Abstract
The Guatemalan strategy for sea turtle conservation was defined by the National Council of Protected Areas (CONAP) in 1989. Hatcheries lie at the core of this strategy: egg collectors are allowed to deliver 20% of a nest to a hatchery in exchange for selling or eating the remaining eggs. Consequently, nearly 100% of nests are collected, with no nests being left on the beaches. Hatchery design promotes shading using roofs made from vegetation. The logic behind this recommendation is that the natural incubation of eggs is supposedly impossible due to the overly high temperatures on the beach. However, changing the incubation temperature of sea turtle eggs can profoundly alter the sex ratio in sea turtles with temperature-dependent sex determination. It can also modify the physiology or behavior of juvenile turtles. Here, we test whether incubation in natural conditions is possible on Guatemalan beaches, and for the first time, we determine the thermal reaction norm of embryo growth to ensure hatching success in sea turtles. We show that incubation in natural conditions is possible since three out of the four monitored nests produced hatchlings. We urge the Guatemala National Council of Protected Areas to reevaluate its strategy for sea turtle conservation in Guatemala in light of these results.
1. Introduction
The olive ridley sea turtle (Lepidochelys olivacea) is the most common species of sea turtle worldwide, as well as locally along the Pacific coast of Guatemala and Mexico [1]. This turtle exhibits polymorphic nesting behaviors: the arribada (synchronous mass nesting), as well as solitary nesting, have made it a species of particular interest to scientists. Olive ridleys are categorized as vulnerable on the IUCN Red List [2]; in the East Pacific, their main threats are egg poaching, female consumption, coastal development, climate change at their nesting sites, and the unintended capture of adult and sub-adult turtles by fisheries operating within this species’ foraging habitats in marine areas [3]. Conservation efforts should, therefore, focus on managing these threats.
Guatemala is of special interest because of its unique national conservation strategy. In 1971, the General Directorate of Forests and Wildlife (DIGEBOS) of the Ministry of Agriculture (MAGA) established the first turtle hatchery in the village of Hawaii in the Santa Rosa Department, which marked the beginning of hatcheries as central elements in the national strategy for the conservation of sea turtles in Guatemala. Approximately 5% of the total number of nests deposited in Guatemala occurred on this beach [4]. The National Council of Protected Areas (CONAP) was established in 1989 as the government’s governing body for biodiversity issues, including the regulation of turtle hatcheries and other sea turtle conservation activities. Over the years, the number of turtle hatcheries operating in Guatemala has fluctuated between 16 and 30, depending on the available resources and sponsors. The management and sponsorship of turtle hatcheries depend on the different NGOs, educational institutions, and government agencies involved in this process. Hatcheries lie at the core of this conservation strategy, with egg collectors authorized to deliver 20% of a nest to a hatchery in exchange for selling or eating the remaining eggs [5]. In this context, nearly 100% of nests are collected, with none being left on the beaches [5].
The hatchery design promoted in Guatemala is of shaded hatcheries, with coconut or saran leaves being used to adjust the percentage of shade (usually 50–80%), with the objective (which is generally not tested) of obtaining a temperature of 29 °C for sea turtle egg incubation [6,7]. The shade method used in Guatemalan hatcheries is promoted based on a report written in 1988 for the Sea Turtle Conservation Program of the Center for Marine and Aquaculture Studies of the University of San Carlos of Guatemala [8]. This report includes very generalized information, without giving any precise experimental design. For example, it is written: “We buried [the eggs] at various depths without a roof and with ample irrigation, but no eggs hatch, and embryos usually do not form inside the shell”; or “Eggs were deposited on the beach in the same place where the turtles lay their eggs and at the same depth, an average of 45 cm, but they do not hatch” (translated from Spanish) [8]. Furthermore, the report did not indicate the number of nests, clutches, or females, or whether the low hatching success is due to sun exposure or overwatering. Based on this 1988 report, it was concluded that “Unshaded hatcheries have disastrous results due to the loss of many eggs” (translated from Spanish) [6]. It is not clear whether these “disastrous results” can be generalized to all Guatemalan beaches, due to the effect of temperature, or whether the results are simply the consequence of non-optimal hatchery management. Nevertheless, it is evident that olive ridleys nested on these beaches long before modern hatchery management practices were developed [9], and that such a design prevents the occurrence of natural selection and adaptation processes.
To understand if natural nest incubation is possible in natural conditions and to determine the influence of natural conditions on the embryonic development of the olive ridley sea turtle (Lepidochelys olivacea), we assessed the variations in incubation temperatures, hatching success, and embryonic development in natural nests of L. olivacea deposited on an eastern Pacific beach in Guatemala. Temperature plays an important role in all phases of the lifecycle of sea turtles [10]. Sand temperature during the incubation period plays a vital role in embryonic development, hatching success [11], hatchling quality [12], and sex ratio in the case of sea turtles with temperature-dependent sex determination [13,14]. For L. olivacea in the East Pacific, the temperature that produces 50% of each sex is 30.46 °C (95% credible interval; 30.26–30.66 °C) and the range of temperatures producing both sexes is 2.24 °C (95% credible interval; 1.72–2.89 °C) [14]. Studying the incubation temperatures in natural nests provides important information about the temperature range that sea turtle embryos can withstand and at which they can successfully hatch [15]. Research to date has not yet determined whether the temperature and stage of embryonic development interact, such that the thermal tolerance of embryos changes during incubation. The aim of this work was to provide a clear response to this issue. First, using new data obtained from nests incubated in natural conditions on a Guatemalan beach, we have been able to establish the thermal reaction norm of embryo growth for a wide range of temperatures. This thermal reaction norm model has been applied to the timeseries of temperatures obtained from the natural nests; this has allowed us to establish the stage progression of embryos for each of these nests. Then, the observations of the number of embryos dead at each of these stages have been established in relation to the mean temperature for the corresponding stage, using a conditional generalized linear mixed model. These results are then considered in the context of rising temperatures due to climate change [16].
