The Blue Tansy Essential Oil–Petra/Osiris/Molinspiration (POM) Analyses and Prediction of Its Corrosion Inhibition Performance Based on Chemical Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mild Steel Composition and Aggressive Solution
2.2. Characterization of Blue Tansy Essential Oil
2.3. Electrochemical Measurements
2.4. Weight Loss Measurements
2.5. Petra/Osiris/Molinspiration (POM) Analyses
3. Results and Discussion
3.1. Chemical Composition of Blue Tansy Essential Oil
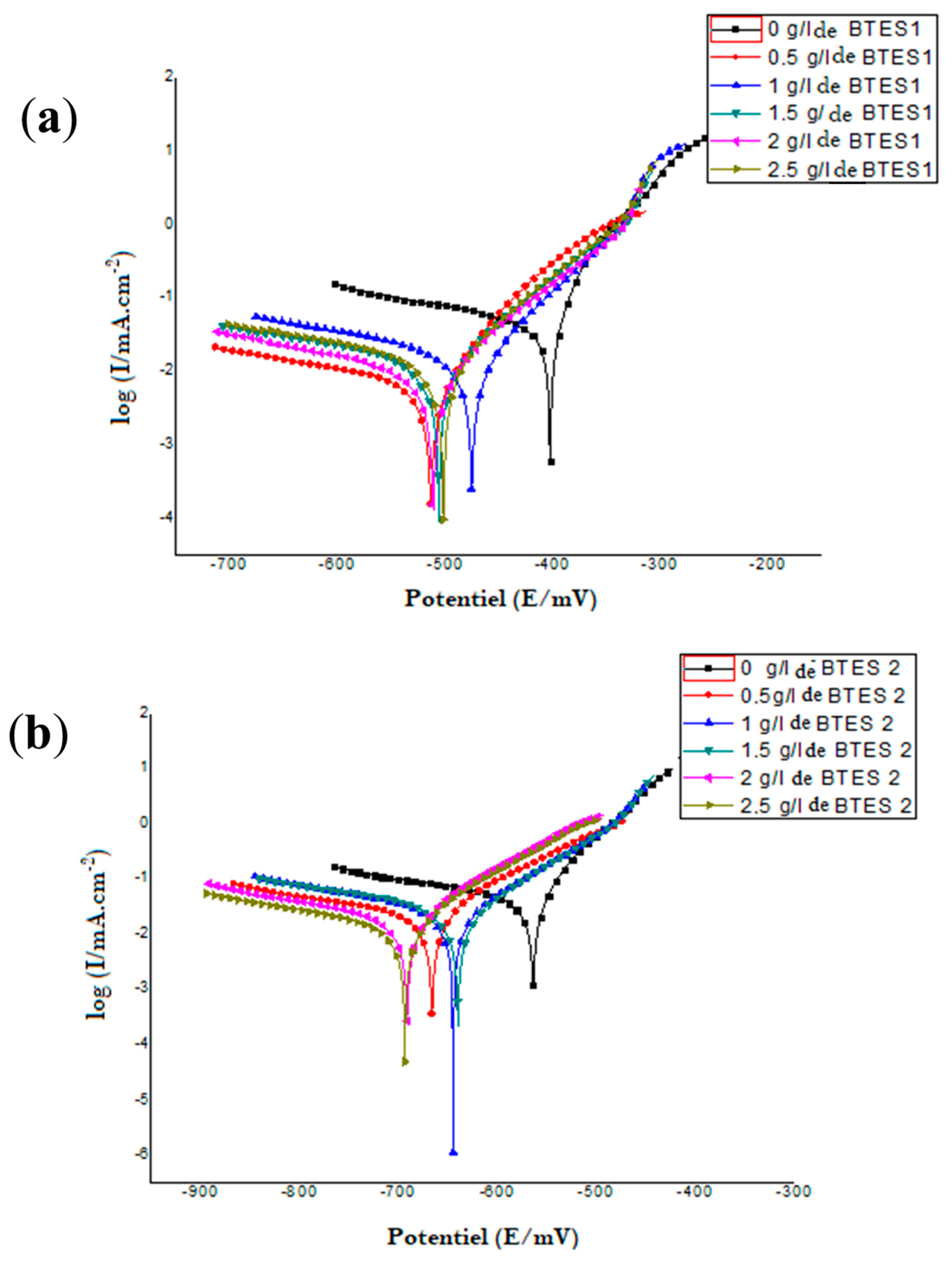
3.2. Electrochemical Measurements
3.3. Weight Loss Measurements
3.4. POM Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Uhlig, H.H.; Revie, R.W. Corrosion and Corrosion Control; John and Wiley and Sons: Hoboken, NJ, USA, 2008; pp. 1–314. [Google Scholar]
- Elayaperumal, K.; Raja, V.S. Corrosion Failures; John and Wiley and Sons: Hoboken, NJ, USA, 2015; pp. 2–70. [Google Scholar]
- Raviprabha, K.; Bhat, R.S. Corrosion inhibition effect of Ethyl 1-(4-chlorophenyl) -5-methyl-1H-1,2,3- triazole-4 carboxylate on aluminium alloy in hydrochloric acid. Protect. Met. Phys. Chem. Surf. 2021, 57, 181–189. [Google Scholar] [CrossRef]
- Raviprabha, K.; Bhat, R.S. Electrochemical and quantum chemical studies of 5-[(4-Chlorophenoxy) methyl] -4H-1,2,4- triazole-3-thiol on the corrosion inhibition of 6061 Al alloy in hydrochloric acid. J Fail. Anal. And Preven. 2020, 20, 1598–1608. [Google Scholar] [CrossRef]
- Ganash, A.; Alsayed, S.; Al-Moubaraki, A.H. Anticorrosive properties of aqueous Cichorium intybus seeds extract as a sustainable-green inhibitor for aluminum corrosion in hydrochloric acid solution: An experimental and DFT/MC/MD theoretical approach. J. Environ. Chem. Eng. 2023, 11, 110227. [Google Scholar] [CrossRef]
- Alao, A.O.; Sanni, O.; Popoola, A.P. Insight into the anti-corrosive performance of Persea americana seed extract as a high-efficiency and sustainable corrosion inhibitor for API 5 L X65 pipeline steel in 1 M HCl solution. Int. J. Electrochem. Sci. 2023, 18, 100248. [Google Scholar] [CrossRef]
- Negm, N.A.; Yousef, M.A.; Tawfik, M. Impact of synthesized and natural compounds in corrosion inhibition of carbon steel and aluminium in acidic media. Recent Pat. Corros. Sci. 2013, 3, 58–68. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Enenebeaku, C.K.; Akalezi, C.O.; Okoro, S.C.; Ayuk, A.A.; Ejike, E.N. Adsorption and corrosion-inhibiting effect of Dacryodis edulis extract on low-carbon-steel corrosion in acidic media. J. Coll. Interf. Sci. 2010, 349, 283–292. [Google Scholar] [CrossRef]
- Singh, W.P.; Bockris, J.O. Toxicity issues of organic corrosion inhibitors: Application of QSAR model. In Corrosion; Paper No. 225; NACE: Houston, TX, USA, 1996. [Google Scholar]
