Role of Experimental, Modeling, and Simulation Studies of Plasma in Sustainable Green Energy
Abstract
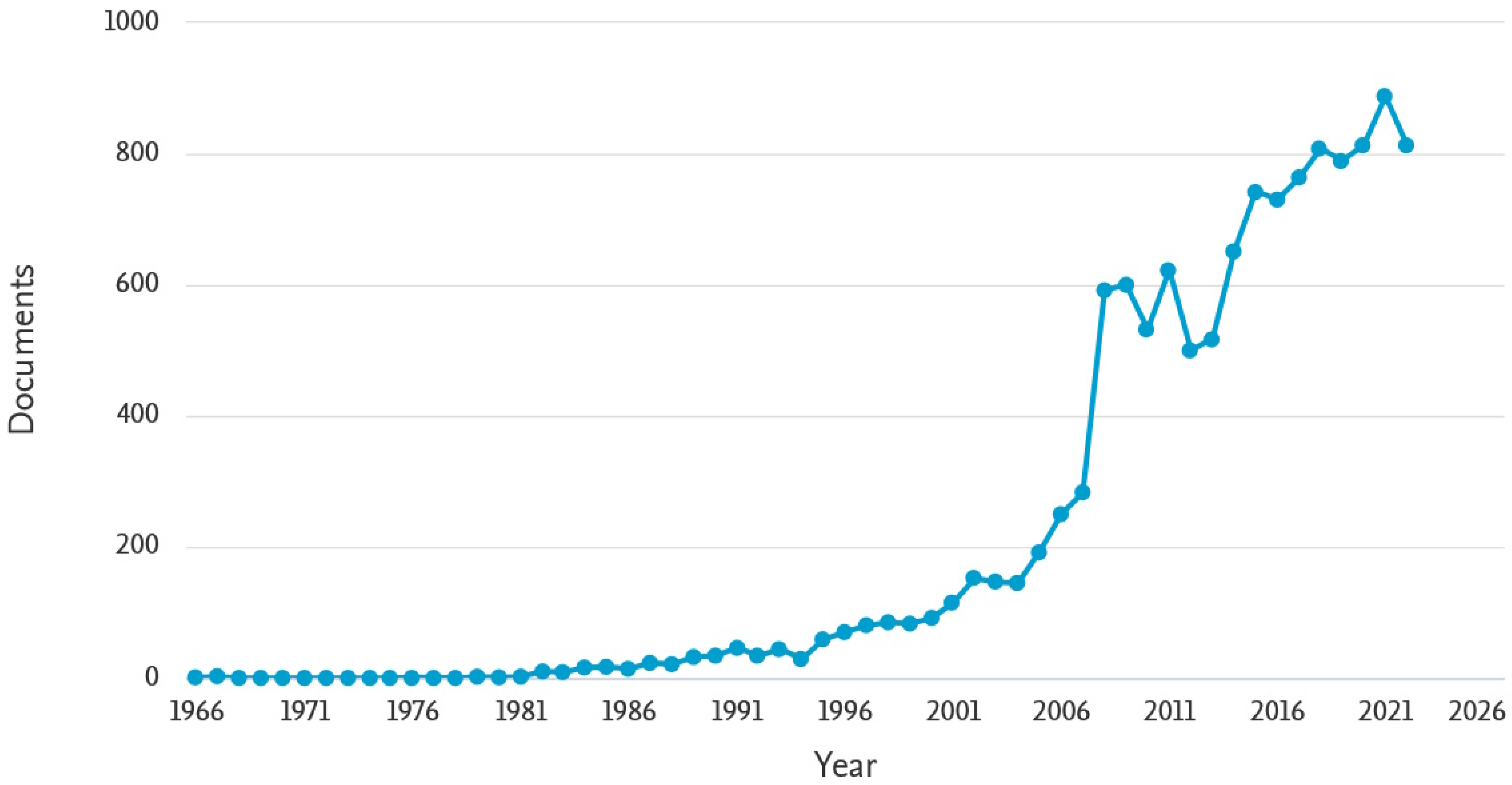
:1. Introduction
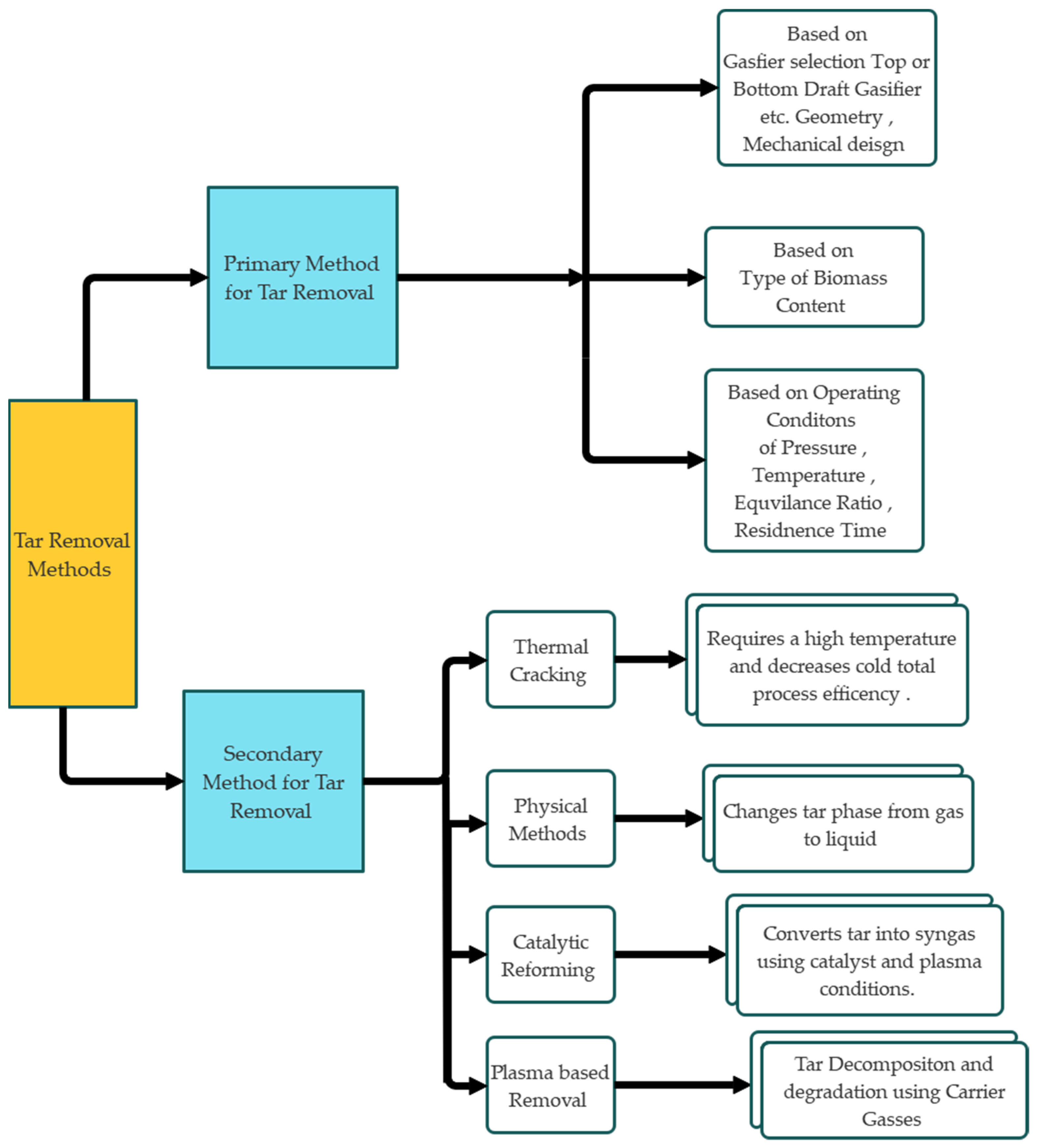
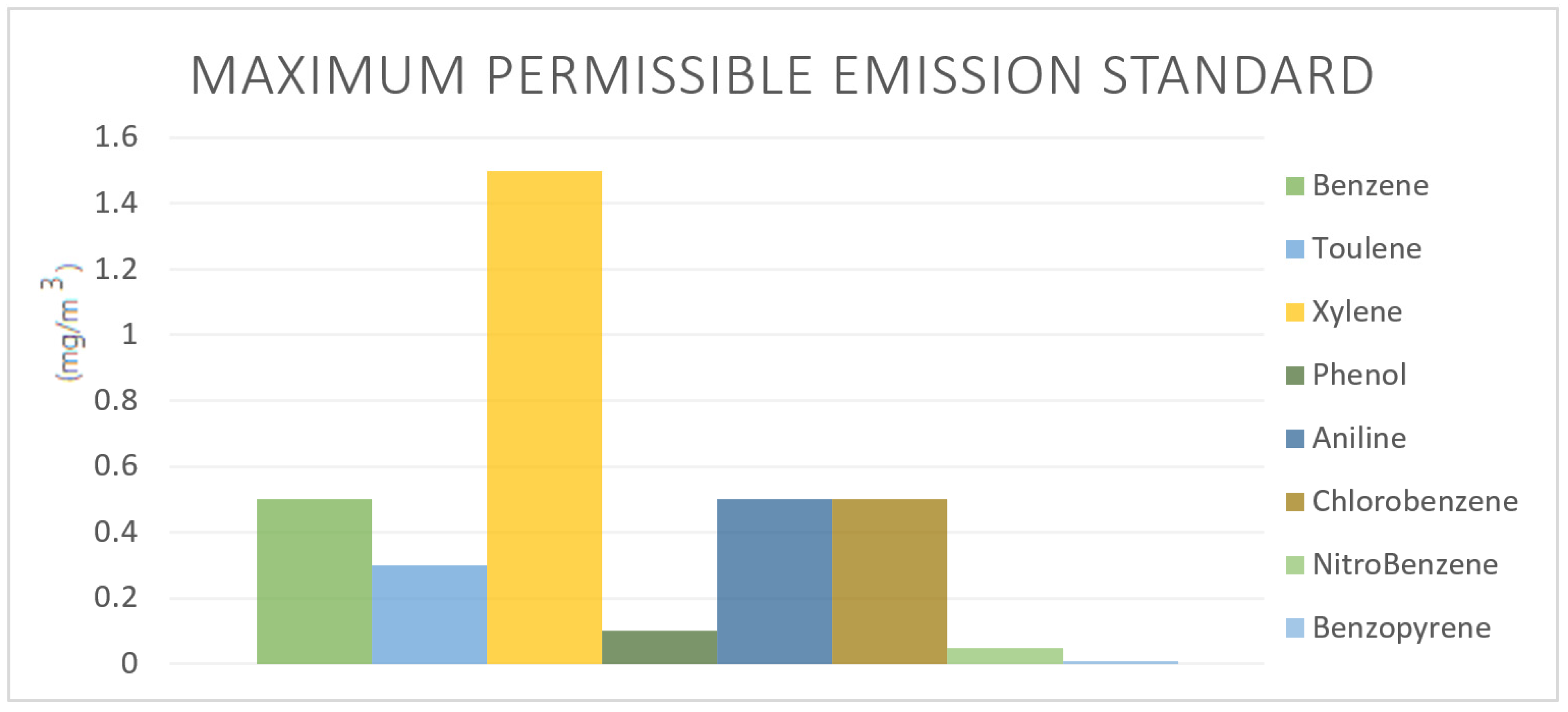
2. Characteristics of Tar in Gasification Processes: Classification, Reactivity, Composition, and Environmental Impact


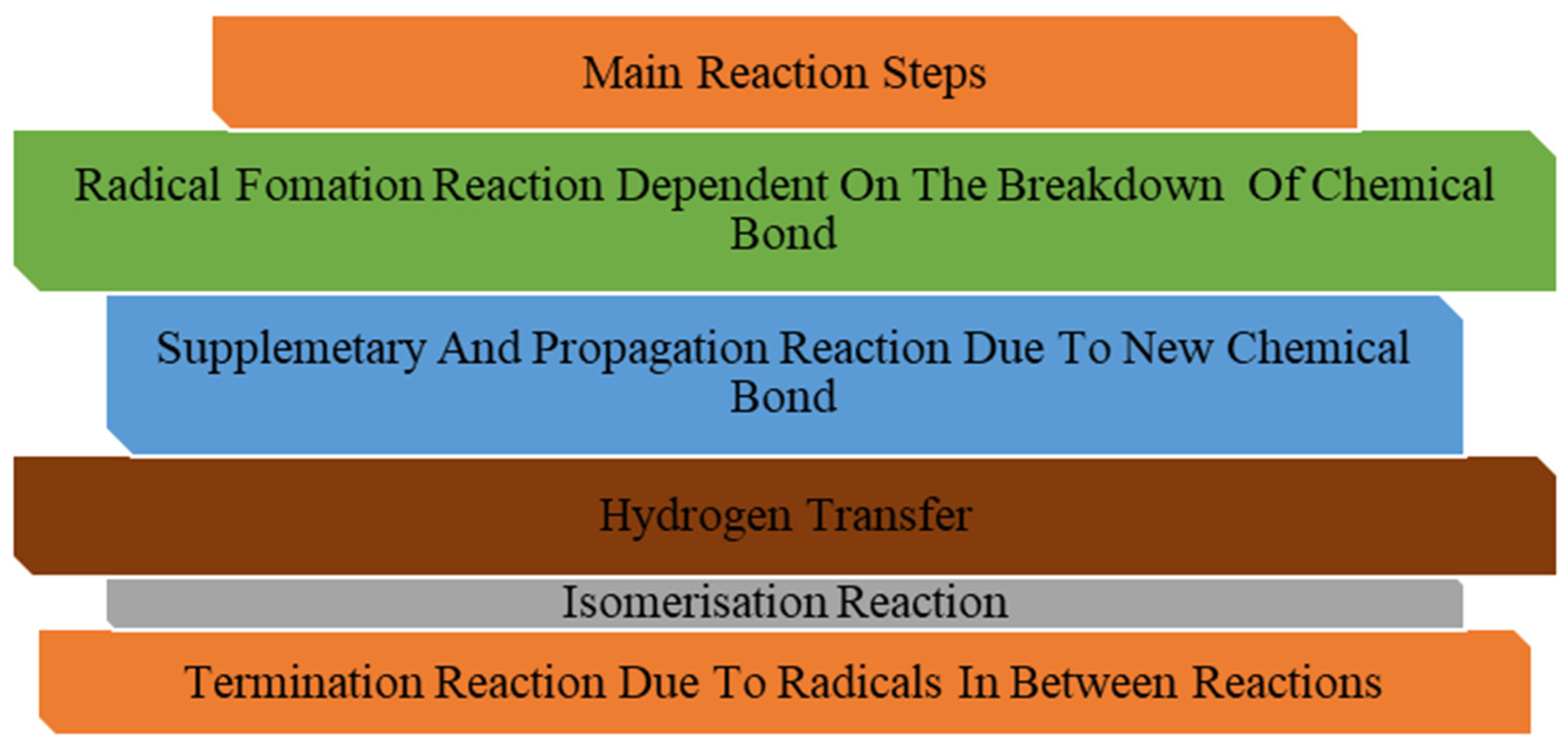
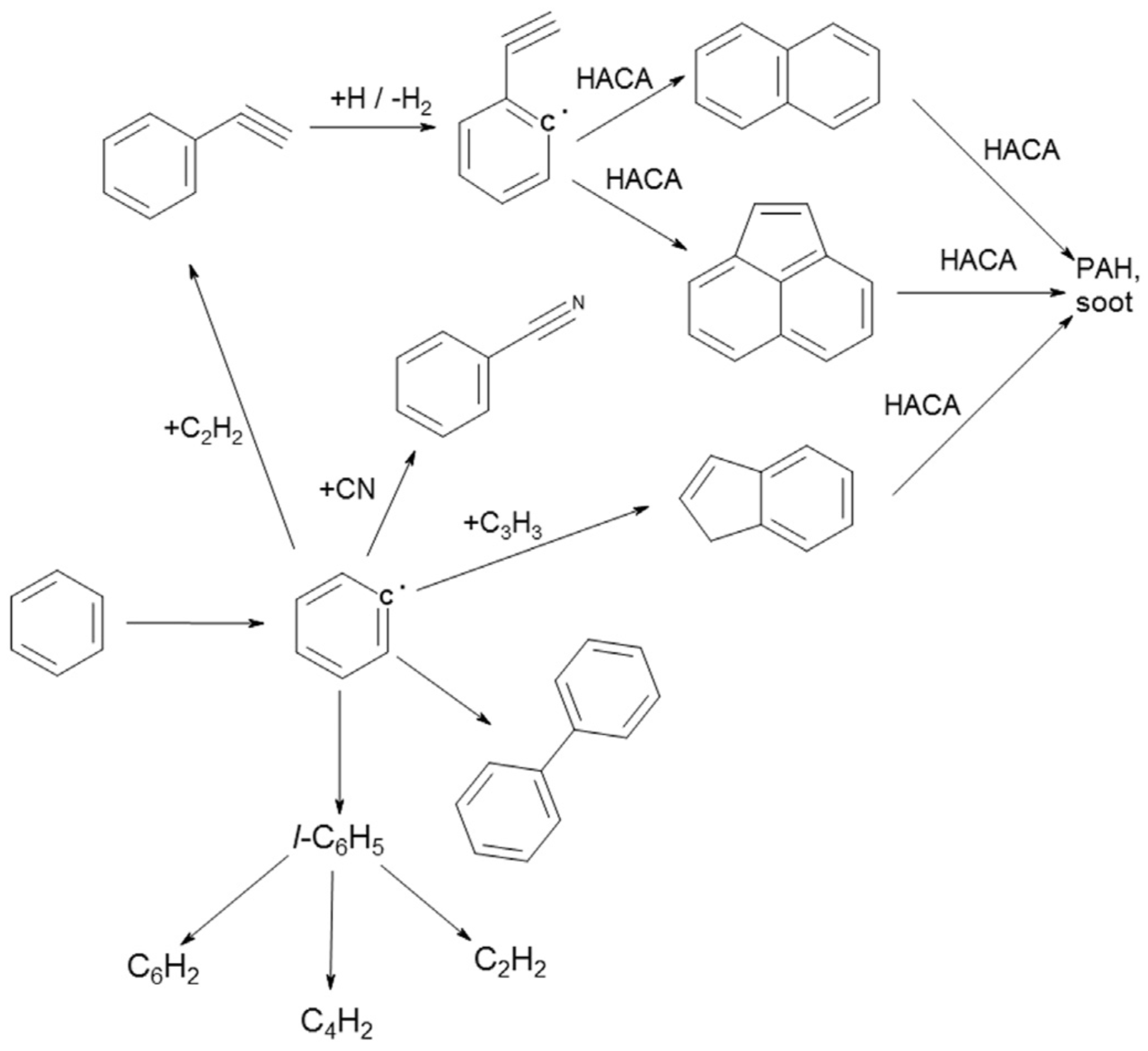
3. Tar Decomposition Mechanisms and Kinetics in Thermal and Plasma Environments
