The Effects of Varying Combinations of Dietary Selenium, Vitamin E, and Zinc Supplements on Semen Characteristics and Antioxidant Enzyme Activity of Spermatozoa in 1-Year-Old Native Turkish Ganders
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Rearing and Feeding
2.3. Sperm Collection and Determination of Quality Characteristics
2.4. Determination of Antioxidative Enzyme Activities
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aral, Y.; Aydın, E. The economic importance of goose breeding and assessment possibility of goose products in Turkey. Turk. J. Vet. Anim. Sci. 2007, 78, 31–38. [Google Scholar]
- Boz, M.A. Effect of classified rearing according to live weight on growth, carcass and some meat quality characteristics in geese. Turk. JAF Sci. Technol. 2019, 7, 1429–1434. [Google Scholar] [CrossRef]
- Boz, M.A.; Erensoy, K.; Sarıca, M. The effects of age and daytime periods on behavioral traits of Turkish geese reared in free-range system. J. Anim. Prod. 2022, 63, 126–135. [Google Scholar]
- Liu, S.J.; Zheng, J.X.; Yang, N. Semen quality factor as an indicator of fertilizing ability for geese. Poult. Sci. 2008, 87, 155–159. [Google Scholar] [CrossRef]
- Tilki, M.; Yazıcı, K.; Sarı, M.; Işık, S.; Saatci, M. Effects of hatching month and sex on slaughter and carcass traits in native Turkish geese. Kafkas Univ. Vet. Fak. Derg. 2011, 17, 831–835. [Google Scholar] [CrossRef][Green Version]
- Tilki, M.; İnal, Ş. Yield traits of geese of different origins reared in Turkey I. Hatching traits. Turk. J. Vet. Anim. Sci. 2004, 28, 149–155. [Google Scholar]
- Onk, K.; Kirmizibayrak, T. The egg production, hatchability, growing, slaughter and carcass characteristics of geese (Anser anser) reared under breeders conditions in Kars Province; I. Egg production and hatchability characteristics. Turk. JAF Sci. Technol. 2019, 7, 543–549. [Google Scholar]
- Arslan, C.; Saatcı, M. Egg yield and hatchability characteristics of native geese in the Kars region. Turk. J. Vet. Anim. Sci. 2003, 27, 1361–1365. [Google Scholar]
- Partyka, A.; Lukaszewicz, E.; Nizanski, W. Lipid peroxidation and antioxidant enzymes activity in avian semen. Anim. Reprod. Sci. 2012, 134, 184–190. [Google Scholar] [CrossRef]
- Qi, X.; Shang, M.; Chen, C.; Chen, Y.; Hua, J.; Sheng, X.; Wang, X.; Xing, K.; Ni, H.; Guo, Y. Dietary supplementation with linseed oil improves semen quality, reproductive hormone, gene and protein expression related to testosterone synthesis in aging layer breeder roosters. Theriogenology 2019, 131, 9–15. [Google Scholar] [CrossRef]
- Akhlaghi, A.; Ahangari, Y.J.; Navidshad, B.; Pirsaraei, Z.A.; Zhandi, M.; Deldar, H.; Rezvani, M.R.; Dadpasand, M.; Hashemi, S.R.; Poureslami, R.; et al. Improvements in semen quality, sperm fatty acids, and reproductive performance in aged Cobb 500 breeder roosters fed diets containing dried ginger rhizomes (Zingiber officinale). Poult. Sci. 2014, 93, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Liu, M.; Niu, Q.J.; Huang, Y.X.; Zhao, L.; Lei, X.G.; Sun, L.H. Both selenium deficiency and excess impair male reproductive system via inducing oxidative stress-activated PI3K/AKT-mediated apoptosis and cell proliferation signaling in testis of mice. Free Radic. Biol. Med. 2023, 197, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Huang, Y.X.; Sun, H.; Liu, M.; Zhao, L.; Sun, L.H. Selenium deficiency dysregulates one-carbon metabolism in nutritional muscular dystrophy of chicks. J. Nutr. 2023, 153, 47–55. [Google Scholar] [CrossRef]
- Yan, W.; Kanno, C.; Oshima, E.; Kuzuma, Y.; Kim, S.W.; Bai, H.; Takahashi, M.; Yanagawa, Y.; Nagano, M.; Wakamatsu, J.I.; et al. Enhancement of sperm motility and viability by turmeric by-product dietary supplementation in roosters. Anim. Reprod. Sci. 2017, 185, 195–204. [Google Scholar] [CrossRef]