- Znini, M.; Majidi, L.; Bouyanzer, A.; Paolini, J.; Desjobert, J.M.; Costa, J.; Hammouti, B. Essential oil of Salvia Aucheri mesatlantica as a green inhibitor for the corrosion of steel in 0.5 M H2SO4. Arab. J. Chem. 2012, 5, 467–474. [Google Scholar] [CrossRef]
- Manssouri, M.; El-Ouadi, Y.; Znini, M.; Costa, J.; Bouyanzer, A.; Desjobert, J.M.; Majidi, L. Adsorption proprieties and inhibition of mild steel corrosion in HCl solution by the essential oil from fruit of Moroccan Ammodaucus leucotrichus. J. Mater. Environ. Sci. 2015, 6, 631–646. [Google Scholar]
- Andreani, S.; De Cian, M.C.; Paolini, J.; Desjobert, J.M.; Costa, J.; Muselli, A. Chemical variability and antioxidant activity of Limbarda crithmoides L. essential oil from Corsica. Chem. Biodivers. 2013, 10, 2061–2077. [Google Scholar] [CrossRef]
- Darriet, F.; Znini, M.; Majidi, L.; Muselli, A.; Hammouti, B.; Bouyanzer, A.; Costa, J. Evaluation of Eryngium maritimum essential oil as environmentally friendly corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 2013, 8, 4328–4345. [Google Scholar] [CrossRef]
- Hmamou, D.B.; Salghi, R.; Zarrouk, A.; Zarrouk, H.; Errami, M.; Hammouti, B.; Afa, L.; Bazzi, L. Adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by Verbena essential oil. Res. Chem. Intermed. 2013, 39, 973–989. [Google Scholar] [CrossRef]
- Gualdron, A.F.; Becerra, E.N.; Pena, D.Y.; Gutierrez, J.C.; Becerra, H.Q. Inhibitory effect of Eucalyptus and Lippia alba essential oils on the corrosion of mild steel in hydrochloric acid. J. Mater. Environ. Sci. 2013, 4, 143–158. [Google Scholar]
- Nabah, R.; Lgaz, H.; Zarrok, H.; Larouj, M.; Benhiba, F.; Ourrak, K.; Cherkaoui, M.; Zarrouk, A.; Touir, R.; Oudda, H. Anticorrosion properties of Niaouli essential oil for tinplate in 3% NaCl medium. J. Mater. Environ. Sci. 2017, 8, 3730–3739. [Google Scholar]
- Boumhara, K.; Tabyaoui, M.; Jama, C.; Bentiss, F. Artemisia mesatlantica essential oil as green inhibitor for carbon steel corrosion in 1 M HCl solution: Electrochemical and XPS investigations. J. Ind. Eng. Chem. 2015, 29, 146–155. [Google Scholar] [CrossRef]
- Rekkab, S.; Zarrok, H.; Salghi, R.; Zarrouk, A.; Bazzi, L.; Hammouti, B.; Kabouche, Z. Green corrosion inhibitor from essential oil of Eucalyptus globulus (Myrtaceae) for C38 steel in sulfuric acid solution. J. Mater. Environ. Sci. 2012, 3, 613–627. [Google Scholar]
- Ivănescu, B.; Tuchiluș, C.; Corciovă, A.; Lungu, C.; Mihai, C.T.; Gheldiu, A.M.; Vlase, L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Loto, R.T.; Solomon, M.M. Application of ginger and grapefruit essential oil extracts on the corrosion inhibition of mild steel in dilute 0.5 M H2SO4 electrolyte. Sci. African 2023, 19, e01489. [Google Scholar] [CrossRef]
