The Release Potential of Microplastics from Face Masks into the Aquatic Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
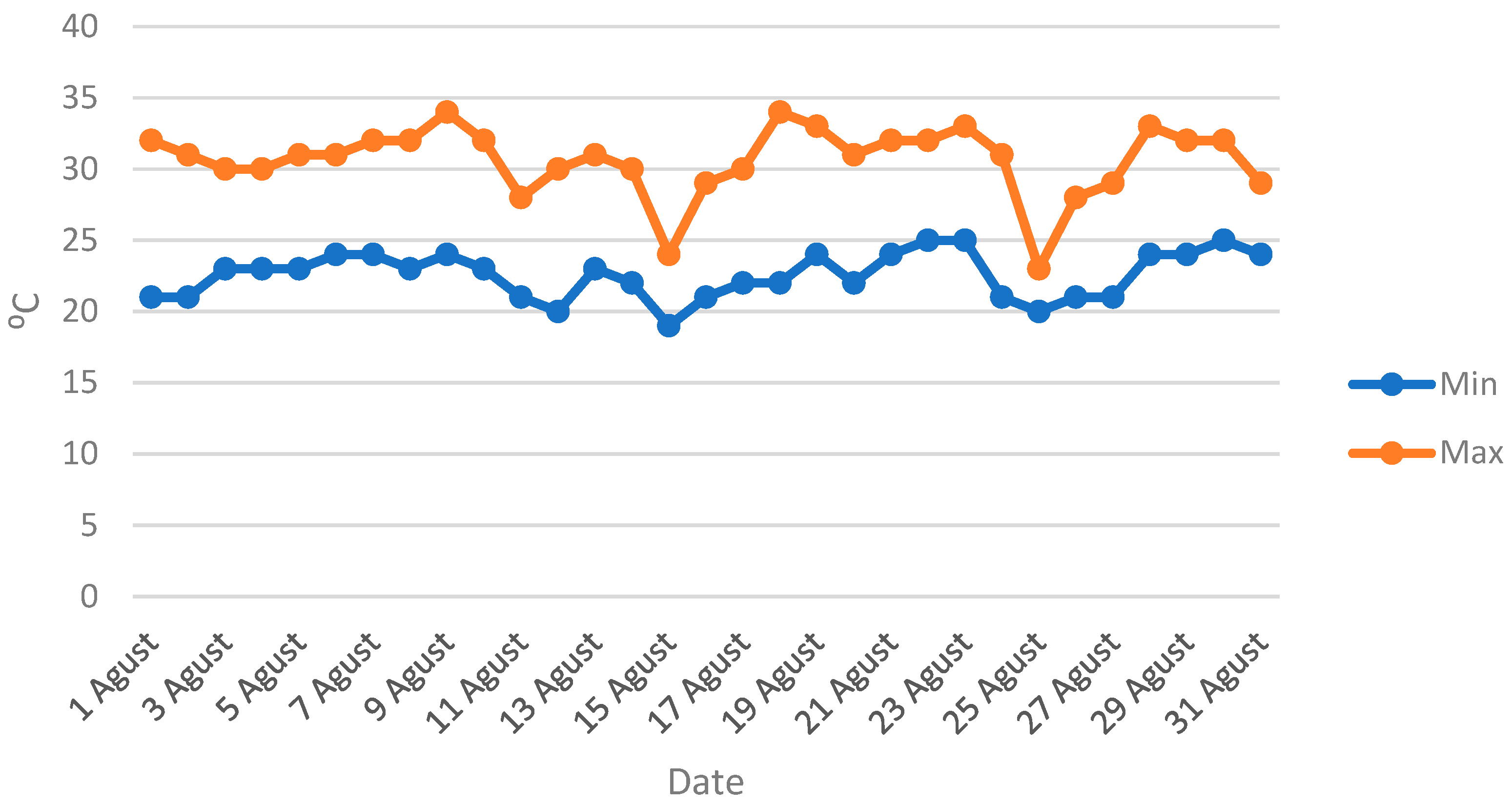
2.2. Experimental Setup
2.3. Instrumental Analysis
2.3.1. Physicochemical Changes of the Face Masks
2.3.2. MP Count
3. Results and Discussion
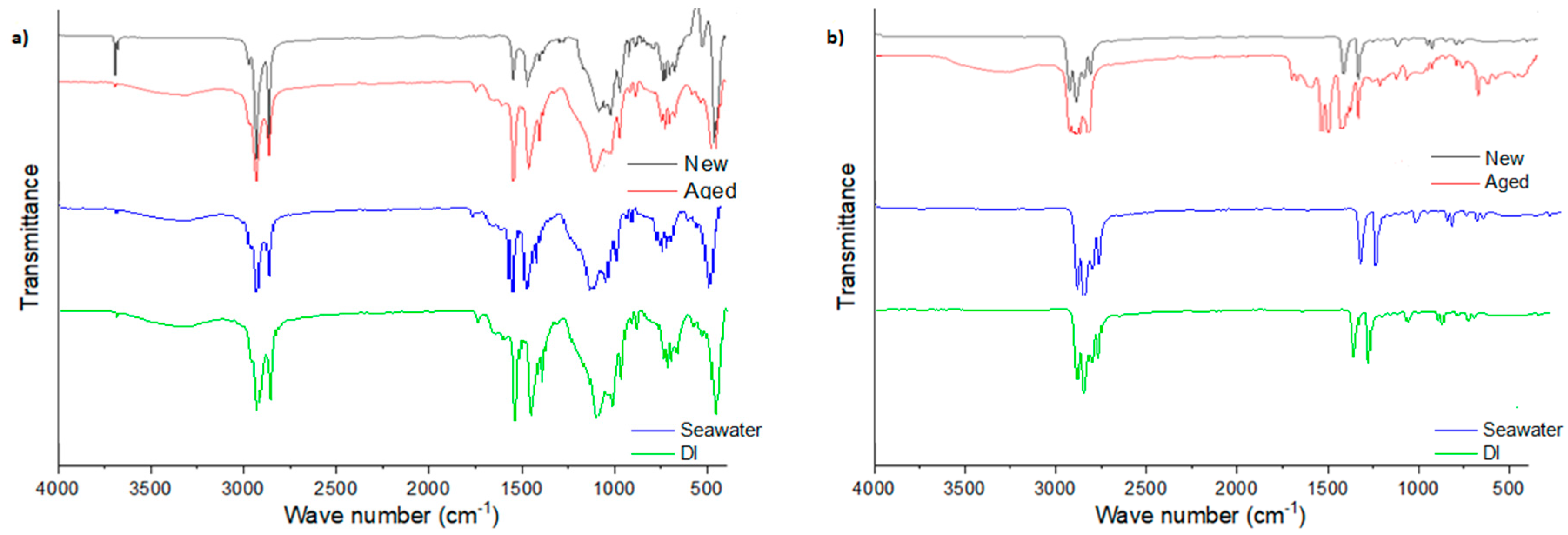
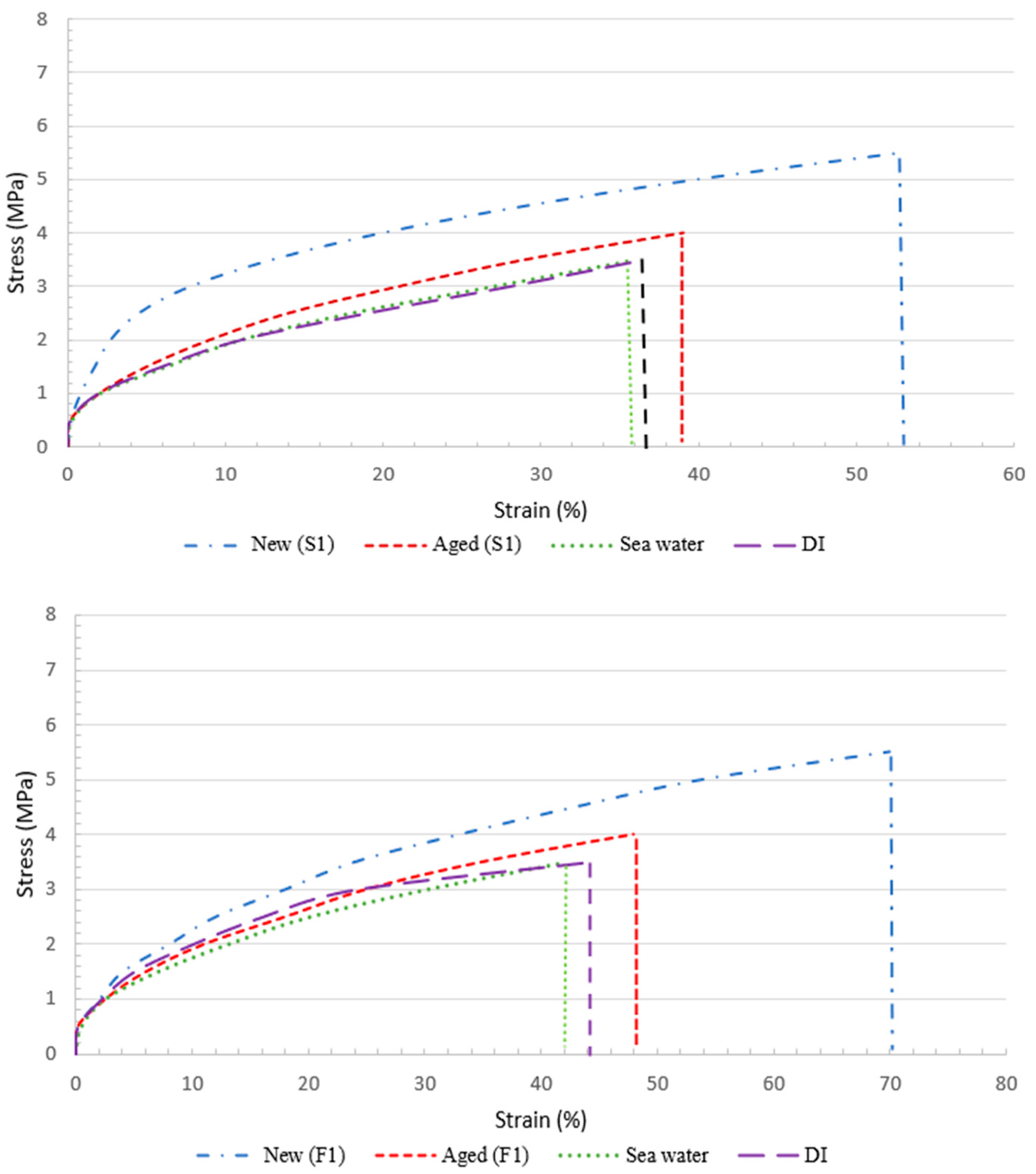
3.1. The Effect of Natural Weathering on the Physicochemical Structure of the Face Masks
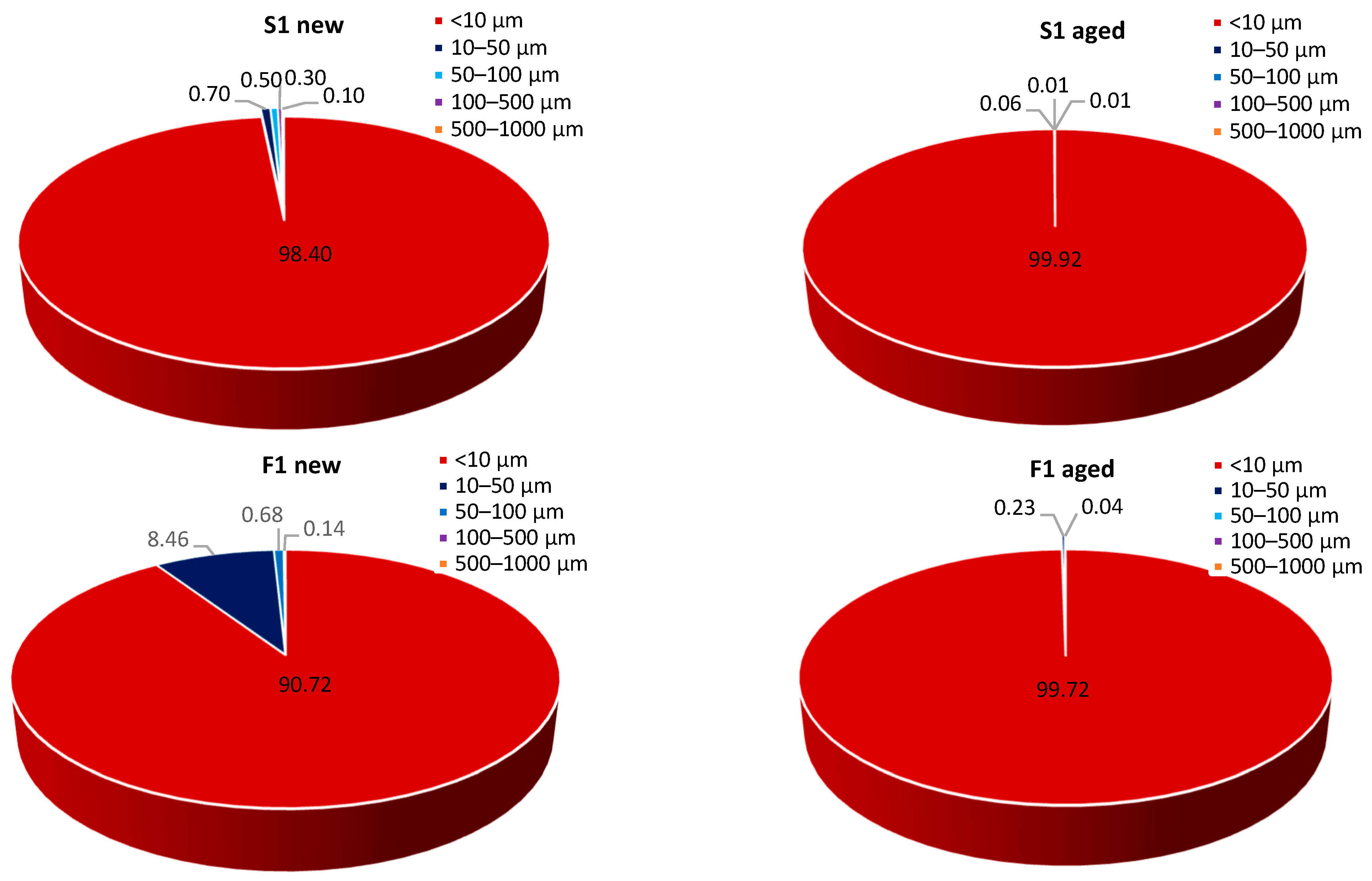
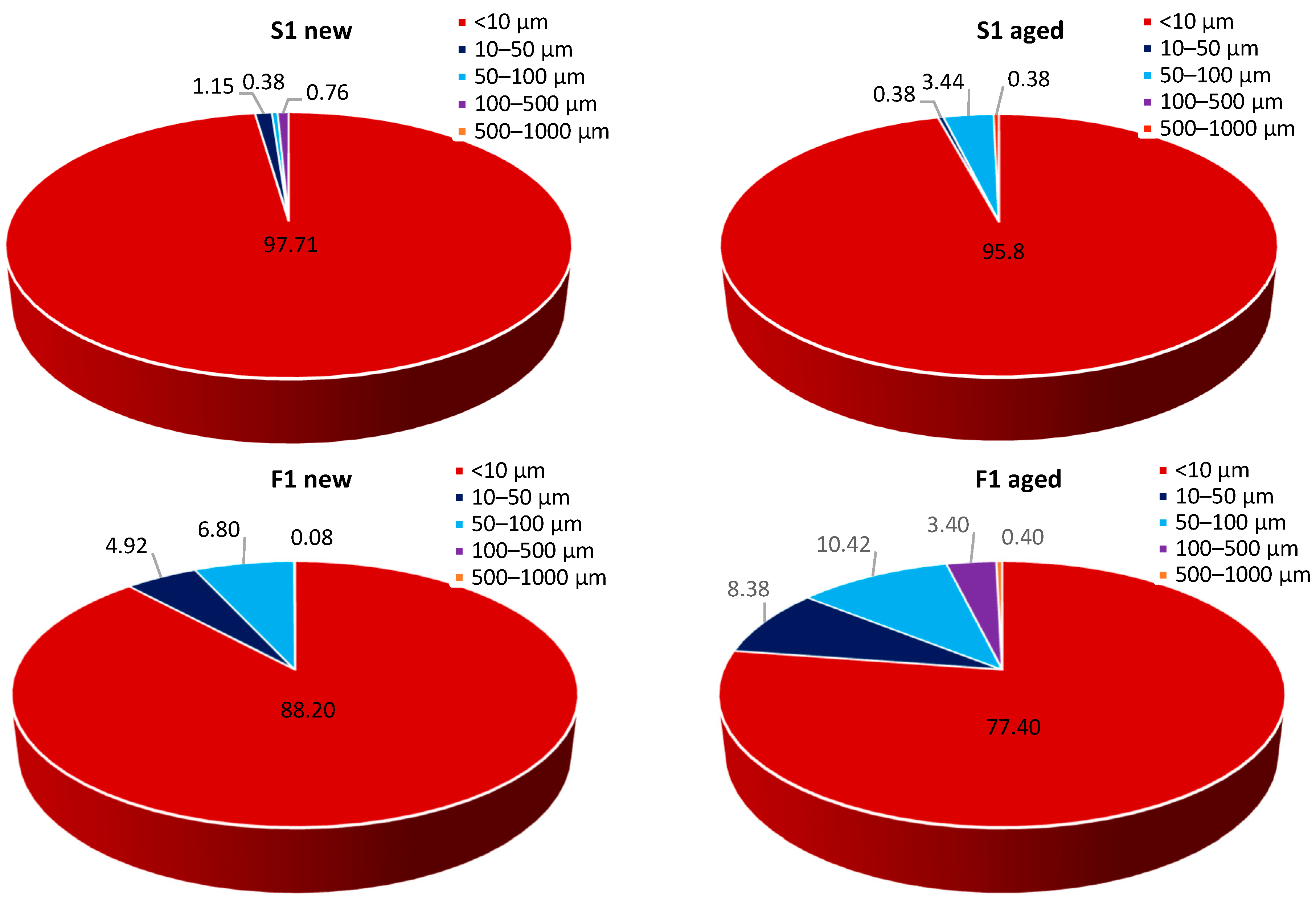
3.2. The Effect of Natural Weathering on MP Release
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the great pacific garbage patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; Volume 43. [Google Scholar] [CrossRef]
- Boucher, J.; Faure, F.; Pompini, O.; Plummer, Z.; Wieser, O.; Alencastro, L.P. (Micro) plastic fluxes and stocks in lake geneva basin. Trends Anal. Chem. 2019, 112, 66–74. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qin, Y.; Li, W.; Yang, W.; Meng, Q.; Yang, J. Microplastic contamination in freshwater: First observation in Lake Ulansuhai, Yellow River Basin, China. Environ. Chem. Lett. 2019, 17, 1821–1830. [Google Scholar] [CrossRef]
- Corradini, F.; Casado, F.; Leiva, V.; Huerta-Lwanga, E.; Geissen, V. Microplastics occurrence and frequency in soils under different land uses on a regional scale. Sci. Total Environ. 2021, 752, 141917. [Google Scholar] [CrossRef]
- Spennemann, D.H.R. COVID-19 Face Masks as a Long-Term Source of Microplastics in Recycled Urban Green Waste. Sustainability 2022, 14, 207. [Google Scholar] [CrossRef]
- Yurtsever, M.; Kaya, A.T.; Çiftçi Bayraktar, S. A research on Microplastic presence in outdoor air. In Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea; Springer Water: Cham, Switzerland, 2018. [Google Scholar]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.H.; Fang, T.; Xu, P.; Zhu, L.X.; Li, D.J. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Reff, R.A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.B.O.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Microplastics are contaminants of emerging concern in freshwater environments: An overview. In Freshwater Microplastics. The Handbook of Environmental Chemistry; Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 58. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Galvão, L.S.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested–a pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yan, Z.; Zhu, Q.; Zhang, Y. Tissue accumulation of microplastics and toxic effects: Widespread health risks of microplastics exposure. In Microplastics in Terrestrial Environments. The Handbook of Environmental Chemistry; He, D., Luo, Y., Eds.; Springer: Cham, Switzerland, 2020; Volume 95. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Yurtsever, M.; Oz, N.; Aksu, A.; Balkis, N.; Altug, G.; Taşkın, Ö.T. Hydrophobic pesticide endosulfan (α + β) and endrin sorption on different types of microplastics. J. Chem. Soc. Pak. 2020, 42, 789–797. [Google Scholar]
- Oz, N.; Kadizade, G.; Yurtsever, M. Investigation of heavy metal adsorption on microplastics. Appl. Ecol. Environ. Res. 2019, 17, 7301. [Google Scholar] [CrossRef]
- Erjavec, A.; Plohl, O.; Zemljič, L.F.; Valh, J.V. Significant Fragmentation of Disposable Surgical Masks—Enormous Source for Problematic Micro/Nanoplastics Pollution in the Environment. Sustainability 2022, 14, 12625. [Google Scholar] [CrossRef]
- Aragaw, T.A. Microplastic pollution in African countries’ water systems: A¬review on¬fndings, applied methods, characteristics, impacts, and¬ managements. SN Appl. Sci. 2021, 3, 629. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, J.; Saturno, J.; Brooks, A.L.; Jacobs, S.; Jambeck, J.R. An emerging source of plastic pollution: Environmental presence of plastic personal protective equipment (PPE) debris related to COVID-19 in a metropolitan city. Environ. Pollut. 2021, 269, 116160. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Nabipour, I.; Tangestani, M.; Abedi, D.; Javanfekr, F.; Jeddi, F.; Zendehboodi, A. Abandoned COVID-19 personal protective equipment along the Bushehr shores, the Persian Gulf: An emerging source of secondary microplastics in coastlines. Mar. Pollut. Bull. 2021, 168, 12386. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int (accessed on 21 December 2020).
- Chowdhury, H.; Chowdhury, T.; Sait, S.M. Estimating marine plastic pollution from COVID-19 face masks in coastal regions. Mar. Pollut. Bull. 2021, 168, 112419. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, F.; Xu, H.; Jiang, H.; Liu, J.; Li, P.; Chen, C.C.; Pan, K. Face masks as a source of nanoplastics and microplastics in the environment: Quantification, characterization, and potential for bioaccumulation. Environ. Pollut. 2021, 288, 117748. [Google Scholar] [CrossRef]
- Wang, Z.; An, C.; Chen, X.; Lee, K.; Zhang, B.; Feng, Q. Disposable masks release microplastics to the aqueous environment with exacerbation by natural weathering. J. Hazard. Mater. 2021, 417, 126036. [Google Scholar] [CrossRef]
- Saliu, F.; Veronelli, M.; Raguso, C.; Barana, D.; Galli, P.; Lasagni, M. The release process of microfibers: From surgical face masks into the marine environment. Environ. Adv. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Shen, M.; Zeng, Z.; Song, B.; Yi, H.; Hu, T.; Zhang, Y.; Zeng, G.; Xiao, R. Neglected microplastics pollution in global COVID-19: Disposable surgical masks. Sci. Total Environ. 2021, 790, 148130. [Google Scholar] [CrossRef]
- D1141-98; Standard Practice for the Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2013.