- Partyka, A.; Nizanski, W. Supplementation of Avian Semen Extenders with Antioxidants to Improve Semen Quality—Is It an Effective Strategy? Antioxidants 2021, 10, 1927. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Blesbois, E.; Grasseau, I.; Chalah, T.; Brillard, J.P.; Wishart, G.; Cerolini, S.; Sparks, N.H.C. Fatty acid composition, glutathione peroxidase and superoxide dismutase activity and total antioxidant activity of avian semen. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1998, 120, 527–533. [Google Scholar] [CrossRef]
- Surai, P.F.; Brillard, J.P.; Speake, B.K.; Blesbois, E.; Seigneurin, F.; Sparks, N.H.C. Phospholipid fatty acid composition, vitamin E content and susceptibility to lipid peroxidation of duck spermatozoa. Theriogenology 2000, 53, 1025–1039. [Google Scholar] [CrossRef]
- Khan, R.U.; Rahman, Z.; Javed, I.; Muhammad, F. Effect of vitamins, probiotics and protein on semen traits in post-molt male broiler breeders. Anim. Reprod. Sci. 2012, 135, 85–90. [Google Scholar] [CrossRef]
- Partyka, A.; Lukaszewicz, E.; Nizanski, W. Effect of cryopreservation on sperm parameters, lipid peroxidation and antioxidant enzymes activity in fowl semen. Theriogenology 2012, 77, 1497–1504. [Google Scholar] [CrossRef]
- Lagares, M.; Ecco, R.; Martins, N.; Lara, L.; Rocha, J.; Vilela, D.; Barbosa, V.; Mantovani, P.; Braga, J.; Preis, I. Detecting reproductive system abnormalities of broiler breeder roosters at different ages. Reprod. Domest. Anim. 2017, 52, 67–75. [Google Scholar] [CrossRef]
- Long, C.; Wang, Z.; Guo, Y.; Sheng, X.; Xing, K.; Ni, H.; Wang, X.; Xiao, L.; Qi, X. Research Note: Dietary supplementation with pyrroloquinoline quinone disodium (PQQ.Na2) improves oxidative status and semen quality in aging layer breeder roosters. Poult. Sci. 2022, 101, 101812. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewicz, E. Effect of semen filtration and dilution rate on morphology and fertility of frozen gander spermatozoa. Theriogenology 2001, 55, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewicz, E.; Kruszynski, W. Evaluation of fresh and frozen-thawed semen of individual ganders by assessment of spermatozoa motility and morphology. Theriogenology 2003, 59, 1627–1640. [Google Scholar] [PubMed]
- Wolc, A.; Barczak, E.; Wężyk, S.; Badowski, J.; Bielińska, H.; Szwaczkowski, T. Genetic evaluation of production and reproduction traits in two selected lines of geese under multitrait animal model. Anim. Sci. Pap. Rep. 2008, 26, 71–78. [Google Scholar]
- Boz, M.A.; Baş, H.; Sarıca, M.; Erensoy, K. The effects of natural mating and artificial insemination on reproductive traits of 1-and 2-year-old domestic Turkish geese. Vet. Res. Commun. 2021, 45, 211–221. [Google Scholar] [CrossRef]
- Baş, H.; Taşkesen, H.O.; Boz, M.A.; Sarıca, M.; Erensoy, K.; Dotas, V.; Symeon, G. The effects of dietary Selenium, Vitamin E and Zinc supplementation and their varying combinations on antioxidant enzyme activity, developmental and histological traits in testicular tissue of 1-year-old native Turkish ganders. Sustainability 2023, 15, 12245. [Google Scholar] [CrossRef]
- Amem, M.H.; Al-Daraji, H.J. Zinc improves egg quality in Cobb500 broiler breeder females. Int. J. Poult. Sci. 2011, 10, 471–476. [Google Scholar] [CrossRef][Green Version]
- Amem, M.H.; Al-Daraji, H.J. Effect of dietary zinc on semen quality of Cobb 500 broiler breeder males. Int. J. Poult. Sci. 2011, 10, 477–482. [Google Scholar] [CrossRef][Green Version]
- Jerysz, A.; Lukaszewicz, E. Effect of Dietary Selenium and Vitamin E on Ganders’ Response to Semen Collection and Ejaculate Characteristics. Biol. Trace Elem. Res. 2013, 153, 196–204. [Google Scholar] [CrossRef]
- Bakst, M.R.; Cecil, H.C. Techniques for Semen Evaluation, Semen Storage, and Fertility Determination; Poultry Science Association: Champaign, IL, USA, 1997. [Google Scholar]
- Bas, H.; Eroglu, H.E.; Dogan, H.; Uskutoglu, T.; Cosge Senkal, B.; Cesur, C. Evaluation of the chemical composition, genotoxic and cytotoxic effects of cocklebur (Xanthium strumarium L.) seed oil on human blood cells. Int. J. Agric. Life Sci. 2022, 6, 1–7. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantative and qualitative characterization of glutathione peroxidase. J. Lab. Med. 1987, 70, 158–165. [Google Scholar]