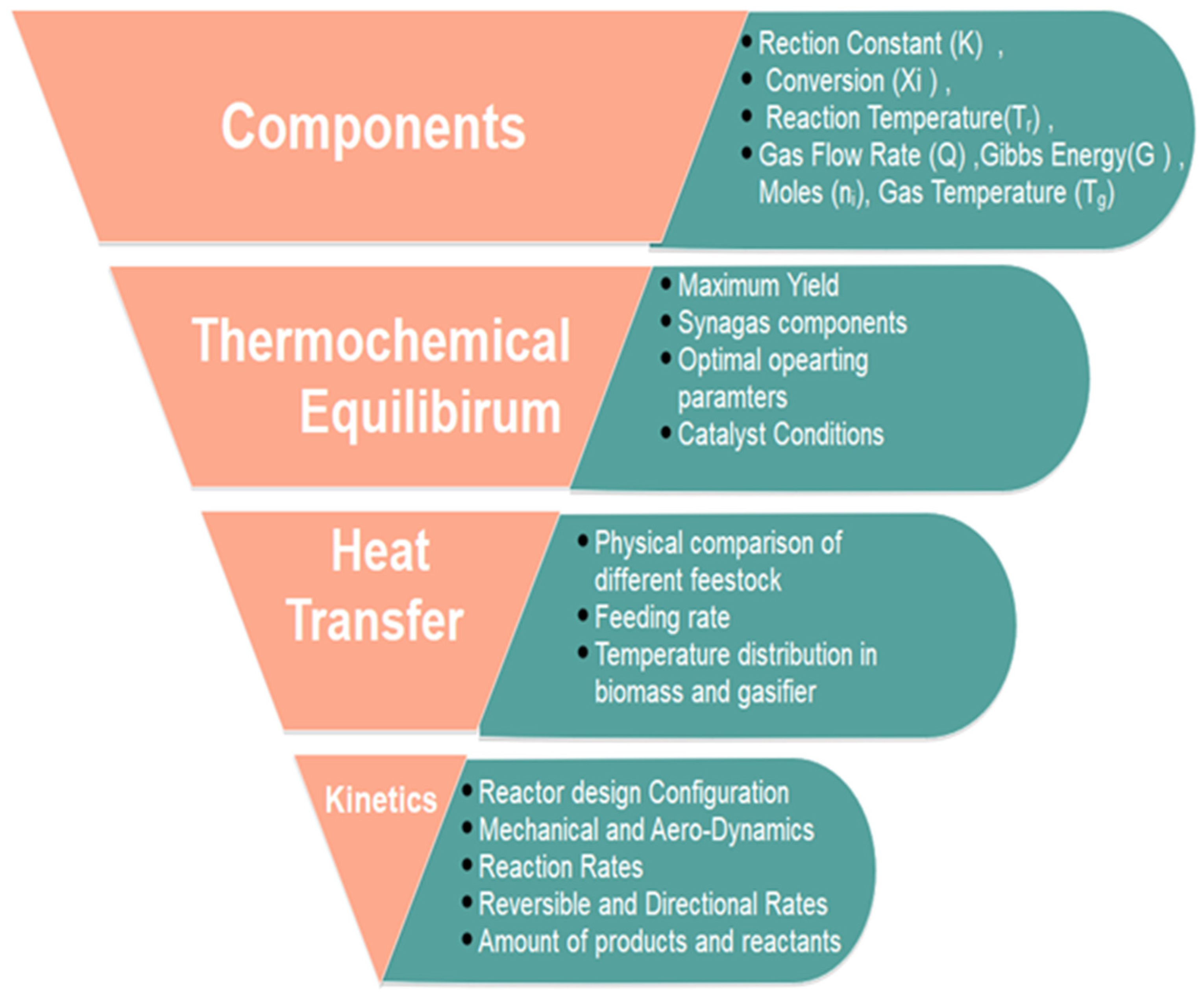
4. Plasma Chemical Kinetic Modeling: Relationship of Reactor Geometry, Energy Yield, and Mass Transfer in Plasma Reactor for Hydrocarbons and Volatile Organic Carbon Conversion
5. Energy-Efficient Removal Kinetics Modeling in Plasma: Investigating Direct Electron Collision and Its Impact on Reactor Configuration and Scaling Up Studies
- Change in reactor configuration and complicated upscaling of modular reactor design.