- Rosliza, R.; Nora’aini, A.; Wan Nik, W.B. Study on the effect of vanillin on the corrosion inhibition of aluminum alloy. J. Appl. Electrochem. 2010, 40, 833–840. [Google Scholar] [CrossRef]
- Aouniti, A.; Elmsellem, H.; Tighadouini, S.; Elazzouzi, M.; Radi, S.; Chetouani, A.; Hammouti, B.; Zarrouk, A. Schiff’s base derived from 2-acetyl thiophene as corrosion inhibitor of steel in acidic medium. J. Taibah Univ. Sci. 2016, 10, 774–785. [Google Scholar] [CrossRef]
- Salih, R.H.H.; Hasana, A.H.; Hussenc, N.H.; Hawaiz, F.E.; Hadda, T.B.; Jamalis, J.; Almalki, F.A.; Adeyinka, A.S.; Coetzee, L.C.; Oyebamiji, A.K. Thiazole-pyrazoline hybrids as potential antimicrobial agent: Synthesis, biological evaluation, molecular docking, DFT studies and POM analysis. J. Mol. Struc. 2023, 1282, 135191. [Google Scholar] [CrossRef]
- Chalkha, M.; El Moussaoui, A.; Hadda, T.B.; Berredjemd, M.; Bouzina, A.; Almalki, F.A.; Saghrouchnif, H.; Bakhoucha, M.; Saadi, M.; El Ammari, L.; et al. Crystallographic study, biological evaluation and DFT/POM/Docking analyses of pyrazole linked amide conjugates: Identification of antimicrobial and antitumor pharmacophore sites. J. Mol. Struc. 2023, 1252, 131818. [Google Scholar] [CrossRef]
- Tariq, M.; Sirajuddin, M.; Ali, S.; Khalid, N.; Tahir, M.N.; Khan, H.; Ansari, T.M. Pharmacological investigations and Petra/Osiris/Molinspiration (POM) analyses of newly synthesized potentially bioactive organotin (IV) carboxylates. J. Photochem. Photobiol. B Biol. 2016, 158, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Benabdellah, M.; Tounsi, A.; Khaled, K.F.; Hammouti, B. Thermodynamic, chemical and electrochemical investigations of 2-rcapto benzimidazole as corrosion inhibitor for mild steel in hydrochloric acid solutions. Arab. J. Chem. 2011, 4, 17–24. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, S.; Xiang, Q.; Tan, W.; Li, W.; Chen, S.; Guo, L. Experimental and theoretical investigation of corrosion inhibition effect of multi-active compounds on mild steel in 1 M HCl. Int. J. Electrochem. Sci. 2019, 14, 6855–6873. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, K.; Liu, B.; Wang, B. Effects of different metal corrosion inhibitors on hydrogen suppression and film formation with Mg–Zn alloy dust in NaCl solutions. Int. J. Hydrogen Energy. 2022, 47, 29172–29183. [Google Scholar] [CrossRef]
- Zerga, B.; Attayibat, A.; Sfaira, M.; Taleb, M.; Hammouti, B.; Ebn Touhami, M.; Radi, S.; Rais, Z. Effect of some tripodal bipyrazolic compounds on C38 steel corrosion in hydrochloric acid solution. J. Appl. Electrochem. 2010, 40, 1575–1582. [Google Scholar] [CrossRef]