2. Materials and Methods
2.1. Natural Nests
Considering that egg collection is legal in Guatemala and is a common practice along the Pacific coast, we solicited support and authorization to enter and use 2 km of private beach along the Guatemalan Pacific Naval Command (CONAPAC, its acronym in Spanish) coast, which is forbidden without a specific permit. In normal times, the eggs are collected by CONAPAC employees and are brought to their own hatchery. As part of the experiment conducted here, the nests were left on the beach in natural conditions, without the risk of the eggs being taken by collectors.
The sand on the Pacific beaches of Central America has a volcanic origin, which means that the beaches have black sand, with iron and other highly heat-conducting metals. The Pacific coastal plain of Guatemala forms a prominent ledge of more than 200 km in length along the North Pacific coast of Central America. This extensive alluvial plain encompasses a series of overlapping sedimentary fans composed mainly of products from Quaternary volcanic activity, such as volcanic sand, gravel, volcanic ash pumice, and lahar deposits [17]. These materials have been deposited along a network of subparallel fluvial channels that descend from the highlands of the Pacific volcanic chain [18]. These beaches exhibit low albedo, so they absorb more solar radiation and, therefore, have higher temperatures [19].
To assess the temperatures and conditions of the nests, a night patrol was carried out for 6 nights in September and October to look for sea turtles that were nesting or about to nest. Patrols were carried out depending on the tides, normally between 9:00 p.m. and 3:00 a.m., in conformity with the official authorization. Once a nesting turtle was found, standard data were collected: time and date, the geographic location of the nesting site, and female morphological parameters. While the female was nesting, a HOBO Pendant® UA-004-64-G datalogger was placed inside the nest to measure the incubation temperature every 3 h throughout embryonic development during the incubation period. Nests 1 to 3 were equipped on 17 October 2022, while nest 4 was equipped on 18 October 2022. Each nest was marked with 4 PVC posts and was enclosed by a plastic warning band (Supplementary Figure S1). At the end of the incubation period, the nest was exhumed, and the dataloggers were retrieved. During the exhumation, the remains of the eggs were characterized, and each egg was photographed. The embryonic stages were determined. The characteristics of the nesting females and the position of the nests on the beach are summarized in Supplementary Table S1.
The four new nests were added to a previous database of 80 olive ridley nests that were incubated in artificial conditions [20]. The temperature of these 80 nests ranged from 27.669 °C to 34.190 °C.
2.2. Table of Development
The development of Lepidochelys olivacea, Caretta caretta, and Chelonia mydas has previously been described in detail [21,22,23]. A total of 210 L. olivacea [21], 1169 C. mydas, and 1882 C. caretta [22,23] embryos were used. The repartition of the embryos among the different stages is not available in the original publications for L. olivacea [21]. Table 2a in Ref. [22] gives wrong information for C. mydas at stages 25 to 30 when compared to the original data in Ref. [23]. Only data from the Ref. [23] have been used. The size of the L. olivacea embryos was only available for two development stages [21]. Nevertheless, crown-rump length (CRL) and straight carapace length (SCL) were available for embryos of C. caretta and C. mydas for 24 stages. Given that L. olivacea, C. mydas, and C. caretta exhibit very similar development [21,22,23,24,25], we used the quantitative information on embryo size in C. caretta and C. mydas to generate a table of equivalent SCL for L. olivacea, using the following cross products:
- The CRL/SCL ratio was determined for both C. caretta and C. mydas.
- When only CRL information was available, the equivalent SCL for the stages of C. caretta and C. mydas development was estimated using the previous cross products. The equivalent SCL is the expected carapace size for an embryonic stage without a carapace, while taking into account the allometry between CRL and SCL.
- The SCL of L. olivacea embryos was then determined using the average of the cross product with the known size of SCL for this species and the equivalent SCL sizes of C. caretta and C. mydas embryos.
2.3. Thermal Reaction Norm of Embryo Growth
The model of embryo growth integrates into a single framework using both the growth rate, which is dependent on temperature, and embryo growth, which is based on the growth rate [26]. The temperature-dependent growth rate and the embryo growth model were fitted using maximum likelihood, and the distribution of parameters was evaluated using the Bayesian Markov chain Monte Carlo method (MCMC). This method is summarized below, with a few changes to the original method.