- The non-equilibrium nature of plasma intrinsically involves spatial and temporal studies complimenting the reaction rates for species conversion and decomposition.
- Single model approach;
- Lumped model;
- Detailed kinetic model approach.
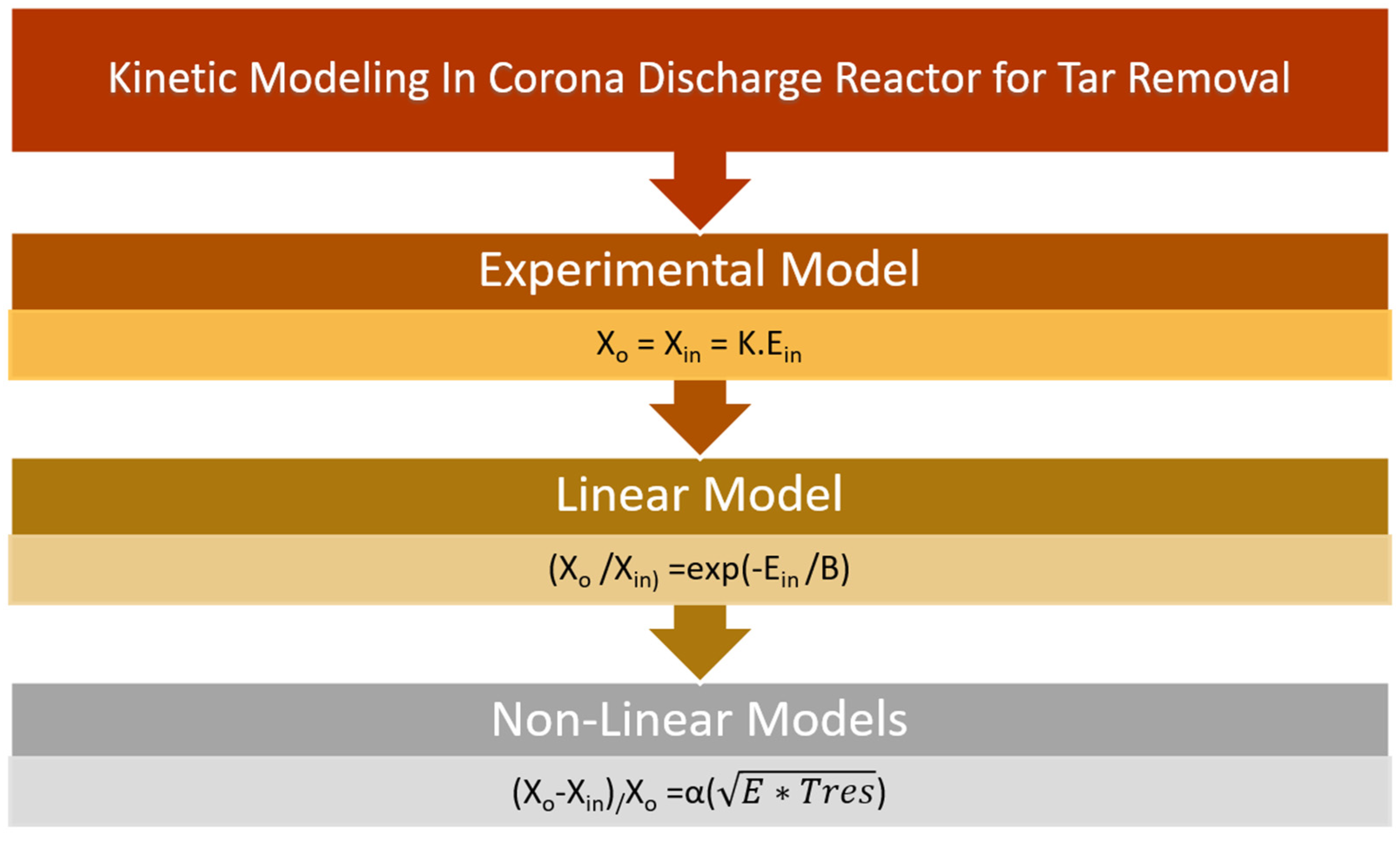
5.1. Zero Effect Radical Termination Kinetics Model
5.2. Reasonable Linear Radical Termination Kinetics Model
5.3. Non-Linear Radical Termination Kinetics Model
6. Thermodynamics in Kinetic Models for Tar Removal in Moisture Content and Equivalent Ratio
7. Development and Validation of Kinetic Models for Hydrocarbon Conversion in Non-Thermal Plasma Reactors
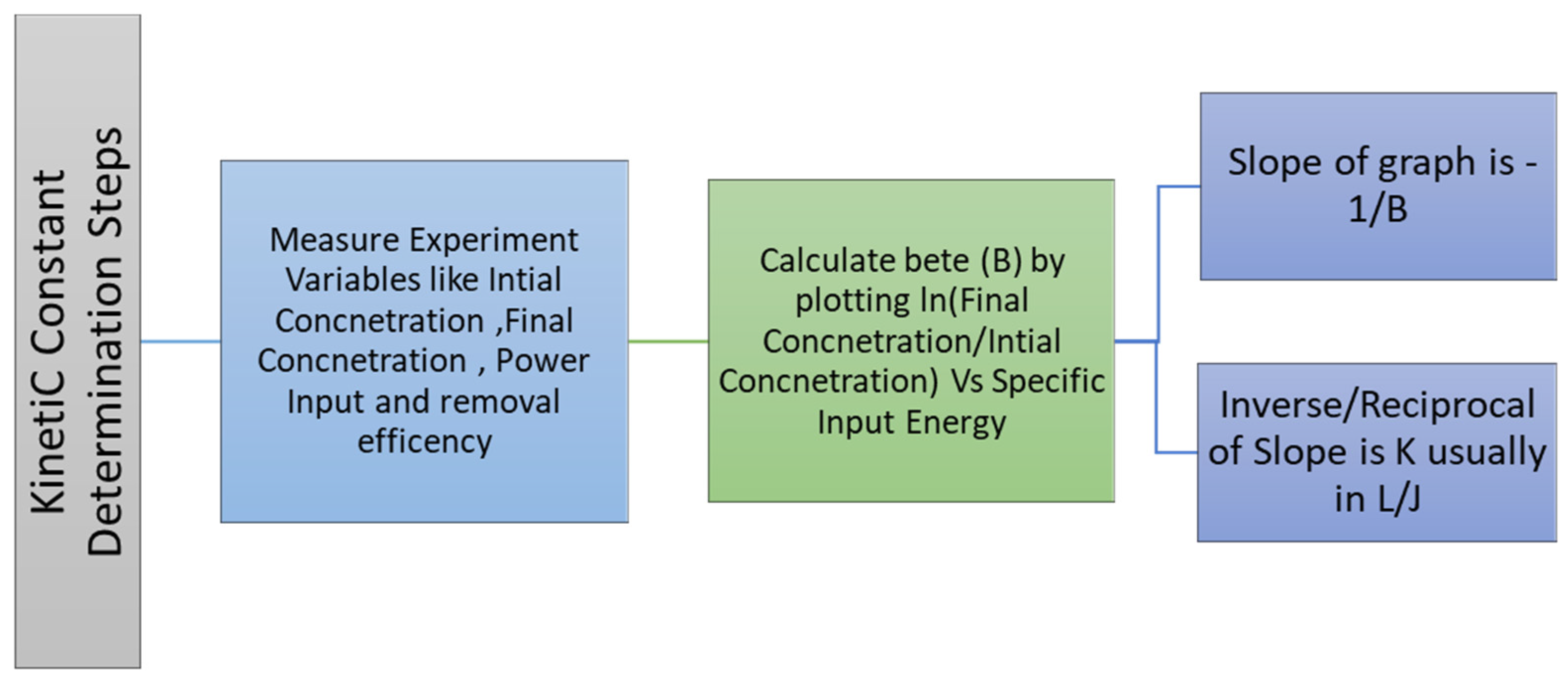
8. Role of Energy Density and Efficiency in Plasma Discharge Kinetics Modeling and for Decomposition Reactions
9. Kinetic Modeling and Energy Efficiency in a Non-Thermal Plasma Reactor for VOC Pollutant Removal and Management
10. Kinetic Modeling for Tar Decomposition in a Plasma Reactor with Effects of Energy Density and Carrier Gases
- Defining chemical reactor discharge and Model Boltzmann kinetics on the basis of the main removal of analogue compound representative chemistry;
- Characterization studies: rotational temperature calculation based on optical emission spectroscopy;
- Average electron energy calculation for the second positive and first negative system discharge calculation for nitrogen molecules has been performed.