- Taylor, C.D.; Li, S.; Samin, A.J. Oxidation versus salt-film formation: Competitive adsorption on a series of metals from first principles. Electrochim. Acta 2018, 269, 93–101. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, J.; Wang, M.; Liu, Q.; Wang, J.; Chong, Y.; Jia, G. Synergistic corrosion inhibition effects of quaternary ammonium salt cationic surfactants and thiourea on Q235 steel in sulfuric acid: Experimental and theoretical research. Corros. Sci. 2022, 199, 110199. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Rahsepar, M. The use of green Bistorta officinalis extract for effective inhibition of corrosion and scale formation problems in cooling water system. J. Alloys Comp. 2019, 770, 669–678. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Barakat, A.; Yousuf, S.; Choudhary, M.I.; Frey, W.; Hadda, T.B.; Mubarak, M.S. Substituted thieno [2,3-b] thiophenes and related congeners: Synthesis, b-glucuronidase inhibition activity, crystal structure, and POM analyses. Substituted thieno [2,3-b] thiophenes and related congeners: Synthesis, b-glucuronidase inhibition activity, crystal structure, and POM analyses. Bioorg. Med. Chem. 2014, 22, 6715–6725. [Google Scholar] [CrossRef] [PubMed]
- Raveen, M.; Morales-Bayuelo, A. Drug Designing against VP4, VP7 and NSP4 of Rotavirus Proteins–Insilico studies. Mor. J. Chem. 2023, 14, 729–741. [Google Scholar] [CrossRef]
- Eziuka, J.E.; Onyeachu, I.B.; Njoku, D.I.; Nwanonenyi, S.C.; Chidiebere, M.A.; Oguzie, E.E. Elucidating the inhibition behavior of Pterocarpus santalinoides leaves extract on mild steel corrosion in H2SO4 solution–GC-MS, FTIR, SEM, Experimental and computational approach. Mor. J. Chem. 2023, 14, 579–593. [Google Scholar]
- Obot, I.B.; Umoren, S.A.; Obi-Egbedi, N.O. Corrosion inhibition and adsorption behaviour for aluminuim by extract of Aningeria robusta in HCl solution: Synergistic effect of iodide ions. J. Mater. Environ. Sci. 2011, 2, 60–71. [Google Scholar]
- Lrhoul, H.; Sekkal, H.; Hammouti, B. Natural Plants as Corrosion Inhibitors: Thermodynamic’s restrictions. Mor. J. Chem. 2023, 14, 689–698. [Google Scholar]
- Eduok, U.M.; Umoren, S.A.; Udoh, A.P. Synergistic inhibition effects between leaves and stem extracts of Sida acuta and iodide ion for mild steel corrosion in 1 M H2SO4 solutions. Arab. J. Chem. 2012, 5, 325–337. [Google Scholar] [CrossRef]
| Injection Volume | 1 µL | ||
| Injector temperature | 250 °C (with split ratio of 5) | ||
| Detector temperature (FID) | 280 °C | ||
| Column | RESTEK (60 m, 0.25 mm ID, 0.25 µm) | ||
| Detector | FID | ||
| Carrier gas | N2, He Air 1.2, 45 et 450 mL/min respectively | ||
| Oven program | °C/min | Temperature (°C) | Maintain (min) |
| … | 50 | 5 | |
| 10 | 200 | 0 | |
| 5 | 240 | 2 | |
