Sustainable Cosmetics: Valorisation of Kiwi (Actinidia deliciosa) By-Products by Their Incorporation into a Moisturising Cream
Abstract
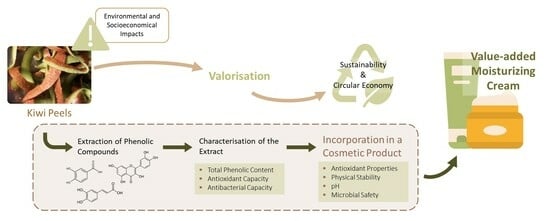
:1. Introduction
| Product | Objectives | Results | Ref. | |
|---|---|---|---|---|
| Food Industry | Flour | Analyse the microbiological and physicochemical properties as well as the BACs of flour made from the KP and bagasse. | Flour made with bagasse presented lower levels of BACs and antioxidant activity compared to flour made with KP. | [23] |
| Chicken meat emulsion | Obtain antioxidant-rich extracts from KP and study its antioxidant efficacy in vivo in chicken meat emulsions. | KP extract (2.5%) improved the sensory and physicochemical properties of meat model systems and extended their shelf-life. | [24] | |
| Food packaging films | Reutilise KP in the preparation of multifunctional sodium alginate-based food packaging films. | KP extracts were able to bio-reduce silver particles, exhibiting strong antibacterial activity and biocompatibility. They also enhanced the antioxidant and antibacterial activities of sodium alginate films. | [25] | |
| Pharmaceutical Industry | ND | Obtain a phenolic-rich extract from KP and explore its capability to modulate inflammatory responses. | The reported findings demonstrated a strong and broad anti-inflammatory profile of the KP extract, which makes it a promising natural ingredient for pharmaceutical formulations, with the potential for prevention and treatment. | [26] |
| Skincare Industry | ND | Summarise the possible cosmetic and medical skincare applications of the different BACs from kiwi by-products, exploring their potential to contribute as active ingredients based on the biological activities reported. | The BACs present in the kiwi by-products contributed to good skin appearance and health, and the addition of these active compounds to the final cosmetic and pharmaceutical products may provide skin-beneficial effects such as anti-ageing, photoprotection, and anti-inflammatory effects, also acting as depigmenting and emollients. | [22] |
2. Materials and Methods
2.1. Samples and Reagents
2.2. Phenolic Compounds Extraction from Kiwi Peels
2.3. Extract Characterisation
2.3.1. Total Phenolic Content
2.3.2. Antioxidant Properties
2.3.3. Antibacterial Properties
2.3.4. Phenolic Composition
2.4. Moisturising Creams Production and Characterisation
2.4.1. Formulations Preparation
2.4.2. Antioxidant Properties
2.4.3. Microbial Contaminations
2.4.4. Formulations Stability
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of the Kiwi Peel Extract
3.2. Characterisation of the Moisturising Creams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Z.; You, C.; Ma, Y.; Zhang, Y.-H.P.J. In vitro synthetic enzymatic biosystems at the interface of the food-energy-water nexus: A conceptual framework and recent advances. Process Biochem. 2018, 74, 43–49. [Google Scholar] [CrossRef]
- Tufail, T.; Ain, H.B.U.; Saeed, F.; Nasir, M.; Basharat, S.; Mahwish; Rusu, A.V.; Hussain, M.; Rocha, J.M.; Trif, M.; et al. A Retrospective on the Innovative Sustainable Valorization of Cereal Bran in the Context of Circular Bioeconomy Innovations. Sustainability 2022, 14, 14597. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World. Repurposing Food and Agricultural Policies to Make Healthy Diets more Affordable; UNICEF: Rome, Italy, 2022; Available online: https://www.fao.org/documents/card/en/c/cc0639en (accessed on 27 June 2023).
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod. Process. 2020, 122, 136–144. [Google Scholar] [CrossRef]
- Ferguson, A.R. New Temperate Fruits: Actinidia chinensis and Actinidia deliciosa. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 342–347. [Google Scholar]
- Morton, J. Kiwifruit. In Fruits of Warm Climates; Morton, J.F., Ed.; Echo Point Books & Media: Miami, FL, USA, 1987; pp. 293–300. [Google Scholar]
- Ward, C.; Courtney, D. Kiwifruit: Taking Its Place in the Global Fruit Bowl. In Advances in Food and Nutrition Research; Boland, M., Moughan, P.J., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 68, pp. 1–14. [Google Scholar] [CrossRef]
- IndexBox. World—Kiwi Fruits—Market Analysis, Forecast, Size, Trends and Insights. 2023. Available online: https://www.indexbox.io/store/world-kiwi-fruit-market-report-analysis-and-forecast-to-2020/ (accessed on 5 July 2023).
- Kiwi Fruit Production in Portugal. Available online: https://knoema.com/data/portugal+agriculture-indicators-production+kiwi-fruit (accessed on 6 April 2023).