11. Efficiency of Tar Removal in Pulse Discharge Reactors using NIST-Based Kinetic Modeling, and Numerical Simulation
12. Effect of Temperature and Gas Composition on Tar Removal Efficiency in NTP and Pulsed Corona Discharge Reactors using Kinetic Modeling and Statistical Analysis
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | alternating current |
| CCS | carbon capture and storage |
| CSTR | continuously stirred tank reactor |
| DBD | dielectric-barrier discharge |
| DC | direct current |
| ECN | Energy Centre of Netherlands (nowadays called TNO) |
| EQR | equivalence ratio |
| EEDF | electron energy distribution function |
| GC-MS | gas chromatography—mass spectroscopy |
| GHSV | Gas Hourly Space Velocity |
| LHV | lower heating value |
| MC | moisture content |
| NIST | national institute of standards and technology |
| NTP | non-thermal plasma |
| PAH | poly-aromatic hydrocarbons |
| PFR | plug flow reactor |
| RSM | response surface methodology |
| SCR | steam to carbon ratio |
| SED | specific energy density |
| SIE | specific input energy |
| VOC | volatile organic compounds |
References
- Tzelepi, V.; Zeneli, M.; Kourkoumpas, D.-S.; Karampinis, E.; Gypakis, A.; Nikolopoulos, N.; Grammelis, P. Biomass Availability in Europe as an Alternative Fuel for Full Conversion of Lignite Power Plants: A Critical Review. Energies 2020, 13, 3390. [Google Scholar] [CrossRef]
- Qvist, S.; Gładysz, P.; Bartela, Ł.; Sowiżdżał, A. Retrofit Decarbonization of Coal Power Plants—A Case Study for Poland. Energies 2021, 14, 120. [Google Scholar] [CrossRef]
- Balode, L.; Zlaugotne, B.; Gravelsins, A.; Svedovs, O.; Pakere, I.; Kirsanovs, V.; Blumberga, D. Carbon Neutrality in Municipalities: Balancing Individual and District Heating Renewable Energy Solutions. Sustainability 2023, 15, 8415. [Google Scholar] [CrossRef]
- Liobikienė, G.; Miceikienė, A. Contribution of the European Bioeconomy Strategy to the Green Deal Policy: Challenges and Opportunities in Implementing These Policies. Sustainability 2023, 15, 7139. [Google Scholar] [CrossRef]
- Piersa, P.; Szufa, S.; Czerwińska, J.; Ünyay, H.; Adrian, Ł.; Wielgosinski, G.; Obraniak, A.; Lewandowska, W.; Marczak-Grzesik, M.; Dzikuć, M.; et al. Pine Wood and Sewage Sludge Torrefaction Process for Production Renewable Solid Biofuels and Biochar as Carbon Carrier for Fertilizers. Energies 2021, 14, 8176. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Szufa, S.; Grzesik, M.; Piotrowski, K.; Janas, R. The Promotive Effect of Cyanobacteria and Chlorella sp. Foliar Biofertilization on Growth and Metabolic Activities of Willow (Salix viminalis L.) Plants as Feedstock Production, Solid Biofuel and Biochar as C Carrier for Fertilizers via Torrefaction Process. Energies 2021, 14, 5262. [Google Scholar]
- Szufa, S.; Piersa, P.; Junga, R.; Błaszczuk, A.; Modliński, N.; Sobek, S.; Marczak-Grzesik, M.; Adrian, Ł.; Dzikuć, M. Numerical modeling of the co-firing process of an in situ steam-torrefied biomass with coal in a 230 MW industrial-scale boiler. Energy 2023, 263, 125918. [Google Scholar] [CrossRef]
- Piersa, P.; Unyay, H.; Szufa, S.; Lewandowska, W.; Modrzewski, R.; Ślężak, R.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Torrefaction Reactors vs. Biomass Pyrolysis—Part 1. Energies 2022, 15, 2227. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Gładysz, P.; Strojny, M.; Bartela, Ł.; Hacaga, M.; Froehlich, T. Merging Climate Action with Energy Security through CCS—A Multi-Disciplinary Framework for Assessment. Energies 2023, 16, 35. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Głuch, S.; Ziółkowski, P.J.; Badur, J. Compact High Efficiency and Zero-Emission Gas-Fired Power Plant with Oxy-Combustion and Carbon Capture. Energies 2022, 15, 2590. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Madejski, P.; Amiri, M.; Kuś, T.; Stasiak, K.; Subramanian, N.; Pawlak-Kruczek, H.; Badur, J.; Niedźwiecki, Ł.; Mikielewicz, D. Thermodynamic Analysis of Negative CO2 Emission Power Plant Using Aspen Plus, Aspen Hysys, and Ebsilon Software. Energies 2021, 14, 6304. [Google Scholar] [CrossRef]
- Stavrakas, V.; Spyridaki, N.-A.; Flamos, A. Striving towards the Deployment of Bio-Energy with Carbon Capture and Storage (BECCS): A Review of Research Priorities and Assessment Needs. Sustainability 2018, 10, 2206. [Google Scholar] [CrossRef]
- Čespiva, J.; Niedzwiecki, L.; Vereš, J.; Skřínský, J.; Wnukowski, M.; Borovec, K.; Ochodek, T. Evaluation of the performance of the cross/updraft type gasification technology with the sliding bed over a circular grate. Biomass Bioenergy 2022, 167, 106639. [Google Scholar] [CrossRef]
- Čespiva, J.; Skřínský, J.; Vereš, J.; Wnukowski, M.; Serenčíšová, J.; Ochodek, T. Solid recovered fuel gasification in sliding bed reactor. Energy 2023, 278, 127830. [Google Scholar] [CrossRef]
- Koido, K.; Takata, E.; Yanagida, T.; Kuboyama, H. Techno-Economic Assessment of Heat Supply Systems in Woodchip Drying Bases for Wood Gasification Combined Heat and Power. Sustainability 2022, 14, 16878. [Google Scholar] [CrossRef]
- Čespiva, J.; Skřínský, J.; Vereš, J.; Borovec, K.; Wnukowski, M. Solid-recovered fuel to liquid conversion using fixed bed gasification technology and a Fischer–Tropsch synthesis unit–Case study. Int. J. Energy Prod. Manag. 2020, 5, 212–222. [Google Scholar] [CrossRef]
- Čespiva, J.; Niedzwiecki, L.; Wnukowski, M.; Krochmalny, K.; Mularski, J.; Ochodek, T.; Pawlak-Kruczek, H. Torrefaction and gasification of biomass for polygeneration: Production of biochar and producer gas at low load conditions. Energy Rep. 2022, 8, 134–144. [Google Scholar] [CrossRef]
- Manga, M.; Aragón-Briceño, C.; Boutikos, P.; Semiyaga, S.; Olabinjo, O.; Muoghalu, C.C. Biochar and Its Potential Application for the Improvement of the Anaerobic Digestion Process: A Critical Review. Energies 2023, 16, 4051. [Google Scholar] [CrossRef]
- Sieradzka, M.; Mlonka-Mędrala, A.; Kalemba-Rec, I.; Reinmöller, M.; Küster, F.; Kalawa, W.; Magdziarz, A. Evaluation of Physical and Chemical Properties of Residue from Gasification of Biomass Wastes. Energies 2022, 15, 3539. [Google Scholar] [CrossRef]
- Výtisk, J.; Čespiva, J.; Jadlovec, M.; Kočí, V.; Honus, S.; Ochodek, T. Life cycle assessment applied on alternative production of carbon-based sorbents—A comparative study. Sustain. Mater. Technol. 2023, 35, e00563. [Google Scholar] [CrossRef]
- Čespiva, J.; Jadlovec, M.; Výtisk, J.; Serenčíšová, J.; Tadeáš, O.; Honus, S. Softwood and solid recovered fuel gasification residual chars as sorbents for flue gas mercury capture. Environ. Technol. Innov. 2023, 29, 102970. [Google Scholar] [CrossRef]
- Sieradzka, M.; Mlonka-Mędrala, A.; Magdziarz, A. Comprehensive investigation of the CO2 gasification process of biomass wastes using TG-MS and lab-scale experimental research. Fuel 2022, 330, 125566. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Analysis of Organic and Inorganic Contaminants in Dried Sewage Sludge and By-Products of Dried Sewage Sludge Gasification. Energies 2014, 7, 462–476. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Castro, C.; Calado, L.; Vilarinho, C. Experimental Analysis of Brewers’ Spent Grains Steam Gasification in an Allothermal Batch Reactor. Energies 2019, 12, 912. [Google Scholar] [CrossRef]
- Wnukowski, M.; Moroń, W. Warm Plasma Application in Tar Conversion and Syngas Valorization: The Fate of Hydrogen Sulfide. Energies 2021, 14, 7383. [Google Scholar] [CrossRef]
- Bityurin, V.; Filimonova, E.; Naidis, G. Mechanisms of Conversion of Heavy Hydrocarbons in Biogas Initiated by Pulsed Corona Discharges. In Plasma Assisted Decontamination of Biological and Chemical Agents; Springer: Dordrecht, The Netherlands, 2008; pp. 135–142. [Google Scholar]
- Filimonova, E.; Naidis, G. Effect of gas mixture composition on tar removal process in a pulsed corona discharge reactor. J. Phys. Conf. Ser. 2010, 257, 012018. [Google Scholar] [CrossRef]
- Zigan, L. Overview of Electric Field Applications in Energy and Process Engineering. Energies 2018, 11, 1361. [Google Scholar] [CrossRef]
- Leonov, S.B. Electrically Driven Supersonic Combustion. Energies 2018, 11, 1733. [Google Scholar] [CrossRef]
- Tholin, F.; Lacoste, D.; Bourdon, A. Influence of fast-heating processes and O atom production by a nanosecond spark discharge on the ignition of a lean H2H2–air premixed flame. Combust. Flame 2014, 161, 1235–1246. [Google Scholar] [CrossRef]
- Zare, S.; Lo, H.W.; Askari, O. Flame Stability in Inverse Coaxial Injector Using Repetitive Nanosecond Pulsed Plasma. J. Energy Resour. Technol. 2020, 142, 082101. [Google Scholar] [CrossRef]
- Lacoste, D.; Moeck, J.; Roberts, W.; Chung, S.; Cha, M.S. Analysis of the step responses of laminar premixed flames to forcing by non-thermal plasma. Proc. Combust. Inst. 2016, 36, 4145–4153. [Google Scholar] [CrossRef]
- Mączka, T.; Pawlak-Kruczek, H.; Niedzwiecki, L.; Ziaja, E.; Chorążyczewski, A. Plasma Assisted Combustion as a Cost-Effective Way for Balancing of Intermittent Sources: Techno-Economic Assessment for 200 MWel Power Unit. Energies 2020, 13, 5056. [Google Scholar] [CrossRef]
- Messerle, V.; Karpenko, E.; Ustimenko, A.; Lavrichshev, O. Plasma preparation of coal to combustion in power boilers. Fuel Process. Technol. 2013, 107, 93–98. [Google Scholar] [CrossRef]
- Bukowski, P.; Kobel, P.; Kordylewski, W.; Mączka, T. Use of cavity plasmatron in pulverized coal muffle burner for start-up of a boiler. Rynek Energii 2010, 86, 132–136. [Google Scholar]
- Karpenko, E.; Messerle, V.; Ustimenko, A. Plasma-aided solid fuel combustion. Proc. Combust. Inst. 2007, 31, 3353–3360. [Google Scholar] [CrossRef]
- Messerle, V.; Karpenko, E.; Ustimenko, A. Plasma assisted power coal combustion in the furnace of utility boiler: Numerical modeling and full-scale test. Fuel 2014, 126, 294–300. [Google Scholar] [CrossRef]
- Nur, M.; Sumariyah, S.; Suseno, A. Removal of emission gas COx, NOx and SOx from automobile using non-thermal plasma. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012085. [Google Scholar]
- Saeed, M.A.; Niedzwiecki, L.; Arshad, M.Y.; Skrinsky, J.; Andrews, G.E.; Phylaktou, H.N. Combustion and Explosion Characteristics of Pulverised Wood, Valorized with Mild Pyrolysis in Pilot Scale Installation, Using the Modified ISO 1 m3 Dust Explosion Vessel. Appl. Sci. 2022, 12, 12928. [Google Scholar] [CrossRef]