- Haboby, A.H.; Hamra, A.H.; Al-Tamemmy, M.J.; Al-Rawi, T.S. Effect of vitamin E and selenium on semen quality, sexual activity, and some blood parameters of Awassi Rams. J. Agric. Investig. 2004, 2, 58–62. [Google Scholar]
- Surai, P.F.; Fujihara, N.; Speake, B.K.; Brillard, J.P.; Wishart, G.J.; Sparks, N.H.C. Polyunsaturated fatty acids, lipid peroxidation and antioxidant protection in avian semen. Asian-Aust. J. Anim. Sci. 2001, 14, 1024–1050. [Google Scholar] [CrossRef]
- Varga, A.; Barna, J.; Almasi, A. Sperm analysis and sexual characteristics of frizzled Hungarian ganders (preliminary study). Allatten Takarman 2003, 2, 167–172. [Google Scholar]
- Lukaszewicz, E. An effective method for freezing White Italian gander semen. Theriogenology 2002, 58, 19–27. [Google Scholar] [CrossRef]
- Malaniuk, P.; Lukaszewicz, E. Effect of feed supplementation with organic selenium and vitamin E on quantitative and qualitative characteristics of Japanese quails (Coturnix japonica). Zesz. Nauk. UP Wroclawiu Biol. Hod Zw. 2006, 548, 99–109. (In Polish) [Google Scholar]
- Edens, F.W.; Sefton, A.E. Sel-Plex® improves spermatozoa morphology in broiler breeder males. Int. J. Poult. Sci. 2009, 8, 853–861. [Google Scholar] [CrossRef]
- Marin-Guzman, J.; Mahan, D.C.; Pate, J.L. Effect of dietary selenium and vitamin E on spermatogenic development in boars. J. Anim. Sci. 2000, 78, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Oldfield, J.E.; Whanger, P.D.; Weswig, P.H. Effect of selenium, vitamin E, and antioxidants on testicular function in rats. Biol. Reprod. 1973, 8, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.A.; Medina, V.; Gravance, C.G.; Baumbe, J. Effect of antioxidants on preservation of motility, viability and acrosomal integrity of equine spermatozoa during storage at 5 °C. Theriogenology 2001, 56, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.F.H.M.; Gadella, B.M. In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic. Biol. Med. 2003, 35, 1382–1391. [Google Scholar] [CrossRef]
- Agarwal, A.; Prabakaran, S.A.; Said, T.M. Prevention of oxidative stress injury to sperm. J. Androl. 2005, 26, 654–660. [Google Scholar] [CrossRef]
- Douard, W.; Hermier, D.; Magistrini, M.; Blesbois, E. Reproductive period affects lipid composition and quality of fresh and stored spermatozoa in turkeys. Theriogenology 2003, 59, 753–764. [Google Scholar] [CrossRef]
- Blesbois, E.; Grasseau, J.; Hermier, D. Changes in lipid content of fowl spermatozoa after liquid storage at 2 to 5 °C. Theriogenology 1999, 52, 325–334. [Google Scholar] [CrossRef]
 : Control,
: Control,  : Se,
: Se,  : Vit E,
: Vit E,  : Zn,
: Zn,  : Se + Vit E,
: Se + Vit E,  : Se + Zn,
: Se + Zn,  : Vit E + Zn,
: Vit E + Zn,  : Se + Vit E + Zn). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day. (**: p < 0.001; *: p < 0.05).
: Se + Vit E + Zn). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day. (**: p < 0.001; *: p < 0.05).
 : Control,
: Control,  : Se,
: Se,  : Vit E,
: Vit E,  : Zn,
: Zn,  : Se + Vit E,
: Se + Vit E,  : Se + Zn,
: Se + Zn,  : Vit E + Zn,
: Vit E + Zn,  : Se + Vit E + Zn). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day. (**: p < 0.001; *: p < 0.05).
: Se + Vit E + Zn). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day. (**: p < 0.001; *: p < 0.05).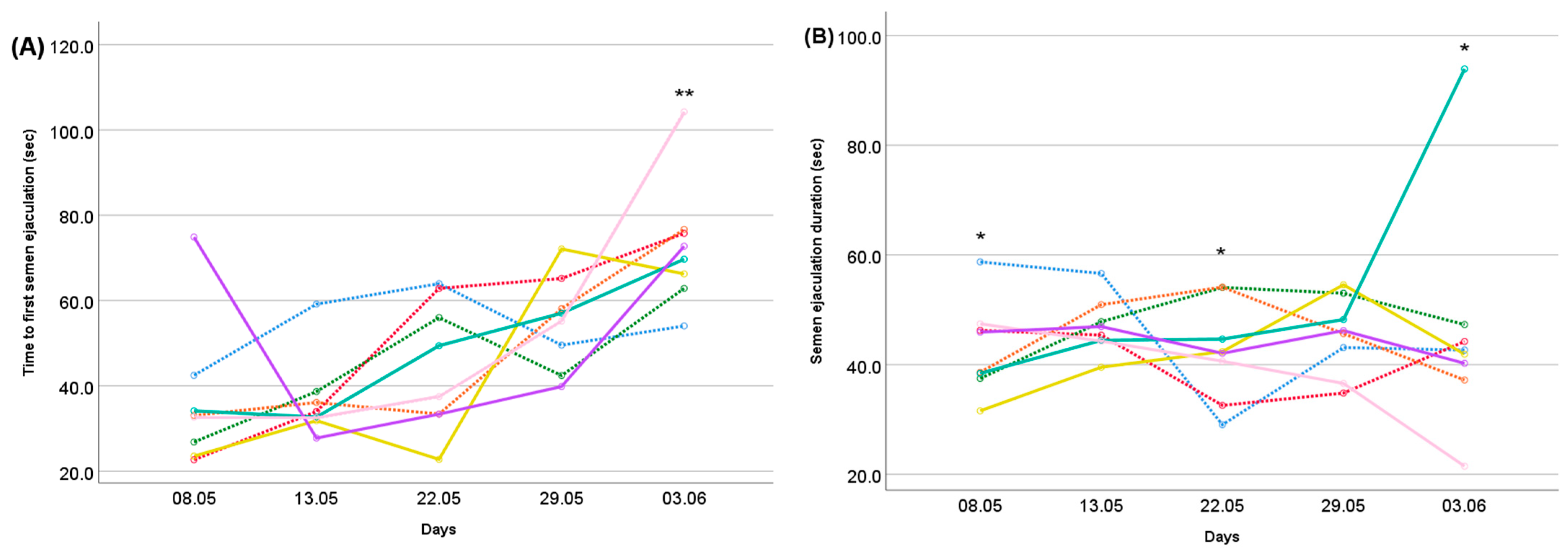
 : Control,
: Control,  : Se,
: Se,  : Vit E,
: Vit E,  : Zn,
: Zn,  : Se + Vit E,
: Se + Vit E,  : Se + Zn,
: Se + Zn,  : Vit E + Zn,
: Vit E + Zn,  : Se + Vit E + Zn). (The age effect was significant at the p < 0.001 level for all traits). 1 Semen volume (mL) was measured by a semen collection cup to the minimum of 10 μL; 2 Sperm concentration (n × 106/mL) was measured by a hemocytometer (Micro Cell counting chamber); 3 Sperm motility (%) was estimated by using the hanging drop method at 400× magnification; 4 SQF = ejaculate semen volume (mL) × sperm concentration (n × 106/mL) × live and normal morphology sperm number (%)/100. Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (**: p < 0.001; *: p < 0.05).