- De Falco, F.; Gullo, M.P.; Gentile, G.; Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnésa, M.; Rovira, A.; Escudero, R.; Villalba, R.; et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2018, 236, 916–925. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless melt-electrospinning of polypropylene nanofibers. J. Nanomater. 2012, 2012, 382639. [Google Scholar] [CrossRef]
- Zhou, S.; Kong, L.; Yan, C.; Zhou, Y.; Qiu, X.; Liu, C. Rhodamine B dye is efficiently degraded by polypropylene-based cerium wet catalytic materials. RSC Adv. 2020, 10, 26813–26823. [Google Scholar] [CrossRef] [PubMed]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.; Sandblom, O.; MacLeod, M. Identification of chain scission products released to water by plastic exposed to ultraviolet light. Environ. Sci. Technol. Lett. 2018, 5, 272–276. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined effects of uv exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, C.; Chen, X.; Jiang, A.; You, Z.; Shah, K.J. Different weathering conditions affect the release of microplastics by masks. Environ. Sci. Pol. Res. 2023, 30, 66102–66112. [Google Scholar] [CrossRef]
- Trevisan, R.; Ranasinghe, P.; Jayasundara, N.; Di Giulio, R.T. Nanoplastics in aquatic environments: Impacts on aquatic species and interactions with environmental factors and pollutants. Toxics 2022, 10, 326. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.L.; Zamparas, M.G.; Kapsalis, V.C. Investigating the human impacts and the environmental consequences of microplastics disposal into water resources. Sustainability 2022, 14, 828. [Google Scholar] [CrossRef]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro-and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.L.; Zamparas, M.; Kapsalis, V.C.; Kalavrouziotis, I. KEutrophication control: The shif to invasive methods managing the internal nutrient loads. a bibliometric analysis. Desalin. Water Treat. 2022, 267, 177–185. [Google Scholar] [CrossRef]
- Cavalcante, J.; Hardian, R.; Szekely, G. Antipathogenic upcycling of face mask waste into separation materials using green solvents. Sustain. Mater. Technol. 2022, 32, e00448. [Google Scholar] [CrossRef]
- Abdelhameed, M.; Elbeh, M.; Baban, N.S.; Pereira, L.; Matula, J.; Song, Y.A.; Ramadi, K.B. High-yield, one-pot upcycling of polyethylene and polypropylene waste into blue-emissive carbon dots. Green Chem. 2023, 25, 1925–1937. [Google Scholar] [CrossRef]






| Face Masks | Specifications |
|---|---|
| S1 | Surgical face mask, Type IIR, TS EN 14683, polypropylene, latex-free, nylon-free, non-sterile, three-ply with ear loop, CE certification, meltblown in three layers. |
| S2 | Surgical face mask, Type IIR, EN 14683, polypropylene, non-sterile, three-ply wit ear loop, CE certification, spunbound (inner and outer layer), meltblown (middle layer) |
| S3 | Surgical face mask, TS EN 14683-AC, Polypropylene, non-sterile, latex-free, glass-fiber-free, three-ply wit ear loop, CE certification |
| S4 | Surgical face mask, Type IIR, TS EN 14683, polypropylene, fiberglass-free, three-ply with ear loop, CE certification, spunbound (inner and outer layer), meltblown (middle layer) |
| F1 | FFP2 face mask, EN 149:2001+A1:2009, latex-free, EU 2016/425, CE certificate |
| F2 | FFP2 face mask, EN 149:2001+A1:2009, latex-free, EU 2016/425, CE certificate |
| F3 | FFP2 face mask, EN 149:2001+A1:2009, latex-free, EU 2016/425, CE certificate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celik, S.O. The Release Potential of Microplastics from Face Masks into the Aquatic Environment. Sustainability 2023, 15, 14293. https://doi.org/10.3390/su151914293
Celik SO. The Release Potential of Microplastics from Face Masks into the Aquatic Environment. Sustainability. 2023; 15(19):14293. https://doi.org/10.3390/su151914293
Chicago/Turabian StyleCelik, Suna Ozden. 2023. "The Release Potential of Microplastics from Face Masks into the Aquatic Environment" Sustainability 15, no. 19: 14293. https://doi.org/10.3390/su151914293
APA StyleCelik, S. O. (2023). The Release Potential of Microplastics from Face Masks into the Aquatic Environment. Sustainability, 15(19), 14293. https://doi.org/10.3390/su151914293








