources, particularly biofuels [2,3]. Therefore, the production and implementation of biofuels has become inevitable [4]. The utilization of biofuels must be integrated with the current fuel market to meet the needs of economic development [5]. In the existing fuel market, hydrogen (H2) is considered the prominent alternative to fossil fuels because of its higher specific energy content [6]. Nevertheless, most of H2 production is from non-renewable sources while bioH2, which can be produced from renewable sources (e.g., biomass) using the metabolic activity of microorganisms, is highly sustainable [7]. Among different bioH2 production techniques, DF (in the absence of light) is highly promising due to the no light or photocatalyst requirement and its’ high yield [8]. During the DF process, H2 is biologically produced through fermentation, in which microbes integrate sugar-rich cellulosic compounds such as galactose and then convert them into H2 [9]. The yield of bioH2 can be optimized through inoculum, pre-treatment (if biomass is lignocellulose), temperature changes, organic loading rate, pH levels, and retention time [10]. On the other hand, bioCH4 can be produced either by AD [11] or thermo-chemical processes such as pyrolysis and gasification [12]. BioCH4 is the major component of biogas, which is extensively used for heating and cooking purposes, but in comparison with bioH2, bioCH4 has an approximately 5–6 times lower energy density and energy content [13,14]. To enhance the energy profile of biogas (mainly bioCH4), new biofuel research has recently introduced biohythane, a gaseous biofuel and a combination of bioH2 and bioCH4. Biohythane produced via two-stage fermentation is a promising direction for sustainable energy recovery from lignocellulosic biomass. Lignocellulosic plant biomass is readily available and is considered a potential feedstock for biohythane [13,14].
The current study was performed using local waste from Pakistan as Pakistan has abundant inexpensive raw materials (e.g., food waste and agricultural residues) to produce biohythane [15,16]. Also, the renewable energy share in Pakistan has increased from 0% to 2% since 2018 [17] and biofuel implementation is being constantly promoted to meet the energy demand [18,19]. The objective of this study was to investigate the production of biohythane using laboratory-scale two-step bioreactors from domestic wastewater sludge and cow dung collected from local sources in Pakistan. The quantification of biohythane was studied at different pH levels (acidic, neutral, and basic conditions) with a mixture of wastewater sludge and cow dung. The modified Gompertz model was used to study the effect of acidic, neutral, and basic pH levels on the biohythane reaction kinetics. Looking at the viability of the gaseous biohythane as a biofuel, the energy density was investigated. The microbiological morphology was studied to identify the type of microorganism present in the reaction mixture influencing the dark fermentation and anaerobic digestion reactions that produce bioH2 and bioCH4/biogas, respectively.
To the author’s best knowledge, this is the first study on biohythane from cow dung and domestic wastewater sludge. Several experimental studies were previously performed on biogas containing bioCH4 and CO2 from cow dung with waste sludge where only bioCH4, CO2 or volatile solid content was used [20,21]. On the other hand, some other studies only focused on bioH2 from cattle wastewater [22] and cattle manure [23]. Based on the yield of these earlier studies, the yield of bioCH4 with CO2 is higher than bioH2, but the energy content of bioH2 (~120 MJ/kg) is higher than bioCH4 (~20–30 MJ/kg). Consequently, biohythane is expected to boost both the biogas yield and energy content. Hence, the novelty of this current study is in implementing both DF and AD within one experimental framework, exploring the possibility of biohythane (a combination of bioH2 and bioCH4) production from cow dung and domestic wastewater sludge in Pakistan and comparing the yield and energy density of biohythane with the single production of bioCH4 or bioH2. The advantages of biohythane over the single production of bioCH4 or bioH2 are: (i) higher output of total biogas, (ii) higher quality biogas in terms of calorific value, and (iii) lower operating costs for separate gas storage and processing for bioCH4 and bioH2 which makes the process more practical and scalable commercially. As agriculture is a crucial sector of Pakistan’s economy where cattle are an essential component of livestock, utilizing cattle manure with wastewater sludge for biohythane production will be an effective pathway to contribute to the energy sector in the country [19,24].
2. Materials and Methods
Through a two-step procedure, biohythane synthesis from organic materials (domestic wastewater sludge and cow dung) was accomplished by DF and AD. DF was used to make the bioH2, whilst AD was used to produce bioCH4. The domestic wastewater sludge was obtained from a wastewater treatment facility at NED University with a daily treatment capacity of 15,000 gal [25], while the cow dung was obtained from a cattle farm located in the Malir district of Karachi. All experimental work was conducted in the Microbiology Laboratory of the Department of Environmental Engineering, NED University of Engineering & Technology, Karachi at a room temperature of 28 °C ± 1 °C.