: Se + Vit E + Zn). (The age effect was significant at the p < 0.001 level for all traits). 1 Semen volume (mL) was measured by a semen collection cup to the minimum of 10 μL; 2 Sperm concentration (n × 106/mL) was measured by a hemocytometer (Micro Cell counting chamber); 3 Sperm motility (%) was estimated by using the hanging drop method at 400× magnification; 4 SQF = ejaculate semen volume (mL) × sperm concentration (n × 106/mL) × live and normal morphology sperm number (%)/100. Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (**: p < 0.001; *: p < 0.05).
 : Control,
: Control,  : Se,
: Se,  : Vit E,
: Vit E,  : Zn,
: Zn,  : Se + Vit E,
: Se + Vit E,  : Se + Zn,
: Se + Zn,  : Vit E + Zn,
: Vit E + Zn,  : Se + Vit E + Zn). (The age effect was significant at the p < 0.001 level for all traits). 1 Semen volume (mL) was measured by a semen collection cup to the minimum of 10 μL; 2 Sperm concentration (n × 106/mL) was measured by a hemocytometer (Micro Cell counting chamber); 3 Sperm motility (%) was estimated by using the hanging drop method at 400× magnification; 4 SQF = ejaculate semen volume (mL) × sperm concentration (n × 106/mL) × live and normal morphology sperm number (%)/100. Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (**: p < 0.001; *: p < 0.05).
: Se + Vit E + Zn). (The age effect was significant at the p < 0.001 level for all traits). 1 Semen volume (mL) was measured by a semen collection cup to the minimum of 10 μL; 2 Sperm concentration (n × 106/mL) was measured by a hemocytometer (Micro Cell counting chamber); 3 Sperm motility (%) was estimated by using the hanging drop method at 400× magnification; 4 SQF = ejaculate semen volume (mL) × sperm concentration (n × 106/mL) × live and normal morphology sperm number (%)/100. Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (**: p < 0.001; *: p < 0.05).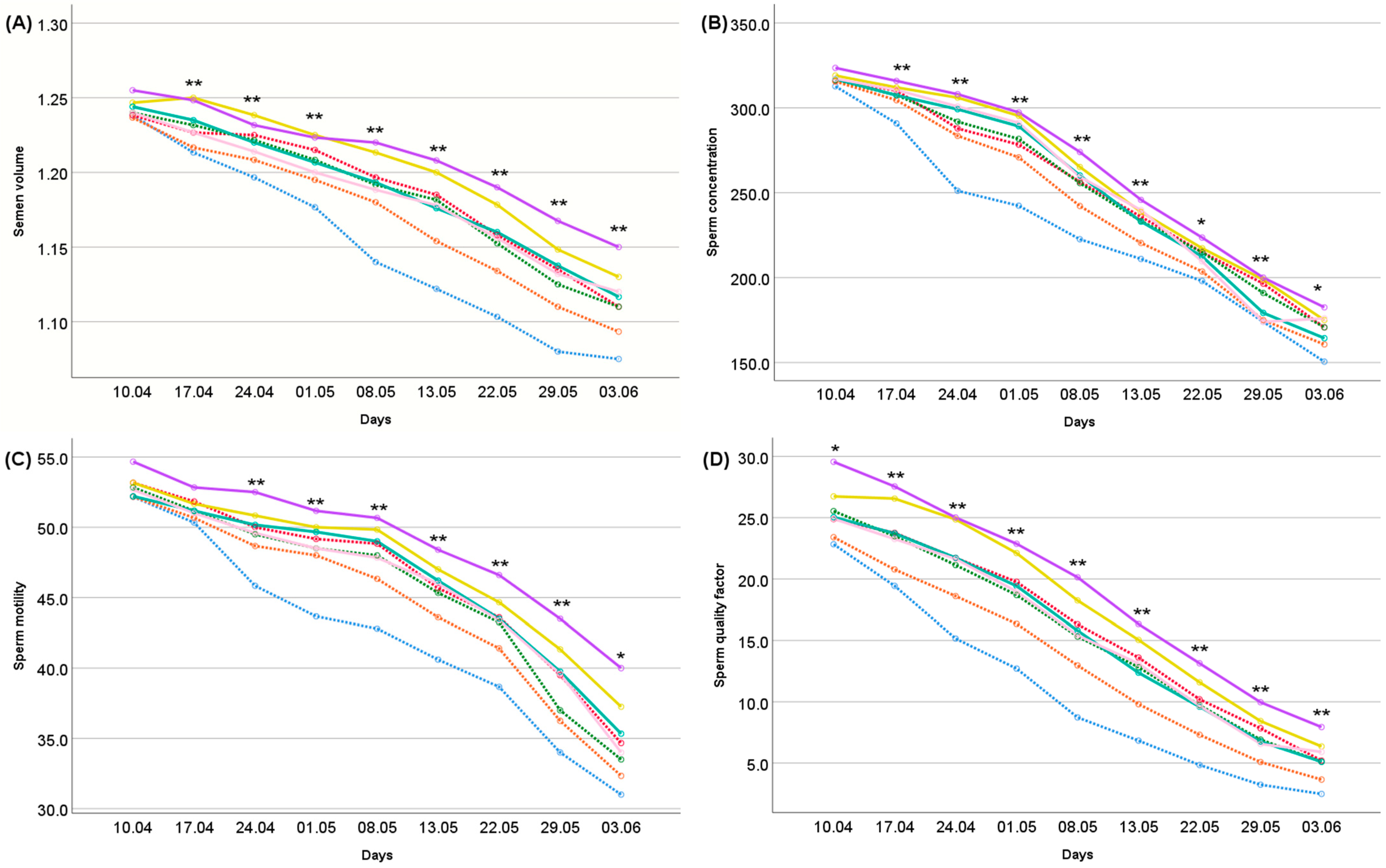
 : Control,