| BTES 1 | BTES 2 | ||||
|---|---|---|---|---|---|
| N° | Molecule | % | N° | Molecule | % |
| 1 | β-Pinene | 2.2 | 1 | Santolina triene | 1.94 |
| 2 | α-Pinene | 2.04 | 2 | α-Pinene | 8.75 |
| 3 | Mycrene | 2.06 | 3 | Camphene | 15.81 |
| 4 | Sabinene | 15.83 | 4 | Sabinene | 3.80 |
| 5 | 1,8-Cineole | 1.26 | 5 | 1,8-Cineole | 2.87 |
| 6 | Gamma-terpinene | 0.83 | 6 | α-terpinene | 3.00 |
| 7 | o-Cymene | 5.86 | 7 | p-Cymene | 0.47 |
| 8 | α-Phellandrene | 4.45 | 8 | β-Phellandrene | 1.00 |
| 9 | β-Caryophyllene | 0.85 | 9 | α-Bisabolol oxide B | 1.17 |
| 10 | β-Eudesmol | 1.66 | 10 | α-Farnesene | 0.93 |
| 11 | β-Farnesene | 0.8 | 11 | β-Farnesene | 0.95 |
| 12 | Terpinene-4-ol | 0.74 | 12 | Terpinene-4-ol | 0.84 |
| 13 | Limonene | 2.33 | 13 | Limonene | 0.70 |
| 14 | Camphor | 10.58 | 14 | Camphor | 3.30 |
| 15 | Chamazulene | 15.61 | 15 | Chamazulene | 13.49 |
| 16 | Not identified | 1.23 | 16 | α-Bisabolol oxide A | 29.03 |
| 17 | Not identified | 0.525 | 17 | α-Terpineol | 0.33 |
| 18 | Not identified | 1.19 | 18 | Linalol | 0.96 |
| 19 | Not identified | 0.66 | 19 | Cis-enyne dicycloether | 0.68 |
| 20 | Not identified | 1.14 | 20 | Trans-enyne dicycloether | 0.91 |
| 21 | α-Bisabolone oxide A | 7.48 | |||
| 22 | Germacrene D | 1.59 | |||
| Inhibitor | Concentration (g/L) | Ecorr (mV/SCE) | βa (mV/dec) | βc (mV/dec) | icorr (mA/cm2) | Rp Ω cm2 | Corrosion µm/Year | IE (%) |
|---|---|---|---|---|---|---|---|---|
| BTES 1 | 0 | −402.9 | 51.5 | −55.04 | 0.0489 | 385.69 | 572.26 | − |
| 0.5 | −513.6 | 73.2 | −72.49 | 0.0091 | 3490 | 106.16 | 81.39 | |
| 1 | −475.5 | 66.5 | −62.72 | 0.019 | 1720 | 222.26 | 61.15 | |
| 2 | −511.5 | 99.1 | −87.02 | 0.0126 | 2850 | 147.14 | 74.23 | |
| 2.5 | −502.1 | 91 | −87.85 | 0.0161 | 2100 | 188.73 | 67.08 | |
| BTES 2 | 0 | −564.4 | 61.1 | −58.56 | 0.0516 | 472.75 | 603.76 | − |
| 0.5 | −666.6 | 109.1 | −114.6 | 0.0225 | 1140 | 262.92 | 56.45 | |
| 1 | −645 | 108.9 | −94.34 | 0.0271 | 1120 | 317.5 | 47.41 | |
| 2 | −691.6 | 100.5 | −97.08 | 0.0198 | 1400 | 231.35 | 61.68 | |
| 2.5 | −639.9 | 92.8 | −88.08 | 0.0121 | 1880 | 141.79 | 76.51 |
| BTES 1 | BTES 2 | ||||
|---|---|---|---|---|---|
| C (g/L) | CR (mg h−1 cm−2) | IE (%) | C (g/L) | CR (mg h−1 cm−2) | IE (%) |
| 0 | 0.12468 | − | 0 | 0.1512 | − |
| 0.5 | 0.02390 | 80.83 | 0.5 | 0.0743 | 50.88 |
| 1 | 0.05400 | 56.69 | 1 | 0.0875 | 42.11 |
| 2 | 0.03500 | 71.93 | 2 | 0.0663 | 56.14 |
| 2.5 | 0.04800 | 61.50 | 2.5 | 0.0424 | 71.93 |
| Molecule | Calculation of Molecule Property | Calculation of Bioactivity Score | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| miLogP | TPSA | Natoms | MW | nON | nOHNH | Nviolations | Nrotb | Volume | GPCR Ligand | ION Channel Modulator | Kinase Inhibitor | Nuclear Receptor Ligand | Protease Inhibitor | Enzyme Inhibitor | |