Biological temperature-dependent rate models, based on Arrhenius’ and Eyring’s equations, were formulated by Sharpe and DeMichele [27]. The original formulation created by Sharpe and DeMichele was modified by Schoolfield et al. [28] to remove the very high correlations of parameter estimators (Equation (1)):
where is the mean development rate at temperature T (time−1), T is the temperature in K (298 K = 24.85 °C), and R is the universal gas constant (J K−1 mol−1). The original model defined R in cal K−1 mol−1, although this has been converted to SI units.
is the development rate at 24.85 °C, assuming no enzyme inactivation (time−1), is the enthalpy of activation of the reaction catalyzed by the enzymes (J mol−1), is the temperature in K at which the enzymes are ½ active and ½ low-temperature inactive, is the change in enthalpy associated with the low-temperature inactivation of the enzymes (J mol−1), is the temperature in K at which the enzymes are ½ active and ½ high-temperature inactive, and is the change in enthalpy associated with the high-temperature inactivation of the enzymes (J mol−1). To ensure that , a new variable was set up, with . Thus, the fitted variables were , , , , , and .
This model can be simplified by taking into account only four parameters [28]:
For Equation (2), the fitted variables were , , , and .
2.4. Modeling Embryo Growth
The growth of embryos was modeled using Laird’s proposed modification of the Gompertz model [29]:
where is the size or mass at nesting time (time = 0), r(T) is the growth rate at the beginning of the curve, and K is the carrying capacity, with . Note that hatching generally occurs before the embryo reaches a size or mass represented by K. The K parameter can be viewed here simply as a way to slow down growth at the end of incubation [26].
r(T) can be calculated with the four or six parameters of the model developed by Schoolfield et al. [28] and an incubation temperature of T. Knowing X(0), K, and a time-series of r(T), the pattern for the change in the size of this nest was evaluated using Equation (3). The mean SCL at hatching for the turtles in Guatemalan Lepidochelys olivacea nests is 40.87 mm (SD 1.82 mm, n = 746) [30].
Parameters were initially estimated using the maximum likelihood, with a Gaussian distribution of SCL and an identity link. Then, Bayesian MCMC using the Metropolis–Hasting algorithm [31,32] was used to estimate the distribution of parameters. Priors were all chosen from the uniform distribution, which was large enough so as not to constrain the posteriors. The number of iterations, burn-ins, and thinning were chosen using the diagnostic methods of Raftery and Lewis [33], after an initial run of 20,000 iterations and 1000 burn-in iterations. The standard deviations used for the new proposals at each timestep were chosen using adaptive MCMC, in order to maintain an acceptance rate that was close to 0.234 [34].
2.5. Hatching Success, Stage, and Age at Death
The exhumation of each nest started with the excavation of each nest to recover the shells, live or dead hatchlings, and unhatched eggs (Supplementary Figure S2). After opening the nest, we counted the number of shells and evaluated the total number of eggs initially laid by the nesting female in the nest. The hatching success was then estimated (the proportion of live juveniles out of the total number of eggs). Then, each unhatched egg was opened and photographed. The embryonic stage of these eggs was characterized as “no visible development” or “with visible development” (Supplementary Figures S3A and S3B, respectively). Eggs with no visible development could include unfertilized or infertile eggs [35,36]. For eggs with visible development, the embryonic stage of death (Supplementary Figure S3C) was determined, based on a comparison with the stages detailed in the tables presented elsewhere by Miller [24] and Crastz [21] (Table 1).

Table 1.
Number of dead embryos at each stage recorded in the four nests that were incubated in natural conditions on a Guatemalan beach. Only stages with at least one embryo are shown. The hatching success is 1 − (∑Dead/∑Eggs). The number of total eggs was inferred from the number of shells counted during excavation. The number of eggs dying at stages < 6 includes unfertilized or infertile eggs [35,36].
2.6. Dynamic Analysis of Hatching Success
The dynamics of the proportion of dead embryos at each stage can be misleading if the overall dynamics are not considered. To illustrate this point, let us take a simple example of a nest with a 100% death rate at stage 15 (the hatchling stage is 31). The number of dead embryos recorded at any stage above stage 15 will be 0, leading to the erroneous conclusion that no death occurs at later stages. Another point to consider is the change in the number of eggs when estimating the confidence interval (CI). To illustrate this point, let us take a simple example of a 100-egg nest with a 50% death rate at stage 15 and then 20 eggs dying at stage 16. The incorrect estimation of the death proportion at stage 16 is 20/100 = 0.2 (95% CI, using the Wilson method [37], 0.13–0.29), whereas the correct estimation is 20/(100–50) = 0.4 (95% CI; 0.27–0.54). Note that the point estimate is different (0.2 vs. 0.4), as is the CI width, due to the change in the size of the binomial distribution (0.16 vs. 0.27).
Let N be the total number of eggs and Di be the number of dead embryos at stage i. The distribution of the number of dead embryos at stage i is, thus, . The number of dead embryos at each stage was modeled using a generalized linear mixed model with a binomial distribution and a canonical logit link function. Fixed factors are the maximum temperature at the stage, the equivalent SCL at the middle of the stage, and their interactions, while the random factor is the nest identity. Model selection with various combinations of fixed factors was based on the Akaike information criterion (AIC) [38].