- Rafique, M.A.; Kiran, S.; Jamal, A.; Anjum, M.N.; Jalal, F.; Munir, B.; Hafiz, I.; Noureen, F.; Ajmal, S.; Ahmad, W.; et al. Green synthesis of copper nanoparticles using Allium cepa (onion) peels for removal of Disperse Yellow 3 dye. Desalin. Water Treat. 2022, 272, 259–265. [Google Scholar] [CrossRef]
- Ramos, A.; Teixeira, C.A.; Rouboa, A. Environmental Assessment of Municipal Solid Waste by Two-Stage Plasma Gasification. Energies 2019, 12, 137. [Google Scholar] [CrossRef]
- Mączka, T. Technologia Plazmowego Zgazowania Biomasy i Odpadów Organicznych dla Wytwarzania Paliw Płynnych; Wydawnictwo Książkowe Instytutu Elektrotechniki: Warsaw, Poland, 2014. [Google Scholar]
- Messerle, V.E.; Mossé, A.L.; Ustimenko, A.B.; Slavinskaya, N.A.; Sitdikov, Z.Z. Recycling of Organic Waste in a Plasma Reactor. J. Eng. Phys. Thermophys. 2020, 93, 987–997. [Google Scholar] [CrossRef]
- Messerle, V.E.; Mossé, A.L.; Paskalov, G.; Sitdikov, Z.Z.; Ustimenko, A.B. Plasma Chemical Conversion of Spent Lubricating Materials. J. Eng. Phys. Thermophys. 2021, 94, 1344–1356. [Google Scholar] [CrossRef]
- Agon, N.; Hrabovský, M.; Chumak, O.; Hlína, M.; Kopecký, V.; Masláni, A.; Bosmans, A.; Helsen, L.; Skoblja, S.; Van Oost, G.; et al. Plasma gasification of refuse derived fuel in a single-stage system using different gasifying agents. Waste Manag. 2016, 47, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Vishwajeet; Pawlak-Kruczek, H.; Baranowski, M.; Czerep, M.; Chorążyczewski, A.; Krochmalny, K.; Ostrycharczyk, M.; Ziółkowski, P.; Madejski, P.; Mączka, T.; et al. Entrained Flow Plasma Gasification of Sewage Sludge–Proof-of-Concept and Fate of Inorganics. Energies 2022, 15, 1948. [Google Scholar] [CrossRef]
- Ivanovska, A.; Milošević, M.; Obradović, B.; Svirčev, Z.; Kostić, M. Plasma Treatment as a Sustainable Method for Enhancing the Wettability of Jute Fabrics. Sustainability 2023, 15, 2125. [Google Scholar] [CrossRef]
- Krochmalny, K.; Pawlak-Kruczek, H.; Skoczylas, N.; Kudasik, M.; Gajda, A.; Gnatowska, R.; Serafin-Tkaczuk, M.; Czapka, T.; Jaiswal, A.K.; Vishwajeet; et al. Use of Hydrothermal Carbonization and Cold Atmospheric Plasma for Surface Modification of Brewer’s Spent Grain and Activated Carbon. Energies 2022, 15, 4396. [Google Scholar] [CrossRef]
- Yar, A.; Arshad, M.Y.; Asghar, F.; Amjad, W.; Asghar, F.; Hussain, M.I.; Lee, G.H.; Mahmood, F. Machine Learning-Based Relative Performance Analysis of Monocrystalline and Polycrystalline Grid-Tied PV Systems. Int. J. Photoenergy 2022, 2022, 3186378. [Google Scholar] [CrossRef]
- Saleem, F.; Abbas, A.; Rehman, A.; Khoja, A.H.; Naqvi, S.R.; Arshad, M.Y.; Zhang, K.; Harvey, A. Decomposition of benzene as a biomass gasification tar in CH4 carrier gas using non-thermal plasma: Parametric and kinetic study. J. Energy Inst. 2022, 102, 190–195. [Google Scholar] [CrossRef]
- Yousaf, A.M.; Aqsa, R. Integrating Circular Economy, SBTI, Digital LCA, and ESG Benchmarks for Sustainable Textile Dyeing: A Critical Review of Industrial Textile Practices. Glob. NEST J. 2023, 25, 39–51. [Google Scholar] [CrossRef]
- Gul, H.; Arshad, M.Y.; Tahir, M.W. Production of H2 via sorption enhanced auto-thermal reforming for small scale Applications-A process modeling and machine learning study. Int. J. Hydrogen Energy 2023, 48, 12622–12635. [Google Scholar] [CrossRef]
- El-Tayeb, A.; El-Dein, A.Z.; Elnaggar, A.Y.; Hussein, E.E. Influence of Temperature in Degradation of Organic Pollution Using Corona Discharge Plasma. Sustainability 2021, 13, 12971. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.; Engelmann, Y.; van‘t Veer, K.; Postma, R.S.; Bogaerts, A.; Lefferts, L. Plasma-driven catalysis: Green ammonia synthesis with intermittent electricity. Green Chem. 2020, 22, 6258–6287. [Google Scholar] [CrossRef]
- Ioannou, I.; D’Angelo, S.C.; Galán-Martín, Á.; Pozo, C.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Process modelling and life cycle assessment coupled with experimental work to shape the future sustainable production of chemicals and fuels. React. Chem. Eng. 2021, 6, 1179–1194. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Wang, Z.; Sun, F.; Dorrell, D.G. A review of supercapacitor modeling, estimation, and applications: A control/management perspective. Renew. Sustain. Energy Rev. 2018, 81, 1868–1878. [Google Scholar] [CrossRef]
- Oliveira, M.; Ramos, A.; Ismail, T.M.; Monteiro, E.; Rouboa, A. A Review on Plasma Gasification of Solid Residues: Recent Advances and Developments. Energies 2022, 15, 1475. [Google Scholar] [CrossRef]
- Rafique, M.; Kiran, S.; Ashraf, A.; Mukhtar, N.; Rizwan, S.; Ashraf, M.; Arshad, M. Effective removal of direct orange 26 dye using copper nanoparticles synthesized from Tilapia fish scales. Glob. NEST J. 2022, 24, 311–317. [Google Scholar]
- Wnukowski, M.; Jamróz, P. Microwave plasma treatment of simulated biomass syngas: Interactions between the permanent syngas compounds and their influence on the model tar compound conversion. Fuel Process. Technol. 2018, 173, 229–242. [Google Scholar] [CrossRef]
- Zhenghui, L.; Zimei, H.; Yaya, S. New media environment, environmental regulation and corporate green technology innovation:Evidence from China. Energy Econ. 2023, 119, 106545. [Google Scholar] [CrossRef]
- Tinghui, L.; Xue, L.; Gaoke, L. Business cycles and energy intensity. Evidence from emerging economies. Borsa Istanb. Rev. 2022, 22, 560–570. [Google Scholar] [CrossRef]
- Valderrama Rios, M.L.; González, A.M.; Lora, E.E.S.; Almazán del Olmo, O.A. Reduction of tar generated during biomass gasification: A review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Rueda, Y.G.; Helsen, L. The role of plasma in syngas tar cracking. Biomass Convers. Biorefinery 2020, 10, 857–871. [Google Scholar] [CrossRef]
- Saleem, F.; Zhang, K.; Harvey, A. Temperature dependence of non-thermal plasma assisted hydrocracking of toluene to lower hydrocarbons in a dielectric barrier discharge reactor. Chem. Eng. J. 2019, 356, 1062–1069. [Google Scholar] [CrossRef]
- Čespiva, J.; Wnukowski, M.; Niedzwiecki, L.; Skřínský, J.; Vereš, J.; Ochodek, T.; Pawlak-Kruczek, H.; Borovec, K. Characterization of tars from a novel, pilot scale, biomass gasifier working under low equivalence ratio regime. Renew. Energy 2020, 159, 775–785. [Google Scholar] [CrossRef]
- Nair, S.; Pemen, A.J.M.; Yan, K.; Heesch, E.J.M.; Ptasinski, K.; Drinkenburg, A.A.H. Chemical Processes in Tar Removal from Biomass Derived Fuel Gas by Pulsed Corona Discharges. Plasma Chem. Plasma Process. 2003, 23, 665–680. [Google Scholar] [CrossRef]
- Mousavi, S.M.A.; Piavis, W.; Turn, S. Reforming of biogas using a non-thermal, gliding-arc, plasma in reverse vortex flow and fate of hydrogen sulfide contaminants. Fuel Process. Technol. 2019, 193, 378–391. [Google Scholar] [CrossRef]
- Schmidt, M.; Jõgi, I.; Holub, M.; Brandenburg, R. Non-thermal plasma based decomposition of volatile organic compounds in industrial exhaust gases. Int. J. Environ. Sci. Technol. 2015, 12, 3745–3754. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Kayamori, A.; Zhang, J. Experimental study and modeling of heavy tar steam reforming. Fuel Process. Technol. 2018, 178, 180–188. [Google Scholar] [CrossRef]
- Saleem, F.; Zhang, K.; Harvey, A. Role of CO2 in the Conversion of Toluene as a Tar Surrogate in a Nonthermal Plasma Dielectric Barrier Discharge Reactor. Energy Fuels 2018, 32, 5164–5170. [Google Scholar] [CrossRef]
- Saleem, F.; Harris, J.; Zhang, K.; Harvey, A. Non-thermal plasma as a promising route for the removal of tar from the product gas of biomass gasification—A critical review. Chem. Eng. J. 2020, 382, 122761. [Google Scholar] [CrossRef]
- Affonso Nobrega, P.; Gaunand, A.; Rohani, V.; Cauneau, F.; Fulcheri, L. Applying chemical engineering concepts to non-thermal plasma reactors. Plasma Sci. Technol. 2018, 20, 065512. [Google Scholar] [CrossRef]
- Mazaheri, N.; Akbarzadeh, A.H.; Madadian, E.; Lefsrud, M. Systematic review of research guidelines for numerical simulation of biomass gasification for bioenergy production. Energy Convers. Manag. 2019, 183, 671–688. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Liang, H.; Wang, G.; Lu, L.; Liu, J. Non-thermal plasma degradation of tar in gasification syngas. Chem. Eng. Process.—Process Intensif. 2019, 145, 107656. [Google Scholar] [CrossRef]
- Wnukowski, M.; Kordylewski, W.; Łuszkiewicz, D.; Leśniewicz, A.; Ociepa, M.; Michalski, J. Sewage Sludge-Derived Producer Gas Valorization with the Use of Atmospheric Microwave Plasma. Waste Biomass Valorization 2020, 11, 4289–4303. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Song, J.; Ahmad, S.; Liang, J.; Sun, Y. Plasma-enhanced steam reforming of different model tar compounds over Ni-based fusion catalysts. J. Hazard. Mater. 2019, 377, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Ohta, N.; Liu, G.; Yoneyama, Y.; Wang, T.; Tsubaki, N. Plasma enhanced catalytic reforming of biomass tar model compound to syngas. Fuel 2013, 104, 53–57. [Google Scholar] [CrossRef]
- Cimerman, R.; Račková, D.; Hensel, K. Tars removal by non-thermal plasma and plasma catalysis. J. Phys. D Appl. Phys. 2018, 51, 274003. [Google Scholar] [CrossRef]
- Chun, Y.N.; Kim, S.C.; Yoshikawa, K. Decomposition of benzene as a surrogate tar in a gliding Arc plasma. Environ. Prog. Sustain. Energy 2013, 32, 837–845. [Google Scholar] [CrossRef]
- Jamróz, P.; Kordylewski, W.; Wnukowski, M. Microwave plasma application in decomposition and steam reforming of model tar compounds. Fuel Process. Technol. 2018, 169, 1–14. [Google Scholar] [CrossRef]
- Saleem, F.; Umer, J.; Rehman, A.; Zhang, K.; Harvey, A. Effect of Methane as an Additive in the Product Gas toward the Formation of Lower Hydrocarbons during the Decomposition of a Tar Analogue. Energy Fuels 2020, 34, 1744–1749. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, Y.; Shen, H.; Ding, N.; Li, Y.; Luo, D. Mechanism and Kinetic Study on the Degradation of Typical Biomass Tar Components (Toluene, Phenol and Naphthalene) by Ozone. Ozone Sci. Eng. 2021, 43, 78–87. [Google Scholar] [CrossRef]
- Nair, S. Corona Plasma for Tar Removal. 2004. Available online: https://pure.tue.nl/ws/files/2272624/200412511.pdf (accessed on 13 September 2023).