: Control,  : Se,
: Se,  : Vit E,
: Vit E,  : Zn,
: Zn,  : Se + Vit E,
: Se + Vit E,  : Se + Zn,
: Se + Zn,  : Vit E + Zn,
: Vit E + Zn,  : Se + Vit E + Zn). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (**: p < 0.001; *: p < 0.05).
: Se + Vit E + Zn). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (**: p < 0.001; *: p < 0.05).
 : Control,
: Control,  : Se,
: Se,  : Vit E,
: Vit E,  : Zn,
: Zn,  : Se + Vit E,
: Se + Vit E,  : Se + Zn,
: Se + Zn,  : Vit E + Zn,
: Vit E + Zn,  : Se + Vit E + Zn). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (**: p < 0.001; *: p < 0.05).
: Se + Vit E + Zn). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (**: p < 0.001; *: p < 0.05).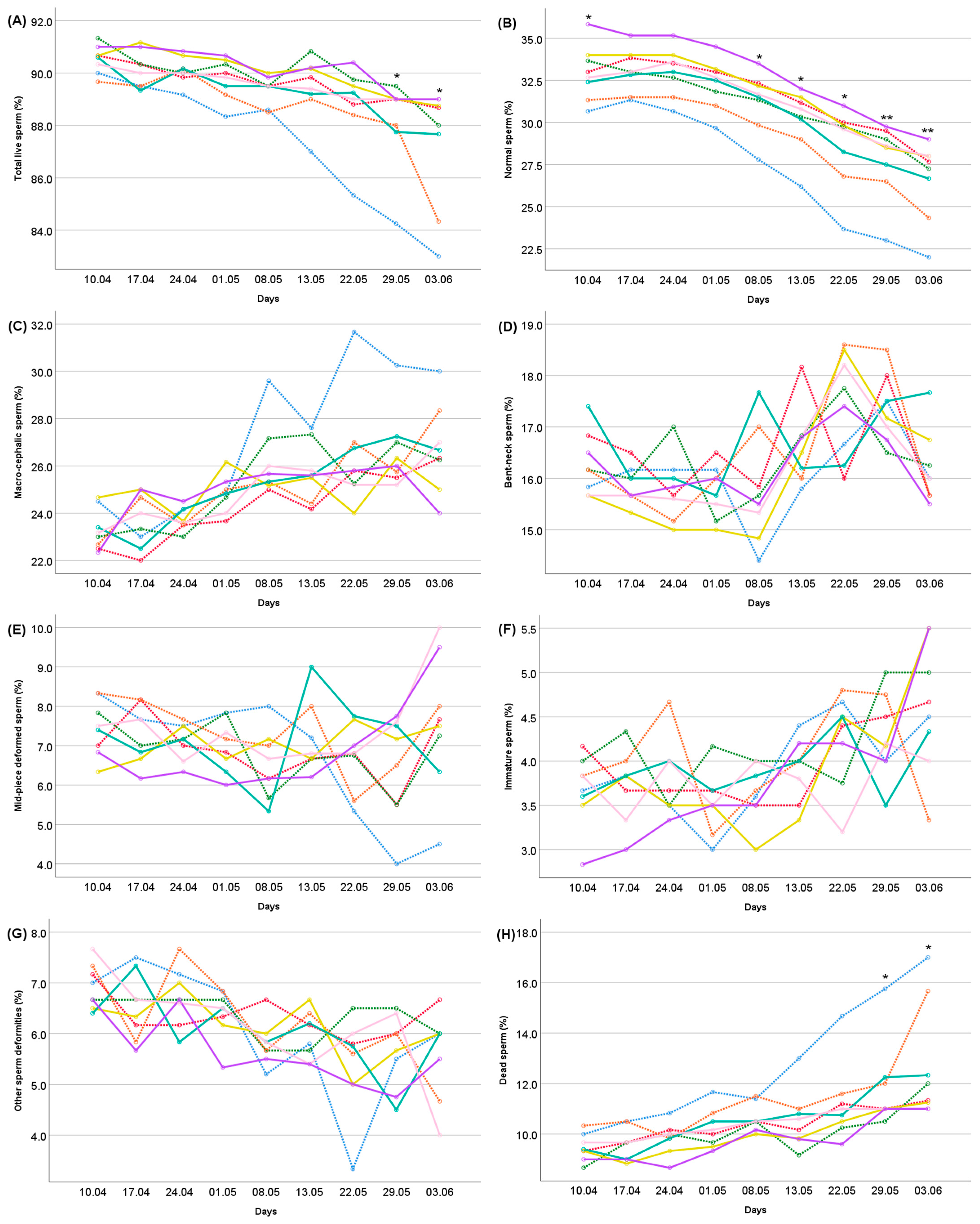
 : Control,
: Control,  : Se,
: Se,  : Vit E,
: Vit E,  : Zn,
: Zn,  : Se + Vit E,
: Se + Vit E,  : Se + Zn,
: Se + Zn,  : Vit E + Zn,
: Vit E + Zn,  : Se + Vit E + Zn). (The age effect was significant at the p < 0.001 level for all traits). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (*: p < 0.05).
: Se + Vit E + Zn). (The age effect was significant at the p < 0.001 level for all traits). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (*: p < 0.05).
 : Control,
: Control,  : Se,
: Se,  : Vit E,
: Vit E,  : Zn,
: Zn,  : Se + Vit E,
: Se + Vit E,  : Se + Zn,
: Se + Zn,  : Vit E + Zn,
: Vit E + Zn,  : Se + Vit E + Zn). (The age effect was significant at the p < 0.001 level for all traits). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (*: p < 0.05).
: Se + Vit E + Zn). (The age effect was significant at the p < 0.001 level for all traits). Differences between treatments indicated by the asterisk are significant according to the ANOVA results within each measurement day (*: p < 0.05).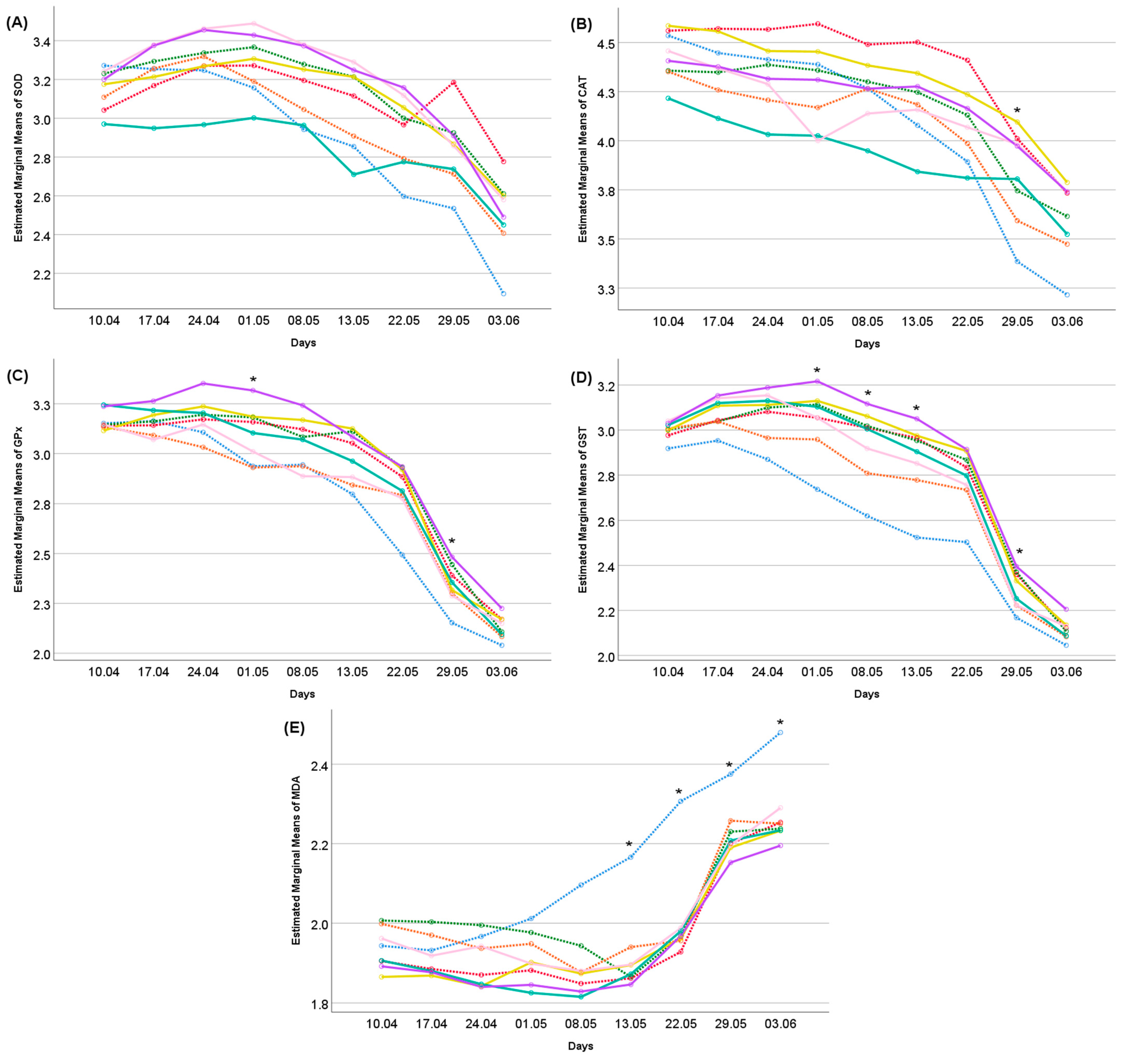
| Ingredient | Unit | Amount |
|---|---|---|
| Corn | % | 57.5 |
| Sunflower seed meal | % | 18.5 |
| Soybean meal (CP 46%) | % | 10.0 |