3. Results
3.1. Table of Development for Lepidochelys olivacea
The quantitative description of olive ridley turtle development and its comparison with Chelonia mydas and Caretta caretta development are shown in Table 2.

Table 2.
Equivalent sizes of straight carapace length in mm in Chelonia mydas, Caretta caretta, and Lepidochelys olivacea. Values in bold are direct measurements, while others are cross-product results. The number of measured embryos is indicated in parentheses.
3.2. Temperatures in Natural Nests
The temperatures recorded in the four natural nests are shown in Figure 1, along with the amplitude of the recorded temperatures measured at the same time. The temperature range of these four nests is from 30.154 °C to 37.935 °C. The amplitude ranges from 0.103 °C to 2.463 °C, with an average of 1 °C (SD 0.36 °C).
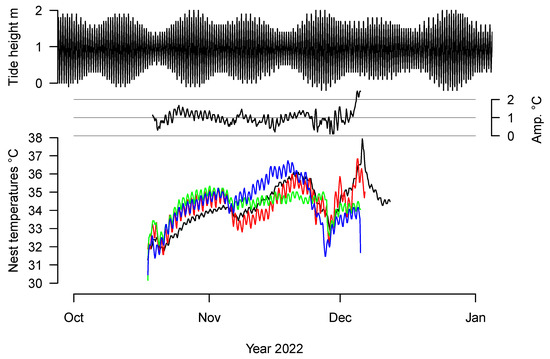
Figure 1.
Temperatures recorded in four natural nests on a Guatemalan beach in 2022. The temperature amplitude was only estimated for the time common to all four nests. Nests 1 to 4 are shown in black, red, green, and blue, respectively. The top graph shows the tide height at the port of San José, located 5 km away from the monitored nesting beach.
3.3. Thermal Reaction Norm of Embryo Growth
An initial MCMC, run with 20,000 iterations, was analyzed using the diagnostic method of Raftery and Lewis [33], showing that at least 418,293 iterations should be run in order to obtain ± 0.005 accuracy. We chose to run 500,000 iterations. The acceptance rates were 0.24, 0.27, 0.27, and 0.26 for , , , and , respectively, which figures are close to the 0.234 optimal acceptance rule [39].
The thermal reaction norm, fitted using the 84 nests (80 from the hatchery, incubated in 2002, and four incubated under natural conditions in 2023), is shown in Figure 2. The temperatures recorded in the four nests incubated under natural conditions are much higher than the temperatures recorded in the hatchery (red vs. white histograms in Figure 2). The development rate increases with temperature until the eggs reach a temperature of 33.45 °C (95% credible interval 33.44–33.46 °C); thereafter, it decreases. When the temperature reaches 37.5 °C, the fitted development rate is 19.4% of the maximum (95% credible interval of 18.6–20.0%).
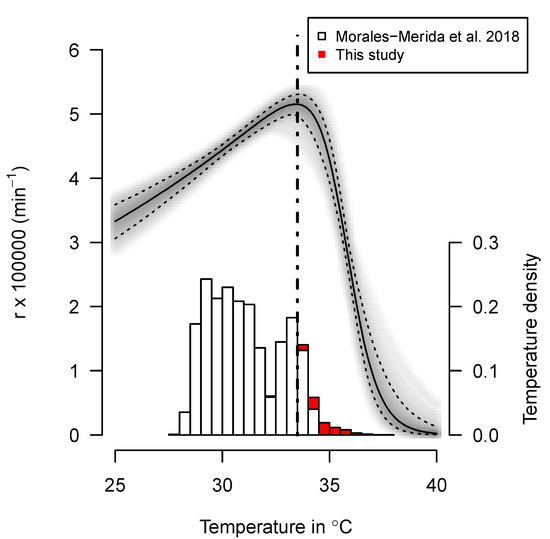
Figure 2.
Density of temperatures recorded in 80 nests incubated in the hatchery (white bars) and in the four natural nests (red bars). The plain line curve shows the median modeled thermal reaction norm of embryo growth, with the 95% credible interval shown in dotted lines. The interrupted vertical line shows the optimal temperature for the rate of embryo growth (i.e., around 33.5 °C) [20].
3.4. Stages of Embryo Death
The four nests incubated in natural conditions show very different patterns regarding the stage of death (Table 1). Nests 1 and 2 show a mortality peak in the last two stages (30 and 31), nest 3 shows a peak at stage 19, and nest 4 shows a peak at stage 30. Overall, mortality is high, and hatching success is only 0.458, 0.557, 0.000, and 0.071 for nests 1 to 4, respectively. The equivalent SCL size for each dead embryo was then determined from Table 1 and 2, and the expected number of days to reach this SCL size was established, based on the embryo growth dynamics (Figure 2 and Figure 3).