- Nair, S.A.; Nozaki, T.; Okazaki, K. Methane oxidative conversion pathways in a dielectric barrier discharge reactor—Investigation of gas phase mechanism. Chem. Eng. J. 2007, 132, 85–95. [Google Scholar] [CrossRef]
- Pemen, A.; Nair, S.; Yan, K.; Heesch, E.J.M.; Ptasinski, K.; Drinkenburg, A. Pulsed Corona Discharges for Tar Removal from Biomass Derived Fuel Gas. Plasmas Polym. 2003, 8, 209–224. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, Z.; Mathieu, S.; Bin, F.; Tu, X. Prediction and evaluation of plasma arc reforming of naphthalene using a hybrid machine learning model. J. Hazard. Mater. 2021, 404, 123965. [Google Scholar] [CrossRef]
- Bogaerts, A.; Eckert, M.; Mao, M.; Neyts, E. Computer modelling of the plasma chemistry and plasma-based growth mechanisms for nanostructured materials. J. Phys. D Appl. Phys. 2011, 44, 174030. [Google Scholar] [CrossRef]
- Jiang, N.; Lu, N.; Li, J.; Wu, Y. Degradation of Benzene by Using a Silent-Packed Bed Hybrid Discharge Plasma Reactor. Plasma Sci. Technol. 2012, 14, 140–146. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Merabet, S.; Wolbert, D. Modeling and simulation of VOCs removal by nonthermal plasma discharge with photocatalysis in a continuous reactor: Synergetic effect and mass transfer. Chem. Eng. J. 2014, 258, 119–127. [Google Scholar] [CrossRef]
- Abdelaziz, A.A.; Seto, T.; Abdel-Salam, M.; Otani, Y. Performance of a surface dielectric barrier discharge based reactor for destruction of naphthalene in an air stream. J. Phys. D Appl. Phys. 2012, 45, 115201. [Google Scholar] [CrossRef]
- Kang, W.; Hur, M.; Song, Y.; Hong, S. Numerical Simulation of Atmospheric-pressure Non-Equilibrium Plasmas: Status and Prospects. Int. J. Plasma Environ. Sci. Technol. 2013, 7, 104–108. [Google Scholar]
- Dors, M.; Kurzyńska, D. Tar Removal by Nanosecond Pulsed Dielectric Barrier Discharge. Appl. Sci. 2020, 10, 991. [Google Scholar] [CrossRef]
- Affonso Nóbrega, P.H.; Rohani, V.; Fulcheri, L. Non-thermal plasma treatment of volatile organic compounds: A predictive model based on experimental data analysis. Chem. Eng. J. 2019, 364, 37–44. [Google Scholar] [CrossRef]
- Font Palma, C. Modelling of tar formation and evolution for biomass gasification: A review. Appl. Energy 2013, 111, 129–141. [Google Scholar] [CrossRef]
- Fazeli, S.M.; Ravari, F.; Bozorgzadeh, H.; Sadeghzadeh Ahari, J. Kinetic model study of dry reforming of methane using cold plasma. Phys. Chem. Res. 2017, 5, 395–408. [Google Scholar] [CrossRef]
- Gagliano, A.; Nocera, F.; Bruno, M.; Cardillo, G. Development of an Equilibrium-based Model of Gasification of Biomass by Aspen Plus. Energy Procedia 2017, 111, 1010–1019. [Google Scholar] [CrossRef]
- Khacef, A.; Cormier, J.-M.; Pouvesle, J.; Le Van, T. Removal of Pollutants by Atmospheric Non Thermal Plasmas. arXiv 2008, arXiv:0810.5432. [Google Scholar]
- Mizuno, A. Industrial applications of atmospheric non-thermal plasma in environmental remediation. Plasma Phys. Control. Fusion 2007, 49, A1–A15. [Google Scholar] [CrossRef]
- Kacprzyk, R.; Mista, W.; Czapka, T. Atmospheric pressure cold plasma reactor with back discharges and parallel gas flow. Int. J. Plasma Environ. Sci. Technol. 2011, 5, 58–61. [Google Scholar]
- Abdelaziz, A.A.; Ishijima, T.; Seto, T. Humidity effects on surface dielectric barrier discharge for gaseous naphthalene decomposition. Phys. Plasmas 2018, 25, 043512. [Google Scholar] [CrossRef]
- Delikonstantis, E.; Scapinello, M.; Stefanidis, G.D. Process Modeling and Evaluation of Plasma-Assisted Ethylene Production from Methane. Processes 2019, 7, 68. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Tsuboi, Y.; Zhang, J.; Liu, J. Steam reforming of tar studied in bench-scale experiments and pilot-scale tests with simulations. Fuel 2021, 290, 120028. [Google Scholar] [CrossRef]
- Arshad, M.Y.; Saeed, M.A.; Tahir, M.W.; Pawlak-Kruczek, H.; Ahmad, A.S.; Niedzwiecki, L. Advancing Sustainable Decomposition of Biomass Tar Model Compound: Machine Learning, Kinetic Modeling, and Experimental Investigation in a Non-Thermal Plasma Dielectric Barrier Discharge Reactor. Energies 2023, 16, 5835. [Google Scholar] [CrossRef]
- Wang, T.C.; Lu, N.; Li, J.; Wu, Y. Degradation of pentachlorophenol in soil by pulsed corona discharge plasma. J. Hazard. Mater. 2010, 180, 436–441. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.W.; Zhu, J.; Feng, W.; Yan, K. Aerosol Formation and Decomposition of Benzene Derivatives by AC/DC Streamer Corona Discharge. Int. J. Plasma Environ. Sci. Technol. 2010, 4, 130–134. [Google Scholar]