2.1. Experimental Lab-Scale Reactor Set-Up
Two reactors each having a capacity of 2 L and made of stainless steel grade (SS304) were used to produce biohythane as per the previous literature [26]. The total length of each reactor was 8 inches with a diameter of 4.5 inches, having a total volume of 127.3 in3 (2 L). The reactors were attached with pressure gauges and water displacement acrylic cylinders with lengths of 40 cm and diameters of 3.6 cm were used for the quantitative measurements of gases produced from each reactor. A manual agitator was fitted to the reactor to mix the organic feedstock on alternate days for AD. Moreover, each reactor was fitted with a 1000 W heater synchronized with a solenoid switch to maintain the required temperature for the reaction. Figure 1 shows the schematic diagram of the two-step DF and AD processes for biohythane production in this study.
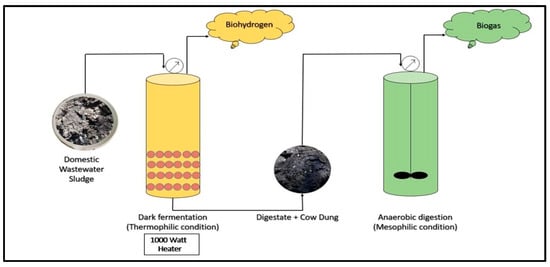
Figure 1.
Schematic diagram of two-step DF and AD processes for biohythane production for this study.
2.2. Physical and Chemical Properties of Organic Feedstock
The domestic wastewater sludge was obtained from the NED University wastewater plant, working on the principle of the activated sludge process, while the fresh cow dung was obtained from a local dairy farm located in Memon Goth, Malir, Karachi. The proximate analyses (moisture content (MC), volatile matter (VM), ash content (AC) and fixed carbon content (FC)) of the domestic wastewater sludge and cow dung were measured as per previous literature following the standard method ASTM D-3172-5 [27]. The total carbon (TC) and total nitrogen (TN) contents were measured using the Organic Elemental Analyzer (ECS 8020, N. C. Technologies, Bussero, Italy) at the Nuclear Institute of Agriculture (NIA), Sindh Agricultural University, Tandojam, Pakistan.
2.3. Experimental Condition of Biohythane Production
The experiments were conducted to produce biohythane from domestic wastewater sludge and cow dung as per the design shown in Table 1. All experiments were performed in duplicates for consistent outputs. The temperature of the reactor was maintained at 55 °C (thermophilic condition) with the help of an electric heater (1000 W). A mixture of 400 mL of domestic wastewater sludge with 800 mL of distilled water was prepared, the mixture was then introduced into a stainless-steel reactor with an initial pH of the sludge found to be 6.9. For bioH2 production, three experimental conditions were run with changes in the pH i.e., acidic (5.5), neutral (7.0) and basic (7.5). The acidic pH was obtained by using 2 M HCl in the mixture of domestic wastewater sludge and distilled water, while the basic pH was adjusted to 7.5 using 1 M NaOH. The hydraulic retention time of 11 days was maintained for bioH2 production. After completion of the retention time, the cumulative bioH2 production reached its peak value. Once the peak value was obtained, the biohydrogen gas concentration was measured using gas measurement sensors MQ-13, MQ-8 and MQ-11 (WAVGAT, Shenzhen, China) for 30 min. These sensors were connected to an Arduino UNO that was programmed to log data over time and measure the gas concentration. The volume of gas was measured using the water displacement method with properly calibrated acrylic cylinders with a length of 45 cm and a diameter of 3.6 cm at a room temperature of 28 °C ± 1 °C as per the method mentioned in earlier studies [28]. After the completion of the bioH2 production reaction, the digestate (1200 mL) from the first reactor was mixed with 100 mL of cow dung and introduced into the second reactor to produce biogas under AD conditions for 25 days. The temperature of the second heater was set at room temperature (i.e., 35 °C, mesophilic condition). The same protocol was used to measure the concentration of bioCH4 produced after the AD reaction.

Table 1.
Experimental design of biohythane production.
2.4. Modelling and Simulation of Biohythane Production
The cumulative production of bioH2 and bioCH4/biogas can be modelled and simulated by the modified Gompertz’s equation, Equation (1) [29]:
where Ps is the cumulative biogas production potential (mL), R is the maximum biogas production rate (mL/h), λ is the lag time (h), t is the cultivation time (h), and e is the constant with a value of 2.71828. The parameters Ps, R, and λ were estimated with a 95% confidence limit using curve fitting in statistical software (IBM SPSS version 26, Chicago, IL, USA). The biohythane production data was modelled through the modified Gompertz’s Equation using statistical analysis software IBM SPSS. The parameters of the model were calibrated via multiple iterations at a 95% confidence interval [26].