- Gaonkar, S.K.; Furtado, I.J. Valorization of low-cost agro-wastes residues for the maximum production of protease and lipase haloextremozymes by Haloferax lucentensis GUBF-2 MG076078. Process Biochem. 2021, 101, 72–88. [Google Scholar] [CrossRef]
- Zhou, W.-W.; Chevalot, I. Frontiers in process biochemistry and biotechnology. Process Biochem. 2023, 130, 566–568. [Google Scholar] [CrossRef]
- Malenica, D.; Kass, M.; Bhat, R. Sustainable Management and Valorization of Agri-Food Industrial Wastes and By-Products as Animal Feed: For Ruminants, Non-Ruminants and as Poultry Feed. Sustainability 2023, 15, 117. [Google Scholar] [CrossRef]
- Remorini, D.; Tavarini, S.; Degl’Innocenti, E.; Loreti, F.; Massai, R.; Guidi, L. Effect of rootstocks and harvesting time on the nutritional quality of peel and flesh of peach fruits. Food Chem. 2008, 110, 361–367. [Google Scholar] [CrossRef]
- Sanz, V.; López-Hortas, L.; Torres, M.D.; Domínguez, H. Trends in kiwifruit and byproducts valorization. Trends Food Sci. Technol. 2021, 107, 401–414. [Google Scholar] [CrossRef]
- Alim, A.; Li, T.; Nisar, T.; Ren, D.; Zhai, X.; Pang, Y.; Yang, X. Antioxidant, antimicrobial, and antiproliferative activity-based comparative study of peel and flesh polyphenols from Actinidia chinensis. Food Nutr. Res. 2019, 63, 1577. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive compounds and in vitro antioxidant activities of peel, flesh and seed powder of kiwi fruit. Int. J. Food Sci. Technol. 2018, 53, 2239–2245. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, N.; Shu, C.; Cheng, S.; Sun, X.; Liu, C.; Xin, G.; Li, B.; Tian, J. Phenolics Profile and Antioxidant Activity Analysis of Kiwi Berry (Actinidia arguta) Flesh and Peel Extracts from Four Regions in China. Front. Plant Sci. 2021, 12, 4148–4155. [Google Scholar] [CrossRef] [PubMed]
- Sun-Waterhouse, D.; Wen, I.; Wibisono, R.; Melton, L.D.; Wadhwa, S. Evaluation of the extraction efficiency for polyphenol extracts from by-products of green kiwifruit juicing. Int. J. Food Sci. Technol. 2009, 44, 2644–2652. [Google Scholar] [CrossRef]
- Cazarin, C.B.B.; Bicas, J.L.; Pastore, G.M.; Marostica Junior, M.R. Introduction. In Bioactive Food Components Activity in Mechanistic Approach; Cazarin, C.B.B., Bicas, J.L., Pastore, G.M., Marostica Junior, M.R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–3. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Wang, Y.; Shi, T.; Zhang, L.; Chen, Y.; Xie, M. Comparison of (poly)phenolic compounds and antioxidant properties of pomace extracts from kiwi and grape juice. Food Chem. 2019, 271, 425–432. [Google Scholar] [CrossRef]
- Silva, A.M.; Costa, P.C.; Delerue-Matos, C.; Latocha, P.; Rodrigues, F. Extraordinary composition of Actinidia arguta by-products as skin ingredients: A new challenge for cosmetic and medical skincare industries. Trends Food Sci. Technol. 2021, 116, 842–853. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Stefanello, F.S.; Huerta, K.d.M.; Monteiro, S.S.; da Rosa, C.S.; Terra, N.N. Characterization of physiochemical and microbiological properties, and bioactive compounds, of flour made from the skin and bagasse of kiwi fruit (Actinidia deliciosa). Food Chem. 2016, 199, 471–478. [Google Scholar] [CrossRef]
- Singh, B.; Wagh, R.V.; Chatli, M.K.; Mehta, N. Optimization of ethanol-assisted extraction of kiwi peel and antioxidant activity in chicken emulsion. Haryana Vet. 2021, 60, 203–207. [Google Scholar]
- Sun, X.; Zhang, H.; Wang, J.; Dong, M.; Jia, P.; Bu, T.; Wang, Q.; Wang, L. Sodium alginate-based nanocomposite films with strong antioxidant and antibacterial properties enhanced by polyphenol-rich kiwi peel extracts bio-reduced silver nanoparticles. Food Packag. Shelf Life 2021, 29, 100741. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Pannucci, E.; Bernini, R.; Campo, M.; Romani, A.; Santi, L.; Velotti, F. In vitro studies on anti-inflammatory activities of kiwifruit peel extract in human THP-1 monocytes. J. Ethnopharmacol. 2019, 233, 41–46. [Google Scholar] [CrossRef]