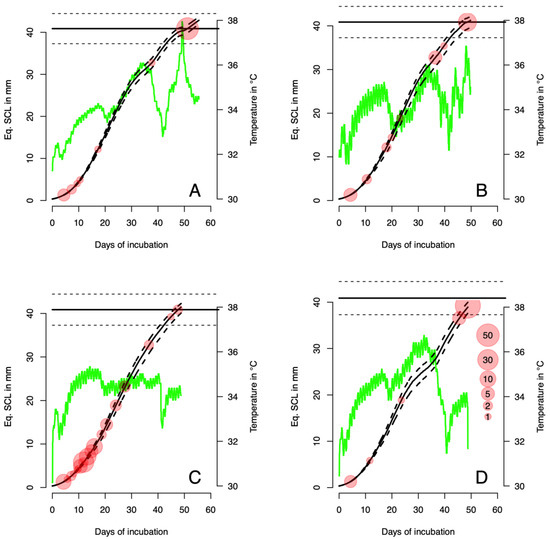
Figure 3.
Modeled growth of embryos in four natural nests (plain line, with the 95% credible interval shown in dashed lines) (left y-axis). The recorded temperature for each of these nests is superimposed (right y-axis). The equivalent SCL size for the dead embryos is shown as points, with the size being proportional to the log of the number of embryos found at the end of the incubation period (the scale is shown in panel (D)). Panels (A–D) show nests 1 to 4, respectively.
3.5. Dynamic Analysis of Hatching Success
The hatching success for the four nests is shown in Table 1. It ranges from 0 (Nest 3) to 0.557 (Nest 2). When the death rate of embryos at each stage is plotted against the maximum incubation temperature at the corresponding stage, a clear pattern emerges (Figure 4A): the death rate increases when the maximum temperature reaches 34 °C. Nest 3, with no hatchling alive at the end of incubation, had an incubation temperature higher than 34 °C during more than half of the incubation period (Figure 3).
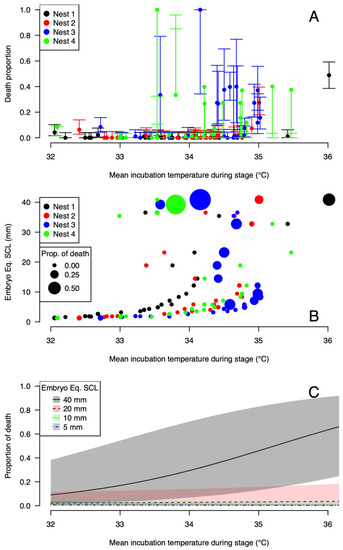
Figure 4.
The observed (A,B) and modeled (C) proportions of dead embryos according to the mean incubation temperature during each stage (A–C) and the developmental stage (equivalent embryo SCL is shown in (B,C)).
The selected model used to link the number of dead embryos at the different stages, the maximum temperature at the corresponding stage, and the equivalent SCL in the middle of the stage, includes both the effects and their interaction (Table 3). This model is strongly supported compared to the simpler models (Akaike weight = 0.93). The fitted parameters show that the proportion of deaths among embryos increases with temperature (Figure 4B,C). The proportion of deaths according to temperature is similar for embryos from 5 to 20 mm but is higher at high temperatures for the late-stage embryos (40 mm). This indicates that late-stage embryos are less resistant to high temperatures.

Table 3.
Generalized linear mixed model, linking the number of dead embryos at the different stages with the mean temperature at the corresponding stage and the equivalent SCL in the middle of the stage. The random factor is the identity of the nest. AICc is the Akaike information criterion corrected for small samples, and the Akaike weight is the probability that the corresponding model is the best among the tested models.
4. Discussion
4.1. Thermal Reaction Norm of Embryo Growth
The previous estimation of the thermal reaction norm of Lepidochelys olivacea only showed an increasing relationship between temperature and the rate of development [20]. The inclusion of four nests incubated under natural conditions expanded the temperature range to 38 °C. We were then able to show that the development rate showed a maximum of around 33.4 °C, with a subsequent decrease. This pattern is similar to the pattern described for Caretta caretta but with a higher temperature for the peak [40]. This indicates that olive ridleys are more resistant than loggerheads to high incubation temperatures, which is unsurprising, given that Caretta caretta nest at a higher latitude compared with Lepidochelys olivacea [41].
4.2. Thermal Reaction Norm for Hatching Success
The thermal limit of egg incubation for sea turtles is described using the concept of maximal lethal temperature [14]. The upper maximal limit for successful incubation is not well defined. Estimates of both 33 °C [42] and 35 °C [43] are frequently cited, although these estimates are based on early studies of natural nest temperatures in the field or artificial incubation experiments at constant temperatures in the laboratory, respectively. More recently, lethal thermal limits of 34 °C or 35 °C were proposed for Lepidochelys olivacea in Java (Indonesia) and Costa Rica [44]. Mean incubation temperatures above 35 °C on Ostional Beach, Costa Rica, did not produce olive ridley hatchlings [44]. However, some olive ridley embryos were reported to survive temperatures exceeding 37 °C for short periods if the mean incubation temperature was below 35 °C [15]. Species differences in terms of the thermal limit have already been observed. For example, incubation at temperatures of up to 35 °C did not reduce hatching success in flatback turtles (Natator depressus) nesting in the Gulf of Carpentaria, Australia, although it did accelerate embryonic development [45].
To further complicate matters, developing embryos may change their thermal tolerance as they grow [15] (see also Figure 4C). Indeed, we are only beginning to understand how exposure to high temperatures in the field influences embryonic development and hatchling production [15].