2.5. Volumetric Energy Density of Biohythane
The total volumetric density of biohythane (MJ/L) was calculated by converting the amount of bioH2 and bioCH4 (mL/g VS) obtained from three different pH conditions into L/kg. Then, we divided the energy value of bioH2 (140 MJ/kg) and bioCH4 (20 MJ/kg) produced with the volume of gas per unit mass (L/kg) as mentioned below in Equation (2).
2.6. Microbiological Morphology
A total of 1 g of sludge was diluted in sterile distilled water up to 10−5 and 1 mL was plated on sterile solidified nutrient agar plates from the last dilution. The plates were incubated at 37 °C and 55 °C for 24–48 h for the development of colonies. Typical colonies from the nutrient agar plates were stained for morphological study. Gram staining was conducted to find out the morphology of bacteria present in the system. Briefly, the bacteria were smeared on a glass slide with 0.85% saline and adjusted on the flame. The smear was stained with crystal violet, the dye was fixed with iodine, decolourized with alcohol, and finally counterstained with safranin. The morphology was observed under an oil immersion objective (100X using a microscope (LB-1500 Digital LCD Polarizing Microscope, Labomed Inc., Los Angeles, CA, USA).
3. Results and Discussion
The physical and chemical properties of domestic wastewater sludge and cow dung were measured and are presented in Table 2. The results showed that both samples had high MC to perform DF and AD sufficiently, the adequate amount of VM, initial CV, TC, TN and cabon/nitrogen (C/N) ratio required to produce bioH2 and bioCH4 in DF and AD processes, respectively. The key indicator for choosing the best procedure for energy recovery is the CV and VMof the primaryfeedstock. The higher VM concentration indicates that biomass has the potential to decompose more easily, resulting in higher yields of vapors, and to process more easily, producing higher yields of liquid and gaseous fuels. The higher FC content suggests that the feedstock is suitable for producing biochar and other solid biofuels with higher yields. When choosing the technique for converting biomass, it is vital to state that the AC is another crucial element of biomass that does not break down during processing. Because it cannot be converted into energy, biomass with a high ACproduces more residual weight than the product [30].

Table 2.
Physico-chemical properties of domestic wastewater sludge and cow dung.
3.1. BioH2 Production
pH is an important factor in bioH2 production because it affects the activities of enzymes involved in the process [22]. If the pH is too low (acidic), the enzymes responsible for bioH2 production may become less effective and cause a reduction in bioH2 production [23]. If the pH is too high (alkaline), the enzymes responsible for bioH2 production may become inactivated and cause a reduction in bioH2 production [19]. Therefore, ideal pH levels for bioH2 production vary depending on the type of microorganism used [28]. The influence of pH plays an important role in the reaction because changes in pH cause reaction imbalances that have an ultimate impact on the bioH2 production. At a low pH, more enzymes are activated very quickly and hence lead to the production of bioH2. An optimal pH level for bioH2 production is also influenced by the type of substrate used, the presence of other microorganisms and other environmental factors [29]. In Figure 2, during bioH2 production at a pH of 5.5, the enzymes responsible for its production may have been more active and the overall efficiency of the bioH2 yield was found to be the highest in the process. Therefore, it is suggested that microorganisms such as Lactobacillus brevis and Clostridium butyricum present in the reaction have evolved to function in an acidic environment, thus producing the highest bioH2 under this pH condition [31]. According to the findings, a pH of 5.5 is ideal for bacterial growth and bioH2 generation. Undoubtedly, a proper pH is advantageous for boosting bacterial activity, while a low or high pH will be damaging to the bacteria. The results indicate that bioH2-producing bacteria can grow better with an appropriate pH [22]. It is also important to note that bioH2 production at a pH of 5.5 may have an impact on the overall yield and efficiency of the process, as well as the stability of the microorganisms used.
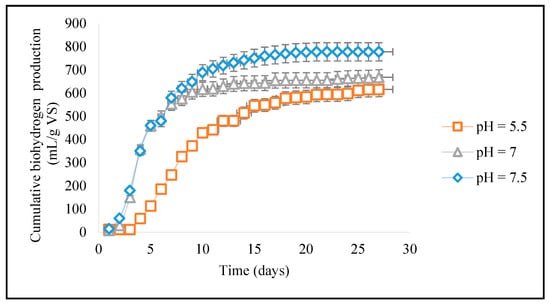
Figure 2.