- Anderson, G.K.; Snyder, H.; Coogan, J. Oxidation of Styrene in a Silent Discharge Plasma. Plasma Chem. Plasma Process. 1999, 19, 131–151. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Study on kinetic model of microwave thermocatalytic treatment of biomass tar model compound. Bioresour. Technol. 2014, 151, 183–190. [Google Scholar] [CrossRef]
- Karatum, O.; Deshusses, M.A. A comparative study of dilute VOCs treatment in a non-thermal plasma reactor. Chem. Eng. J. 2016, 294, 308–315. [Google Scholar] [CrossRef]
- Mei, D.; Tu, X. Conversion of CO2 in a cylindrical dielectric barrier discharge reactor: Effects of plasma processing parameters and reactor design. J. CO2 Util. 2017, 19, 68–78. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Zhao, F.; Xu, D. The chemistry of gaseous benzene degradation using non-thermal plasma. Environ. Sci. Pollut. Res. 2021, 28, 1565–1573. [Google Scholar] [CrossRef]
- Abdelaziz, A.A.; Seto, T.; Abdel-Salam, M.; Otani, Y. Influence of N2/O2 Mixtures on Decomposition of Naphthalene in Surface Dielectric Barrier Discharge Based Reactor. Plasma Chem. Plasma Process. 2014, 34, 1371–1385. [Google Scholar] [CrossRef]
- Bityurin, V.A.; Filimonova, E.A.; Naidis, G.V. Simulation of Naphthalene Conversion in Biogas Initiated by Pulsed Corona Discharges. IEEE Trans. Plasma Sci. 2009, 37, 911–919. [Google Scholar] [CrossRef]
- Lorcet, H.; Guenadou, D.; Latge, C.; Brothier, M.; Mariaux, G.; Vardelle, A. Kinetics modeling of biomass gasification under thermal plasma conditions. Application to a refractory species: The methane. In International Symposium on Plasma Chemistry, Germany; International Union of Pure and Applied Chemistry: Research Triangle Park, NC, USA, 2022. [Google Scholar]
- Yan, K.; van Heesch, E.J.M.; Pemen, A.J.M.; Huijbrechts, P.A.H.J. From Chemical Kinetics to Streamer Corona Reactor and Voltage Pulse Generator. Plasma Chem. Plasma Process. 2001, 21, 107–137. [Google Scholar] [CrossRef]
- Nair, S.A.; Yan, K.; Pemen, A.J.M.; van Heesch, E.J.M.; Ptasinski, K.J.; Drinkenburg, A.A.H. Tar Removal from Biomass-Derived Fuel Gas by Pulsed Corona Discharges. A Chemical Kinetic Study. Ind. Eng. Chem. Res. 2004, 43, 1649–1658. [Google Scholar] [CrossRef]
- Filimonova, E.A.; Amirov, R.H.; Kim, H.T.; Park, I.H. Comparative modelling of NOx and SO2 removal from pollutant gases using pulsed-corona and silent discharges. J. Phys. D Appl. Phys. 2000, 33, 1716–1727. [Google Scholar] [CrossRef]
- Blin-Simiand, N.; Jorand, F.; Magne, L.; Pasquiers, S.; Postel, C.; Vacher, J.R. Plasma Reactivity and Plasma-Surface Interactions During Treatment of Toluene by a Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2008, 28, 429–466. [Google Scholar] [CrossRef]
- Mariana, F.M.; Antonio, M.C.; Raúl, V.A.; Régulo, L.C.; Samuel, B.D.; Rosendo, P.E.; Arturo, M.C.; Bethsabet, J.S. NOx Chemical Reduction in a Cylindrical DBD Reactor: Theoretical and Experimental Analysis. Conf. Fr. Electrost. 2008, 3, 98. [Google Scholar]
- Ratkiewicz, A.; Truong, T. A canonical form of the complex reaction mechanism. Energy 2012, 43, 64–72. [Google Scholar] [CrossRef]
- Nair, S.A.; Yan, K.; Pemen, A.J.M.; van Heesch, E.J.M.; Ptasinski, K.J.; Drinkenburg, A.A.H. Tar Removal from Biomass Derived Fuel Gas by Pulsed Corona Discharges: Chemical Kinetic Study II. Ind. Eng. Chem. Res. 2005, 44, 1734–1741. [Google Scholar] [CrossRef]
- Kraus, M.; Egli, W.; Haffner, K.; Eliasson, B.; Kogelschatz, U.; Wokaun, A. Investigation of mechanistic aspects of the catalytic CO2 reforming of methane in a dielectric-barrier discharge using optical emission spectroscopy and kinetic modeling. Phys. Chem. Chem. Phys. 2002, 4, 668–675. [Google Scholar] [CrossRef]
- Bityurin, V.A.; Filimonova, E.A.; Kerst, R.A.B.P.; Naidis, G.V.; Pemen, A.J.M. The modeling of tar removal from biogas stimulated by a pulse corona discharge. Vacuum 2006, 2, N2. [Google Scholar]
- Heesch, B.E.J.M.V.; Pemen, G.U.A.J.M.; Keping, Y.; Paasen, S.V.B.V.; Ptasinski, K.J.; Huijbrechts, P.A.H.J. Pulsed corona tar cracker. IEEE Trans. Plasma Sci. 2000, 28, 1571–1575. [Google Scholar] [CrossRef]
- Kim, H.-H.; Ogata, A.; Futamura, S. Atmospheric plasma-driven catalysis for the low temperature decomposition of dilute aromatic compounds. J. Phys. D Appl. Phys. 2005, 38, 1292–1300. [Google Scholar] [CrossRef]