| α-Bisabolol oxide A | 3.41 | 29.46 | 17 | 238.37 | 2 | 1 | 0 | 1 | 252.29 | −0.07 | 0.40 | −0.70 | 0.39 | −0.11 | 0.76 |
| Sabinene | 3.10 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 1 | 152.37 | −1.15 | −0.33 | −1.79 | −0.69 | −0.78 | −0.60 |
| Camphene | 3.33 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 0 | 152.37 | −1.02 | −0.55 | −1.85 | −1.15 | −1.40 | −0.82 |
| Chamazulene | 4.84 | 0.00 | 14 | 184.28 | 0 | 0 | 0 | 1 | 194.52 | −0.33 | −0.34 | −0.67 | −0.40 | −0.72 | 0.04 |
| Camphor | 2.16 | 17.07 | 11 | 152.24 | 1 | 0 | 0 | 0 | 159.86 | −0.79 | −0.56 | −2.12 | −1.21 | −0.95 | −0.52 |
| α-Pinene | 3.54 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 0 | 151.81 | −0.48 | −0.43 | −1.50 | −0.62 | −0.85 | −0.34 |
| α-Bisabolone oxide A | 3.41 | 29.46 | 17 | 238.37 | 2 | 1 | 0 | 1 | 252.29 | −0.07 | 0.40 | −0.70 | 0.39 | −0.11 | 0.76 |
| α-terpinene | 3.36 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 1 | 156.74 | −0.96 | −0.24 | −1.29 | −0.24 | −1.52 | −0.11 |
| p-Cymene | 3.90 | 0.00 | 10 | 134.22 | 0 | 0 | 0 | 1 | 150.55 | −1.18 | −0.61 | −1.40 | −1.21 | −1.42 | −0.78 |
| β-Phellandrene | 3.58 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 1 | 157.32 | −0.99 | −0.48 | −1.55 | −0.28 | −1.31 | −0.27 |
| α-Bisabolol oxide B | 3.41 | 29.46 | 17 | 238.37 | 2 | 1 | 0 | 2 | 252.29 | −0.05 | −0.03 | −0.56 | 0.32 | −0.33 | 0.45 |
| α-Farnesene | 5.82 | 0.00 | 15 | 204.36 | 0 | 0 | 1 | 6 | 239.27 | −0.30 | 0.14 | −0.62 | 0.17 | −0.63 | 0.45 |
| β-Farnesene | 5.84 | 0.00 | 15 | 204.36 | 0 | 0 | 1 | 7 | 239.82 | −0.44 | −0.05 | −0.77 | 0.07 | −0.68 | 0.27 |
| 1,8-Cineole | 1.80 | 29.46 | 12 | 170.25 | 2 | 1 | 0 | 0 | 174.71 | −0.60 | 0.50 | −1.24 | −0.32 | −0.35 | 0.36 |
| Germacrene D | 5.43 | 0.00 | 15 | 204.36 | 0 | 0 | 1 | 1 | 234.90 | −0.30 | −0.11 | −0.81 | 0.32 | −0.67 | 0.26 |
| Terpinene-4-ol | 2.60 | 20.23 | 11 | 154.25 | 1 | 1 | 0 | 1 | 170.65 | −0.56 | −0.04 | −1.68 | −0.20 | −0.92 | 0.06 |
| Limonene | 3.62 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 1 | 157.30 | −0.91 | −0.27 | −2.01 | −0.34 | −1.38 | −0.21 |
| Linalol | 3.21 | 20.23 | 11 | 154.25 | 1 | 1 | 0 | 4 | 175.59 | −0.73 | 0.07 | −1.26 | −0.06 | −0.94 | 0.07 |
| Cis-enyne dicycloether | 3.50 | 18.47 | 15 | 200.24 | 2 | 0 | 0 | 0 | 191.51 | −0.56 | 0.12 | −0.71 | 0.04 | −0.51 | 0.06 |
| Trans-enyne dicycloether | 3.50 | 18.47 | 15 | 200.24 | 2 | 0 | 0 | 0 | 191.51 | −0.56 | 0.12 | −0.71 | 0.04 | −0.51 | 0.06 |
| Santolina triene | 4.20 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 3 | 162.03 | −1.27 | −0.19 | −1.76 | −0.67 | −1.24 | −0.38 |