BioH2 production as a function of pH.
The enzymes responsible for bioH2 production are likely to be the most active and efficient. For example, some bacteria like Clostridium pasteurianum, Clostridium acetobutylicum and Enterobacter aerogenes have been shown to produce bioH2 efficiently at a neutral pH of 7. These bacteria are facultative anaerobes, meaning that they can survive in both anaerobic and aerobic environments, which makes them well-suited for the production of bioH2. Therefore, in the current study, bioH2 production at a pH of 7 can be considered as an active condition for most microorganisms as it allows enzymes to work efficiently, and it is a neutral condition that most microorganisms can tolerate. The present research showed that the bioH2 yield was lower than that with a pH of 5.5 but was higher than at the basic condition of a pH of 7.5.
BioH2 production at a pH of 7.5 is considered slightly alkaline and the enzymes responsible for bioH2 production may be less active compared to a neutral pH of 7. However, some microorganisms have been reported to tolerate slightly alkaline conditions and produce bioH2 efficiently at this pH level. To illustrate, microorganisms like Thermotoga maritima, Caldicellulosiruptor bescii, and Saccharomyces cerevisiae have been reported to produce higher bioH2 at a pH of 7.5 [32]. Similar to some earlier research studies, Caldicellulosiruptor bescii, a hyperthermophilic cellulolytic bacteria was used and the fermentation of cow manure was performed as the single carbon source in combination with switchgrass and wastewater biosolids. The greatest output reported from DF on cattle manure was 82.5 N mL/g VS when cattle dung was employed as the only carbon source [23]. Therefore, it can be worth noting that these microorganisms are thermophilic indicating their ability to thrive in elevated temperatures and withstand mildly alkaline environment. Another research study also determinedthis phenomenon of higher bioH2 yields ranging from 56 to 135 mL/g VS under thermophilic conditions, while lower yields were obtained from 36.5 to 113 mL/g VS under mesophilic circumstances [22].
Furthermore, it was also evident that both mesophilic and thermophilic microbes can produce bioH2 through earlier studies [24]. For various reasons, the metabolic pathways for bioH2-generating microorganisms prefer thermophilic conditions to initiate metabolic reactions, and accelerates the rates of chemical and enzymatic reactions gradually which is directly proportional to temperature. Besides, higher temperatures make the bioH2 generation pathway of microbes more thermodynamically favorable and thermophilic conditions are important in reducing the thermodynamic restriction on bioH2 gas production [24]. A previous study presented that the initial pH has an impact on the yield and rate of bioH2 production using a pure anaerobic bacterium of Clostridium beijerinckii and glucose substrate content [20]. The optimal pH range for methanogenic microbes is between 6.0 and 7.5, whereas bioH2-generating microorganisms operate better below a pH of 6. When the pH was maintained at ~6.0, the production of bioH2 was found to be higher, but a microenvironment with a pH of ≤4.5 made it difficult for the microbial population to generate bioH2. For the generation of bioH2 with DF as well as for the suppression of solvogenesis and methanogenesis, a pH range of 5.5–6.0 is advantageous. Solvogenesis is more likely to occur in fermentative pathways in an alkaline environment. Additionally, reduced substances (including aldehydes, alcohols, and reducing sugars) and changed membrane potentials are produced by the H+ that shuttles between metabolic intermediates, which slows down cellular growth. During the DF process, volatile fatty acids build up and cause a reduction in the pH of the system. This phenomenon lowers the system’s buffering ability, which ultimately stops bioH2 production. For the generation of bioH2 with DF as well as the suppression of solventogenesis and methanogenesis, a pH range of 5.5–6.0 was found to be highly favourable [21].