- Sun, X.; Jia, P.; Bu, T.; Zhang, H.; Dong, M.; Wang, J.; Wang, X.; Zhe, T.; Liu, Y.; Wang, L. Conversional fluorescent kiwi peel phenolic extracts: Sensing of Hg(2+) and Cu(2+), imaging of HeLa cells and their antioxidant activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 244, 118857. [Google Scholar] [CrossRef]
- Bringheli, I.; Brindisi, G.; Morelli, R.; Marchetti, L.; Cela, L.; Gravina, A.; Pastore, F.; Semeraro, A.; Cinicola, B.; Capponi, M.; et al. Kiwifruit’s Allergy in Children: What Do We Know? Nutrients 2023, 15, 3030. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Gomes, S.M.; Santos, L. A Novel Approach in Skin Care: By-Product Extracts as Natural UV Filters and an Alternative to Synthetic Ones. Molecules 2023, 28, 2037. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.M.; Leitão, A.; Alves, A.; Santos, L. Incorporation of Moringa oleifera Leaf Extract in Yoghurts to Mitigate Children’s Malnutrition in Developing Countries. Molecules 2023, 28, 2526. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. A Potential Valorization Strategy of Wine Industry by-Products and Their Application in Cosmetics-Case Study: Grape Pomace and Grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzaman, N.; Yusop, S.M. Determination of stability of cosmetic formulations incorporated with water-soluble elastin isolated from poultry. J. King Saud Univ. Sci. 2021, 33, 101519. [Google Scholar] [CrossRef]
- Kim, S.H.; Jung, E.Y.; Kang, D.H.; Chang, U.J.; Hong, Y.-H.; Suh, H.J. Physical stability, antioxidative properties, and photoprotective effects of a functionalized formulation containing black garlic extract. J. Photochem. Photobiol. B 2012, 117, 104–110. [Google Scholar] [CrossRef]





| Ingredients | Function | % (w/w) | ||||
|---|---|---|---|---|---|---|
| NC | PC | KPC | Mix | |||
| Aqueous Phase | Water | Solvent | 73.60 | 73.10 | 73.10 | 73.10 |
| Glycerine | Humectant | 7.60 | 7.60 | 7.60 | 7.60 | |
| Xanthan gum | Thickener | 0.60 | 0.60 | 0.60 | 0.60 | |
| Oil Phase | Coconut oil | Emollient | 7.60 | 7.60 | 7.60 | 7.60 |
| Sweet almond oil | Emollient | 6.80 | 6.80 | 6.80 | 6.80 | |
| Betaine | Emulsifier | 2.70 | 2.70 | 2.70 | 2.70 | |
| Soy lecithin | Emulsifier | 1.10 | 1.10 | 1.10 | 1.10 | |
| Additives | BHT | Synthetic antioxidant | - | 0.50 | - | 0.25 |
| KP extract | Natural antioxidant | - | - | 0.50 | 0.25 |
| Antioxidant Activity | Antibacterial Activity | ||||
|---|---|---|---|---|---|
| IC50 (mg/L) | dhalo (mm) | ||||
| DPPH | ABTS | SA | E. coli | S. aureus | S. epidermidis |
| 243.94 ± 6.79 | 58.29 ± 4.00 | ND | ND | 8.0 ± 0.8 | ND |
| Standard | Concentration |
|---|---|
| (mgstandard/gextract) | |
| Caffeic acid | 0.15 |
| Catechin | 0.36 |
| Chlorogenic acid | 0.40 |
| Epicatechin | 0.32 |
| Formulation | pH | |
|---|---|---|
| t0 | t2 | |
| NC | 6.04 ± 0.03 c | 5.96 ± 0.02 c |
| PC | 6.05 ± 0.05 c,B | 5.93 ± 0.08 c,A |
| KPC | 4.11 ± 0.05 a,B | 4.02 ± 0.02 a,A |
| Mix | 4.46 ± 0.01 b | 4.53 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, S.M.; Miranda, R.; Santos, L. Sustainable Cosmetics: Valorisation of Kiwi (Actinidia deliciosa) By-Products by Their Incorporation into a Moisturising Cream. Sustainability 2023, 15, 14059. https://doi.org/10.3390/su151914059
Gomes SM, Miranda R, Santos L. Sustainable Cosmetics: Valorisation of Kiwi (Actinidia deliciosa) By-Products by Their Incorporation into a Moisturising Cream. Sustainability. 2023; 15(19):14059. https://doi.org/10.3390/su151914059
Chicago/Turabian StyleGomes, Sandra M., Rita Miranda, and Lúcia Santos. 2023. "Sustainable Cosmetics: Valorisation of Kiwi (Actinidia deliciosa) By-Products by Their Incorporation into a Moisturising Cream" Sustainability 15, no. 19: 14059. https://doi.org/10.3390/su151914059
APA StyleGomes, S. M., Miranda, R., & Santos, L. (2023). Sustainable Cosmetics: Valorisation of Kiwi (Actinidia deliciosa) By-Products by Their Incorporation into a Moisturising Cream. Sustainability, 15(19), 14059. https://doi.org/10.3390/su151914059