For the first time, the interplay between incubation temperature and developmental stage is deciphered herein. We show that embryo sensitivity to high temperatures, and its relation to hatching success, are similar for all embryonic stages, except for the late embryonic stages, when the embryos are close to pipping. Indeed, late-stage embryos are more sensitive to high temperatures. This observation is important because incubation temperatures often increase during development due to metabolic heating [46,47].
We agree with Howard, Bell, and Pike [15] that “the exact lethal limit of sea turtles will most likely never be known.” A solution may be found by recognizing that hatching success is the outcome of various factors. In this context, hatching success is not an all-or-nothing phenomenon but rather a continuous effect, meaning that the unique species-specific “thermal limit” does not exist. The thermal limit is rather the consequence of reaction norms involving many physiological and environmental components.
The statistical tools that we used here should be tested with more nests and other species to test for the possibility of generalizing these conclusions.
4.3. Implications for the Conservation Strategy in Guatemala
The conservation strategy in Guatemala is based on the premise that the natural incubation of sea turtle eggs is impossible. However, the presence of nesting females on Guatemalan beaches and natal philopatry indicate that at least some natural incubations must have produced live hatchlings. The authorized collection of sea turtle eggs for human consumption along with the delivery of 20% of nests to shaded hatcheries has been promoted for several decades. However, the consequences of this management approach on the viability of turtle populations are not well understood. For example, the consequences for population dynamics of the current value of 20% of nests collected for hatcheries should be tested using a population dynamic model. Bioethical principles regarding the procedures for moving eggs from their natural position to hatcheries and the use of unnatural shade have never been considered. In addition, how might the control of these conditions affect the sexual ratio of the species in the medium and long term, due to the manipulation of the eggs and their natural incubation conditions? For example, the impact on sex determination, which is influenced by the incubation temperature of the eggs [14], has not been studied in Guatemala, except for specific cases [30].
Several alternative proposals have been discussed, such as a total ban on sea turtle egg collection, a partial ban (1 day or 1 week per year), or the establishment of 200-meter protection zones around each turtle farm to promote natural nesting beaches, which is viewed as an optimal conservation mechanism [5]. As shown here, the natural incubation of olive ridley eggs is viable in Guatemala, even if hatching success is low. Nevertheless, the hatching success seen in hatcheries depends on their management, which is an unknown variable. Taking into account the broad influence of environmental factors, including temperature, on the physiology and behavior of hatchlings, we urge the Guatemalan government to reevaluate its national strategy for sea turtles and, notably, to allow some nests to incubate in natural conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su151914196/s1, Figure S1: Natural nests after the nesting process and before the emergence of hatchlings; Figure S2: Exhumation process: (A) counting the shells; (B) hatchlings found alive; Figure S3: Characterization of embryos; Table S1: Characteristics of the nesting females and the position of the nests on the beach.
Author Contributions
Conceptualization, B.A.M.-M.; field investigation, B.A.M.-M., A.M.-C. and C.C.; data curation, B.A.M.-M. and M.G.; statistics and models: M.G.; writing—original draft preparation, M.G.; writing—review and editing, M.G., B.A.M.-M., A.M.-C. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this research was funded by a grant from the University of Paris-Saclay to B.A.M.-M.
Institutional Review Board Statement
The animal study protocol was conducted under the I013-2012 CONAP researcher license number delivered to B.A.M.-M.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data are available on request to the corresponding author.
Acknowledgments
We would like to thank the Guatemalan Pacific Naval Command for allowing us to work at their site and supporting us, especially Alferez Martinez Canahuí, Orellana and Blanco for their special attention. We also acknowledge our support team in the field: Estefany Ordoñez, María Renee Contreras, and Pilar Velásquez, as well as the offsite team of Airam López and Ricardo Gill. Three anonymous referees are thanked for their constructive comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hart, C.E.; Maldonado-Gasca, A.; Ley-Quiñonez, C.P.; Flores-Peregrina, M.; de Jesús Romero-Villarruel, J.; Aranda-Mena, O.S.; Plata-Rosas, L.J.; Tena-Espinoza, M.; Llamas-González, I.; Zavala-Norzagaray, A.A.; et al. Status of olive ridley sea turtles (Lepidochelys olivacea) after 29 years of nesting rookery conservation in Nayarit and Bahía de Banderas, Mexico. Chelonian Conserv. Biol. 2018, 17, 27–36. [Google Scholar] [CrossRef]