| Limestone | % | 8.0 |
| Cotton seed meal (CP 26%) | % | 5.0 |
| Salt | % | 0.75 |
| Vitamin premix | % | 0.25 |
| Analyzed nutrient content * | ||
| Dry Matter | % | 88.76 |
| Crude Protein (CP) | % | 15.50 |
| ME | MJ/kg | 10.29 |
| Crude oil | % | 3.30 |
| Crude fiber | % | 7.14 |
| Crude Ash | % | 11.68 |
| Se | mg/kg | 0.15 |
| Zn | mg/kg | 60 |
| Vit E | mg/kg | 30 |
| Groups | Se (mg/kg) | Vit E (mg/kg) | Zn (mg/kg) |
|---|---|---|---|
| Control | 0.15 | 30 | 60 |
| Se | 0.45 | 30 | 60 |
| Vit E | 0.15 | 130 | 60 |
| Zn | 0.15 | 30 | 160 |
| Se + Vit E | 0.45 | 130 | 60 |
| Se + Zn | 0.45 | 30 | 160 |
| Zn + Vit E | 0.15 | 130 | 160 |
| Se + Vit E + Zn | 0.45 | 130 | 160 |
| Dietary Treatments | Time to First Semen Ejaculation (s) | Semen Ejaculation Duration (s) | Total n of Ganders Producing or Not-Producing Semen | |
|---|---|---|---|---|
| Not-Producing | Producing | |||
| Control | 53.8 | 46.0 | 11 (20.4%) | 43 (79.6%) |
| Se | 52.1 | 40.7 | 8 (14.8%) | 46 (85.2%) |
| Vit E | 45.4 | 47.9 | 8 (14.8%) | 46 (85.2%) |
| Zn | 47.5 | 45.3 | 7 (13.0%) | 47 (87.0%) |
| Se + Vit E | 43.3 | 42.0 | 2 (3.7%) | 52 (96.3%) |
| Se + Zn | 48.6 | 53.9 | 9 (16.7%) | 45 (83.3%) |
| Vit E + Zn | 52.4 | 38.1 | 9 (16.7%) | 45 (83.3%) |
| Se + Vit E + Zn | 49.7 | 44.3 | 8 (14.8%) | 46 (85.2%) |
| SEM | 1.511 | 1.354 | ||
| F values | 0.803 | 1.438 | Pearson’s chi-square, χ2 = 7.156 | |
| df | 7,153 | 7,153 | 7 | |
| p values | 0.586 | 0.194 | 0.413 | |
| Dietary Treatments | Semen Volume (mL) | Sperm Concentration (n × 106 mL−1) | Sperm Motility (%) | SQF |
|---|---|---|---|---|
| Control | 0.15 e | 228.12 d | 42.12 d | 10.69 e |
| Se | 0.19 bc | 251.87 b | 46.27 b | 15.92 bc |
| Vit E | 0.18 c | 251.44 b | 45.45 b | 15.41 c |
| Zn | 0.17 d | 241.80 c | 44.38 c | 13.10 d |
| Se + Vit E | 0.20 b | 258.59 b | 47.31 b | 17.77 b |
| Se + Zn | 0.19 c | 251.32 b | 46.33 b | 15.50 c |