| α-Terpineol | 2.60 | 20.23 | 11 | 154.25 | 1 | 1 | 0 | 1 | 170.65 | −0.51 | 0.15 | −1.45 | −0.02 | −0.78 | 0.14 |
| Beta-Pinene | 3.33 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 0 | 152.37 | −0.53 | −0.32 | −1.45 | −0.50 | −0.80 | −0.34 |
| gamma-terpinene | 3.36 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 1 | 156.74 | −0.90 | −0.24 | −1.37 | −0.33 | −1.55 | −0.07 |
| o-Cymène | 3.38 | 0.00 | 10 | 134.22 | 0 | 0 | 0 | 1 | 150.55 | −1.09 | −0.54 | −1.35 | −1.14 | −1.37 | −0.71 |
| alpha-Phellandrene | 3.79 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 1 | 156.77 | −1.00 | −0.40 | −1.40 | −0.32 | −1.38 | −0.15 |
| Mycrene | 3.99 | 0.00 | 10 | 136.24 | 0 | 0 | 0 | 4 | 162.24 | −1.11 | −0.33 | −1.51 | −0.45 | −1.31 | −0.07 |
| Beta-Caryophyllene | 5.17 | 0.00 | 15 | 204.36 | 0 | 0 | 1 | 0 | 229.95 | −0.34 | 0.28 | −0.78 | 0.13 | −0.60 | 0.19 |
| Beta-Eudesmol | 4.01 | 20.23 | 16 | 222.37 | 1 | 1 | 0 | 1 | 243.86 | −0.02 | 0.43 | −0.62 | 0.60 | −0.10 | 0.48 |
| Molecule | 2D Structure/Canonical SMILES | Calculation of Toxicity Risks, Drug-likeness and Drug Score | Molecule | 2D Structure/Canonical SMILES | Calculation of Toxicity Risks, Drug-likeness, and Drug Score |
|---|---|---|---|---|---|
| Santolina triene | 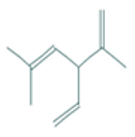 CC(=CC(C=C)C(=C)C)C | 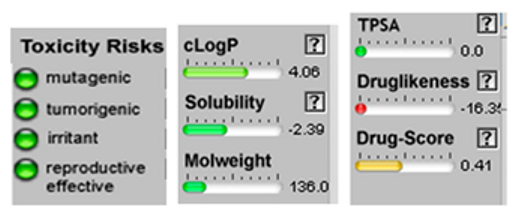 | α-Bisabolol oxide A |  CC1=CCC(CC1)C2(CCC(C(O2)(C)C)O)C | 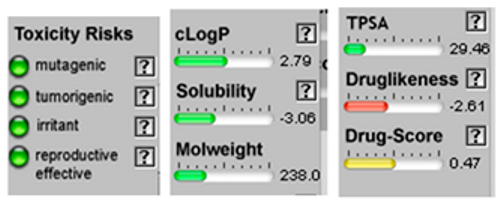 |
| α-Pinene | 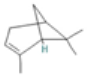 CC1=CCC2CC1C2(C)C | 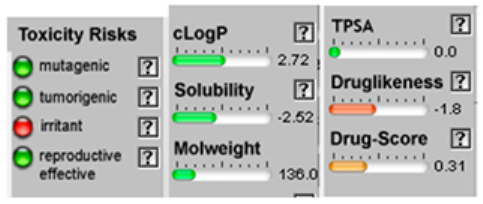 | α-Terpinene | 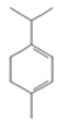 CC1=CC=C(CC1)C(C)C |  |
| Camphene | 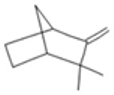 CC1(C2CCC(C2)C1=C)C | 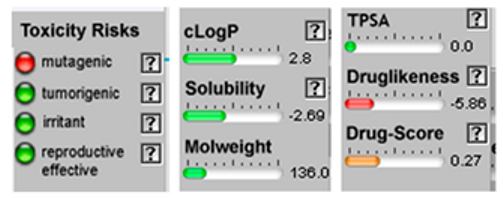 | P-Cymene |  CC1=CC=C(C=C1)C(C)C | 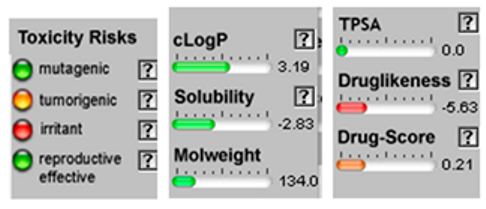 |
| Sabinene | 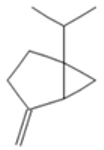 CC(C)C12CCC(=C)C1C2 | 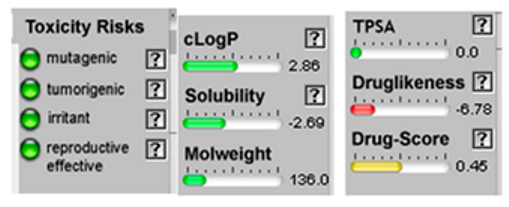 | β-Phellandrene | 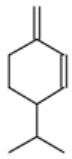 CC(C)C1CCC(=C)C=C1 | 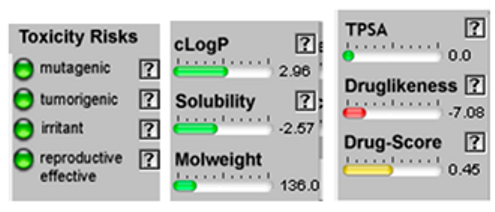 |
| 1.8-Cineole |  CC1(C2CCC(O1)(C(C2)O)C)C |  | α-Bisabolol oxide B | 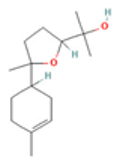 CC1=CCC(CC1)C2(CCC(O2)C(C)(C)O)C |  |
| β-Farnesene | 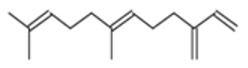 CC(=CCCC(=CCCC(=C)C=C)C)C | 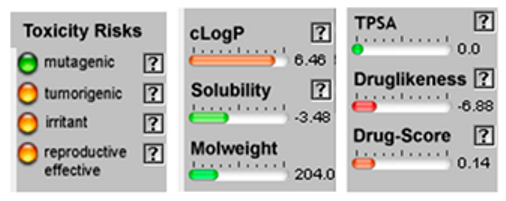 | α –Farnesene | 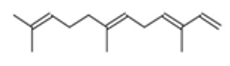 CC(=CCC/C(=C/C/C=C(\C)/C=C)/C)C |  |
| Germacrene D | 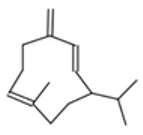 CC1=CCCC(=C)C=CC(CC1)C(C)C |  | Camphor |  CC1(C2CCC1(C(=O)C2)C)C | 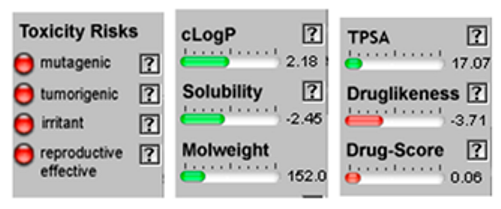 |
| Terpinene-4-ol |  CC1=CCC(CC1)(C(C)C)O | 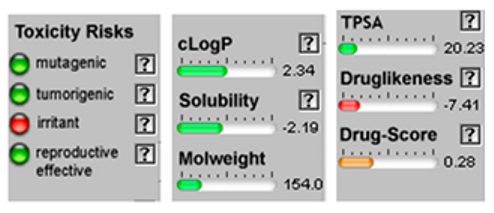 | Linalol |  CC(=CCCC(C)(C=C)O)C | 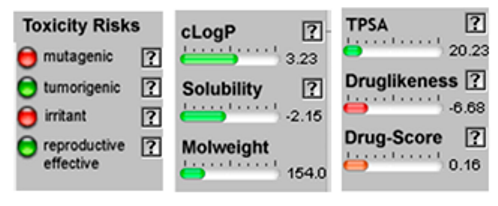 |