3.2. Biogas (CH4 Mixed with CO2) Production
Figure 3 shows that the biogas yield at a pH of 5.5 in the acidic condition was found to be lower as compared to the basic condition with a pH of 7.5. At this acidic pH level, the production of biogas is comparatively lower than in basic pH conditions because specific enzymes that are responsible for the breakdown of organic matter to produce biogas are less active in the acidic pH range [30]. These microorganisms can survive and function in acidic environments, such as the fermentation of organic acids, and thus can produce biogas efficiently at this pH; but most of the microorganisms in biogas cannot survive in an acidic environment and hence are acid sensitive, which influences the microbial population at a low pH of 5.5. The production of biogas is strongly influenced by changes in pH because they affect the rate and efficiency of biogas production, with lower or higher pH levels potentially inhibiting bacterial activity. The optimal pH for biogas production from wastewater sludge is typically around 7.5. At pH levels beyond 7.5, the activity of these bacteria can be inhibited, leading to decreased biogas production. However, the optimum pH depends upon the type of feedstock and microorganism [33]. More carbon content sources are given to cattle manure in the form of biomass (agricultural residues) to increase the carbon content and decrease the quantity of ammonia produced during the methanogenic activity, which limits the generation of bioCH4. For instance, the co-digestion of dairy manure and wasted mushrooms at a ratio of 1:3 produced the maximum biogas yield, which was 4 times more than that obtained without co-digestion [19]. Theoretically, primary and waste-activated sludge can produce between 210 and 650 mL bioCH4/g volatile solids (VS) of methane. However, the presence of hazardous chemicals in the sludge causes retardation in the anaerobic digestion process causing a reduction in the biogas yield [23]. However, in the current investigation, the biogas yield (768.56 mL CH4/g VS) was higher due to the absence of heavy metals or hazardous chemicals that would have decreased the sludge’s ability to biodegrade and increased the amount of biogas produced.
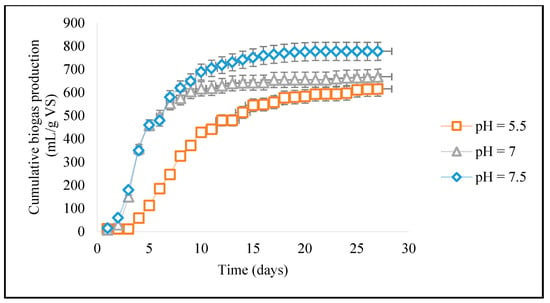
Figure 3.
Biogas production as a function of pH.
3.3. Biohythane Modelling through Modified Gompertz’s Equation
Figure 4 shows the cumulative bioH2 and biogas/bioCH4 production at two incubation temperatures (thermophilic and mesophilic conditions). BioH2 was found to be highest in acidic conditions, while basic conditions produced the lowest value during DF. During the basic conditions of AD, the biogas cumulative value was the highest. The lowest cumulative biogas was obtained in acidic AD conditions. The data for the AD yield of bioCH4 fluctuated a little due to the temperature change of the reactor during the day (28 °C) and night (23 °C) in the laboratory; while in the case of DF, the temperature was kept constant (55 °C) using a solenoid switch-operated heater.
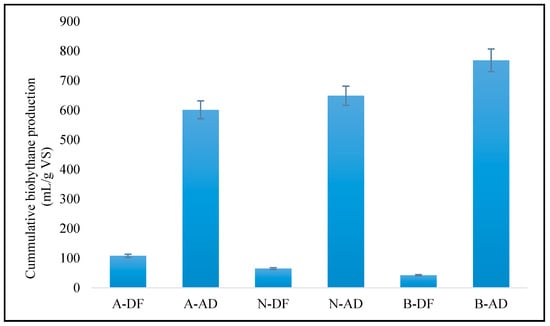
Figure 4.
The overall output of cumulative biohythane production.
By fitting Equation (1) to the experimental data, the kinetic parameters, such as the maximum gas production rate (Ps), the lag phase (λ) and gas production rate (R), were determined using IBM SPSS software v. 26 during the batch scale experiment using the yield of bioH2 and biogas at different experimental conditions (see Table 3). The model was solved using a non-linear multiple regression function. The data set generated using this model was plotted and compared with the experimental data set with R2 values found to be close to unity, showing statistically significant bioH2 and bioCH4 yields concerning variations in pH values.

Table 3.
Effect of pH on the production of H2 production (by DF) and CH4 production (by AD).
3.4. Volumetric Energy Density of Biohythane
The volumetric energy density of biohythane produced with varying pH conditions was calculated and is presented in Table 4. It has been observed that basic pH conditions yielded a higher volumetric energy density of biohythane (3.316 MJ/L). This shows that biohythane produced from a basic pH level occupies a higher energy density per unit volume of the mixture of biohydrogen and biogas as compared to the other two pH conditions (i.e., acidic and neutral). Moreover, the standard volumetric energy density of bioH2 is 8 MJ/L, while biogas has 0.028 MJ/L showing that bioH2 has more influence on the final product gas energy output. A comprehensive techno-economic analysis and life cycle assessment (LCA) of this target product (biohythane) and its by-products is highly recommended for further study to determine the economic feasibility and positive environmental impact, respectively. The economic feasibility of the biohythane production from cattle manure and domestic wastewater sludge will benchmark the process with the existing biofuel technologies in the market, while LCA will determine the quantitative details of the total water and carbon footprint.

Table 4.
The volumetric energy density of biohythane at different pH conditions.