- Abreu-Grobois, A.; Plotkin, P.T. Lepidochelys olivacea; IUCN SSC Marine Turtle Specialist Group: Gland, Switzerland, 2008. [Google Scholar]
- Rguez-Baron, J.M.; Kelez, S.; Liles, M.; Zavala-Norzagaray, A.; Torres-Suárez, O.L.; Amorocho, D.F.; Gaos, A.R. (Eds.) Sea Turtles in the East Pacific Ocean Region; IUCN-SSC Marine Turtle Specialist Group: Gland, Switzerland, 2019; p. 237. [Google Scholar]
- Morales-Mérida, B.A.; Muccio, C.; Girondot, M. Validating trends in olive ridley nesting track counts in Guatemala in the light of a national hatchery protection strategy. Oryx 2023, 57, 48–54. [Google Scholar] [CrossRef]
- CONAP. Estrategia Nacional de Manejo y Conservación de Tortugas Marinas de Guatemala; Consejo Nacional de Áreas Protegidas: Guatemala city, Guatamala, 2015; p. 58. [Google Scholar]
- Juarez, R.; Muccio, C. Sea turtle conservation in Guatemala. Mar. Turt. Newsl. 1997, 77, 15–17. [Google Scholar]
- Muccio, C.; Ortiz, L.; Martinez, J. Manual para la Conservación de las Tortugas Marinas en Guatemala, con un Enfasis en el Manejo de Tortugarios; ARCAS and CNOAP: Guatemala city, Guatemala, 2009; p. 57. [Google Scholar]
- Higginson, J.; Orantes, R. Manejo de Tortugas Marinas; Universidad de San Carlos de Guatemala: Guatemala City, Guatemala, 1988. [Google Scholar]
- Milne Edwards, H.; Vaillant, L. Recherches Zoologiques Pour Servir à L’histoire de la Faune de l’Amérique Centrale et du Mexique; Imprimerie Nationale: Paris, France, 1870–1909; p. 1012. [Google Scholar]
- Taylor, E.N.; Diele-Viegas, L.M.; Gangloff, E.J.; Hall, J.M.; Halpern, B.; Massey, M.D.; Rödder, D.; Rollinson, N.; Spears, S.; Sun, B.j.; et al. The thermal ecology and physiology of reptiles and amphibians: A user’s guide. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 335, 13–44. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.S.; Ruiz-García, N.A.; García-Gasca, A.; Abreu-Grobois, F.A. Best swimmers hatch from intermediate temperatures: Effect of incubation temperature on swimming performance of olive ridley sea turtle hatchlings. J. Exp. Mar. Biol. Ecol. 2019, 519, 151186. [Google Scholar] [CrossRef]
- Maulany, R.I.; Booth, D.T.; Baxter, G.S. The effect of incubation temperature on hatchling quality in the olive ridley turtle, Lepidochelys olivacea, from Alas Purwo National Park, East Java, Indonesia: Implications for hatchery management. Mar. Biol. 2012, 159, 2651–2661. [Google Scholar] [CrossRef]
- Fuentes, M.M.P.B.; Limpus, C.; Hamann, M. Vulnerability of sea turtle nesting grounds to climate change. Glob. Change Biol. 2011, 17, 140–153. [Google Scholar] [CrossRef]
- Abreu-Grobois, F.A.; Morales-Mérida, B.A.; Hart, C.E.; Guillon, J.-M.; Godfrey, M.H.; Navarro, E.; Girondot, M. Recent advances on the estimation of the thermal reaction norm for sex ratios. PeerJ 2020, 8, e8451. [Google Scholar] [CrossRef]
- Howard, R.; Bell, I.; Pike, D.A. Thermal tolerances of sea turtle embryos: Current understanding and future directions. Endanger. Species Res. 2014, 26, 75–86. [Google Scholar] [CrossRef]
- Laloë, J.-O.; Cozens, J.; Renom, B.; Taxonera, A.; Hays, G.C. Effects of rising temperature on the viability of an important sea turtle rookery. Nat. Clim. Change 2014, 4, 513–518. [Google Scholar] [CrossRef]
- Short, N.M.; Blair, R.W., Jr. (Eds.) Geomorphology from Space: A Global Overview of Regional Landforms; NASA, Scientific and Technical Branch: Washington, DC, USA, 1986; p. 717.
- Marshall, J.S. The geomorphology and physiographic provinces of Central America. In Central America: Geology, Hazards, & Resources; Bundschuh, J., Alvarado, G.E., Eds.; Taylor-Francis: London, UK, 2007; Volume 1, pp. 75–121. [Google Scholar] [CrossRef]
- Ariano-Sánchez, D.; Nesthus, A.; Rosell, F.; Reinhardt, S. Developed black beaches—Too hot to emerge? Factors affecting sand temperatures at nesting grounds of olive ridley sea turtles (Lepidochelys olivacea). Clim. Change Ecol. 2023, 5, 100074. [Google Scholar] [CrossRef]
- Morales-Merida, B.A.; Bustamante, D.M.; Monsinjon, J.; Girondot, M. Reaction norm of embryo growth rate dependent on incubation temperature in the Olive Ridley sea turtle, Lepidochelys olivacea, from Pacific Central America. J. Embryol. 2018, 1, 12–24. [Google Scholar]
- Crastz, F. Embryological stages of the marine turtle Lepidochelys olivacea (Eschscholtz). Rev. De Biol. Trop. 1982, 30, 113–120. [Google Scholar]