| Limonene |  CC1=CCC(CC1)C(=C)C | 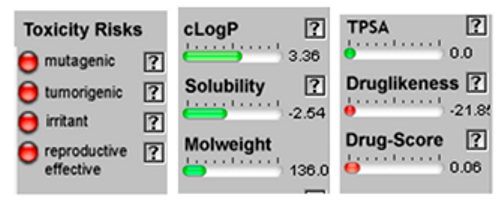 | Chamazulene | 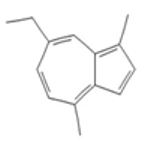 CCC1=CC2=C(C=CC2=C(C=C1)C)C | 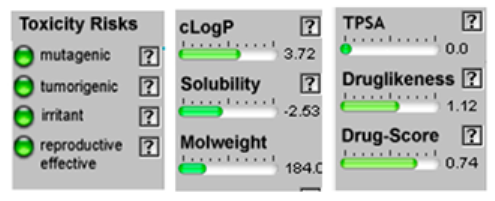 |
| α-Terpineol |  CC1=CCC(CC1)C(C)(C)O | 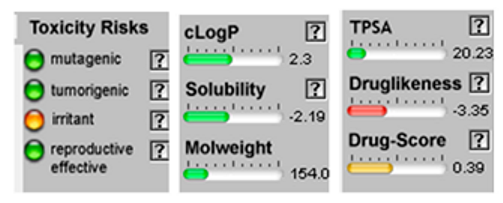 | Trans-enyne dicycloether | 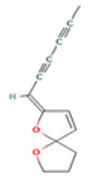 CC#CC#CC=C1C=CC2(O1)CCCO2 | 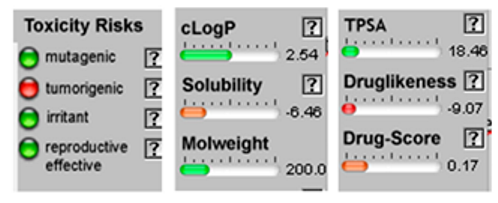 |
| Cis-enyne dicycloether | 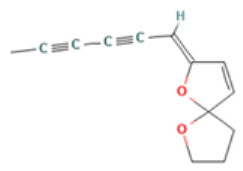 CC#CC#CC=C1C=CC2(O1)CCCO2 | 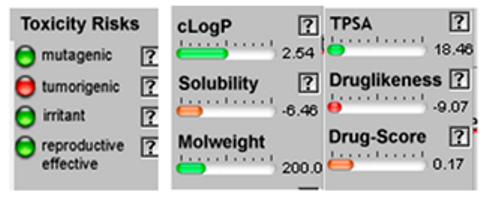 | Gamma-terpinene | 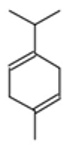 CC1=CCC(=CC1)C(C)C |  |
| α-Bisabolone oxide A | 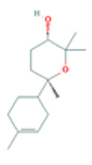 CC1=CCC(CC1)C2(CCC(C(O2)(C)C)O)C |  | o-Cymene |  CC1=CC=CC=C1C(C)C | 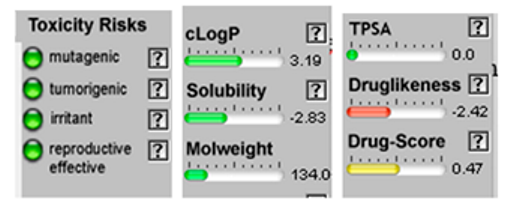 |
| β-Pinene | 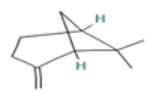 CC1(C2CCC(=C)C1C2)C |  | α-Phellandrene | 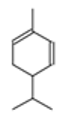 CC1=CCC(C=C1)C(C)C | 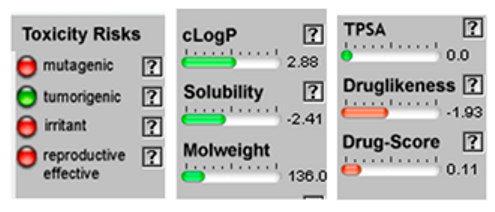 |
| Mycrene | 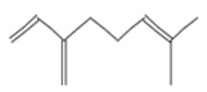 CC(=CCCC(=C)C=C)C | 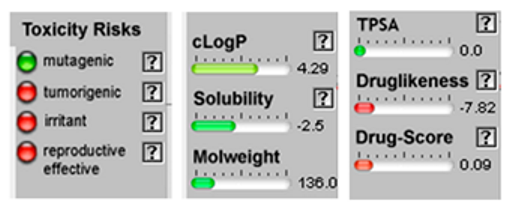 | beta-Caryophyllene |  CC1=CCCC(=C)C2CC(C2CC1)(C)C | 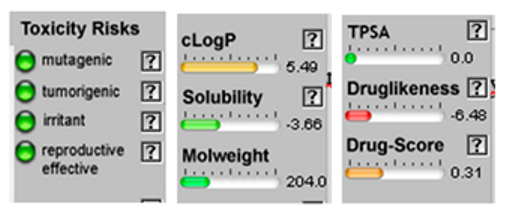 |
| Beta –Eudesmol |  CC12CCCC(=C)C1CC(CC2)C(C)(C)O |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zriouel, W.; Bentis, A.; Majid, S.; Hammouti, B.; Gmouh, S.; Umoren, P.S.; Umoren, S.A. The Blue Tansy Essential Oil–Petra/Osiris/Molinspiration (POM) Analyses and Prediction of Its Corrosion Inhibition Performance Based on Chemical Composition. Sustainability 2023, 15, 14274. https://doi.org/10.3390/su151914274
Zriouel W, Bentis A, Majid S, Hammouti B, Gmouh S, Umoren PS, Umoren SA. The Blue Tansy Essential Oil–Petra/Osiris/Molinspiration (POM) Analyses and Prediction of Its Corrosion Inhibition Performance Based on Chemical Composition. Sustainability. 2023; 15(19):14274. https://doi.org/10.3390/su151914274
Chicago/Turabian StyleZriouel, Wafaa, Aziz Bentis, Sanaa Majid, Belkheir Hammouti, Said Gmouh, Peace S. Umoren, and Saviour A. Umoren. 2023. "The Blue Tansy Essential Oil–Petra/Osiris/Molinspiration (POM) Analyses and Prediction of Its Corrosion Inhibition Performance Based on Chemical Composition" Sustainability 15, no. 19: 14274. https://doi.org/10.3390/su151914274
APA StyleZriouel, W., Bentis, A., Majid, S., Hammouti, B., Gmouh, S., Umoren, P. S., & Umoren, S. A. (2023). The Blue Tansy Essential Oil–Petra/Osiris/Molinspiration (POM) Analyses and Prediction of Its Corrosion Inhibition Performance Based on Chemical Composition. Sustainability, 15(19), 14274. https://doi.org/10.3390/su151914274