3.5. Microbiological Morphology
Figure 5 depicts the bacterial morphology isolated from sludge after gram straining. Presence of different shaped bacteria have been observed where Figure 5a presents gram-positive small and long rods scattered in the arrangement representing gram positive bacilli, Figure 5b shows gram-positive short rods representing gram positive bacilli, Figure 5c shows gram-positive long rods scattered shapes with a chain of 2–3 cells and small rods representing gram positive bacilli, Figure 5d shows gram-positive long rods with a chain of 2–3 cells representing gram positive cocci, Figure 5e shows gram-positive bacterial colony with oval shapes representing gram positive cocci/ gram positive bacilli/gram negative diplococci/gram negative coccobacilli, and Figure 5f shows gram-positive long rods scattered bacterial colony representing gram positive bacilli.. The diverse shapes of bacteria indicate the presence of various strains of Lactobacilli, Clostridium and Enterobacter species in the sludge of which are inevitable for the production of biogas. Efficient microbial fermentation boosts the overall yield of bioH2, especially the gram-positive long rods of the Clostridium species, responsible for high yield of bioH2 production [10]. Also, organic matter decomposition involves several bacteria and the predominant is Clostridia strains that produce biogas. Strainsof Enterobacteriales and Clostridiales are metabolically active during bioH2 production from the organic substrate [27]. Many strains of Clostridium evolve production of bioH2 and CO2 from organic matter, such as cellulose. The high yield of biogas was achieved in this study due to the presence of these bacteria. They have diverse fermentation pathways and play a vital role in the microbial community when the complex substrate is present in the digestor. Strainsof Actinobacteria (gram-positive, rod-shaped bacteria) can help other microflora produce biogas from oligosaccharides [34]. The current study is one of the initial attempts throughout literature to perform the experiments forthe production of biohythane from mixed waste sources of domestic wastewater sludge andcow dung and it shows significant promise of overall biohythane yield.
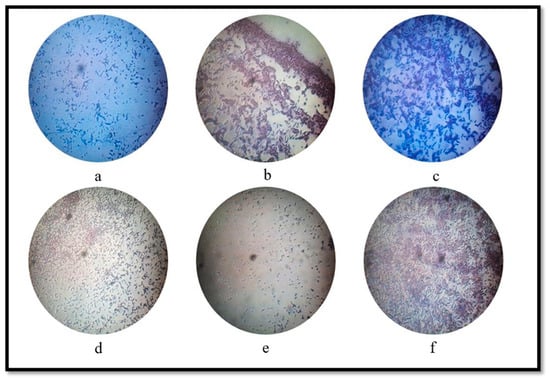
Figure 5.
Morphology of bacteria isolated from sludge after gram-staining where (a) gram+ small and long rod shaped bacterial, (b) gram+ short rod-shaped bacteria, (c) gram+ scattered long rod-shaped bacteria, (d) gram+ chained long rod-shaped bacteria, (e) gram+ oval-shaped bacteria and (f) gram+ scattered long rod-shaped bacteria.
4. Conclusions
Biohythane was produced from domestic wastewater sludge and cow dung using a two-step anaerobic fermentation process in locally fabricated reactors. The study investigated bioH2 production through DF at thermophilic conditions (55 °C), and various pH conditions, acidic (5.5), neutral (7.0) and basic (7.5). The highest concentration of bioH2 (108.04 mL bioH2/g VS) was obtained in acidic conditions, while the lowest (42.54 mL bioH2/g VS) was obtained with a basic pH level. The highest amount of biogas produced at a pH level of 7.5 was found (768.56 mL bioCH4/g VS), whereas a lower biogas concentration was found (601.35 mL bioCH4/g VS) with an acidic pH condition. The production of biohythane was modelled through a modified Gompertz’s equation with a 95% confidence interval which showed the R2 values coming close to unity being statistically significant with the variation in pH values. The biohythane produced from basic pH conditions has a higher volumetric energy density (3.316 MJ/L). A microbiological test of the sludge that specifically included the bacterial morphology observed the presence of microorganisms that support and favor the reaction pathways to produce bioH2 and biogas/ bioCH4 in thermophilic DF and mesophilic AD conditions, respectively. Untreated sludge from domestic wastewater treatment facilities can be used to produce value-added sustainable biohythane (bioH2 and bioCH4) as a source of alternative fuel and its digestate as a bio-fertilizer or soil conditioner.