| Vit E + Zn | 0.18 c | 253.15 b | 45.84 b | 15.49 c |
| Se + Vit E + Zn | 0.21 a | 263.40 a | 48.93 a | 19.16 a |
| SEM | 0.001 | 0.415 | 0.099 | 0.099 |
| F values | 83.798 | 83.463 | 51.056 | 86.301 |
| p values | <0.001 | <0.001 | <0.001 | <0.001 |
| Dietary Treatments | Total Live | Normal | Macro-Cephalic | Bent-Neck | Mid-Piece Deformed | Immature | Other Deformities | Dead |
|---|---|---|---|---|---|---|---|---|
| Control | 87.2 c | 27.2 d | 27.3 a | 16.2 | 6.6 | 3.9 | 6.0 | 12.8 a |
| Se | 89.6 ab | 31.6 ab | 24.2 b | 16.6 | 6.9 | 4.0 | 6.3 | 10.4 bc |
| Vit E | 90.0 a | 31.0 bc | 25.2 ab | 16.4 | 6.9 | 4.2 | 6.3 | 10.0 bc |
| Zn | 88.5 bc | 29.2 cd | 25.2 ab | 16.5 | 7.4 | 4.0 | 6.2 | 11.5 ab |
| Se + Vit E | 90.1 a | 31.8 ab | 25.1 ab | 16.1 | 7.0 | 3.9 | 6.2 | 9.9 bc |
| Se + Zn | 89.3 ab | 30.4 bc | 25.2 ab | 16.7 | 7.1 | 3.9 | 6.0 | 10.7 bc |
| Vit E + Zn | 89.6 ab | 31.3 ab | 24.9 ab | 16.1 | 7.4 | 3.8 | 6.1 | 10.4 bc |
| Se + Vit E + Zn | 90.3 a | 32.9 a | 24.9 ab | 16.2 | 6.9 | 3.8 | 5.6 | 9.7 c |
| SEM | 0.103 | 0.127 | 0.169 | 0.145 | 0.121 | 0.060 | 0.079 | 0.105 |
| F values | 11.275 | 23.430 | 3.179 | 0.350 | 0.594 | 0.633 | 1.108 | 11.031 |
| p values | <0.001 | <0.001 | 0.003 | 0.930 | 0.797 | 0.729 | 0.358 | <0.001 |
| Dietary Treatments | SOD (U/mg Protein) | CAT (mmol/mg Protein) | GPx (mmol/mg Protein) | GST (mmol/mg Protein) | MDA (mmol/mg Protein) |
|---|---|---|---|---|---|
| Control | 2.88 ± 0.055 bc | 4.06 ± 0.051 bc | 2.75 ± 0.032 c | 2.59 ± 0.033 c | 2.14 ± 0.026 a |
| Se | 3.11 ± 0.054 ab | 4.38 ± 0.050 a | 2.91 ± 0.031 ab | 2.82 ± 0.032 ab | 1.95 ± 0.026 b |
| Vit E | 3.13 ± 0.053 ab | 4.16 ± 0.049 bc | 2.92 ± 0.031 ab | 2.84 ± 0.032 ab | 2.02 ± 0.025 ab |
| Zn | 2.97 ± 0.051 bc | 4.05 ± 0.047 cd | 2.79 ± 0.029 c | 2.73 ± 0.030 bc | 2.01 ± 0.024 ab |
| Se + Vit E | 3.10 ± 0.047 ab | 4.32 ± 0.044 ab | 2.93 ± 0.027 bc | 2.86 ± 0.028 ab | 1.95 ± 0.023 b |