- Jiang, N.; Lu, N.; Shang, K.; Li, J.; Wu, Y. Effects of electrode geometry on the performance of dielectric barrier/packed-bed discharge plasmas in benzene degradation. J. Hazard. Mater. 2013, 262, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, S.; Ma, W.; Austin, K.; Kumar, A. A wet packed-bed scrubber for removing tar from biomass producer gas. Fuel Process. Technol. 2019, 193, 197–203. [Google Scholar] [CrossRef]
- Materazzi, M.; Lettieri, P.; Mazzei, L.; Taylor, R.; Chapman, C. Reforming of tars and organic sulphur compounds in a plasma-assisted process for waste gasification. Fuel Process. Technol. 2015, 137, 259–268. [Google Scholar] [CrossRef]

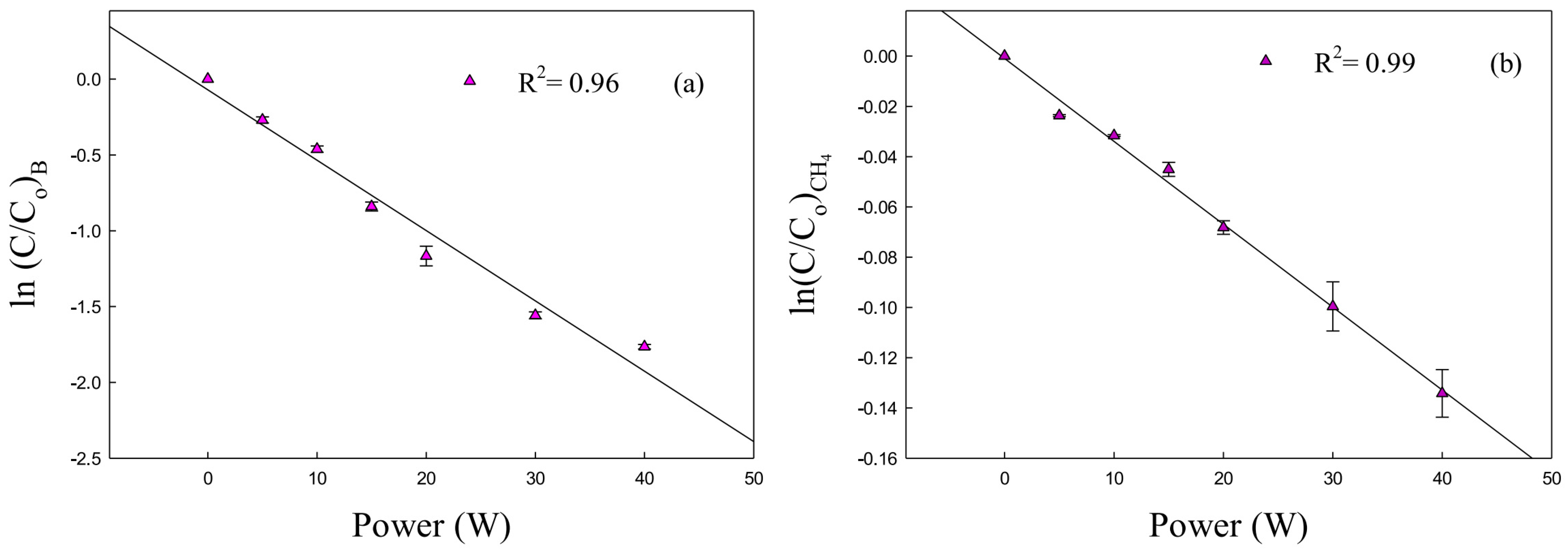



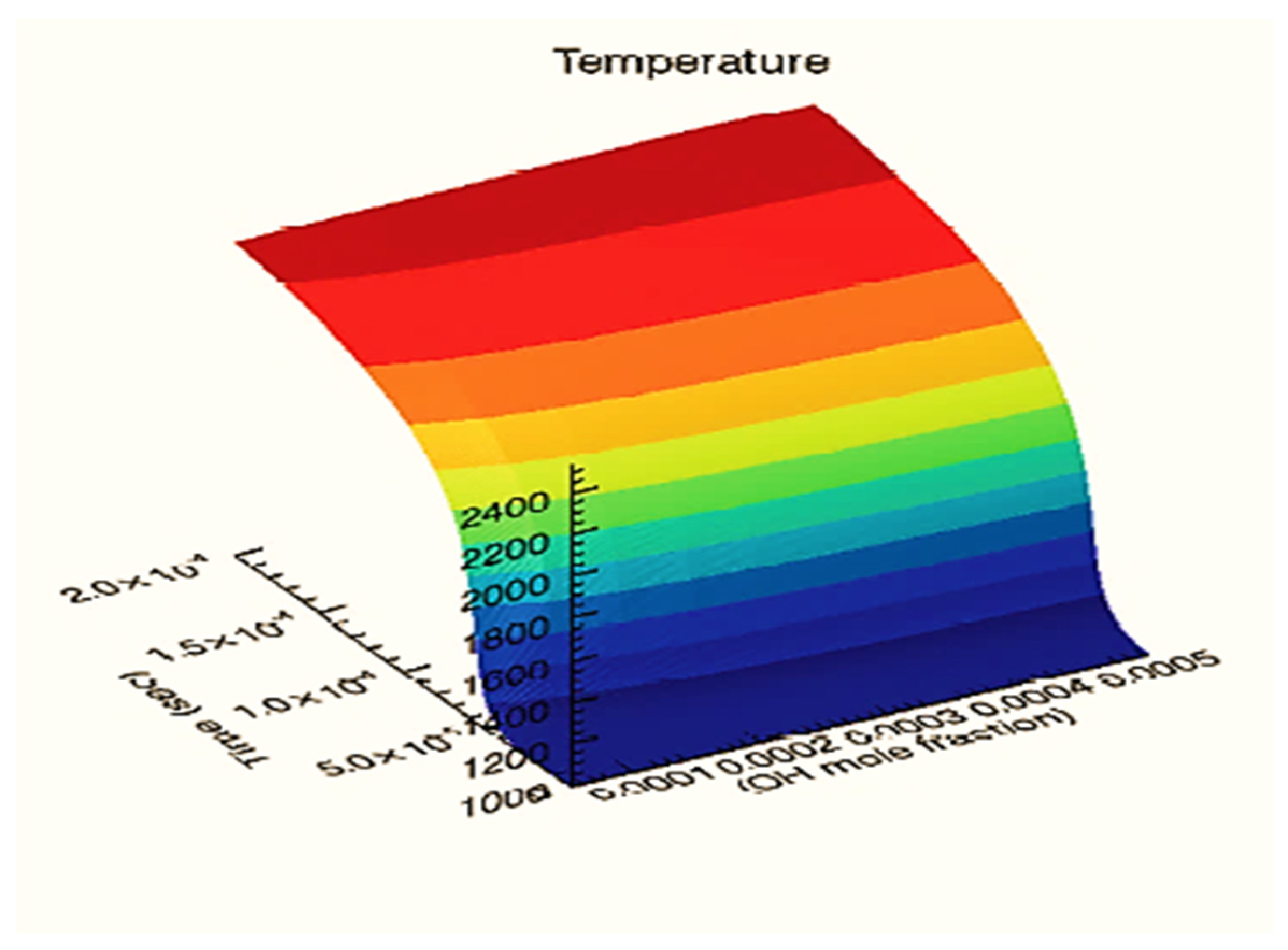

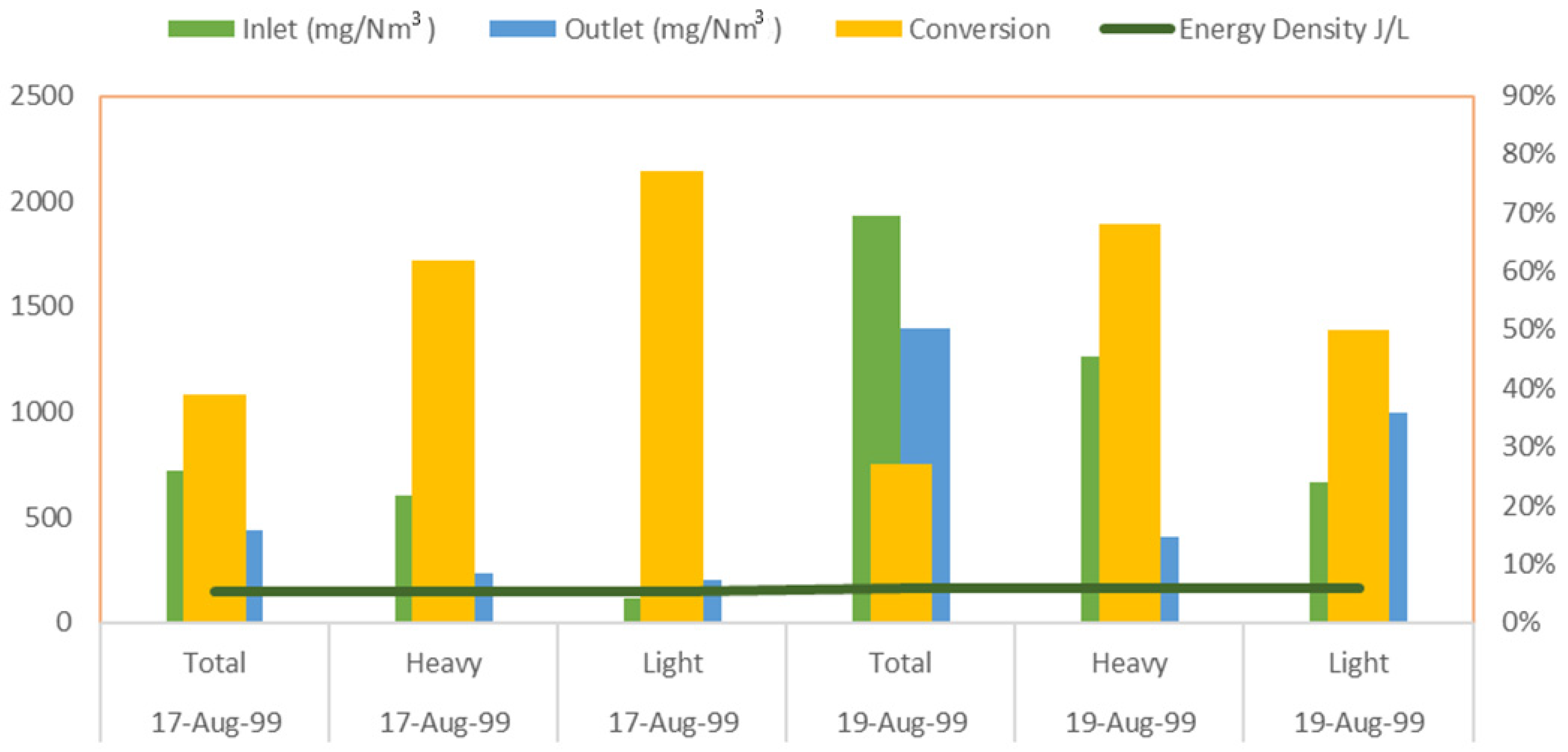
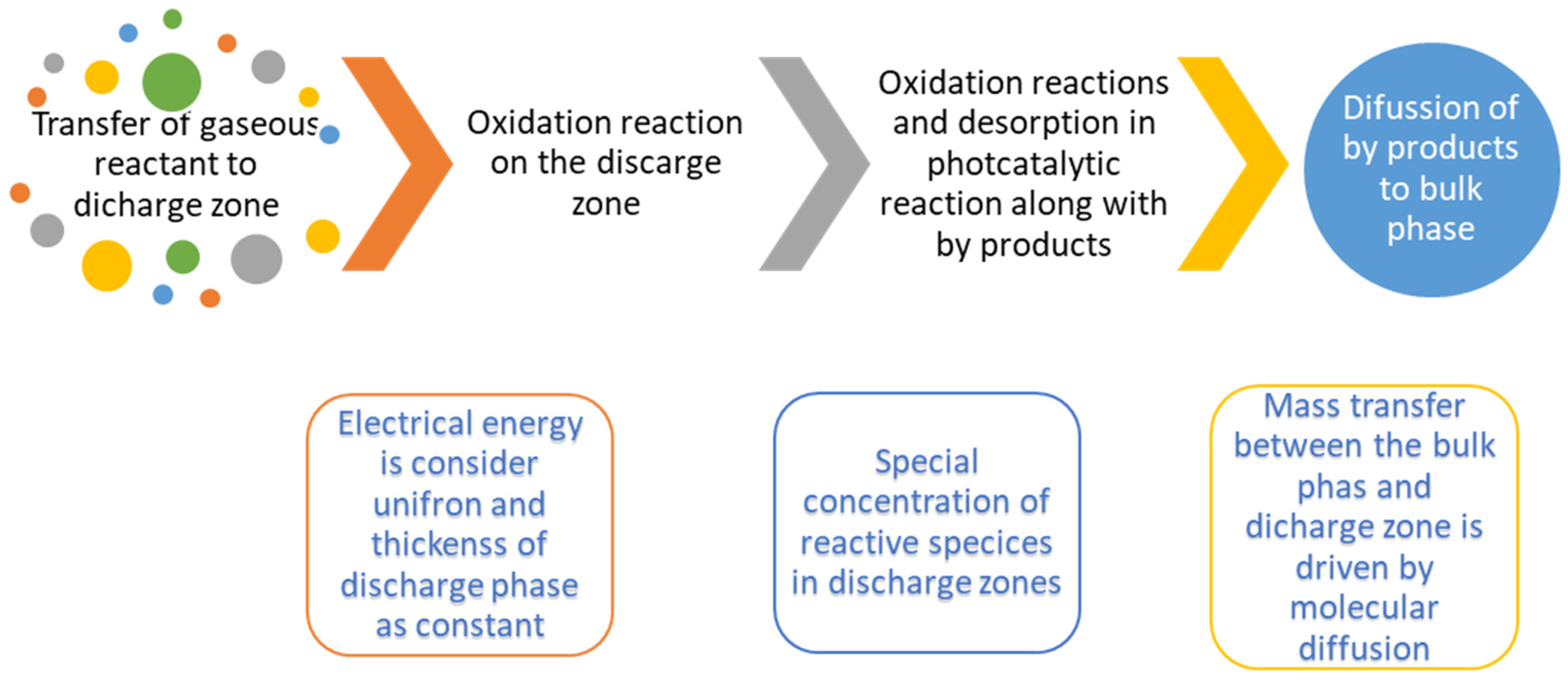

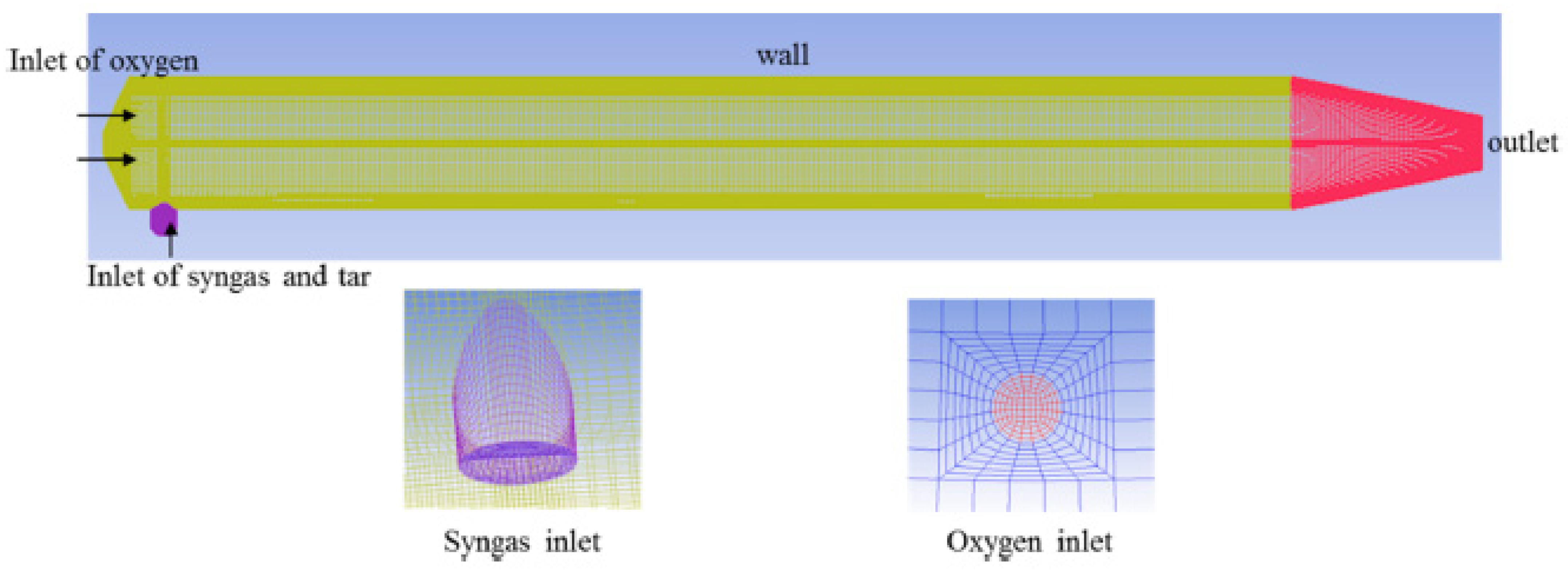

| Reactor Type | Batch Reactor | CSTR Reactor | PFR Reactor |
|---|---|---|---|
| Generalized Equation | T) | T) | |
| Space Time | T | T | |
| Conversion X, n = 1 | Conversion is greater than CSTR for first order reaction | Conversion is less for first order reaction than PFR | Conversion is greater for first order reaction |
| Reactant Density p | Usually, fluid density is kept constant | Usually, fluid density is kept constant | Usually, fluid density is kept constant |
| Variable Order of Reaction | Conversion is greater than CSTR if n > 0, T | Conversion is less than PFR if n < 0, T | Conversion is greater than CSTR if n > 0, T |
| At n = 0 | Conversion is the same as another reactor | Conversion is the same as another reactor | Conversion is the same as another reactor |
| Molecules | Temperature (°C) | β (J/L) | Energy Cost eV/Molecule | Reactor Type |
|---|---|---|---|---|
| Methanol (100 ppm) | 25.00 | 195.00 | 283.00 | Pulsed Corona Reactor |
| Benzene (150 ppm) | 25.00 | 671.00 | 1038.00 | Pulsed Corona Reactor |
| Benzene (150 ppm) | 300.00 | 138.00 | 214.00 | Pulsed Corona Reactor |
| Dichloromethane (160 ppm) | 25.00 | 1488.00 | 2159.00 | Pulsed Corona Reactor |
| Dichloromethane (160 ppm) | 300.00 | 46.00 | 67.00 | Pulsed Corona Reactor |
| Methanol (400 ppm) | 300.00 | 75.00 | 44.00 | Pulsed Corona Reactor |
| Acetone (800 ppm) | 25.00 | 3543.00 | 1028.00 | Pulsed Corona Reactor |
| Acetone (800 ppm) | 300.00 | 285.00 | 83.00 | Pulsed Corona Reactor |
| ChloroBenzene (244 ppm) | 180.00 | 102.00 | 97.00 | Silent discharge Plasma Reactor |
| Styrene (407 ppm) | 300.00 | 53.60 | 30.60 | Silent discharge Plasma Reactor |
| Trichloroethylene (500 ppm) | 25.00 | 12.40 | 5.80 | Silent discharge Plasma Reactor |
| Styrene (814 ppm) | 300.00 | 115.00 | 32.80 | Silent discharge Plasma Reactor |
| Styrene (1627 ppm) | 300.00 | 299.00 | 42.70 | Silent discharge Plasma Reactor |
| Styrene (2441 ppm) | 300.00 | 461.00 | 43.80 | Silent discharge Plasma Reactor |
| Benzene (80 ppm) | 25.00 | 277.00 | 83.00 | Pulsed Corona Reactor |
| Toluene (100 ppm) | 180.00 | 500.00 | 32.80 | Pulsed Corona Reactor |
| Styrene (200 ppm) | 300.00 | 1660.00 | 2159.00 | Silent discharge Plasma Reactor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, M.Y.; Saeed, M.A.; Tahir, M.W.; Raza, A.; Ahmad, A.S.; Tahir, F.; Borkowski, B.; Mączka, T.; Niedzwiecki, L. Role of Experimental, Modeling, and Simulation Studies of Plasma in Sustainable Green Energy. Sustainability 2023, 15, 14193. https://doi.org/10.3390/su151914193
Arshad MY, Saeed MA, Tahir MW, Raza A, Ahmad AS, Tahir F, Borkowski B, Mączka T, Niedzwiecki L. Role of Experimental, Modeling, and Simulation Studies of Plasma in Sustainable Green Energy. Sustainability. 2023; 15(19):14193. https://doi.org/10.3390/su151914193
Chicago/Turabian StyleArshad, Muhammad Yousaf, Muhammad Azam Saeed, Muhammad Wasim Tahir, Ahsan Raza, Anam Suhail Ahmad, Fasiha Tahir, Bartłomiej Borkowski, Tadeusz Mączka, and Lukasz Niedzwiecki. 2023. "Role of Experimental, Modeling, and Simulation Studies of Plasma in Sustainable Green Energy" Sustainability 15, no. 19: 14193. https://doi.org/10.3390/su151914193
APA StyleArshad, M. Y., Saeed, M. A., Tahir, M. W., Raza, A., Ahmad, A. S., Tahir, F., Borkowski, B., Mączka, T., & Niedzwiecki, L. (2023). Role of Experimental, Modeling, and Simulation Studies of Plasma in Sustainable Green Energy. Sustainability, 15(19), 14193. https://doi.org/10.3390/su151914193