- Kaska, Y.; Downie, R. Embryological development of sea turtles (Chelonia mydas, Caretta caretta) in the Mediterranean. Zool. Middle East 1999, 19, 55–69. [Google Scholar] [CrossRef]
- Kaska, Y. Studies on the Embryology, Ecology and Evolution of Sea Turtles in the Eastern Mediterranean. Ph.D. Thesis, University of Glasgow, Glasgow, Walles, 1998. [Google Scholar]
- Miller, J.D. Embryology of marine turtles. In Biology of the Reptilia; Gans, C., Billet, F., Maderson, P.F., Eds.; Wiley-Liss: New-York, NY, USA, 1985; pp. 270–328. [Google Scholar]
- Miller, J.D.; Mortimer, J.A.; Limpus, C.J. A field key to the developmental stages of marine turtles (Cheloniidae) with notes on the development of Dermochelys. Chelonian Conserv. Biol. 2017, 16, 111–122. [Google Scholar] [CrossRef]
- Girondot, M.; Kaska, Y. A model to predict the thermal reaction norm for the embryo growth rate from field data. J. Therm. Biol. 2014, 45, 96–102. [Google Scholar] [CrossRef]
- Sharpe, P.J.H.; DeMichele, D.W. Reaction kinetics of poikilotherm development. J. Theor. Biol. 1977, 64, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Schoolfield, R.M.; Sharpe, P.J.; Magnuson, C.E. Non-linear regression of biological temperature-dependent rate models based on absolute reaction-rate theory. J. Theor. Biol. 1981, 88, 719–731. [Google Scholar] [CrossRef]
- Laird, A.K. Dynamics of tumor growth. Br. J. Cancer 1964, 18, 490–502. [Google Scholar] [CrossRef]
- Morales Mérida, A. Relationship between Incubation Length and the Sex Ratio of the Sea Turtles Lepidochelys olivacea in the Natural Reserve of Multiple Uses, in the Pacific Coast of Guatemala; Universidad de San Carlos de Guatemala: Guatemala City, Guatemala, 2012. [Google Scholar]
- Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E. Equations of state calculations by fast computing machines. J. Chem. Phys. 1953, 21, 1087–1092. [Google Scholar] [CrossRef]
- Hastings, W.K. Monte Carlo sampling methods using Markov chains and their applications. Biometrika 1970, 57, 97–109. [Google Scholar] [CrossRef]
- Raftery, A.E.; Lewis, S.M. The number of iterations, convergence diagnostics and generic Metropolis algorithms. In Practical Markov Chain Monte Carlo; Gilks, W.R., Spiegelhalter, D.J., Richardson, S., Eds.; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Rosenthal, J.S. Optimal proposal distributions and adaptive MCMC. In MCMC Handbook; Brooks, S., Gelman, A., Jones, G., Meng, X.-L., Eds.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2011; pp. 93–112. [Google Scholar]
- Phillott, A.D.; Godfrey, M.H. Assessing the evidence of « infertile » sea turtle eggs. Endanger. Species Res. 2020, 41, 329–338. [Google Scholar] [CrossRef]
- Phillott, A.D.; Godfrey, M.H.; Avens, L.I. Distinguishing between fertile and infertile sea turtle eggs. Mar. Turt. Newsl. 2021, 162, 18–21. [Google Scholar]
- Wilson, E.B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Sherlock, C. Optimal scaling of the random walk Metropolis: General criteria for the 0.234 acceptance rule. J. Appl. Probab. 2013, 50, 1–15. [Google Scholar] [CrossRef]
- Monsinjon, J.; Jribi, I.; Hamza, A.; Ouerghi, A.; Kaska, Y.; Girondot, M. Embryonic growth rate thermal reaction norm of Mediterranean Caretta caretta embryos from two different thermal habitats, Turkey and Libya. Chelonian Conserv. Biol. 2017, 16, 172–179. [Google Scholar] [CrossRef]
- Wallace, B.P.; DiMatteo, A.D.; Hurley, B.J.; Finkbeiner, E.M.; Bolten, A.B.; Chaloupka, M.Y.; Hutchinson, B.J.; Abreu-Grobois, F.A.; Amorocho, D.; Bjorndal, K.A.; et al. Regional management units for marine turtles: A novel framework for prioritizing conservation and research across multiple scales. PLoS ONE 2010, 5, e15465. [Google Scholar] [CrossRef]
- Miller, J.D. Reproduction in sea turtles. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: New York, NY, USA, 1997; pp. 51–81. [Google Scholar]
- Ackerman, R.A. The nest environment and the embryonic development of sea turtles. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: New York, NY, USA, 1997; pp. 83–106. [Google Scholar]
- Valverde, R.A.; Wingard, S.; Gómez, F.; Tordoir, M.T.; Orrego, C.M. Field lethal incubation temperature of olive ridley sea turtle Lepidochelys olivacea embryos at a mass nesting rookery. Endanger. Species Res. 2010, 12, 77–86. [Google Scholar] [CrossRef]
- Howard, R.; Bell, I.; Pike, D.A. Tropical flatback turtle (Natator depressus) embryos are resilient to the heat of climate change. J. Exp. Biol. 2015, 218, 3330–3335. [Google Scholar] [CrossRef]
- Sönmez, B. Relationship between metabolic heating and nest parameters in green turtles (Chelonia mydas, L. 1758) on Samandağ Beach, Turkey. Zool. Sci. 2018, 35, 243–248. [Google Scholar] [CrossRef]
- Zbinden, J.A.; Margaritoulis, D.; Arlettaz, R. Metabolic heating in Mediterranean loggerhead sea turtle clutches. J. Exp. Mar. Biol. Ecol. 2006, 334, 151–157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).