| Se + Zn | 2.83 ± 0.052 c | 3.92 ± 0.048 d | 2.89 ± 0.030 bc | 2.82 ± 0.031 ab | 1.95 ± 0.025 b |
| Vit E + Zn | 3.20 ± 0.059 a | 4.13 ± 0.055 bc | 2.81 ± 0.034 bc | 2.80 ± 0.035 ab | 1.99 ± 0.028 b |
| Se + Vit E + Zn | 3.18 ± 0.053 a | 4.20 ± 0.049 bc | 3.01 ± 0.031 a | 2.91 ± 0.031 a | 1.93 ± 0.025 b |
| F values | 6.701 | 9.633 | 7.939 | 9.583 | 6.504 |
| p values | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taşkesen, H.O.; Baş, H.; Boz, M.A.; Sarıca, M.; Erensoy, K.; Dotas, V.; Symeon, G. The Effects of Varying Combinations of Dietary Selenium, Vitamin E, and Zinc Supplements on Semen Characteristics and Antioxidant Enzyme Activity of Spermatozoa in 1-Year-Old Native Turkish Ganders. Sustainability 2023, 15, 14083. https://doi.org/10.3390/su151914083
Taşkesen HO, Baş H, Boz MA, Sarıca M, Erensoy K, Dotas V, Symeon G. The Effects of Varying Combinations of Dietary Selenium, Vitamin E, and Zinc Supplements on Semen Characteristics and Antioxidant Enzyme Activity of Spermatozoa in 1-Year-Old Native Turkish Ganders. Sustainability. 2023; 15(19):14083. https://doi.org/10.3390/su151914083
Chicago/Turabian StyleTaşkesen, Hulüsi Ozan, Hatice Baş, Mehmet Akif Boz, Musa Sarıca, Kadir Erensoy, Vassilios Dotas, and George Symeon. 2023. "The Effects of Varying Combinations of Dietary Selenium, Vitamin E, and Zinc Supplements on Semen Characteristics and Antioxidant Enzyme Activity of Spermatozoa in 1-Year-Old Native Turkish Ganders" Sustainability 15, no. 19: 14083. https://doi.org/10.3390/su151914083
APA StyleTaşkesen, H. O., Baş, H., Boz, M. A., Sarıca, M., Erensoy, K., Dotas, V., & Symeon, G. (2023). The Effects of Varying Combinations of Dietary Selenium, Vitamin E, and Zinc Supplements on Semen Characteristics and Antioxidant Enzyme Activity of Spermatozoa in 1-Year-Old Native Turkish Ganders. Sustainability, 15(19), 14083. https://doi.org/10.3390/su151914083