Author Contributions
F.S.: Conceptualization, Methodology, Formal Analysis, Writing—Original Draft; M.A. and S.K.: Supervision, Writing—Review and Editing; N.H.: Writing—Review and Editing, Funding Acquisition for APC. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
Acknowledgments
The NED University of Engineering and Technology provided the lab resources necessary to carry out this research, which the authors gratefully acknowledge.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Noori, M.T.; Min, B. Fundamentals and recent progress in bioelectrochemical system-assisted biohythane production. Bioresour. Technol. 2022, 361, 127641. [Google Scholar] [CrossRef]
- Trowbridge, J.; Goin, D.E.; Abrahamsson, D.; Sklar, R.; Woodruff, T.J. Fossil fuel is the common denominator between climate change and petrochemical exposures, and effects on women and children’s health. Int. J. Gynecol. Obstet. 2023, 160, 368–371. [Google Scholar] [CrossRef]
- Yang, M.; Chen, L.; Wang, J.; Msigwa, G.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Circular economy strategies for combating climate change and other environmental issues. Environ. Chem. Lett. 2023, 21, 55–80. [Google Scholar] [CrossRef]
- Soares, J.F.; Confortin, T.C.; Todero, I.; Mayer, F.D.; Mazutti, M.A. Dark fermentative biohydrogen production from lignocellulosic biomass: Technological challenges and future prospects. Renew. Sustain. Energy Rev. 2020, 117, 109484. [Google Scholar] [CrossRef]
- Yousaf Raza, M.; Lin, B. Oil for Pakistan: What are the main factors affecting the oil import? Energy 2021, 237, 121535. [Google Scholar] [CrossRef]
- Mannarino, G.; Sarrion, A.; Diaz, E.; Gori, R.; De la Rubia, M.A.; Mohedano, A.F. Improved energy recovery from food waste through hydrothermal carbonization and anaerobic digestion. Waste Manag. 2022, 142, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kumar, G.; Chen, W.-H.; Khanal, S.K. Renewable hydrogen production from biomass and wastes (ReBioH2-2020). Bioresour. Technol. 2021, 331, 125024. [Google Scholar] [CrossRef] [PubMed]
- Melitos, G.; Voulkopoulos, X.; Zabaniotou, A. Waste to Sustainable Biohydrogen Production Via Photo-Fermentation and Biophotolysis—A Systematic Review. Renew. Energy Environ. Sustain. 2021, 6, 45. [Google Scholar] [CrossRef]
- Uggetti, E.; Passos, F.; Solé, M.; Garfí, M.; Ferrer, I. Recent Achievements in the Production of Biogas from Microalgae. Waste Biomass Valorization 2017, 8, 129–139. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Shanmugam, S.; Sekar, M.; Mathimani, T.; Incharoensakdi, A.; Kim, S.-H.; Parthiban, A.; Edwin Geo, V.; Brindhadevi, K.; Pugazhendhi, A. Insights on biological hydrogen production routes and potential microorganisms for high hydrogen yield. Fuel 2021, 291, 120136. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.; Rahman, S. A review of process parameters influence in solid-state anaerobic digestion: Focus on performance stability thresholds. Renew. Sustain. Energy Rev. 2022, 167, 112756. [Google Scholar] [CrossRef]
- Thakur, N.; Salama, E.S.; Sharma, M.; Sharma, P.; Sharma, D.; Li, X. Efficient utilization and management of seaweed biomass for biogas production. Mater. Today Sustain. 2022, 18, 100120. [Google Scholar] [CrossRef]
- Boro, M.; Verma, A.K.; Chettri, D.; Yata, V.K.; Verma, A.K. Strategies involved in biofuel production from agro-based lignocellulose biomass. Environ. Technol. Innov. 2022, 28, 102679. [Google Scholar] [CrossRef]
- Javed, F.; Aslam, M.; Rashid, N.; Shamair, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U.; et al. Microalgae-based biofuels, resource recovery and wastewater treatment: A pathway towards sustainable biorefinery. Fuel 2019, 255, 115826. [Google Scholar] [CrossRef]
- Ghosh, S.; Kar, D. Biohythane: A Potential Biofuel of the Future. Appl. Biochem. Biotechnol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Liaquat, R.; Husain Khoja, A.; Safdar, U. A comparison of energy policies of Pakistan and their impact on bioenergy development. Sustain. Energy Technol. Assess. 2021, 46, 101246. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Ud Din, Z.; Sabah, N.U.; Jamil, M.A.; Mujtaba, M.A.; Abid, A. The potential of sustainable biogas production from biomass waste for power generation in Pakistan. J. Clean. Prod. 2021, 307, 127250. [Google Scholar] [CrossRef]
- Abdullah, A.; Ahmed, A.; Akhter, P.; Razzaq, A.; Hussain, M.; Hossain, N.; Bakar, M.S.A.; Khurram, S.; Majeed, K.; Park, Y.-K. Potential for sustainable utilisation of agricultural residues for bioenergy production in Pakistan: An overview. J. Clean. Prod. 2021, 287, 125047. [Google Scholar] [CrossRef]
- Chowdhury, T.; Chowdhury, H.; Hossain, N.; Ahmed, A.; Hossen, M.S.; Chowdhury, P.; Thirugnanasambandam, M.; Saidur, R. Latest advancements on livestock waste management and biogas production: Bangladesh’s perspective. J. Clean. Prod. 2020, 272, 122818. [Google Scholar] [CrossRef]
- Skonieczny, M.T.; Yargeau, V. Biohydrogen production from wastewater by Clostridium beijerinckii: Effect of pH and substrate concentration. Int. J. Hydrogen Energy 2009, 34, 3288–3294. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.J.; Lee, D.W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed]
- Sołowski, G.; Shalaby, M.S.; Abdallah, H.; Shaban, A.M.; Cenian, A. Production of hydrogen from biomass and its separation using membrane technology. Renew. Sustain. Energy Rev. 2018, 82, 3152–3167. [Google Scholar] [CrossRef]
- Lotfi Aski, A.; Borghei, A.; Zenouzi, A.; Ashrafi, N.; Taherzadeh, M.J. Steam explosion pretreatment of sludge for pharmaceutical removal and heavy metal release to improve biodegradability and biogas production. Fermentation 2020, 6, 34. [Google Scholar] [CrossRef]
- Ersoy, Ş.; Akaçin, İ.; Güngörmüşler, M. Comparative evaluation of the biohydrogen production potential of thermophilic microorganisms isolated from hot springs located in Izmir. Int. J. Hydrogen Energy 2023, 48, 22897–22908. [Google Scholar] [CrossRef]
- Ali, M.; Masood, A.; Saleem, M. Microalgae cultivation in wastewater for simultaneous nutrients removal and biomass production. Int. J. Energy Environ. Eng. 2021, 12, 475–485. [Google Scholar] [CrossRef]
- Ghimire, A.; Luongo, V.; Frunzo, L.; Lens, P.N.L.; Pirozzi, F.; Esposito, G. Biohythane production from food waste in a two-stage process: Assessing the energy recovery potential. Environ. Technol. 2022, 43, 2190–2196. [Google Scholar] [CrossRef]
- Dauptain, K.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Carrere, H. Role of indigenous bacteria in dark fermentation of organic substrates. Bioresour. Technol. 2020, 313, 123665. [Google Scholar] [CrossRef]
- Ali, M.; Niazi, F.; Siddiqui, M.A.; Saleem, M. Comparative Study on Oven and Solar Drying of Agricultural Residues and Food Crops. Int. J. Renew. Energy Dev. 2022, 11, 14. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Zheng, J.; Wang, B.; Liang, H.; Phulpoto, I.A.; Habiyakare, T.; Zhou, D. Microbial electrohydrogenesis cell and dark fermentation integrated system enhances biohydrogen production from lignocellulosic agricultural wastes: Substrate pretreatment towards optimization. Renew. Sustain. Energy Rev. 2021, 145, 111078. [Google Scholar] [CrossRef]
- Liew, Y.X.; Chan, Y.J.; Manickam, S.; Chong, M.F.; Chong, S.; Tiong, T.J.; Lim, J.W.; Pan, G.-T. Enzymatic pretreatment to enhance anaerobic bioconversion of high strength wastewater to biogas: A review. Sci. Total Environ. 2020, 713, 136373. [Google Scholar] [CrossRef]
- Manuel, C.-R.; Carlos, Q.-F.; Carmen, P.-C.; Marisela, V.-D.L.; Iván, M.-A. Fungal solid-state fermentation of food waste for biohydrogen production by dark fermentation. Int. J. Hydrogen Energy 2022, 47, 30062–30073. [Google Scholar] [CrossRef]
- Pradhan, N.; Dipasquale, L.; d’Ippolito, G.; Panico, A.; Lens, P.N.L.; Esposito, G.; Fontana, A. Hydrogen Production by the Thermophilic Bacterium Thermotoga neapolitana. Int. J. Mol. Sci. 2015, 16, 12578–12600. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Trabold, T.A. Anaerobic Digestion of Food Waste with Unconventional Co-Substrates for Stable Biogas Production at High Organic Loading Rates. Sustainability 2019, 11, 3875. [Google Scholar] [CrossRef]
- Wirth, R.; Kovács, E.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnol. Biofuels 2012, 5, 41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).